Abstract
Background
The gut microbiota has been shown to be involved in the development and severity of type 2 diabetes. The aim of the present study was to test the effect of 4-week functional food ingredient feeding, alone or in combination, on the gut microbiota composition in diabetic rats.
Methods
Streptozotocin (STZ)-induced diabetic rats were treated for 4 weeks with (1) native taro starch, (2) modified taro-starch, (3) beet juice, (4) psicose, (5) the probiotic L. plantarum IS-10506, (6) native starch combined with beet juice, (7) native starch to which beet juice was adsorbed, (8) modified starch combined with beet juice or (9) modified starch to which beet juice was adsorbed, to modulate the composition of the gut microbiota. This composition was evaluated by sequencing the PCR amplified V3–V4 region of the 16S rRNA gene.
Results
The next-generation sequencing showed beneficial effects particularly of taro-starch feeding. Operational taxonomic units (OTUs) related to health (e.g. correlating with low BMI, OTUs producing butyrate) were increased in relative abundance, while OTUs generally correlated with disease (e.g. Proteobacteria) were decreased by feeding taro-starch.
Conclusion
The results of study show that a 4-week intervention with functional food ingredients, particularly taro-derived starch, leads to a more healthy gut microbiota in rats that were induced to be diabetic by induction with STZ.
Similar content being viewed by others
Background
Type 2 diabetes (T2D) increases at a dramatic pace worldwide. The global incidence of T2D is predicted to reach 360 million cases by the year 2030 [1], on a projected world population of 8.5 billion [2]. Although it is commonly accepted that an increase in energy intake and a decrease in energy expenditure are the leading causes of obesity and associated with that T2D, metabolic syndrome and cardiovascular disease, recently the gut microbiota has been shown to play a role as well [3,4,5,6]. The gut microbiota composition and/or activity can be changed using functional food ingredients. The vital role of food for prevention and treatment of T2D needs proper attention, such as in the development of dietary components that positively influence postprandial glycaemia and through this their potential to reduce the impact of T2D. On top of that, effects through the modulation of the gut microbiota need to be considered.
Indonesia is rich in biodiversity, including a variety of local tubers, which are currently underutilized, despite their potential functional properties, and widespread use in the past. One of these interesting tubers is Cocoyam or taro, which belongs to the monocotyledonous family Araceae (the aroids). Taro used to be an important ethnic root crop throughout Asia. It was cultivated and used as a staple crop in various parts of the humid tropics and sub-tropics, as it adapts well to different agro-climatic conditions [7,8,9]. Its utilization is also related to the culture of a region, hence, taro is very important for community-life [10]. Keirke et al. [11] reported that Indonesia has the highest taro diversity in the world, and can be found in areas in Borneo, Java, Sumatra, and Sulawesi [12]. Taro traditionally was used as an alternative carbohydrate source to reduce dependence on rice. We have shown recently that taro contains resistant starch (RS) (manuscript submitted for publication), which reaches the colon and can contribute to the modulation of the gut microbiota.
Recently, in an attempt to produce functional foods aimed at low calorie and less sugar intake for T2D, D-psicose, a rare monosaccharide also known as D-allulose, is considered as a substitute for sugar with proven antihyperglycemic, antihyperlipidemic, and anti-inflammatory effects [13, 14].
Probiotics are defined as “life microorganisms, which when administered in adequate amounts, have a beneficial effect on the host” [15]. Probiotic strains have been isolated from dadih, a traditional fermented buffalo milk, produced in West Sumatra [16]. Dadih has been shown to reduce adiposity, weight gain and adiposity inflammation in high fat induced obese rats [17]. The microbes present in dadih, amongst which L. plantarum IS-10506 [16, 18, 19], may contribute to this beneficial effect.
Beet juice is rich in including phenolic acids, flavonoids and betalains [20], and beet juice has a high total antioxidant capacity and total polyphenol content [21]. Polyphenols including flavonoids, phenolic acids, proanthocyanidins and tannins have been suggested to be able to modify postprandial (hyper)glycaemia [22, 23] by inhibiting carbohydrate digestion, reducing glucose absorption in the intestines, stimulation of insulin release from pancreatic β-cells, modulation of hepatic glucose output, activation of insulin receptors, and/or modulation of glucose uptake in insulin-sensitive cells [24, 25]. Polyphenols are not very well absorbed in the small intestine, and reach the colon where the can modulate the gut microbiota. Therefore, beet juice is an interesting food model to investigate any influence of its bioactive components on the glycaemic response, either by direct inhibition of glucose uptake or by indirect action affecting insulin sensitivity, whether or not through modulation of the gut microbiota.
In a previous study (submitted for publication), we reported the effect of (modified) taro starch, psicose, the probiotic L. plantarum IS-10506 and phytonutrients of beet juice, alone or in combination on the gut microbiota composition in streptozotocin-induced T2D rats, in a pilot-study of 1 week of feeding. The aim of the current study is to extend the data with results after 4 weeks of feeding these functional food ingredients.
Materials and methods
Materials
Streptozotocin (STZ; catalogue number ALX-380-010-G001) was purchased from Enzo Life Science (NY, USA). Psicose was from Merck (Darmstadt, Germany; catalogue number P8043). L. plantarum IS-10506 was cultured and prepared as described before [16].
In vivo animal trial
Animals and housing
Because in Indonesia only the Ministry of Health and universities with a medical faculty have ethical committees (and this excludes the universities participating in this study), the ethical committee of the Faculty of Medicine of the University of Indonesia (the most reputable committee) was chosen to assess the animal trial. All animal care procedures were conducted under the animal protocol approved by the ethical committee (Ref: 1196/UN2.F1/ETIK/2018) under approval number 18-09-1045. Male Sprague Dawley rats were purchased from the Animal Experimental Laboratory National Agency of Drug and Food Control (Jakarta, Indonesia) at 6 weeks of age and were allowed to adapt for 14 days. They were housed in individual cages and maintained with a 12-h light/dark cycle, at 21–23 °C and 55% ± 5% humidity. All rodents were given ad libitum access to water and commercially available rat normal pellet diet (NPD) purchased from local market, during a 14 days acclimatization period, prior to the dietary manipulation. Subsequently, purified Rodent Diet AIN-93 M, a modified AIN-76A standard diet (American Institute of Nutrition) [26] was provided to the rats as a control.
Diets
Native taro starch “HASIL BUMIKU” was purchased from a local supplier (Kusuka Ubiku) in Bantul, Yogyakarta, Central Java, Indonesia. Modified taro-starch was manufactured by the autoclave-cooling method according to a modification of the method of Zhao and Lin [27]. In brief, taro starch was blended with distilled water based on the ratio 1:3.5, and the blend was then gelatinized using pressure-heated instrument at 121 °C for 30 min and cooled to 4 °C, with a repetition of two cycles. Afterwards, the retrogradaded starch was dried using a fan-assisted oven at 60 °C for 16 h after which it was allowed to cool at room temperature for 24 h, and subsequently grinded, and sieved using a 60 mesh.
Beet juice was adsorbed to both native and modified taro starch by absorbing beetroot juice, at a ratio of 1:1, and then drying in an oven at 40 °C for 16 h. These were prepared and fed according to the dose of beet juice of 6 ml/day, but in adsorbed beet juice form.
Beet juice (6 ml/day) was also provided separately, as well as in combination with native and modified starch (non-adsorbed). The probiotic L. plantarum IS-10506 was given by gavage at 1010 colony forming units/day.
Rats were first provided modified AIN-93M by replacing corn starch with the respective taro starch, and replacing sucrose and cellulose with maltodextrin. This ration was provided for 3 days, with 25% incremental increasing dose of the modified AIN (at 75, 50 and 25% commercial rat pellet diet and 25, 50, and 75% dietary intervention formulation for the 3 days, respectively). Beet juice and probiotic were added to AIN-93M diet where applicable by gavage.
Development of type 2 diabetes by STZ-treatment
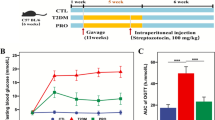
Four rats in each group were allocated to the dietary treatments. Then the rats were injected intraperitoneally (i.p.) with 120 mg kg−1 nicotinamide in 0.9% NaCl, followed after 15 min by STZ in citrate buffer pH 4.4 (70 mg kg−1), and four days after the STZ injection, fasting and postprandial blood glucose levels were measured using a Freestyle glucose meter (Easy Touch GCU 3 in 1) from a puncture at the tip of the tail. The rats with a fasting glucose of ≥ 100 mg dl−1 and/or postprandial blood glucose levels of ≥ 140 mg dl−1 were considered as type 2 diabetic, and those rats which had not yet developed T2D within these 4 days were injected for the second time with 120 mg kg−1 nicotinamide and STZ in citrate buffer pH 4.4 (70 mg kg−1).
After confirmation of T2D, the 8 week old rats of 190–220 g were divided into 10 groups of n = 4 each, namely:
-
(1)
AIN-93 M (control),
-
(2)
AIN-93 M with two times in a day 3 ml psicose by gavage (psicose),
-
(3)
AIN-93 M with two times in a day 3 ml beetroot juice by gavage (beet juice),
-
(4)
native taro starch (native starch),
-
(5)
modified taro starch (modified starch),
-
(6)
native taro starch with beetroot juice adsorbed (native beet adsorbed),
-
(7)
modified taro starch with beetroot juice adsorbed (modified beet adsorbed),
-
(8)
native taro starch combined with two times in a day 3 ml beetroot juice by gavage (native + beet),
-
(9)
modified taro starch combined with two times in a day 3 ml beetroot juice by gavage (modified + beet), and
-
(10)
L. plantarum IS-10506 (probiotic).
The rats were allowed to continue to feed on their respective diets until the end of the study. Food and water intake were monitored every day. Bodyweight was monitored weekly. The average food intake per rat was calculated. Fecal pellets were collected at the end of 1 week of feeding, as well as after 4 weeks.
Extraction of nucleic acids
DNA extraction of feces samples was performed using the Quick-DNA™ Fecal/Soil Microbe Miniprep Kit (Zymo Research) according to manufacturer’s instructions, using the Precellys 24 tissue homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France), applying 3 cycles of 30 s each, with 5 min cooling on ice in between.
PCR-amplifying the V3-V4 region of the 16S rRNA gene and next generation sequencing
Illumina 16S rRNA gene amplicon libraries were generated and sequenced at BaseClear (Leiden, the Netherlands). In short, barcoded amplicons from the V3-V4 region of 16S rRNA genes were generated using a 2-step PCR. 10–25 ng isolated genomic DNA was used as template for the first PCR with a total volume of 50 μl using the 341F (5′-CCTACGGGNGGCWGCAG-3′) and the 785R (5′-GACTACHVGGGTATCTAATCC-3′) primers appended with Illumina adaptor sequences (Illumina, San Diego, CA, USA). PCR products were purified and the size of the PCR products were checked on Fragment analyzer (Advanced Analytical Technologies, Heidelberg, Germany) and quantified by fluorometric analysis. Purified PCR products were used for the 2nd PCR in combination with sample-specific barcoded primers (Nextera XT index kit, Illumina). Subsequently, PCR products were purified, checked on a Fragment analyzer (Advanced Analytical Technologies) and quantified, followed by multiplexing, clustering, and sequencing on an Illumina MiSeq with the paired-end (2x) 300 bp protocol and indexing.
Sequence processing and analyses
The sequencing run was analyzed with the Illumina CASAVA pipeline (v1.8.3) with demultiplexing based on sample-specific barcodes. The raw sequencing data produced was processed removing the sequence reads of too low quality (only "passing filter" reads were selected) and discarding reads containing adaptor sequences or PhiX control with an in-house filtering protocol. A quality assessment on the remaining reads was performed using the FASTQC quality control tool version 0.10.0. Subsequently, the sequences were further analyzed using the Quantitative Insights Into Microbial Ecology (QIIME) software pipeline, version 1.9.1 [28] for α- and β-diversity and (un)weighted principal coordinate analysis. The software package R (3.5.0) (R Core Team 2013) was used to determine correlations between OTUs and treatments. Statistical analyses were performed with RStudio (1.0.153). Kruskal–Wallis correlations were calculated between OTUs and non-continuous values (treatments). Multiple comparison was corrected using the false discovery rate (FDR), and q values (adjusted p values) were considered significantly different at q < 0.05.
Data availability
The datasets are available from the corresponding author on reasonable request. Raw sequences have been deposited in the European Nucleotide Archive under submission number PRJEB39722: (https://www.ebi.ac.uk/ena/browser/view/PRJEB39722).
Results and discussion
STZ induced diabetes in the rats, as indicated by the average fasting blood glucose concentrations of 149 ± 6.3 mg/dl. At this point, rats were randomly divided over the 10 groups, and fed their respective diet for 4 weeks. After 4 weeks of treatment with the different diets, the microbiota composition was clearly different than that at baseline (Fig. 1a). The principal coordinate analysis (PCoA) plot of the unweighted UniFrac β-diversity analysis (Fig. 1a) shows that even after 1 week there are already differences in the gut microbiota, as reported by us before (manuscript submitted). We will focus here on the week 4 data. The same PCoA plot, but then with the baseline and week 1 data removed, shows a separation at week 4 by treatment (Fig. 1b), but especially when the treatments are clustered by containing starch or not (Fig. 1c). This is also the case if only the samples of week 4 are plotted in the unweighted UniFrac β-diversity analysis (Fig. 1d, e for treatment and starch or not, respectively).
Principal coordinate analysis of unweigthed UniFrac of a baseline, week 1 and week 4 samples; b the same plot, but with baseline and week 1 samples removed, clustered according to treatment; c same as b but then clustered according to whether rats were fed starch in their diet or not. Focusing on the week 4 data graphs d, e the unweighted UniFrac clustered according to treatment and whether rats were fed starch in their diet or not, respectively
Kruskal–Wallis analysis revealed the significant OTUs (corrected for multiple comparisons; q value < 0.05) that were different between treatment (Fig. 2; Table 1A) or whether rats were fed starch or not (Fig. 3; Table 1B). Six of the OTUs that were significantly different when tested against treatment (Table 1A), were also different when tested for starch, indicating that starch treatment drove the separation between the samples for these 6 OTUs. The relative abundance (RA) of these 6 OTUs, Christensenellaceae R-7 group, Prevotella 9, an unknown species of the Prevotellaceae family, Prevotellaceae UCG-001, and Ruminococcaceae UCG-005; and Ruminococcus 1, were all increased after starch feeding (Fig. 3a, h–k, m, respectively). However, in most cases the stimulation was higher after modified starch feeding (irrespective of the additional feeding or adsorption of beet juice) than after native starch feeding (Fig. 2a, f–j, respectively). Starch also increased the RA of Oscillibacter, Phascolarctobacterium, and an uncultured species of the Ruminococcaceae family (Fig. 3e, g, l, respectively). These were not significantly different when tested between treatments (although the p values were < 0.05 for Phascolarctobacterium and the Ruminococcaceae species, after multiple correction the q value was not). Christensenellaceae has been shown to be enriched in individuals with low body mass index [29]. Members of the Ruminococcaceae family (including Oscillibacter) have been well known to be involved in the production of butyrate, an important health promoting microbial metabolite [30] produced by the gut microbiota, particularly known to be produced upon starch feeding [31, 32]. Members of Prevotellaceae are commonly found in the digestive tracts of people who maintain a diet low in animal fats and high in carbohydrates [33]. Phascolarctobacterium has been associated with low C-reactive protein in premenopausal women [34].
Box-plot representation of OTUs that were significantly different (q < 0.05; Table 1A) at the genus of family level after Kruskal–Wallis analysis when compared for dietary treatment. a Christensenellaceae R-7 group; b uncultured species of the Christensenellaceae family; c Clostridium sensu stricto 11; d Holdemania; e uncultured species of the Peptococcaceae family; f Prevotella 9; g unknown species of the Prevotellaceae family; h Prevotellaceae UCG-001; i Ruminococcaceae UCG-005; j Ruminococcus 1
Box-plot representation of OTUs that were significantly different (q < 0.05; Table 1B) at the genus or family level after Kruskal–Wallis analysis when compared whether rats were fed starch in their diet or not. a Christensenellaceae R-7 group; b uncultured species of the Erysipelotrichaceae family; c Escherichia-Shigella; d Klebsiella; e Oscillibacter; f unknown species of the Peptostreptococcaceae family; g Phascolarctobacterium; h Prevotella 9; i unknown species of the Prevotellaceae family; j Prevotellaceae UCG-001; k Ruminococcaceae UCG-005; l uncultured species of the Ruminococcaceae family; m Ruminococcus 1; n Turicibacter
Starch feeding led to a reduction in RA of an uncultured species of the Erysipelotrichaceae family, Escherichia-Shigella, Klebsiella, an unknown species of the Peptostreptococcaceae family, and Turicibacter. Members of the family Erysipelotrichaceae (incl. Turicibacter) have been shown to be increased in the mouse gut microbiota for mice switched to diets high in fat, and appear to be highly immunogenic and can potentially flourish post-treatment with broad spectrum antibiotics [35, 36]. Therefore, a reduction in these OTUs is interpreted to be beneficial. Similarly, the reduction of the RA of the Proteobacteria Klebsiella and Escherichia-Shigella is considered to be beneficial. Moreover, a reduction in Peptostreptococcaceae, which has been shown to be over-represented in the guts of colorectal cancer patients [37], appears to be beneficial too.
Overall, the changes in relative abundances discussed above induced by taro-starch feeding seem to be beneficial for health, although currently most of the benefits described have been through associations and not cause-and-effect relationships yet.
In addition to the OTUs that were significantly different in abundance upon starch feeding, Fig. 2 list 4 more OTUs (in addition to the 6 that were also significant when testing for starch feeding) that are significantly different when comparing overall treatments. One of these, an uncultured species of the Christensenellaceae family (Fig. 2b) seemed to be mostly stimulated by starch, but primarily by modified taro-starch, which may be why it is not significant when comparing for starch feeding versus not. The other 3 are Clostridium sensu stricto 11, Holdemania and an uncultured species of the Peptococcaceae. Clostridium sensu stricto 11 (Fig. 2c) is primarily stimulated by native starch (in the presence and absence of beet juice), but also by modified starch with beet juice adsorbed, as well as by feeding L. plantarum IS-10506. For Holdemania no clear health effects have been described. It is reduced (compared to AIN) in native starch and native starch combined with beet juice, and when psicose is fed (Fig. 2d). Members of the family Peptococcaceae have been associated with stress in pregnant women: higher RA correlated with reduced prenatal stress [38]. The RA of the uncultured OTU was primarily increased by modified taro-starch.
Conclusion
In conclusion, particularly taro-starch feeding led to changes in the gut microbiota of the rats with induced diabetes that could be related to beneficial changes to health.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIN:
-
American Institute of Nutrition
- BMI:
-
Body mass index
- FDR:
-
False discovery rate
- OTU:
-
Operational taxonomic unit
- PCoA:
-
Principal coordinate analysis
- PCR:
-
Polymerase chain reaction
- QIIME:
-
Quantitative insights into microbial ecology
- RA:
-
Relative abundance
- RS:
-
Resistant starch
- STZ:
-
Streptozotocin
- T2D:
-
Type 2 diabetes
References
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53.
UN-News. https://news.un.org/en/story/2015/07/505352-un-projects-world-population-reach-85-billion-2030-driven-growth-developing (2015). Accessed 29 Mar 2020.
Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–79.
Shapiro H, Suez J, Elinav E. Personalized microbiome-based approaches to metabolic syndrome management and prevention. J Diabetes. 2017;9(3):226–36.
Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60.
Onwueme IC. The tropical tuber crops—yams, cassava, sweet potato and cocoyams. Chichester: Wiley; 1978.
Asha DA. Genetic diversity analysis in taro using molecular markers—an overview. J Root Crops. 2012;38(1):15–25.
Solossa AH, Sastrahidayat IR, Hakim L. Home gardens of the local community surrounding Lake Ayamaru, West Papua province, and its consequences for tourism development and lake conservation. J Biodiv Environ Sci. 2017;3(3):1–11.
Iskandar J, Iskandar BS. Various plants of traditional rituals: ethnobotanical research among the Baduy community. Biosaintifika J Biol Biol Educ. 2017;9(1):114–25.
Kreike CM, Van Eck HJ, Lebot V. Genetic diversity of taro, Colocasia esculenta (L.) Schott, in Southeast Asia and the Pacific. Theor Appl Genet. 2004;109(4):761–8.
Prana MS, Kuswara T. Budidaya Talas: Diversifikasi untuk Menunjang Ketahanan Pangan Nasional (Taro cultivation: diversification to support national food security). Bogor: Medikom Pustaka Mandiri; 2002.
Chen J, Huang W, Zhang T, Lu M, Jiang B. Anti-obesity potential of rare sugar d-psicose by regulating lipid metabolism in rats. Food Funct. 2019;10(5):2417–25.
Hossain A, Yamaguchi F, Matsuo T, Tsukamoto I, Toyoda Y, Ogawa M, et al. Rare sugar D-allulose: potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol Ther. 2015;155:49–59.
FAO. Joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food: health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria; 2001.
Surono I, Verhoeven J, Verbruggen S, Venema K. Microencapsulation increases survival of the probiotic Lactobacillus plantarum IS-10506, but not Enterococcus faecium IS-27526 in a dynamic, computer-controlled in vitro model of the upper gastrointestinal tract. J Appl Microbiol. 2018;124(6):1604–9.
Kusuma RJ, Azzyati F, Purbarani G, Sulistyorini R, Nofiartika F, Huriyati E. Effect of traditional fermented buffalo milk (Dadih) on body weight, adipose tissue mass and adiposity inflammation in high fat-induced obese rats. EC Nutr. 2015;1(3):106–14.
Prakoeswa CRS, Herwanto N, Prameswari R, Astari L, Sawitri S, Hidayati AN, et al. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef Microbes. 2017;8(5):833–40.
Surono IS, Martono PD, Kameo S, Suradji EW, Koyama H. Effect of probiotic L. plantarum IS-10506 and zinc supplementation on humoral immune response and zinc status of Indonesian pre-school children. J Trace Elem Med Biol. 2014;28(4):465–9.
Kujala TS, Vienola MS, Klika KD, Loponen JM, Pihlaja K. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. Eur Food Res Technol. 2002;214(6):505–10.
Wootton-Beard PC, Ryan L. Short communication: a beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. J Funct Foods. 2011;3:329–34.
Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54(7):1720–5.
Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed). 1984;288(6428):1401–4.
Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59(7):365–73.
Ishikawa A, Yamashita H, Hiemori M, Inagaki E, Kimoto M, Okamoto M, et al. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J Nutr Sci Vitaminol (Tokyo). 2007;53(2):166–73.
Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr. 1993;123(11):1923–31.
Zhao X-H, Lin Y. The impact of coupled acid or pullulanase debranching on the formation of resistant starch from maize starch with autoclaving–cooling cycles. Eur Food Res Technol. 2009;230:179.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6.
Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99.
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–19.
Kovatcheva-Datchary P, Egert M, Maathuis A, Rajilic-Stojanovic M, de Graaf AA, Smidt H, et al. Linking phylogenetic identities of bacteria to starch fermentation in an in vitro model of the large intestine by RNA-based stable isotope probing. Environ Microbiol. 2009;11(4):914–26.
Rose DJ, Venema K, Keshavarzian A, Hamaker BR. Starch-entrapped microspheres show a beneficial fermentation profile and decrease in potentially harmful bacteria during in vitro fermentation in faecal microbiota obtained from patients with inflammatory bowel disease. Br J Nutr. 2010;103(10):1514–24.
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8.
Citronberg JS, Curtis KR, White E, Newcomb PA, Newton K, Atkinson C, et al. Association of gut microbial communities with plasma lipopolysaccharide-binding protein (LBP) in premenopausal women. ISME J. 2018;12(7):1631–41.
Greiner T, Backhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab. 2011;22(4):117–23.
Kaakoush NO. Insights into the role of erysipelotrichaceae in the human host. Front Cell Infect Microbiol. 2015;5:84.
Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(24):1907–11.
Hechler C, Borewicz K, Beijers R, Saccenti E, Riksen-Walraven M, Smidt H, et al. Association between psychosocial stress and fecal microbiota in pregnant women. Sci Rep. 2019;9(1):4463.
Acknowledgements
The authors wish to thank Cheng Long for help with statistical analyses in R.
Funding
The study was funded through a grant from Insentif Riset Sistem Inovasi Nasional (INSINAS) 2019; Grant Number l8/lNS-2/PPK/84/2018. The study was also partly funded by the Centre for Healthy Eating & Food Innovation (HEFI) of Maastricht University – campus Venlo. This research has been made possible with the support of the Dutch Province of Limburg with a grant to HEFI.
Author information
Authors and Affiliations
Contributions
ISS, PW and BS initiated the study, and supervised the animal trial; AAW prepared the taro-starch samples; BS collected the fecal samples; JS and KV analyzed the microbiota composition in fecal samples; KV performed the bioinformatic analyses and did the statistical analyses; ISS and KV wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animal procedures undertaken were approved by Ethic committee of the Faculty of Medicine University of Indonesia (Ref: 1196/UN2.F1/ETIK/2018).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Surono, I.S., Wardana, A.A., Waspodo, P. et al. Effect of functional food ingredients on gut microbiota in a rodent diabetes model. Nutr Metab (Lond) 17, 77 (2020). https://doi.org/10.1186/s12986-020-00496-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-020-00496-2