Abstract
Objective
We explored the dietary effects of replacing normal dietary staple foods with supplementary nutritional protein powder, dietary fiber, and fish oil on several metabolic parameters. We examined weight loss, glucose and lipid metabolism, and intestinal flora in obese individuals when compared with individuals on a reduced staple food low carbohydrate diet.
Methods
From inclusion and exclusion criteria, 99 participants (28 kg/m2 ≤ body mass index (BMI) ≤ 35 kg/m2) were recruited and randomly assigned to control and intervention 1 and 2 groups. Physical examinations and biochemical indices were performed/gathered before the intervention and at 4 and 13 weeks post intervention. After 13 weeks, feces was collected and 16s rDNA sequenced.
Results
After 13 weeks, when compared with controls, body weight, BMI, waist circumference, hip circumference, systolic blood pressure, and diastolic blood pressure values in intervention group 1 were significantly reduced. In intervention group 2, body weight, BMI, waist circumference, and hip circumference were significantly reduced. Triglyceride (TG) levels in both intervention groups were significantly reduced. Fasting blood glucose, glycosylated hemoglobin, glycosylated albumin, total cholesterol, and apolipoprotein B levels in intervention group 1 were decreased, while high density lipoprotein cholesterol (HDL-c) decreased slightly. Glycosylated albumin, TG, and total cholesterol levels in intervention group 2 decreased, while HDL-c decreased slightly, High sensitive C-reactive protein, MPO, Ox-LDL, LEP, TGF-β1, IL-6, GPLD1, pro NT, GPC-4, and LPS levels in both intervention groups were lower when compared with controls. Adiponectin (ADPN) levels in intervention groups were higher when compared with controls. Tumor necrosis factor-α (TNF-α) levels in intervention group 1 were lower when compared with controls. There is no obvious difference in α diversity and β diversity between intestinal flora of 3 groups. Among the first 10 species of Phylum, only the control group and the intervention group 2 had significantly higher Patescibacteria than the intervention group 1. Among the first 10 species of Genus, only the number of Agathobacter in intervention group 2 was significantly higher than that in control group and intervention group 1.
Conclusions
We showed that an LCD, where nutritional protein powder replaced some staple foods and dietary fiber and fish oil were simultaneously supplemented, significantly reduced weight and improved carbohydrate and lipid metabolism in obese individuals when compared with an LCD which reduced staple food intake.
Similar content being viewed by others
Introduction
Obesity is a rapidly growing public health problem in both developed and developing countries [1]. Obesity increases the morbidity risks of hypertension, cardiovascular disease, diabetes, high cholesterol, cancer, respiratory disease, and musculoskeletal disease, with mortality risks gradually increasing once the overweight threshold is exceeded [2, 3].
A low carbohydrate diet (LCD) limits carbohydrates and replaces them with fat and/or protein. Both protein and fat cause satiety and reduce blood glucose fluctuations that lead to hunger. Molecularly, this occurs via insulin-mediated signal pathways that send appetite signals to the brain [4]. When compared with low-fat diets, individuals on LCD only need to reduce carbohydrate intake, fat and protein are consumed normally which improves LCD compliance,while those on low-fat diets limit calories [4].
Crowd compliance is critical for weight loss. One method improving compliance is to use partially controlled conventional foods or meal substitutes that provide predetermined food and calorie quantities. Partial diet control appears to improve dietary compliance by reducing participant tendencies to underestimate calorie intake [5, 6]. In particular, meal substitutes reduce the complexity associated with meal-planning and food preparation, reduce cognitive needs and decision-making, and reduce the implications of overeating [7]. Additionally, substitute meals support adherence to calorie goals via sensory-specific satiety [5].
Research now shows that increasing soybean protein intake reduces body fat rates, total serum cholesterol, and low-density lipoprotein cholesterol (LDL-c) levels [8]. Dietary fiber regulates blood lipids and sugars via physical effects, immune regulation, anti-inflammatory effects, and prebiotic effects so as to prevent and treat obesity. Fish oil combined with weight-reducing diets can significantly reduce waist circumference and waist hip ratios [9]. Cereals are rich in carbohydrates, with Chinese populations mainly eating rice and flour (wheat). Therefore, in obese individuals, we explored the advantages/disadvantages of replacing some staple foods with nutritional protein powders, dietary fiber, and fish oil when compared with an LCD where staple food intake was reduced. We hypothesized this scientific and healthy nutritional intervention diet could help obese individuals reduce weight.
Methods
Participants
Inclusion criteria
(1) Males or females aged between 18 and 65 years old with 28 kg/m2 ≤ BMI ≤ 35 kg/m2. (2) No serious liver, kidney, digestive tract, cardiovascular, cerebrovascular, or mental diseases. (3) No drug/dietary supplements lowering blood lipids, blood glucose, or weight levels within the previous 3 months: participants agreed to not use supplements/drugs during the intervention. (4) Dietary control/guidance acceptance by participants. (5) After information was provided, participants volunteered and signed consent forms.
Exclusion criteria
(1) Participants aged < 18 or > 65, BMI < 28 kg/m2 or BMI > 35 kg/m2. (2) Pregnant or lactating females. (3) Participants with serious diseases such as liver, kidney, digestive tract, cardiovascular, cerebrovascular, or psychosis. (4) In the previous 3 months, participants had taken drugs related to lowering blood lipids, blood glucose, or weight levels, and also dietary supplements potentially impacting study outcomes. (5) Signs of alcoholism. (6) Participants with special eating habits: vegetarians, ketogenic eaters. (7) Participants who could not follow study requirements.
Groups and interventions
According to the formula N = 2 (Z1−α/2+Zβ) 2σ2/d2 and the literature, each group had 28 individuals, which was based on a 10% follow-up rate loss. Using aforementioned criteria, 99 participants were recruited from Nanjing and Beijing. Excel spreadsheets (Microsoft) were used to generate random allocation sequences for groups. Participants were randomly divided into control, intervention 1 and intervention 2 groups, with 33 participants/group. The intervention lasted 13 weeks. Interventions are outlined (Table 1).
Ethics approval
The study was approved by the Ethics Committee of China Clinical Registration Trials (ethics review number: ChiECRCT20200292 and clinical registration number: ChiCTR2100050070). Participants signed consent forms before study commencement.
Test indices
Before inclusion and after 4 and 13 weeks of the intervention, participants underwent physical examinations.
Before the intervention
(1) Physical examination: height, weight, waist circumference, hip circumference, and blood pressure. (2) Blood indicators: blood glucose, blood lipids, liver function, kidney function, and inflammation risk indicators. (3) DEXA analysis to assess body fat.
Four weeks into the intervention
1). Physical examination as described. 2) Blood indicators as described.
Thirteen weeks into the intervention
(1) Physical examination as described; (2) Blood indicators as described plus cell adipose indicators. (3) DEXA analysis as described; (4) Feces collection for intestinal flora analyses.
Statistical analyses
We used SPSS 23.0 software to process and analyze data. If data conformed to a normal distribution, they were expressed as the mean ± standard deviation (X ± S). If data had a skewed distribution, they were expressed as the median ± interquartile interval (Me ± IQR). For data conforming to normality and variance homogeneity, single factor analysis of variance was used for comparisons between groups. For data not conforming to normality or variance homogeneity, rank sum tests were used. A P < 0.05 value was considered statistically significant.
Results
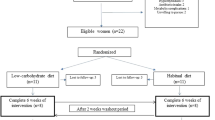
Flow chart
A study flow chart is shown (Fig. 1).
Participant compliance and loss to follow-up
In this study, 99 participants who met inclusion and exclusion criteria were randomized into three study groups, with 33/group. During follow-up, four participants in the control group failed to participate due to personal reasons; loss rate = 12.12%. Four participants in intervention group 1 were lost to follow-up due to personal reasons; follow-up rate = 12.12%. Two participants in intervention group 2 were lost to follow-up due to personal reasons, one to diarrhea and two to gout attack; loss rate = 15.15%. No statistical differences in follow-up loss rates were recorded between groups (Chi square test and P > 0.05).
Participant characteristics
Data analyses showed that participant traits such as age, gender, nationality, labor intensity, education level, marital status, smoking, drinking, and height were balanced and comparable, with no statistical differences between groups (Table 2).
Participant dietary status before and after intervention
Participant diets across all groups are shown (Table 3). At baseline, the daily intake of energy, carbohydrate, protein, and fat across groups was similar, with no statistical differences. During the initial 1–4 week intervention, when compared with controls, the daily carbohydrate intake in intervention groups was significantly lower when compared with controls. The daily protein intake of intervention group 1 was higher when compared with controls, but no statistical differences were recorded. The daily protein intake of intervention group 2 was significantly higher when compared with controls. During the 5–13 week intervention, when compared with controls, the daily carbohydrate intake of intervention groups was significantly lower when compared with controls. Daily protein intake was significantly higher when compared with controls.
Participant changes in anthropometric indicators before and after intervention
Changes in anthropometric indicators across groups at baseline, weeks 4 and 13 of the intervention, and also 4- and 13-week variations are shown (Table 4). At baseline, weight, BMI, waist circumference, hip circumference, systolic blood pressure, and diastolic blood pressure across groups were similar, with no statistical differences.
After week 13, waist circumference, hip circumference and systolic blood pressure in controls had decreased, while weight, BMI, and diastolic blood pressure increased slightly. When compared with controls, body weight, BMI, waist circumference, hip circumference, systolic blood pressure, and diastolic blood pressure values in intervention group 1 were significantly reduced. When compared with controls, weight, BMI, waist circumference, and hip circumference values in intervention group 2 were significantly reduced. When compared with controls, systolic and diastolic blood pressure in intervention group 2 had decreased, but no statistical differences were recorded.
Participant fat rate changes before and after intervention
Fat rate changes across groups at baseline, week 13, and 13-week variations are shown (Table 5). At baseline, whole body, head, left upper limb, right upper limb, trunk, left lower limb, and right lower limb fat rates across groups were similar, with no significant differences. After week 13, when compared with controls, body, head, left upper limb, trunk, and right lower limb fat rates in intervention group 1 decreased, but no statistical differences were recorded. When compared with controls, total body, head, left upper, trunk, left lower limb, and right lower limb fat rates in intervention group 2 was decreased, but no statistical differences were recorded. During the intervention period, the lean tissue content of the three groups of subjects changed slightly, and there was no significant decrease.
Participant blood glucose and blood lipid index changes before and after intervention
Changes in blood glucose and blood lipid indices across groups at baseline and weeks 4 and 13, and also 4- and 13-week variations are shown (Table 6). At baseline, fasting blood glucose, glycosylated hemoglobin, glycosylated albumin, fasting insulin, triglyceride (TG), total cholesterol, high-density lipoprotein cholesterol (HDL-c), LDL-c, apolipoprotein A1, and apolipoprotein B levels across groups were similar, with no statistical differences.After week 13, fasting blood glucose, glycosylated albumin, fasting insulin, total cholesterol, HDL-c, and LDL-c levels in controls decreased, while HbA1c was unchanged. TG, apolipoprotein A1, and apolipoprotein B levels increased slightly. Furthermore, fasting blood glucose, glycosylated hemoglobin, glycosylated albumin, fasting insulin, TG, total cholesterol, HDL-c, LDL-c, and apolipoprotein B levels in intervention group 1 decreased, while apolipoprotein A1 increased slightly.
Fasting blood glucose, glycosylated albumin, fasting insulin, TG, total cholesterol, and HDL-c levels were decreased in intervention group 2, while HbA1c remained unchanged. LDL-c, apolipoprotein A1, and apolipoprotein B levels increased slightly. Among variables, when compared with controls, TGs in both groups were significantly reduced. When compared with controls, fasting blood glucose, glycosylated hemoglobin, glycosylated albumin, total cholesterol, and apolipoprotein B levels in intervention group 1 decreased, but no statistical differences were observed. When compared with controls, HDL-c in intervention group 1 decreased slightly, with no statistical differences.When compared with controls, glycosylated albumin, TG, and total cholesterol levels in intervention group 2 decreased, but no statistical difference was observed. When compared with controls, HDL-c in intervention group 2 decreased slightly, with no statistical differences.
Participant liver and kidney function indices before and after intervention
Changes in liver and kidney function indices across groups at baseline and weeks 4 and 13, and also 4- and 13-week variations are shown (Table 7). At baseline, total bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, lactate dehydrogenase, urea, and creatinine levels across groups were similar, with no statistical differences. After week 13, when compared with controls, total bilirubin in the intervention group 1 had increased significantly but remained in the normal range with no clinical significance. Importantly, liver and kidney function indices showed no significant changes with no statistical differences during the intervention.
Participant inflammatory risk, adipocytokines, and other indicators before and after intervention
Inflammation risk and adipocyte factor indicators across groups are shown (Table 8). After week 13, high sensitive C-reactive protein (CRP), myeloperoxidase (MPO), oxidized LDL (Ox-LDL), leptin (LEP), transforming growth factor-β1 (TGF-β1), interleukin-6 (IL-6), glycosylphosphatidylinositol specific phospholipase D1 (GPLD1), preneurotensin (pro NT), recombinant glypican 4 (GPC-4), and lipopolysaccharide (LPS) in both intervention groups were lower when compared with controls, but no statistical differences were recorded. Adiponectin (ADPN) levels in both groups were higher when compared with controls, with no statistical differences. Tumor necrosis factor-α (TNF- α) in intervention group 1 was lower when compared with controls, with no statistical differences.
Fecal intestinal flora analyses
Differences between groups
Phyla
As shown (Fig. 2), the top 10 phyla in intestinal flora across groups were Firmicutes, Bacteroidota, Actinobacteria, Proteobacteria, Verrucomicrobiota, Fusobacteriota, Desulfobacterota, Cyanobacteria, Patescibacteria, and Campylobacterota, of which, Firmicutes, Bacteroidota, and Actinobacia were dominant. As shown (Fig. 3), among the top 10 bacteria, except that the Patescibacteria in the control group and the intervention group 2 were significantly higher than that in the intervention group 1 (P < 0.05), the other nine kinds of bacteria had no statistical difference among the three groups. Thus, on the whole, no significant phyla differences were observed among groups.
Genera
As indicated (Fig. 4), the top 10 genera across groups were Bacteroides, Faecalibacterium, Prevotella_9, Bifidobacterium, Streptococcus, Escherichia-Shigella, Megamonas, Subdoligranulum, Agathobacter, and Monoglobus, of which, Bacteroides, Faecalibacterium, and Prevotella_9 were dominant. As shown (Fig. 5), among the top 10 bacterial species, except that the Agathobacter in the intervention group 2 was significantly higher than that in the control group and the intervention group 1 (P < 0.05), there was no statistical difference among the other nine species of bacteria in the three groups. Therefore, on the whole, no significant differences in genera were identified among groups.
Discussion
After a 13 week dietary intervention, when compared with controls, body weight, BMI, waist circumference, hip circumference, systolic blood pressure, and diastolic blood pressure values in intervention group 1 were statistically significantly reduced. Body weight, BMI, waist circumference, and hip circumference in intervention group 2 were statistically significantly reduced. TGs in both groups were statistically significantly reduced.
Typically, LCDs have a carbohydrate energy supply ratio ≤ 40%, fat energy supply ratio ≥ 30%, relatively increased protein levels, and limited or unlimited total energy intake [10, 11]. Individuals on LCDs tend to have faster weight loss [12]. Sun et al. [13] examined the combination of energy unlimited LCD and exercise to intervene for 4 weeks in overweight Chinese women and found that participant weight, waist circumference, and hip circumference were significantly reduced.
Food substitutes can generate sustainable weight-loss effects by reducing food types and controlling food portions, thereby improving obesity-related disease risk factors and minimizing lean weight loss so as to maintain strength, physical function, and weight over extended periods [14]. System evaluations also showed that weight-loss effects, by substituting foods, improved within 1 year, and may be used as effective obesity management strategies for community and health care institutions [15]. Also, dietary substitution was effective for managing obesity and type 2 diabetes [16,17,18]; the approach improved fat quality, blood pressure, glycosylated hemoglobin, insulin, and other indicators [19, 20].
A recent systematic review and meta-analysis reported that in obese patients (BMI = 36–43 kg/m²), weight loss was 8.9–15.0 kg after very low (< 800 kcal/day) or low calorie (> 800 kcal/day) liquid-meal substitutes were used [21]. We hypothesize that weight-loss differences in this meta-analysis were possibly due to differences in daily calorie intake. Studies have shown that moderate and sustained weight loss reduces the risk of long-term adverse outcomes when compared with rapid weight loss [22]. Therefore, in our study, we selected a more modest daily energy intake target (800–1500 kcal/day), which increased participant compliance and reduced follow-up losses.
Research has also shown that when compared with traditional energy-limiting diets, soybean protein-based energy limiting diets significantly reduce serum TC and LDL-C levels and body fat content in overweight adults [8]. A soybean protein based high-protein diet is more acceptable and improves weight loss, body composition, and cardiac health indicators [23]. In our study, we explored differences between an LCD (reduced staple food intake) with an LCD (some staple foods replaced with nutritional protein powder (soybean protein)) while supplementing with dietary fiber and fish oil. After week 13, when compared with controls, both intervention groups showed significant weight loss and reduced blood pressure and blood lipids, consistent with Clinton et al. [24]. Thus, our LCD (staple food replacement with nutritional protein powder and dietary fiber and fish oil) had greater effects on weight loss, blood pressure, and blood lipid reduction, amongst others.
Obesity is a complex multifactorial disease and is defined as “abnormal or excessive fat accumulation in adipose tissue” [25, 26]. Adipose tissue is important for energy storage and endocrine and immune regulation. Many cytokines, hormones, extracellular matrix proteins, and other bioactive factors are synthesized and released by adipose tissue and are known as adipokines [27]. To a large extent, adipose tissue dysfunction in obese patients is manifested by imbalanced proinflammatory and anti-inflammatory adipose factor expression [28], which initiates chronic inflammation in adipose tissue and causes insulin resistance and multiple metabolic disorders [27]. Research now shows that TNF-α and IL-6 are fat factors which induce insulin resistance [29, 30]. ADPN is also a fat factor which improves insulin resistance [31]. Research also indicates that adipose tissue is a target tissue of ADPN, which increases insulin sensitivity and resists macrophage infiltration and inflammatory factor expression caused by obesity [32]. ADPN also inhibits TNF-α in many cell types and exerts anti-inflammatory effects [33]. GPLD1 is a phospholipase which cleaves GPI [34] and may cleave GPC4 [35]. Serum GPC4 levels are speculated to be positively correlated with BMI and body fat levels [36, 37]. Our study data are generally consistent with the literature.
Current evidence also suggests close relationships between intestinal flora and obesity, with intestinal microorganisms having important roles in food digestion and metabolic regulation [38, 39]. Intestinal flora metabolic activity impacts nutrient absorption, which in turn affects energy balance during energy storage and consumption by promoting the energy metabolism of dietary components [40, 41]. We showed that intestinal flora levels across all study groups exhibited α- and β-diversity, but no distinct diversity differences were recorded. In the top 10 bacterial species, except for Patescibacteria in controls and intervention group 2, which were significantly higher than intervention group 1 (P < 0.05), the remaining nine bacterial species showed no statistical differences. Among the first 10 species of bacteria, except that the Agathobacter in the intervention group 2 was significantly higher than that in the control group and the intervention group 1 (P < 0.05), no statistical differences were identified in the remaining nine bacterial species across groups. Dietary fiber are edible carbohydrate polymers comprising three or more monomeric units are resistant to endogenous digestive enzymes and are neither hydrolyzed nor absorbed in the small intestine [42]. Firmicutes and Actinomycetes are the main dietary fiber responders [43]. In our intervention groups, we replaced some staple foods with nutritional protein powder (soybean protein) and dietary fiber and fish oil at the same time. However, our small sample size, large individual differences, and short intervention times may have contributed to many non-significant differences in our data.
After 4 weeks, weight loss and metabolic improvement effects in intervention group 2 were much better when compared with intervention group 1. But after week 13, intervention group 1 effects were better when compared with intervention group 2. Nutritional protein powder, dietary fiber, and fish oil doses in intervention group 1 in the first 4 weeks were low, but doubled in the next 9 weeks. The total intake in intervention group 2 in 13 weeks was the same as intervention group 1 in the later 9 weeks. Thus, a dose-doubling intervention for intervention group 1 improved participant compliance and weight loss effects.
Our study had some limitations. First, the sample size was small. Second, we did not conduct regular follow-up checks after the study to assess diet sustainability. Finally, as the weight-loss intervention period spanned autumn/winter, the weather was getting colder, thus if a spring/ summer study was conducted, weight-loss effects may have improved.
Conclusions
We showed that an LCD, where nutritional protein powder was used to replace some staple foods, and dietary fiber and fish oils were simultaneously supplemented, significantly reduced weight and improved carbohydrate and lipid metabolism in obese individuals was observed when compared with an LCD that reduced staple food intake. Our study provides a scientific and healthy nutritional intervention for obese individuals who wish to lose weight.
Data Availability
All data generated or analyzed during this study are included in this published article.
Change history
10 May 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12986-023-00747-y
References
SUN S, HE J, SHEN B et al. Obesity as a “self-regulated epidemic”: coverage of obesity in Chinese newspapers [J]. Eating and Weight Disorders-Studies on Anorexia Bulimia and Obesity, 2021, 26(2):569–84.
JENSEN MD, RYAN D H, APOVIAN C M, et al. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the obesity society [J]. J Am Coll Cardiol. 2014;63(25):2985–3025.
TCHANG B G, SAUNDERS K H. IGEL L I. Best Practices in the management of overweight and obesity [J]. Med Clin North Am. 2021;105(1):149–74.
FEINMAN RD, POGOZELSKI W K, ASTRUP A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base [J]. Nutrition. 2015;31(1):1–13.
CHAO A M, QUIGLEY K M. WADDEN T A. Dietary interventions for obesity: clinical and mechanistic findings [J].Journal of Clinical Investigation, 2021, 131(1)
WADDEN T A, BUTRYN M L, BYRNE K J. Efficacy of lifestyle modification for long-term weight control [J]. Obes Res. 2004;12:151S–62S.
WING R R. JEFFERY R W. Food provision as a strategy to promote weight loss [J]. Obes Res. 2001;9:271S–5S.
LIAO F-H, SHIEH M-J, YANG S-C, et al. Effectiveness of a soy-based compared with a traditional low-calorie diet on weight loss and lipid levels in overweight adults [J]. Nutrition. 2007;23(7–8):551–6.
DU S, JIN J, FANG W, et al. Does Fish Oil have an anti-obesity effect in Overweight/Obese adults? A Meta-analysis of Randomized Controlled trials [J]. PLoS ONE. 2015;10(11):e0142652.
BRAY GA, HEISEL W E, AFSHIN A, et al. The science of obesity management: an endocrine Society Scientific Statement [J]. Endocr Rev. 2018;39(2):79–132.
VAN ZUUREN E J, FEDOROWICZ Z, KUIJPERS T, et al. Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: a systematic review including GRADE assessments [J]. Am J Clin Nutr. 2018;108(2):300–31.
JOHNSTON B C, KANTERS S, BANDAYREL K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis [J]. JAMA. 2014;312(9):923–33.
SUN S, KONG Z, SHI Q et al. Non-Energy-Restricted Low-Carbohydrate Diet Combined with Exercise Intervention Improved Cardiometabolic Health in Overweight Chinese Females [J].Nutrients, 2019, 11(12)
COLEMAN C D, KIEL J R, MITOLA A H et al. Effectiveness of a Medifast meal replacement program on weight, body composition and cardiometabolic risk factors in overweight and obese adults: a multicenter systematic retrospective chart review study [J]. Nutrition journal, 2015, 14(77.
15] ASTBURYNM, HARTMANN-BOYCE J PIERNASC, et al. Obes reviews: official J Int Association Study Obes. 2019;20(4):569–87. A systematic review and meta-analysis of the effectiveness of meal replacements for weight loss [J].
LEANME, LESLIE W S, BARNES A C, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial [J]. Lancet (London England). 2018;391(10120):541–51.
STEVEN S, HOLLINGSWORTH K G, AL-MRABEH A, et al. Very low-calorie Diet and 6 months of Weight Stability in Type 2 diabetes: pathophysiological changes in responders and nonresponders [J]. Diabetes Care. 2016;39(5):808–15.
KEMPF K, SCHLOOT N C, GäRTNER B et al. Meal replacement reduces insulin requirement, HbA1c and weight long-term in type 2 diabetes patients with > 100 U insulin per day [J].Journal of human nutrition and dietetics: the official journal of the British Dietetic Association, 2014, 27 Suppl 2(21 – 7.
KöNIG D, KOOKHAN S, SCHAFFNER D, et al. A meal replacement regimen improves blood glucose levels in prediabetic healthy individuals with impaired fasting glucose [J]. Nutrition. 2014;30(11–12):1306–9.
KEMPF K, ALTPETER B, BERGER J, et al. Efficacy of the telemedical lifestyle intervention program TeLiPro in Advanced Stages of type 2 diabetes: a randomized controlled trial [J]. Diabetes Care. 2017;40(7):863–71.
LESLIE W S, TAYLOR R et al. HARRIS L, Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes: systematic review and meta-analysis [J]. International journal of obesity (2005), 2017, 41(1): 96–101.
STEFAN N, HäRING HU. SCHULZE M B. metabolically healthy obesity: the low-hanging fruit in obesity treatment? [J]. Volume 6. The lancet Diabetes & endocrinology; 2018. pp. 249–58. 3.
SPEAKER K J, SAYER R D, PETERS JC, et al. Effects of consuming a high-protein diet with or without soy protein during weight loss and maintenance: a non-inferiority, randomized clinical efficacy trial [J]. Volume 4. Obesity science & practice; 2018. pp. 357–66. 4.
DAHLBERG C J, OU J J, BABISH J G, et al. A 13-week low glycemic load diet and lifestyle modification program combining low glycemic load protein shakes and targeted nutraceuticals improved weight loss and cardio-metabolic risk factors [J]. Can J Physiol Pharmacol. 2017;95(12):1414–25.
SUN S, HE J, SHEN B, et al. Obesity as a “self-regulated epidemic”: coverage of obesity in chinese newspapers [J]. Eat weight disorders: EWD. 2021;26(2):569–84.
HEBEBRAND J, HOLM J C WOODWARDE, et al. A proposal of the European Association for the study of obesity to improve the ICD-11 diagnostic criteria for obesity based on the three Dimensions Etiology, Degree of Adiposity and Health risk [J]. Obes Facts. 2017;10(4):284–307.
UNAMUNO X, GóMEZ-AMBROSI J, RODRíGUEZ A, et al. Adipokine dysregulation and adipose tissue inflammation in human obesity [J]. Eur J Clin Invest. 2018;48(9):e12997.
OUCHI N, PARKER J L, LUGUS JJ, et al. Adipokines in inflammation and metabolic disease [J]. Nat Rev Immunol. 2011;11(2):85–97.
MRAZ M, HALUZIK M. The role of adipose tissue immune cells in obesity and low-grade inflammation [J]. J Endocrinol. 2014;222(3):R113–27.
JUNG U J, CHOI MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease [J]. Int J Mol Sci. 2014;15(4):6184–223.
WHITEHEAD JP, RICHARDS A A, HICKMAN I J, et al. Adiponectin–a key adipokine in the metabolic syndrome [J]. Volume 8. Diabetes, obesity & metabolism; 2006. pp. 264–80. 3.
KIM J Y, VAN DE WALL E, LAPLANTE M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue [J]. J Clin Investig. 2007;117(9):2621–37.
MAEDA N, SHIMOMURA I, KISHIDA K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30 [J]. Nat Med. 2002;8(7):731–7.
GRAY D L, O’BRIEN K D, D’ALESSIO D A, et al. Plasma glycosylphosphatidylinositol-specific phospholipase D predicts the change in insulin sensitivity in response to a low-fat but not a low-carbohydrate diet in obese women [J]. Metab Clin Exp. 2008;57(4):473–8.
BRUNNER G, METZ C N, NGUYEN H, et al. An endogenous glycosylphosphatidylinositol-specific phospholipase D releases basic fibroblast growth factor-heparan sulfate proteoglycan complexes from human bone marrow cultures [J]. Blood. 1994;83(8):2115–25.
USSAR S, BEZY O, BLüHER M, et al. Glypican-4 enhances insulin signaling via interaction with the insulin receptor and serves as a novel adipokine [J]. Diabetes. 2012;61(9):2289–98.
GESTA S, BLüHER M, YAMAMOTO Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution [J]. Proc Natl Acad Sci USA. 2006;103(17):6676–81.
BäCKHED F, DING H, WANG T, et al. The gut microbiota as an environmental factor that regulates fat storage [J]. Proc Natl Acad Sci USA. 2004;101(44):15718–23.
TURNBAUGH PJ, GORDON JI. The core gut microbiome, energy balance and obesity [J]. J Physiol. 2009;587(Pt 17):4153–8.
TURNBAUGH PJ, LEY R E, MAHOWALD MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest [J]. Nature. 2006;444(7122):1027–31.
TREMAROLI V, BäCKHED F. Functional interactions between the gut microbiota and host metabolism [J]. Nature. 2012;489(7415):242–9.
STEPHEN A M, CHAMP M M CLORANSJ, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health [J]. Nutr Res Rev. 2017;30(2):149–90.
DEEHAN E C, DUAR R M, ARMET A M et al. Modulation of the Gastrointestinal Microbiome with Nondigestible Fermentable Carbohydrates To Improve Human Health [J].Microbiology spectrum, 2017, 5(5)
Funding
Open Access funding enabled and organized by Projekt DEAL.
This work was supported by the13th Five Year National Key R&D Program (2016YFD0400604).
Author information
Authors and Affiliations
Contributions
Yulian Zhong, Ximin Chen, Shaokang Wang, and Guiju Sun developed the concept and designed the study. Chao Huang, Yuexiao Chen, Fengyi Zhao, Runhua Hao, and Niannian Wang conducted physical examinations and data analyses. Yulian Zhong, Wang Liao, Hui Xia, and Ligang Yang drafted the manuscript. Shaokang Wang and Guiju Sun provided critical review and revised the manuscript. All authors agreed to the final submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors report no conflict of interest.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of China Clinical Registration Trials (ethics review number: ChiECRCT20200292 and clinical registration number: ChiCTR2100050070). Participants signed consent forms before study commencement.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: table 1 has been updated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhong, Y., Chen, X., Huang, C. et al. The effects of a low carbohydrate diet combined with partial meal replacement on obese individuals. Nutr Metab (Lond) 20, 18 (2023). https://doi.org/10.1186/s12986-023-00740-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-023-00740-5