Abstract
Background
Fat percentage and distribution in pigs are associated with their productive efficiency and meat quality. Dietary branched-chain amino acids (BCAA) regulate fat metabolism in weanling piglets with unknown mechanism. It is reported that N6-methyl-adenosine (m6A) is involved in fat metabolism in mice. The current study was designed to investigate the relationship between dietary branched-chain amino acids and fat metabolism through N6-methyl-adenosine (m6A) in weanling piglets.
Methods
A total of 18 healthy crossbred weaned piglets (Duroc × Landrace × Large White, 10.45 ± 0.41 kg) were divided into 3 treatments and were fed the low BCAA dose diet (L-BCAA), the normal dose BCAA diet (N-BCAA), or the high dose BCAA (H-BCAA) diet for 3 weeks.
Results
Our results show that compared with the N-BCAA group, the L-BCAA group had higher concentration of serum leptin (P < 0.05), while the H-BCAA group had lower concentration of serum adiponectin (P < 0.05). Fatty acid synthesis in pigs from the H-BCAA group was lower than those from the N-BCAA group with the down-regulation of lipogenic genes (ACACA, FASN, PPAR-r, SREBP-1c in ventral and dorsal fat, SREBP-1c in liver) and up-regulation of lipolysis genes (HSL, ATGL, CPT-1A, FABP4 in ventral fat, HSL in liver) (P < 0.05). Similarly, fatty acid synthesis in pigs from the L-BCAA group was also lower than those from the N-BCAA group with the decrease of lipogenic genes (ACACA in ventral, ACACA and FASN in dorsal fat, ACACA, FASN, SREBP-1c in liver) and the increase of lipolysis genes (ATGL, CPT-1A CD36, FABP4 in ventral fat and HSL, ATGL, CPT-1A in dorsal fat, CPT-1A) (P < 0.05). Feeding H-BCAA diet significantly reduced total m6A levels in ventral and dorsal fat and liver tissues (P < 0.05). The decrease of total m6A is associated with down-regulation of METTL3, METTL14 and FTO in dorsal fat and METTL3 and FTO in liver (P < 0.05). Decreased m6A modification of ACACA and FASN in ventral and dorsal adipose tissues was observed in pig fed with excessive BCAA.
Conclusion
These results suggest that insufficient or excessive BCAA decreased the fat deposition by increasing lipolysis and deceasing lipogenesis in adipose and liver tissues. Dietary excessive BCAA might regulate the process of lipid metabolism partly through the m6A RNA methylation.
Similar content being viewed by others
Introduction
Feeding pigs with protein-restricted diet is a strategic way to save the cost of protein ingredients and decrease the emission of nitrogen in animal husbandry [1]. However, pigs fed with low-protein diet tend to deposit thicker back fat during growing and fastening periods, which eventually affect carcass characteristics [2, 3]. Decreasing dietary crude protein level by 2–4% from the NRC (1998) recommendation is the most classical method to make protein-restricted diets for pigs. In order to compensate the deficiency of essential amino acids, four crystalline amino acids (L-lysine, DL-methionine, L-threonine, and L-tryptophan) are usually supplemented to the low protein diets [1]. However, there might be a shortage of other functional amino acids which participated in protein synthesis and lipid metabolism in low-protein diets. Branched chain amino acids (BCAAs) are considered as important functional amino acids to improve carcass characteristics of pigs due to its potential to enhance protein synthesis through activating the Sestrin2/mTORC1 (mammalian target of rapamycin complex 1) pathway [4, 5].
Recent studies have shown that BCAAs also play essential roles in energy homeostasis and lipid metabolism [6]. Growing evidence have found that shortage of BCAAs reduces fat deposition in mice. A significant reduction of ventral fat mass is observed in mice consuming a leucine-deficient diet, which is partly caused by the increase of energy expenditure [7]. Furthermore, expression of genes involved in lipogenesis and lipolysis is decreased and increased in these mice, respectively [7]. Similarly, when mice are fed isoleucine- or valine-deficient diets, ventral and whole body fat mass are also decreased along with the up-regulated lipolytic genes [8]. Interestingly, excessive BCAAs also inhibit fat accumulation. Decreased body fat mass is observed in rats fed a high level of leucine diet [9]. Leucine over-supplementation also suppresses fat synthesis in murine adipocytes in vitro [10]. In recent years, studies in growing pigs have demonstrated that different dietary BCAA ratios modulate lipid metabolism in intramuscular fat and adipose tissues, indicating their vital role in fat metabolism in pigs [11, 12].
Lipogenesis and lipolysis are well-regulated pathways and a number of genes have been shown to be involved in these processes. The Fat Mass and Obesity-associated (FTO) is one of the genes associated with body mass index and obesity in children and adult [13]. FTO has been originally reported to contribute to human risk of obesity, mainly through the regulation of food intake [14]. Recently, some studies have demonstrated that FTO knockout in mouse significantly disrupts the progress of adipogenesis, while FTO overexpression in mouse increases adipogenic differentiation in primary adipocytes and mouse embryonic fibroblasts [15]. In addition, an intimate relationship between FTO expression and fat deposition has also been observed in pigs [16]. It is worth mentioning that FTO is also an important enzyme involved in N6-Methyladenosine (m6A) RNA modification [17]. m6A modification, which promotes mRNA stability, splicing, export, and translation, is considered as the most abundant post-transcriptional modification in eukaryotic mRNA and long noncoding RNA [18]. The biological process of m6A is reversible, which is regulated by methyltransferases and demethylases [19]. Methyltransferases, which add methyl groups to m6A, include Wilms’ tumor 1-associating protein (WTAP), methyltransferase like 3 (METTL3), and methyltransferase like 14 (METTL14). These methyl groups from m6A can be removed by demethylases [FTO and α-ketoglutarate-dependent dioxygenase alkB homolog 5 (ALKBH5)]. The execution of the function of m6A requires the recognition of m6A by readers like: YTH domain family (YTHDF) proteins and the heterogeneous nuclear ribonucleoprotein (HNRNP) proteins.
In the current study, we hypothesized that dietary BCAAs might dose-dependently regulate fat metabolism through m6A methylation partially through the regulate of the FTO. To test our hypothesis, weanling pigs were assigned to one of the following diets: BCAA-deficient, −normal, or -excessive diets. Changes of rate-limiting enzymes involved in lipolysis, lipogenesis and m6A RNA modification in subcutaneous adipose tissue, ventral adipose tissue, and liver were determined in this study. These results provide important information regarding the relationship between dietary BCAAs and carcass characteristics in pigs and its underlying mechanism. Our study also extends our knowledge that m6A methylation might act as a novel and critical bridge between dietary nutrients and obesity in human.
Materials and methods
Animals and experimental diets
All procedures outlined in this study were conducted under the protocol (SCAU-AEC-2010-0416) approved by the South China Agricultural University Animal Care and Use Committee. In our experiments, a total of 18 healthy crossbred weaned piglets (Duroc × Landrace × Large White, 10.45 ± 0.41 kg) were divided into 3 treatments using a completely randomized design. All piglets were kept in metabolic cages (1.40 × 0.68 × 0.90 m3). The formula of three treatments are shown in Table 1. Pigs in the L-BCAA group were fed the low-protein (LP) diet containing 17.05% crude protein (CP) supplemented with L-lysine, L-methionine, L-threonine, and L-serine. N-BCAA and H-BCAA groups were designed based on the L-BCAA group. In N-BCAA group, extra crystal BCAAs were supplemented as recommended by the National Research Council (NRC) (2012) (0.13% L-isoleucine, 0.09% L-leucine, and 0.23% L-valine) to meet the requirement of standardized ileal digestible amino acids (SID AAs). In the H-BCAA diet, BCAAs were supplemented to reach the 150% SID AA requirement according to the NRC (2012). Pigs had free access to water via a nipple drinker throughout the process of this experiment.
Sample collection
Blood samples were collected from pigs in heparin-free vacutainer tubes at the end of the experiment (fasting state). After blood collection, all samples were centrifuged at 3000 g for 15 min at 4 °C. The serum was obtained and stored at − 80 °C immediately for later analysis. All piglets were sacrificed by electrocution immediately after blood sampling. Ventral, subcutaneous adipose and dorsal subcutaneous adipose were excised from the left side of the carcasses between the sixth and seventh ribs. Liver samples were consistently dissected from right side of whole liver. Adipose and liver tissues were immediately frozen in liquid nitrogen and then stored at − 80 for further analysis.
Serum biochemical analysis
The concentrations of total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), glucose and triglyceride in serum were measured using corresponding commercial available kits (Nanjing Jiancheng Biochemical Reagent Co., Nanjing, China) through the automatic microplate reader (Thermo Scientific™ Multiskan™ GO, USA). In addition, the concentrations of leptin, insulin and adiponectin in serum samples were measured by the commercial ELISA kits purchased from Cusabio Biotech Co., Ltd. (Wuhan, China).
RNA extraction and purification
Cytoplasmic RNA was isolated from ventral subcutaneous adipose, dorsal subcutaneous adipose, and liver of piglets using the cytoplasmic & nuclear RNA purification kit according to the manufacturer’s protocol (NORGEN, Canada, North American). The concentration of extracted RNA was measured by Nano Drop spectrophotometer (Nano Drop Technologies, Wilmington, DE, USA). We also checked the integrity of mRNA by 1% agarose gel electrophoresis. The mRNA was reverse-transcribed to complementary DNA (cDNA) with the PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Dalian, Liaoning, China) according the manufacturer’s protocol. After that, the synthesized cDNA was stored at − 20 °C for further real-time PCR analysis.
Gene expression using RT-PCR
The real-time polymerase chain reaction (RT-PCR) was conducted using an ABI Prism 7500 sequence detection system (Applied Biosystems, Carlsbad, CA). This procedure was performed in a 20 μL reaction volume, containing 10 μL SYBR Green PCR Master Mix (Takara, Dalian, Liaoning, China), 2 μL cDNA, 0.8 μL of each PCR primer (10 μM), 0.4 μL ROX (Dalian, Liaoning, China), and 6 μL dd H2O. The cycling conditions for polymerase chain reaction were as follows: (1) incubation for 5 min at 94 °C, followed by (2) 40 repeated cycles of 94 °C for 30 s, (3) annealing at 60 °C for 30 s and extension at 72 °C for 20 s. The mRNA expression level of the target genes was calculated through the 2−ΔΔCt method. Gene-specific primer sequences used for the RT-PCR detection are listed in Table 2, which were synthesized by Sangon Biotech Co. Ltd. (Shanghai, China).
Western blotting analysis
Western blotting analysis was conducted to detect relative protein levels for METTL3, METTL14, FTO, ALKBH5 and YTHDF2 which are associated with RNA methylation. First, all proteins samples were homogenized on ice with RIPA Lysis Buffer (Beyotime, Shanghai, China), which contains 150 mM NaCl, 50 mM Tris-HCl (pH = 7.4), 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS and some specific protease inhibitors. A BCA Protein Assay Kit (Beyotime, Shanghai, China) was used to detected total protein concentrations of each sample. Subsequently, a total of 30 μg of protein from each tissue was separated by electrophoresis on SDS-PAGE gels. After transferring to nitrocellulose membranes, all strips were blocked in 5% skimmed milk for 1 h at room temperature. Next, blots were incubated with the following primary antibodies at 4 °C overnight with gently shaking: METTL3, ALKBH5 and MTTL14 (1:1000; Abcam, Cambridge, MA, USA), FTO and β-actin (1:1000; Santa Cruz Biotechnology, USA), YTHDF2 (1:1000, Millipore, Bedford, MA, USA). Subsequently, the membranes were incubated with the corresponding secondary antibody (1: 5000 dilution) (Thermo Fisher Scientific, Rockford, IL, USA) for 1 h at room temperature. Finally, proteins on membrane were developed by the ECL Plus chemiluminescence detection kit (Applygen Technologies, Beijing, China) and analyzed by Image Processing Software (Image Pro Plus 6.0) (Rockville, MD, USA).
Measurement of m6A content
The total content of m6A in adipose and liver tissues were determined by an EpiQuik™ m6A RNA methylation quantification kit (Epigentek Group Inc. USA) following the recommended procedures. The total content of m6A was calculated using the formula: m6A % = {[(OD Sample –OD NC)/S] ÷ [(OD PC – OD NC)]} × 100%, and S, PC and NC represents the total amount of inputting RNA, positive and negative controls, respectively.
m6A immunoprecipitation QPCR of target genes
In order to measure the m6A modification of genes involved in lipogenesis and lipolysis, m6A immunoprecipitation was performed. Briefly, m6A antibody (Abcam, Cambridge, MA, USA) and Normal rabbit IgG (Abclonal, Wuhan China) were conjugated to protein A/G mixed magnetic beads (Bio-rad Laboratories, Hercules, CA, USA) at 4 °C overnight. RNA was fragmented and incubated with the magnetic beads in immunoprecipitation buffer (10 mM Tris, 0.5% NP-40, and 150 mM NaCl) supplemented with RNase inhibitor at 4 °C for 3 h. After washing twice with IP buffer, mRNA was eluted from the beads by elution buffer (5 mM Tris·HCl, 1 mM EDTA, and 0.05% SDS). After extraction and precipitation, the input RNA and eluted RNA were reverse transcribed, and its abundance was determined by RT-PCR.
Statistical analysis
Data of this experiments were analyzed with one-way ANOVA according to the procedure GLM of SAS (SAS Institute, Cary, NC, USA). Differences among treatment means were determined using Student-Newman-Keuls (SNK) multiple range test. All results are presented as means with their standard error of mean (SEM). P value less than 0.05 was considered as significant and effects were considered as tendency when 0.05 ≤ P ≤ 0.10.
Result
Lipid-related hormone levels in serum
Plasma hormone levels of insulin, leptin and adiponectin are shown in Table 3. Compared with pigs fed the N-BCAA diet, plasma leptin levels were significantly increased in pigs from the L-BCAA groups (P < 0.05). In comparison with the N-BCAA diet, plasma adiponectin levels did not change in the L-BCAA group, but significantly increased in the H-BCAA group (P < 0.05). There was no difference in plasma insulin level among different groups (P > 0.05).
Serum biochemical parameters
Serum biochemical parameters are shown in Table 4. Compared with the L-BCAA group, serum concentrations of T-CHO were lower in the H-BCAA group (P < 0.05). Both serum concentrations of HDL-C and LDL-C were obviously lower in pigs fed the L-BCAA diet (P < 0.05) compared with those fed the N-BCAA diet. However, no difference was found in plasma TG and glucose levels among different BCAA dose groups (P > 0.05).
Expression of genes involved in fat metabolism in adipose and liver tissues
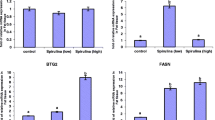
Figure 1 shows the expression level of genes associated with lipid metabolism in adipose and liver tissues. In ventral subcutaneous adipose tissue, the mRNA level of ACACA in L-BCAA treatment was lower than that in the N-BCAA treatment (P < 0.05) (Fig. 1a). Also, the mRNA expression of ACACA, FASN, PPAR-γ and SREBP-1c were lower in the H-BCAA group when compared with the N-BCAA group (P < 0.05) (Fig. 1a). Furthermore, the mRNA expression of ATGL and CPT-1A involved in fat hydrolysis (P < 0.05) (Fig. 1b) as well as FABP4 and CD36 (P < 0.05) (Fig. 1c) involved in fatty acid transport were significantly increased in piglets fed the L-BCAA diet compared with those fed the N-BCAA diet. Similarly, the mRNA expression of HSL, ATGL, CPT-1A (P < 0.05) (Fig. 1b) and FABP4 (P < 0.05) (Fig. 1c) were higher in the H-BCAA group than the N-BCAA group. In dorsal subcutaneous adipose tissue, the L-BCAA group significantly inhibited ACACA and FASN mRNA expression level (P < 0.05) (Fig. 1d) when compared with the N-BCAA group. Also, the H-BCAA group have lower mRNA expression of ACACA, FASN, DGAT1, PPAR-r and SREBP1c than the N-BCAA group (P < 0.05) (Fig. 1d). In addition, when compared with the N-BCAA group, pigs from the L-BCAA group have significantly higher mRNA expression of HSL, ATGL, CPT-1A (P < 0.05) (Fig. 1e) and FABP4 (P < 0.05) (Fig. 1f). In liver tissue samples, pigs fed the L-BCAA diet had lower expression of lipogenic genes (ACACA and SREBP-1c) (P < 0.05) (Fig. 1g) and higher expression of lipolysis genes (CPT-1A) (P < 0.05) (Fig. 1h) than those fed the N-BCAA diet. While pigs fed with H-BCAA diet have lower level of SREBP-1c (P < 0.05) (Fig. 1g) and FABP4 (P < 0.05) (Fig. 1i).
Effect of dietary BCAA level on the expression of genes involved in fat metabolism in ventral subcutaneous adipose (a, b, c), dorsal subcutaneous adipose (d, e, f) and liver (g, h, i). Data are shown as mean ± SEM (n = 4). Different letters indicate a significant difference among different treatments (P < 0.05)
Total methylation levels in adipose and liver tissues
As shown in (Fig. 2), in ventral subcutaneous adipose tissue and dorsal subcutaneous adipose tissue, compared with the N-BCAA group, total amount of m6A was significantly reduced in the H-BCAA group (P < 0.05) (Fig. 2a and b), In liver, compared with the N-BCAA group, total amount of m6A were higher in the L-BCAA group (P < 0.05) (Fig. 2c).
mRNA expression of m6A related enzymes
The expression of m6A related enzymes are listed in (Fig. 3). In ventral subcutaneous adipose tissue, the mRNA levels of FTO and ALKBH5 (P < 0.05) (Fig. 3a, b, and c) were significantly increased in the H-BCAA group when compared with the N-BCAA group. Compared with the N-BCAA group, L-BCAA group increased mRNA expression of METTL3, MELLT14, WTAP as well as YTHDF1 and decreased mRNA expression of YTHDF2 and YTHDF3 in the ventral subcutaneous adipose tissue (Fig. 3a, b, and c). In dorsal subcutaneous adipose, piglets fed with the H-BCAA diet had lower mRNA expression of METTL3, METTL14, WTAP, FTO, ALKBH5, YTHDF2 and YTHDF3 when compared with pigs fed with the N-BCAA diet (Fig. 3d, e and g). However, piglets fed with the L-BCAA diet have higher mRNA levels of METTL14, FTO, ALKBH5, and YTHDF1 than the piglets fed with the N-BCAA diet (Fig. 3d, e and g). In liver tissue, piglets fed the H-BCAA diet showed higher mRNA levels for METTL14, WTAP, FTO, YTHDF2 (P < 0.05) (Fig. 3g) compared to those fed with the N-BCAA diet. In addition, compared with the N-BCAA group, pigs fed the L-BCAA group significantly decreased FTO and ALKBH5 expression, but increased METTLE3, METLE14, YTH family protein 1, 2, and 3 expression in liver tissue.
Effect of dietary BCAA level on the expression of genes related to m6A RNA methylation in ventral subcutaneous adipose (a, b, c), dorsal subcutaneous adipose (d, e, f) and liver (g, h, i). Data are shown as mean ± SEM (n = 6). Different letters indicate a significant difference among different treatments (P < 0.05)
Protein abundance of m6A related enzymes
The proteins abundance of METTL3, METTL14, FTO, ALKBH5 and YTHDF2 in adipose and liver tissues are presented in (Fig. 4). In ventral subcutaneous adipose tissue, protein levels of METTL14 and ALKBH5 were higher in the L-BCAA group than the N-BCAA group. The protein levels of MELLT14, FTO and YTHDF2 were higher in the H-BCAA group than the N-BCAA group. (P < 0.05) (Fig. 4a). In dorsal subcutaneous fat tissue, the protein amounts of METTL3 and METTL14 were obviously decreased (P < 0.05) (Fig. 4b) in the H-BCAA group, when compared with the N-BCAA group. Notably, the protein abundance of ALKBH5 in the L-BCAA group was higher than that in the N-BCAA group (P < 0.05) (Fig. 4b). In liver tissue, compared with the N-BCAA group, pigs fed the L-BCAA diet had higher protein levels of METTL3 and METTL14 (P < 0.05) (Fig. 4c), while pigs fed the H-BCAA diet inhibited the protein abundance of METTL3 (P < 0.05) (Fig. 4c).
m6A modification on lipid metabolism associated genes in adipose tissue
The effect of excessive BCAA on m6A modification of genes involved in lipogenesis and lipolysis in adipose tissues is presented in Fig. 5. Compared with the N-BCAA group, the H-BCAA group significantly decreased the m6A modification of ACACA and FASN in ventral adipose tissue (P < 0.05) and ACACA, FASN and DGAT1 in dorsal adipose tissue (P < 0.05).
Discussion
BCAAs are traditionally considered as functional amino acids that regulate protein synthesis via mTORC1 [6]. Recently, BCAAs have been reported to participate in fatty acid metabolism [7]. However, the underlying mechanism is still unclear. In the current research, the BCAA dose-dependent experiment was conducted to explore the effect of dietary BCAA deficient or excess on fat metabolism in fat and liver and its related mechanism.
Lipid related hormones are affected by different dietary BCAA levels in our study. Adipose tissue is not only an organ for energy storage, but also an endocrine organ which secretes different biologically active molecules into the circulation. Leptin and adiponectin are the two most important fat-derived hormones that participate in energy metabolism [20, 21]. Plasma leptin and adiponectin concentrations are positively and negatively correlated with body fat storage, respectively [22, 23]. In our study, piglets fed the BCAA deficient diet significantly increased the level of leptin, while a lower level of adiponectin was observed from the piglets fed the BCAA excessive diet. In consistent with our results, Li, Wei [24] also found plasma leptin concentration decreases in the BCAA-deficient group. Although the relationship between plasma adiponectin and dietary BCAA level is unclear, an intimate relationship was found between them in the current study. Duan, Li [12] reported that plasma adiponectin concentrations change with different dietary BCAA ratios. Furthermore, leucine metabolite β-hydroxy-β-methylbutyrate regulates fat metabolism partly through adiponectin. Together, these results indicate that unbalanced BCAA might disrupt the whole-body energy metabolism through the modification of leptin and adiponectin secretion. In addition, HDL-C and LDL-C that participated in fat metabolism are also regulated by different dietary BCAA concentrations in our study.
To further study the effect of dietary BCAA on fat metabolism in piglets, genes related to lipolysis and lipogenesis were analyzed in our study. Lipogenesis consists of a series of enzymes, such as ACACA, FASN, DGAT1, SREBPs and PPARs. First of all, acetyl CoA (converted from glucose) is carboxylated into malonyl-CoA by acetyl-CoA carboxylase (ACACA) [25]. Subsequently, fatty acids are synthesized by fatty acid synthase (FASN) with acetyl-CoA, malonyl-CoA and NADPH [26]. Finally, three free fatty acids and one glycerol are combined into a triglyceride under diacylglycerol acyltransferase (DAGT) catalysis as the final step of lipogenesis [27]. This lipogenesis process is regulated by several important regulators. For instance, sterol regulatory element–binding proteins (SREBPs), a membrane-bound transcription factor, can strongly increase the expression of genes involved in synthesis and uptake of fatty acids [28]. Furthermore, nuclear receptor peroxisome proliferator-activated receptors (PPARs) also enhance fatty acid synthesis by increasing the expression of fatty acid transport protein and acyl-CoA synthetase [29]. Lipolysis is also a well-regulated process, which includes fatty acid mobilization and oxidation. Free fatty acids are released from triacylglycerol by hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) [30, 31] and are then transported into the cell by FABP4 and FAT/CD36 [32,33,34]. After the production of acyl CoA from free fatty acid, the step-limiting enzyme carnitine Palmitoyl Transferase-1 (CPT1) transports acyl-CoA into mitochondria for β-oxidation [35].
In this study, decreased lipogenesis and enhanced lipolysis gene expression was observed in piglets fed both BCAA deficient and excess diets. The inhibition of fat synthesis in adipose tissue have also been reported by many other groups using animal models or cell lines in BCAA deficient situation [7, 8, 36, 37] or excessive situation [9, 10, 38, 39]. Similar effects are also observed in the liver. In broiler chicken, low BCAA decreased the lipogenesis and increased lipolysis in the liver partly through AMPK-mTOR-FoxO1 pathway [40]. In addition, a research conducted in mice reveals that supplementation of BCAA decreases the hepatic TG and lipid droplet size and inhibits the expression of lipogenesis gene [41]. Three interesting observations in the current study are worth noting. First of all, more lipid metabolism genes were changed in piglets fed BCAA excess diet than deficient diet. For example, genes participated in lipogenesis (ACACA, FASN, DAGT1, PPAR-γ and SREBP-1c) were significantly decreased both in fat tissues when piglets fed BCAA excess diet, while only ACACA was down-regulated when piglets fed BCAA deficient diet. In addition, the degree of the decrease in gene expression was greater in the BCAA excess group than in the BCAA deficient group. Finally, the effects of BCAA on gene expression were tissue dependent. For instance, although both ventral subcutaneous adipose tissue and dorsal subcutaneous adipose tissue are belonging to fat, pigs fed BCAA deficient diet only increased the lipolysis genes (HSL, ATGL, and CPT-1A) in ventral subcutaneous adipose tissue, but not in dorsal subcutaneous adipose tissue. However, the underlying mechanism of this phenomenon is still unknown and needs further research.
To test the possible role of m6A RNA modification in this experiment, the total m6A methylation level and critical enzymes involved in m6A RNA modification were tested in our study. We found that only high level of BCAA regulated the m6A RNA methylation in adipose tissues. Interestingly, our study also observed that BCAA dose-dependently decreased the m6A RNA methylation in the liver. FTO, a m6A demethylase, has been found to have positive correlation with fat deposition [42]. The expression of FTO in subcutaneous fat and liver were decreased in the H-BCAA group, which is consistent with the inhibition of the fatty acid deposition in this research. Intriguingly, we observed that down-regulated FTO is accompanied with the decrease of the total m6A methylation levels in subcutaneous fat and liver in piglets. The seemingly contradictory results may imply that in addition to FTO, other methyltransferases and demethylases might be involved in the process and need further investigation. We further measured other enzymes and found methyltransferases METTL3 (liver and dorsal subcutaneous adipose tissue), MELLT14 (dorsal subcutaneous adipose tissue) also significantly decreased in the H-BCAA group. The decreased expression of methyltransferases may explain the decreased level of m6A methylation. Interestingly, in this study, although excess BCAA diet inhibited lipogenesis in ventral subcutaneous adipose tissue, the expression of FTO was unexpected up-regulated for unknown reason. Collectively, the present study indicates: 1. high dose BCAA treatment decrease the level of m6A RNA methylation in adipose tissue and liver, 2. The effect of BCAA on demethylase and methyltransferase is tissue specific. m6A RNA methylation is commonly considered to be regulated by dietary methyl donors like: methionine, choline and betaine [43]. To our knowledge, our study was first to report that BCAAs affect m6A RNA methylation which might further regulate fat metabolism. Furthermore, decreased m6A modification of ACACA and FASN in ventral and dorsal adipose tissues was observed in pig fed with excessive BCAA. Future studies are needed to clarify the changes of m6A methylation level of other lipid metabolism associated genes which might participated in fat metabolism.
Conclusion
BCAA deficient or excess diet decrease the fat deposition by increasing lipolysis and lipogenesis in adipose tissue and the liver. m6A RNA methylation is partially involved in the regulation of fat metabolism in diet supplemented with excessive but not deficient BCAA.
Availability of data and materials
All data used in the current study are available from the corresponding author on reasonable request.
Abbreviations
- AAs:
-
Amino acids
- BCAA:
-
Branched-chain amino acids
- CP:
-
Crude protein
- H-BCAA:
-
High dose BCAA
- HDL-C:
-
High-density lipoprotein-cholesterol
- HNRNP:
-
Heterogeneous nuclear ribonucleoprotein proteins
- L-BCAA:
-
Low BCAA dose diet
- LDL-C:
-
Low-density lipoprotein-cholesterol
- LP:
-
Low-protein
- m6A:
-
N6-methyl-adenosine
- N-BCAA:
-
Normal dose BCAA diet
- NRC:
-
National Research Council
- RT-PCR:
-
Real-time polymerase chain reaction
- SID:
-
Standardized ileal digestible
- TC:
-
Total cholesterol
References
Wang Y, Zhou J, Wang G, Cai S, Zeng X, Qiao S. Advances in low-protein diets for swine. J Anim Sci Biotechnol. 2018;9:60. https://doi.org/10.1186/s40104-018-0276-7.
Tuitoek K, Young L, De Lange C, Kerr B. The effect of reducing excess dietary amino acids on growing-finishing pig performance: an elevation of the ideal protein concept. J Anim Sci. 1997;75:1575–83. https://doi.org/10.2527/1997.7561575x.
Hinson R, Schinckel A, Radcliffe J, Allee G, Sutton A, Richert B. Effect of feeding reduced crude protein and phosphorus diets on weaning-finishing pig growth performance, carcass characteristics, and bone characteristics. J Anim Sci. 2009;87:1502–17. https://doi.org/10.2527/jas.2008-1325.
Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43–8 www.sciencemag.org/content/351/6268/43/suppl/DC1.
Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, et al. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science. 2016;351:53–8 www.sciencemag.org/content/351/6268/53/suppl/DC1.
Zhang S, Zeng X, Ren M, Mao X, Qiao S. Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol. 2017;8:10. https://doi.org/10.1186/s40104-016-0139-z.
Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes. 2010;59:17–25. https://doi.org/10.2337/db09-0929.
Du Y, Meng Q, Zhang Q, Guo F. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids. 2012;43:725–34. https://doi.org/10.1007/s00726-011-1123-8.
Vianna D, Resende GFT, Torres-Leal FL, Pantaleão LC, Donato J Jr, Tirapegui J. Long-term leucine supplementation reduces fat mass gain without changing body protein status of aging rats. Nutrition. 2012;28:182–9. https://doi.org/10.1016/j.nut.2011.04.004.
Sun X, Zemel MB. Leucine and calcium regulate fat metabolism and energy partitioning in murine adipocytes and muscle cells. Lipids. 2007;42:297–305. https://doi.org/10.1007/s11745-007-3029-5.
Duan Y, Duan Y, Li F, Li Y, Guo Q, Ji Y, et al. Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs. Amino Acids. 2016;48:2131–44. https://doi.org/10.1007/s00726-016-2223-2.
Duan Y, Li F, Guo Q, Wang W, Zhang L, Wen C, et al. Branched-chain amino acid ratios modulate lipid metabolism in adipose tissues of growing pigs. J Funct Foods. 2018;40:614–24. https://doi.org/10.1016/j.jff.2017.12.004.
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. https://doi.org/10.1126/science.1141634.
Church C, Moir L, Mcmurray F, Girard C, Banks GT, Teboul L, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42(12):1086–92. https://doi.org/10.1038/ng.713.
Merkestein M, Laber S, Mcmurray F, Andrew D, Sachse G, Sanderson J, et al. FTO influences adipogenesis by regulating mitotic clonal expansion. Nat Commun. 2015;6:6792. https://doi.org/10.1038/ncomms7792.
Fu Y, Li L, Ren S. Effect of FTO expression and polymorphism on fat deposition in Suzhong pigs. Asian-Australas J Anim Sci. 2013;26:1365–73. https://doi.org/10.5713/ajas.2013.13055.
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–7. https://doi.org/10.1038/nchembio.687.
Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m 6 A RNA methylation. Nat Rev Genet. 2014;15:293–306. https://doi.org/10.1038/nrg3724.
Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42. https://doi.org/10.1038/nrm.2016.132.
Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51. https://doi.org/10.1210/er.2005-0005.
Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–25. https://doi.org/10.1373/clinchem.2004.032482.
Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–32. https://doi.org/10.1016/S1043-2760(00)00301-5.
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J-I, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. https://doi.org/10.1006/bbrc.1999.0255.
Li Y, Wei H, Li F, Duan Y, Guo Q, Yin Y. Effects of low-protein diets supplemented with branched-chain amino acid on lipid metabolism in white adipose tissue of piglets. J Agric Food Chem. 2017;65:2839–48. https://doi.org/10.1021/acs.jafc.7b00488.
Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N. De novo lipogenesis in health and disease. Metabolism. 2014;63:895–902. https://doi.org/10.1016/j.metabol.2014.04.003.
Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–77. https://doi.org/10.1038/nrc2222.
Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu Y-H. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–89. https://doi.org/10.1172/JCI30565.
Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. https://doi.org/10.1172/JCI15593.
Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37:907–25. https://doi.org/10.1016/0929-7855(95)00062-3.
Yeaman SJ. Hormone-sensitive lipase-a multipurpose enzyme in lipid metabolism. Biochim Biophys Acta (C), Mol Cell Res. 1990;1052:128–32. https://doi.org/10.1016/0167-4889(90)90067-N.
Li Y-C, Zheng X-L, Liu B-T, Yang G-S. Regulation of ATGL expression mediated by leptin in vitro in porcine adipocyte lipolysis. Mol Cell Biochem. 2010;333:121–8. https://doi.org/10.1007/s11010-009-0212-4.
Hertzel AV, Bernlohr DA. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol Metab. 2000;11:175–80. https://doi.org/10.1016/S1043-2760(00)00257-5.
Bonen A, Campbell SE, Benton CR, Chabowski A, Coort SL, Han X-X, et al. Regulation of fatty acid transport by fatty acid translocase/CD36. Proc Nutr Soc. 2004;63:245–9. https://doi.org/10.1079/PNS2004331.
Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJ, Glatz JF, Bonen A. A novel function for fatty acid translocase (FAT)/CD36 involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem. 2004;279:36235–41. https://doi.org/10.1074/jbc.M400566200.
Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients. 2015;7:9453–74. https://doi.org/10.3390/nu7115475.
Freudenberg A, Petzke KJ, Klaus S. Dietary L-leucine and L-alanine supplementation have similar acute effects in the prevention of high-fat diet-induced obesity. Amino Acids. 2013;44:519–28. https://doi.org/10.1007/s00726-012-1363-2.
Lyman R, Cook C, Williams MA. Liver lipid accumulation in isoleucine-deficient rats. J Nutr. 1964;82:432–8. https://doi.org/10.1093/jn/82.4.432.
Bong HY, Kim JY, Jeong HI, Moon MS, Kim J, Kwon O. Effects of corn gluten hydrolyzates, branched chain amino acids, and leucine on body weight reduction in obese rats induced by a high fat diet. Nutr Res Pract. 2010;4:106–13. https://doi.org/10.4162/nrp.2010.4.2.106.
Zhang Y, Guo K, Leblanc RE, Loh D, Schwartz GJ, Yu Y-H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–54. https://doi.org/10.2337/db07-0123.
Bai J, Greene E, Li W, Kidd MT, Dridi S. Branched-chain amino acids modulate the expression of hepatic fatty acid metabolism-related genes in female broiler chickens. Mol Nutr Food Res. 2015;59:1171–81. https://doi.org/10.1002/mnfr.201400918.
Honda T, Ishigami M, Luo F, Lingyun M, Ishizu Y, Kuzuya T, et al. Branched-chain amino acids alleviate hepatic steatosis and liver injury in choline-deficient high-fat diet induced NASH mice. Metabolism. 2017;69:177–87. https://doi.org/10.1016/j.metabol.2016.12.013.
Kang H, Zhang Z, Yu L, Li Y, Liang M, Zhou L. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation. J Cell Biochem. 2018;119:5676–85. https://doi.org/10.1002/jcb.26746.
Wang X, Wang Y. From histones to RNA: role of methylation in signal proteins involved in adipogenesis. Curr Protein Peptide Sci. 2017;18:589–98. https://doi.org/10.2174/1389203717666160627082444.
Funding
This study was supported by National Natural Science Foundation of the P. R. of China (No. 31802067 and 31872364) and the Natural Science Foundation of Guangdong Province (No. 2018A030310201) and the National Key R&D Program of China (No. 2018YFD0500600 and 2018YFD0501000).
Author information
Authors and Affiliations
Contributions
JH, SZ, WG and FC designed the experiment and supervised the project. JH, ZW, MT and JC performed the experiments and conducted the lab work. JH, ZW and HS conducted the statistical analysis. JH and SZ wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal use and care protocols were approved by the Committee of the South China Agricultural University Animal Care and Use (20110107–1).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Heng, J., Wu, Z., Tian, M. et al. Excessive BCAA regulates fat metabolism partially through the modification of m6A RNA methylation in weanling piglets. Nutr Metab (Lond) 17, 10 (2020). https://doi.org/10.1186/s12986-019-0424-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-019-0424-x