Abstract
Background
Coffee consumption is a known inducer of cytochrome P450 1A2 (CYP1A2) enzyme activity. We recently observed that a group of type-2 diabetes patients consumed more caffeine (coffee) on a daily basis than non-type-2 diabetes controls. Here, we investigated whether type-2 diabetes cases may metabolize caffeine faster than non-type-2 diabetes controls.
Methods
To estimate CYP1A2 enzyme activity, an established marker of caffeine metabolism, we quantified the paraxanthine/caffeine concentration ratio in saliva in 57 type-2 diabetes and 146 non-type-2 diabetes participants in a case–control field study. All participants completed validated questionnaires regarding demographic status, health and habitual caffeine intake, and were genotyped for the functional -163C > A polymorphism of the CYP1A2 gene.
Results
In the diabetes group, we found a larger proportion of participants with the highly inducible CYP1A2 genotype. Furthermore, the paraxanthine/caffeine ratio, time-corrected to mitigate the impact of different saliva sampling times with respect to the last caffeine intake, was higher than in the control group. Participants who reported habitually consuming more caffeine than the population average showed higher CYP1A2 activity than participants with lower than average caffeine consumption. Multiple regression analyses revealed that higher caffeine intake was potentially an important mediator of higher CYP1A2 activity.
Conclusions
Estimated CYP1A2 enzyme activity, and thus speed of caffeine metabolism, was higher in our type-2 diabetes group; this was possibly due to higher intake of caffeine, a known inducer of CYP1A2 enzyme activity. Given the fairly small sample sizes, the results need to be considered as preliminary and require validation in larger populations.
Similar content being viewed by others
Background
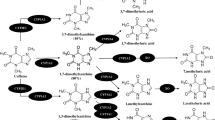
Caffeine is almost completely metabolized in the body by cytochrome P450 1A2 (CYP1A2). This enzyme accounts for the metabolism of caffeine to its principal metabolite, paraxanthine [1]. In vivo, CYP1A2 activity exhibits a significant degree of inter-individual variation (see [2] for review). Inter-individual variability in CYP1A2 enzyme activity is typically between 5- and 15-fold in healthy humans [3, 4], possibly due to environmental and genetic factors. For example, coffee consumption and cigarette smoking both induce CYP1A2 activity, in a dose-dependent manner [4]. Interestingly, rodent models demonstrate that the blood-glucose-regulatory hormone insulin also acts as an inducer of CYP1A2 activity [5]. While this relationship has not been directly assessed in humans, a correlational study revealed a positive relationship between CYP1A2 activity and endogenous insulin levels in premenopausal women [6]. Also functional variations in the CYP1A2 gene may contribute to inter-individual differences in enzyme activity [7]. Indeed, a single nucleotide polymorphism (SNP) (−163C > A) of CYP1A2 has been associated with increased enzymatic activity in smokers [8].
Systemic caffeine clearance is considered the gold-standard approach to estimating CYP1A2 activity [9], which reflects the combined effects of genetic, environmental and endogenous factors [10]. However, this method requires extensive blood sampling, which is expensive, invasive and time consuming [2]. A validated alternative is to determine the concentration ratio of paraxanthine to caffeine in a saliva sample collected 6 h post caffeine dose [9, 11].
In a case–control field study, we recently found that type-2 diabetes patients consumed more caffeine than non-type-2 diabetes controls, possibly to attenuate daytime sleepiness typically associated with the disease [12]. Based on the above presented evidence that caffeine and insulin act as possible inducers of CYP1A2 activity, we hypothesized that the type-2 diabetes patient group would show higher CYP1A2 enzyme activity than the non-type-2 diabetes control group. To our knowledge, only one study has previously assessed CYP1A2 enzyme activity in type-2 diabetes patients, and no difference was found between case and control groups [13]. The findings, however, are limited by the small sample size (n = 16 patients and controls), the long time frame between caffeine ingestion and provision of saliva (8 h) [9, 11], and the lack of data regarding habitual caffeine/coffee intake, smoking, and CYP1A2 genotype. All these factors may impact CYP1A2 activity in patients and controls.
Methods
Subjects
A total of 445 study participants were recruited. Two type-1 diabetes participants were excluded due to the different pathophysiology of type-1 and type-2 diabetes; 7 participants of non-European descent were excluded due to genetic variation between populations of different ethnic origin. In addition, 179 participants who did not report a 3–12 h time interval between their final caffeine portion and saliva sampling, were excluded. This is because the paraxanthine/caffeine ratio only reliably measures CYP1A2 enzyme activity when there is a 3–12 h time interval between caffeine intake and provision of the saliva sample, due to the non-linear kinetics of caffeine metabolism [14]. Two participants were excluded due to missing saliva. In 17 participants, the HPLC measurements were below the quantification limit (BQL) of caffeine (BQL = 0.077 μg/ml; n = 5), paraxanthine (BQL = 0.024 μg/ml; n = 5) or both analytes (n = 7); and in 23 participants, the corrected paraxanthine/caffeine ratio was negative (see below). Finally, 14 participants were excluded because of technical difficulties with the HPLC quantification. The final sample comprised 203 participants (57 type-2 diabetes cases and 146 non-type-2 diabetes controls).
The type-2 diabetes status was determined by an affirmative response to the question: “Over the past 12 months, have you suffered from type-2 diabetes?”. As well as, an answer to the question: “If you suffer from type-2 diabetes, when did you receive your diagnosis?”; and a report of a diabetes-appropriate treatment regime of oral medication and/or insulin.
Questionnaire assessment
Questionnaires gathered information regarding demographic status, health and habitual caffeine intake. The survey was completed either online (2ask® survey software) or in paper form. Habitual caffeine intake was assessed using an extended version of the caffeine intake questionnaire of the sleep laboratory of the University of Zürich [15]. Participants were asked to report how frequently (per day or per week) they usually consumed a given range of caffeine-containing foods, drinks, medications and supplements. Additional file 1: Table S1 displays the estimated caffeine content (mg/serving) of each item in the questionnaire. These data were used to calculate participants’ daily habitual caffeine intake.
Saliva sampling
Participants gave two samples of saliva at home and then posted them back to the laboratory in a pre-paid envelope (Tyvek® material; DuPont). Beforehand, participants were posted a parcel containing detailed information, a checklist (to record time/date of sampling and caffeinated products consumed that day), and two saliva receptacles [1) Salivette® swab (Sarstedt, Nümbrecht, Germany) to determine caffeine and paraxanthine concentrations; 2) Oragene® DNA kit (DNA Genotek Inc., Ottawa, Canada) for DNA extraction and genotyping]. Participants were instructed to give both saliva samples at bedtime, and without eating, drinking, chewing gum or smoking in the thirty minutes beforehand; and also to complete the checklist. Contact details of the research team were available in case participants needed assistance.
Genomic assessment with salivary DNA
Oragene receptacles were stored at room temperature until genomic DNA was extracted from saliva according to DNA Genotek Inc.’s instructions. Participants were genotyped for the functional rs762551 polymorphism of the CYP1A2 gene, a demonstrated determinant of inducible CYP1A2 activity [8, 16], and labelled ‘highly inducible’ or ‘less inducible’ caffeine metabolizers (A/A genotype = ‘highly inducible’; A/C and C/C genotypes = ‘less inducible’). All genetic analyses were replicated at least once for independent confirmation of the results. Experimental protocols are described in Additional file 1.
HPLC assessment of salivary caffeine and paraxanthine
The saliva samples were delivered to the laboratory at room temperature. Upon receipt, the salivettes were stored immediately at −20 °C. The stability of salivary caffeine and paraxanthine concentrations over 14 days at room temperature has previously been confirmed [17].
After thawing, saliva was extracted from the Salivette® according to the manufacturer’s instructions (centrifugation for 2 min at 1,000 g). Salivary caffeine and paraxanthine concentrations were quantified by HPLC, coupled to a UV detector, essentially as described by Fuhr and Rost [11], but with minor modifications as described in Additional file 1.
The stability of salivary caffeine and paraxanthine concentrations during long-term storage at −20 °C was confirmed in a sub-sample (n = 7). Saliva was analyzed at two time points, ten months apart. Statistical comparisons revealed that there was no significant difference between the caffeine concentrations at the two time points: 2.590 ± 1.573 (SD) vs. 2.523 ± 1.351 μg/ml (p > 0.8; paired-sample t-test). There was also no significant difference between the paraxanthine concentrations at the two time points: 0.920 ± 0.461 (SD) vs. 0.789 ± 0.341 μg/mL (p > 0.2).
Determination of corrected paraxanthine/caffeine ratio
The present field study participants reported varied caffeine consumption on the day of saliva sampling, and varied time intervals between their last caffeine intake and the saliva sampling. While Perera and colleagues [18] demonstrated that CYP1A2 activity can be reliably assessed without a 24-h period of caffeine abstinence, assessment of CYP1A2 phenotypes is very time-dependent [19–22]. Correlation analyses between immunoreactive CYP1A2 in the liver, intrinsic clearance for caffeine-3-demethylation to paraxanthine, and various plasma, saliva, and urine based CYP1A2 metrics showed that the saliva paraxanthine/caffeine ratio 6 h after caffeine intake had the best correlation to intrinsic caffeine-to-paraxanthine clearance, which is the “gold standard” for CYP1A2 activity assessment [9]. That is, six hours post caffeine dose, the molar concentration ratio of salivary paraxanthine to caffeine provides the most valid estimate of CYP1A2 enzyme activity [9, 11]. We therefore developed a method to adjust the CYP1A2 activity ratio values to the optimal 6-h post-dose sampling time point, and to thus allow direct comparison within and between groups.
Spigset and colleagues [14] investigated the relationship between sampling time and individual salivary paraxanthine/caffeine ratios, after intake of a single oral dose of 200 mg caffeine, in 12 healthy, young men in a controlled, laboratory setting. Based on inspection of Fig. 1 in their publication, the lowest and highest paraxanthine/caffeine ratio, at each time point, was recorded. The mean of the lowest and highest ratio was then calculated and entered in GraphPad Prism (La Jolla, California, USA). After fitting a curve to the data set, the equation y = 0.016 + (0.141 * x) + (−0.004 * x 2 ) was used to estimate the participants’ paraxanthine/caffeine ratio (‘y’), if the time span between last caffeine intake and provision of saliva (‘x’) was known. A time span of 6 h equates to a mean paraxanthine/caffeine ratio of 0.725.
Relationship between sampling time and salivary paraxanthine/caffeine ratio. Solid line: mean observed ratio estimates based on data of Spigset et al. [14]. Error bars show standard deviation across the mean of observed ratio data (n = 12). Dotted line: ratio based on fitted curve (dotted line) using a second-order polynomial model: Y = A + (B x X) + (C x X 2 ). Best-fit values (95 % confidence intervals): A = 0.016 (-0.206 - 0.238); B = 0.141 (0.090 - 0.191); C = -0.004 (-0.006 - -0.002). Equation: Y = 0.016 + (0.141 x X) + (-0.004 x X 2 ); Y = paraxanthine/caffeine ratio; X = time interval between final caffeine intake and saliva sampling
Figure 2 shows the relationship between the time interval between caffeine intake and saliva sampling, and the paraxanthine/caffeine ratio for both the mean observed ratios based on the data of Spigset et al. [14], and the ratio based on the fitted curve using the above-mentioned equation. The fitted curve explained roughly 70 % (R2 = 0.702) of the variance in the observed ratio data.
Paraxanthine/caffeine ratios in type-2 diabetes patient and non-type-2 diabetes control groups. Boxplots represent paraxanthine/caffeine ratios corrected to an “ideal” time interval between last caffeine intake and saliva sampling of 6 h (box: 25th percentile, median and 75th percentile; whiskers = 10th to 90th percentiles; dots: individual data points outside of the whisker range). Statistics compared type-2 diabetes patient (n = 57) and non-type-2 diabetes control (n = 146) groups by independent samples t-test on square-rooted data (2-tailed; equal variances assumed). Statistical analysis with the non-parametric Mann-Whitney U-test on non-transformed corrected paraxanthine/caffeine ratios confirmed the robustness of the result: T2D vs. non-T2D: mean rank 120.93 vs. 94.61; exact sig. 2-tailed: p = 0.004)
To estimate the participants’ paraxanthine/caffeine ratio adjusted to the ‘ideal’ time interval of 6 h, several steps were taken. First, based on the molar concentrations of paraxanthine and caffeine, the ‘actual’ paraxanthine/caffeine ratio was calculated for each participant. Next, the Spigset-derived equation was used to estimate the ‘correct’ mean ratio, based on participants’ reported time interval between caffeine intake and saliva sampling. The difference between the ‘actual’ ratio and the ‘correct’ mean ratio was then calculated. Finally, participants’ time-corrected paraxanthine/caffeine ratio, to assume a 6 h ‘ideal’ time span, was determined by adding this difference to the equation’s estimate of the paraxanthine/ caffeine ratio at a 6-h time interval (0.725). Graphically spoken, the individual ratio value was shifted on a curve with the slope of the Spigset data-derived mean curve to the ideal time of 6 h. The paraxanthine/caffeine ratio data, derived from our time-correction technique, ranged from 0.00 to 2.85 (mean: 0.577 ± 0.411 [SD]; n = 203).
Statistical analyses
Analyses were performed with Microsoft Excel 2010 (Microsoft Corp., Seattle, USA) and IBM SPSS Statistics 22 (IBM Corp., Armonk, USA), and adhered to documented statistical principles [23]. Mean values (± standard deviations) of raw data are reported and significance was set at α < 0.05. Continuous variables that were not normally distributed (based on visual inspection of histogram and SPSS-derived skewness score −1 > x <1) were transformed to approximate a normal distribution. Table legends indicate the successful transformation method. If data were missing for a variable, the smaller sample size for that variable was reported with the results. Data from type-2 diabetes and non-type-2 diabetes groups were compared by Fisher’s Exact Test (nominal data) and independent samples t-test (normally distributed continuous data). Independent samples t-tests were used to compare the corrected paraxanthine/caffeine ratio data grouped by variables that were previously reported to influence CYP1A2 enzyme activity, including age (≤ mean age of 59.3 years vs. > mean age), body mass index (underweight/healthy ≤ 24.9 vs. overweight/obese > 24.9), habitual caffeine intake [lower/normal habitual caffeine intake (≤ Swiss average of 288 mg/day) vs. higher habitual caffeine intake (> Swiss average)], contraceptive pill (no vs. yes), CYP1A2 -163C > A genotype (A/C and C/C allele carriers vs. A/A allele carriers), gender (male vs. female), insulin administration (no vs. yes), long-term medication (no vs. yes) and smoking (non smoking vs. smoking). Within the type-2 diabetes group, the corrected paraxanthine/caffeine ratio data was compared between selected binary covariates (habitual caffeine intake, CYP1A2 inducibility, gender, insulin administration, smoking) by independent samples t-test on raw data that approximated a normal distribution.
If there was a statistical difference in the results of the independent samples t-tests across the whole sample, which compared the corrected ratio data grouped by variables previously reported to influence CYP1A2 activity, then that covariate was included in a multiple regression analysis (simultaneous entry). The regression model tested the association between the selected variables and the paraxanthine/caffeine ratio across the whole group. The outcome variable was the corrected paraxanthine/caffeine ratio, transformed by square root to approximate a normal distribution. The two predictors were type-2 diabetes status (nominal; binary) and high (> Swiss average of 288 mg/day) caffeine intake (nominal; binary). ‘Insulin’ was not included as a predictor variable since it was only administered by type-2 diabetes patients, and thus its inclusion would confound the results. The model was tested to ascertain that it met the statistical assumptions of multiple regression [23, 24].
Results
Table 1 reports the characteristics of the type-2 diabetes cases and the non-type-2 diabetes controls. The groups differed in age, gender, body mass index, long-term medication intake and oral contraceptive use.
The total self-reported habitual caffeine intake was 96.5 mg higher per day in the type-2 diabetes group (Table 1). Coffee was the major source of caffeine for both groups. Despite the shorter time interval between saliva sampling and the final portion of caffeine intake in the patients, the mean salivary concentration of paraxanthine was significantly higher in the type-2 diabetes patient group than in the control group (Table 1).
The -163C > A allele frequencies of the CYP1A2 gene were similar in type-2 diabetes and non-type-2 diabetes groups (Table 1). However, compared to the control group, there was a higher proportion of diabetes participants with the highly-inducible A/A genotype and a lower proportion of diabetes participants with the less-inducible A/C and C/C genotypes (Table 1).
As illustrated in Fig. 1, the mean time-corrected paraxanthine/caffeine ratio was significantly higher in type-2 diabetes cases than in non-type-2 diabetes controls (type-2 diabetes patients: 0.700 ± 0.426; non-type-2 diabetes controls: 0.529 ± 0.396; p = 0.010, two-tailed t-test). This finding indicates a higher mean CYP1A2 enzyme activity in the group of type-2 diabetes patients. When those patients who reported to take insulin were excluded, the difference was no longer significant (0.664 vs. 0.529; p = 0.121). Indeed, CYP1A2 enzyme activity was significantly faster in participants who administered insulin (Table 2). When only type-2 diabetes patients were assessed, however, the difference was not significant (0.664 vs. 0.770; p = 0.382).
It is estimated that the Swiss population consumes roughly 288 mg caffeine per capita and day [12, 25]. Participants who reported habitually consuming higher amounts of caffeine (>288 mg/day), showed significantly faster CYP1A2 enzyme activity, compared to participants consuming less caffeine (≤288 mg/day) (Table 2). Within the type-2 diabetes sample, patients who reported habitually consuming more caffeine than the population average showed a numerically faster CYP1A2 activity (0.763 vs. 0.638), but not to a significant degree (p = 0.276).
The CYP1A2 genotype, gender and smoking status had no significant effect on the mean time-corrected paraxanthine/caffeine ratio (Table 2).
Multiple regression analysis was used to predict the paraxanthine/caffeine ratio across the whole sample (Table 3). The two selected predictors of CYP1A2 enzyme activity, i.e., type-2 diabetes status and higher caffeine intake (see Methods), significantly predicted the paraxanthine/caffeine ratio (F2,200 = 5.580, p = 0.004). While they accounted for only 5.3 % of the variation in the data, both made statistically significant contributions to the prediction (T2D status: Beta = 0.146; p = 0.040; higher caffeine intake: Beta = 0.146; p = 0.041). When the regression model was run with caffeine intake as a continuous variable, the model also predicted the paraxanthine/caffeine ratio (ANOVA: F2,200 = 5.298, p = 0.006), with type-2 diabetes being a significant predictor (Beta = 0.145; p = 0.044) and caffeine intake exhibiting a strong trend to predict the ratio (Beta = 0.137; p = 0.056).
Discussion
In support of our hypothesis, the main finding of this field study was that CYP1A2 enzyme activity was significantly higher in a type-2 diabetes group compared to a control group. Since caffeine is almost completely metabolized by CYP1A2 [1], this faster enzyme activity indicates a faster metabolism of caffeine in the type-2 diabetes participants. Indeed, patients’ salivary concentrations of paraxanthine, caffeine’s major metabolite [1], were significantly higher at bedtime. The results indicate that the previously described inducing effect of caffeine on its own CYP1A2-mediated metabolism may also be present in type-2 diabetes patients.
We used a novel correction technique to adjust participants’ paraxanthine/caffeine ratios, an established marker of CYP1A2 enzyme activity, to account for the varied reported time intervals between last caffeine intake and saliva sampling. Based on the equation we derived from published data [14], the paraxanthine/caffeine ratio of the present participants was adjusted to reflect a ratio that would stem from the ‘ideal’ time interval of 6 h [9, 11]. The time-corrected paraxanthine/caffeine ratios obtained with this method were comparable to published reports [11], which supports the validity of our approach.
In accordance with previous research [4], participants who habitually consumed higher amounts of caffeine showed higher paraxanthine/caffeine ratios, and thus faster CYP1A2 enzyme activity. Participants administering insulin also showed faster CYP1A2 activity. While this relationship has previously not been directly assessed in humans, rodent models demonstrate that insulin induces CYP1A2 activity [5]. Moreover, an observational study in humans linked higher CYP1A2 activity with higher endogenous insulin levels [6]. These results further help to support the validity of our correction technique. Nevertheless, our technique needs to be validated in larger and stringently controlled samples, alongside comparison with systemic caffeine clearance data. If these future studies are successful, the correction could be applied, for example, in epidemiological settings where varied time frames between caffeine intake and saliva sampling are allowed. Here we used this correction only for data of participants who reported caffeine consumption within the time window of 3 to 12 h before saliva sampling. This is because, compared to the ‘gold standard’ approach of estimating CYP1A2 enzyme activity (systemic caffeine-to-paraxanthine clearance from blood) [9], the salivary paraxanthine/caffeine ratio only accurately reflects CYP1A2 activity during this time interval [14].
Interestingly, we found that the time interval between the final caffeine portion and saliva sampling was shorter in the patient group than in the control group. Thus, if both groups had equal CYP1A2 enzyme activity, a lower amount of caffeine would have been metabolized in the patients by the time of saliva sampling and a smaller salivary paraxanthine concentration should have been observed. By contrast, the paraxanthine concentration was higher in the type-2 diabetes participants, consistent with our conclusion that CYP1A2 activity was higher in the patients than in the controls.
The time-corrected paraxanthine/caffeine ratio was higher in study participants who reported higher caffeine consumption than the mean Swiss caffeine intake of 288 mg/day. Furthermore, statistical modelling revealed that high habitual caffeine intake was a significant predictor of faster CYP1A2 enzyme activity in our study sample. While type-2 diabetes status also contributed to the prediction of the paraxanthine/caffeine ratio, we suggest that out of the two predictor variables, caffeine intake was potentially the stronger mediator of faster caffeine metabolism. This is because caffeine is a known inducer of CYP1A2 activity [4, 26, 27], and the diabetes patients of the present study consumed larger amounts of caffeine (Table 1). Nineteen out of 57 patients administered insulin that has also been described as an inducer of CYP1A2 activity. When only type-2 diabetes patients were assessed, however, there were no significant differences in CYP1A2 activity between insulin users and non-users. This result suggests that insulin may not be a key driver of CYP1A2 activity in our study participants. Nevertheless, larger samples are needed in future studies to corroborate the existence of higher CYP1A2 activity in the type-2 diabetes patient population, and that this higher activity is due primarily to high caffeine intake.
We found no effect of age and BMI on CYP1A2 enzyme activity (Table 2). This finding was in-line with previous research [4]. Furthermore, there was no significant difference between CYP1A2 enzyme activity of participants who reported taking medication over the long-term, compared to participants that were not taking medications. This finding may reflect that medication has varying effects on CYP1A2 activity (inhibition, induction, or no effect), which are drug-specific [2]. Because we did not collect information regarding the specific medications of participants, it is impossible to further qualify this result.
In contrast to previous studies [4, 8], female gender, contraceptive pill use, smoking, and CYP1A2 genotype also revealed no significant effect on CYP1A2 activity. Female gender has only a small influence on the paraxanthine/caffeine ratio [4], and our sample was probably not large enough to show a significant effect. In addition, the numbers of participants who reported taking oral contraceptives (n = 9) and smoking (n = 21), two fairly strong modulators of CYP1A2 activity [4], were low. These low participant numbers may explain why significant effects of these covariates were not seen. The paucity of smokers may also explain why, in the present data set, the CYP1A2 -163 A > C genotype had no significant effect on CYP1A2 enzyme activity and speed of caffeine metabolism; since the more pronounced increase in CYP1A2 activity caused by this genetic variation is only observed in current smokers [7, 8].
The exact mechanism that links caffeine intake to speed of caffeine clearance is not yet fully understood. Animal studies have shown increased liver microsome CYP1A2 activity and mRNA levels in rats on very high doses of caffeine [26, 27]. This observation indicates an auto-induction of caffeine on CYP1A2 [4]. Support also comes from epidemiological studies, where a 1.45-fold higher CYP1A2 activity was observed per daily liter of coffee intake [2, 4]. Another suggestion is that persons with existing high CYP1A2 activity may consume more coffee because they metabolize it more quickly [28]. Coffee is a complex blend of organic compounds and therefore, constituent substances, aside from caffeine, may also contribute to its inducing effect [4]. For example, coffee beans are roasted at high temperatures, and thus may contain compounds similar to those found in tobacco smoke or chargrilled meats - known inducers of CYP1A2 activity [29]. Moreover, coffee’s diverse composition roots the existing controversy between coffee and caffeine consumption and risk of type-2 diabetes (see [30] for review). The limitations of this study include the reliance on self-reports to determine the timing of saliva sampling and the lack of information regarding habitual consumption of some dietary components known to influence CYP1A2 activity, e.g., chargrilled meat, as well as the intake of specific medications. Also habitual caffeine intake was measured by self-report questionnaire. While the validity of this method is established [12, 31], variability exists in the amount of caffeine per serving [32]. Therefore, caffeine use may have been under- or overestimated. The correction technique applied to the paraxanthine/caffeine ratios needs further, external validation. The fitted curve explained roughly 70 % of the variance in the Spigset data set [14], leaving 30 % unexplained. However, Fig. 1 suggests that this proportion of unexplained variance lies at time points greater than 12 h. We used the equation-derived paraxanthine/caffeine ratio at the 6 h time point. Finally, despite the regression model significantly predicting the paraxanthine/caffeine ratio, its explanatory capacity was low. This indicates that other, unknown or unmeasured predictor variables were also influencing CYP1A2 activity in our study sample. Previously, it has been noted that a large proportion of CYP1A2 activity is currently unexplained [2].
Conclusions
In conclusion, while various factors probably influence CYP1A2 activity, high caffeine intake likely plays an important role. Here, we provide evidence that a positive association between caffeine consumption and CYP1A2 activity is present in our type-2 diabetes patient sample. Future studies are warranted to establish whether higher CYP1A2 enzyme activity is indeed causally related to high caffeine intake.
Abbreviations
- BMI:
-
Body mass index
- BQL:
-
Below quantification limit
- CYP1A2:
-
Enzyme cytochrome P450 1A2
- CYP1A2 :
-
Gene cytochrome P450 1A2
- HPLC:
-
High performance liquid chromatography
- SNP:
-
Single nucleotide polymorphism
- T2D:
-
Type-2 diabetes
References
Gu L, Gonzalez FJ, Kalow W, Tang BK. Biotransformation of caffeine, paraxanthine, theobromine and theophylline by cDNA-expressed human CYP1A2 and CYP2E1. Pharmacogenetics. 1992;2(2):73–7.
Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97:125–34.
Schrenk D, Brockmeier D, Mörike K, Bock KW, Eichelbaum M. A distribution study of CYP1A2 phenotypes among smokers and non-smokers in a cohort of healthy Caucasian volunteers. Eur J Clin Pharmacol. 1998;53:361–7.
Tantcheva-Poór I, Zaigler M, Rietbrock S, Fuhr U. Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharmacogenetics. 1999;9(2):131–44.
Barnett CR, Wilson J, Wolf CR, Flatt PR, Ioannides C. Hyperinsulinemia causes a preferential increase in hepatic P4501A2 activity. Biochem Pharmacol. 1992;43(6):1255–61.
Hong CC, Tang BK, Hammond GL, Tritchler D, Yaffe M, Boyd NF. Cytochrome P450 1A2 (CYP1A2) activity and risk factors for breast cancer: a cross-sectional study. Breast Cancer Res. 2004;6:R352–65.
Sachse C, Bhambra U, Smith G, Lightfoot TJ, Barrett JH, Scollay J, Garner RC, Boobis AR, Wolf CR, Gooderham NJ. Polymorphisms in the cytochrome P450 CYP1A2 gene (CYP1A2) in colorectal cancer patients and controls: allele frequencies, linkage disequilibrium and influence on caffeine metabolism. Br J Clin Pharmacol. 2003;55:68–76.
Sachse C, Brockmöller J, Bauer S, Roots I. Functional significance of a C to A polymorphism in intron 1 of the CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47(4):445–9.
Fuhr U, Rost KL, Engelhardt R. Evaluation of caffeine as a test drug for CYP1A2. NAT2 and CYP2E1 phenotyping in man in vivo versus in vitro correlations. Pharmacogenetics. 1996;6:159–76.
Streetman DS, Bertino Jr JS, Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216.
Fuhr U, Rost KL. Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and saliva. Pharmacogenetics. 1994;4:109–16.
Urry E, Jetter A, Holst SC, Berger W, Spinas GA, Langhans W, Landolt HP. Relationships among type 2 diabetes, sleepiness, and habitual caffeine intake: a case–control field study. J Psychopharmacol. 2016. In press. doi:10.1177/0269881116668595.
Matzke GR, Reginald FF, John JE, Robert JS, Stanley WC. Evaluation of the influence of diabetes mellitus on antipyrine metabolism and CYP1A2 and CYP2D6 activity. Pharmacotherapy. 2000;20(2):182–90.
Spigset O, Hägg S, Söderström E, Dahlqvist R. The paraxanthine:caffeine ratio in serum or in saliva as a measure of CYP1A2 activity: when should the sample be obtained? Pharmacogenetics. 1999;9:409–12.
Rétey JV, Adam M, Khatami R, et al. Genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81:692–8.
Han XM, Ou-Yang DS, Lu PX, et al. Plasma caffeine metabolite ratio (17X/137X) in vivo associated with G-2964A and C734A polymorphisms of human CYP1A2. Pharmacogenetics. 2001;11(5):429–35.
Perera V, Gross AS, McLachlan AJ. Caffeine and paraxanthine HPLC assay for CYP1A2 phenotype assessment using saliva and plasma. Biomed Chromatogr. 2010;24:1136–44.
Perera V, Gross AS, Xu H, McLachlan AJ. Pharmacokinetics of caffeine in plasma and saliva, and the influence of caffeine abstinence on CYP1A2 metrics. J Pharm Pharmacol. 2011;63:1161–8.
Grant DM, Tang BK, Kalow W. Variability in caffeine metabolism. Clin Pharmacol Ther. 1983;33:591–602.
Kalow W, Tang BK. The use of caffeine for enzyme assays: a critical appraisal. Clin Pharmacol Ther. 1993;53:503–14.
Rostami-Hodjegan A, Nurminen S, Jackson PR, Tucker GT. Caffeine urinary metabolite ratios as markers of enzyme activity: a theoretical assessment. Pharmacogenet. 1996;6:121–49.
Labedzki A, Buters J, Jabrane W, Fuhr U. Differences in caffeine and paraxanthine metabolism between human and murine CYP1A2. Biochem Pharmacol. 2002;63:2159–67.
Field A. Discovering statistics using IBM SPSS statistics. London: Sage; 2013.
Babyak MA. What You See May Not Be What You Get: A Brief, Nontechnical Introduction to Overfitting in Regression-Type Models. Psychosom Med. 2004;66(3):411–21.
Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51(1):83–133.
Chen L, Bondoc FY, Lee MJ, Hussin AH, Thomas PE, Yang CS. Caffeine induces cytochrome P4501A2: induction of CYP1A2 by tea in rats. Drug Metab Dispos. 1996;24:529–33.
Goasduff T, Dréano Y, Guillois B, Menez JF, Berthou F. Induction of liver and kidney CYP1A1/1A2 by caffeine in rat. Biochem Pharmacol. 1996;52:1915–9.
Landi MT, Zocchetti C, Bernucci I, Kadlubar FF, Tannenbaum S, Skipper P, Bartsch H, Malaveille C, Shields P, Caporaso NE, Vineis P. Cytochrome P4501A2: enzyme induction and genetic control in determining 4-aminobiphenyl-hemoglobin adduct levels. Cancer Epidemiol Biomarkers Prev. 1996;5:693–8.
Fontana RJ, Lown KS, Paine MF, Fortlage L, Santella RM, Felton JS, Knize MG, Greenberg A, Watkins PB. Effects of a chargrilled meat diet on expression of CYP3A, CYP1A, and P-glycoprotein levels in healthy volunteers. Gastroenterology. 1999;117:89–98.
Palatini P, Benetti E, Mos L, Garavelli G, Mazzer A, Cozzio S, Fania C, Casiglia E. Association of coffee consumption and CYP1A2 polymorphism with risk of impaired fasting glucose in hypertensive patients. Eur J Epidemiol. 2015;30(3):209–17.
Addicott MA, Yang LL, Peiffer AM, Laurienti PJ. Methodological considerations for the quantification of self-reported caffeine use. Psychopharmacology. 2009;203:571–8.
Bracken MB, Triche E, Grosso L, Hellenbrand K, Belanger K, Leaderer BP. Heterogeneity in assessing self-reports of caffeine exposure: implications for studies of health effects. Epidemiology. 2002;13:165–71.
Acknowledgements
The authors thank all study participants, as well as colleagues at the Institutes of Pharmacology and Toxicology and of Medical Molecular Genetics (Director: Prof. Wolfgang Berger) at the University of Zürich. Specifically, Dr. Roland Dürr (data collection and analysis infrastructure), Ms. Daria Erkhova (advertising, recruitment, saliva and data handling), Mr. Thomas Müller and Mr. Simon Vogt (HPLC laboratory assistance) are gratefully acknowledged for their help in data collection and analyses.
Funding
The study was supported by the Clinical Research Priority Program Sleep & Health of the University of Zürich and the Swiss National Science Foundation (grant # 320030_135414).
Availability of data and materials
All relevant data and material are presented in the main paper and Additional file 1, and will be made publicly available upon publication of the paper.
Authors’ contributions
The present study has been conceived and designed by HPL, AJ, and EU. The experiments were performed by EU. Samples and data were analyzed by AJ and EU. Data were interpreted by AJ, EU, and HPL. The manuscript was written, revised and approved by EU, HPL, and AJ. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the review board of the ethics committee at the ETH, Zürich (ethics number: EK 2012-N-53). Experimental protocols were conducted according to the principles of the Declaration of Helsinki. Participants were recruited via advertisements at hospitals, in magazines and at public seminars throughout German-speaking Switzerland. The data were collected after written informed consent was obtained, analyzed anonymously and individual results kept confidential.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
(Caffeine content of products available for consumption in German-speaking Switzerland) and experimental protocols (genomic assessment with salivary DNA; HPLC assessment of salivary caffeine and paraxanthine). (DOCX 39 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Urry, E., Jetter, A. & Landolt, HP. Assessment of CYP1A2 enzyme activity in relation to type-2 diabetes and habitual caffeine intake. Nutr Metab (Lond) 13, 66 (2016). https://doi.org/10.1186/s12986-016-0126-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-016-0126-6