Abstract
Background
Human papillomavirus (HPV) is among the leading cause of sexually transmitted infections, particularly prevalent among sexually active individuals. While many HPV infections clear up over time, some may progress to various cancers such as anal cancer, cervical cancer and, vaginal cancer. This study examines the prevalence of different HPV genotypes, classified as high-risk (HR) and low-risk (LR), among females of various age groups who visited the laboratory in Karaj.
Material and methods
Genital specimens were gathered from the individuals involved in the study and subjected to DNA extraction (DNA/RNA extraction AmpliSense, Moscow, Russia) followed by amplification using Real-Time PCR. HR- and LR-HPV genotypes were identified using the GenoFlow HPV Array test kit (GenoFlow; DiagCor Bioscience, Hong Kong) and homemade HPV genotyping kit. Demographic information such as age, was examined alongside statistical virological data.
Results
Overall, 367 (17%) out of the 2109 (100%) female cases tested positive for HPV. Among these, 219 (46.2%) were classified as low-risk, 44 (9.3%) as potentially high-risk, and 211 (44.5%) as high-risk. The highest percentage of positive test results was detected in individuals under 30 years old (35%) and those aged 40–50 (18%). Individuals in the < 30 age group were primarily infected with HR genotypes. The most commonly identified genotypes overall were HPV-16 (11.7%), HPV-54 (10.3%), HPV-56 (8.4%), HPV-40 (8.1%). The lowest frequency was observed for HPV-70, HPV-71, HPV-82, and HPV-90, each recorded in only a single case.
Conclusion
Our results highlight the notable occurrence of HPV among females who visited the laboratory in Karaj, especially in the < 30 age group. Identifying HPV-16 as the most prevalent genotype in our examination highlights the necessity of tailored interventions for specific age ranges. While HPV-16 is covered by vaccination programs, HPV-54 and HPV-56 are not, emphasizing the need for effective screening and preventive plans to manage the consequences of HPV-related diseases in future.
Similar content being viewed by others
Introduction
Human Papillomavirus (HPV) is a double-stranded DNA virus which belongs to Papillomaviridae family. This virus is among the predominant causes of sexually transmitted infections in both women and men, with an estimated nearly all sexually active individuals being infected by the virus at some point in their lives [1, 2]. Globally, around 690,000 new cancer diagnoses annually are linked to HPV infection [3]. While the majority of HPV infections are transitory and benign, certain infections can endure for extended periods and carry significant risk for developing various cancers, including cervical cancer, head and neck cancer, oropharyngeal squamous cell carcinomas, penile cancer and, anal cancers [4, 5].
Over 200 genotypes of HPV have been recognized to date, with nearly 40 capable of infecting genital regions. These genotypes are additionally categorized into low-risk HPV (LR-HPV) and high-risk HPV (HR-HPV) according to their potential for causing cancer. LR-HPV types (including HPVs 6, 11, 40, 42, 43, 44, 55, 61, 81, 83) are robustly associated with diseases but not cancer and are commonly found in benign or mildly abnormal cervical tissue as well as genital warts [6, 7]. As well, the International Agency for Research on Cancer (IARC) categorizes twelve HPV genotypes as HR-HPV, including HPVs 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 [8]. It is widely recognized that persistent infection with HR-HPV is the primary contributor to cervical cancer, with HPV-16 and HPV-18 specifically linked to over 70% of cases [9, 10]. Worldwide, cervical cancer ranks as the fourth most frequently occurring cancer in women [11]. Despite a noticeable decrease seen globally in recent decades, cervical cancer continues to have high incidence and mortality rates among women, particularly prevalent in low- and middle-income countries [12].
There are some known methods to address HPV-related cancer. The initial preventive measure is the HPV vaccine, while the second is screening programs. Since 2006, there has been advancement in the global expansion of HPV vaccination, with approximately two-thirds (127 out of 194) of countries implementing HPV vaccination programs (as of 2022) [13]. Currently, three HPV vaccines are commonly used: the bivalent vaccine (Cervarix), offering defense against HPV-16 and HPV-18; the quadrivalent vaccine (Gardasil), safeguarding against four HPV strains (6, 11, 16, and 18); and the nonavalent (Gardasil-9) vaccine, which prevents infection by HPV genotypes 6, 11, 16, 18, 31, 33, 45, 52, and 58 [14]. Even though the vaccine provides immunity against a substantial portion of HPV infections, there remains a potential risk of HPV-related diseases from the remaining strains, even after vaccination [15]. Furthermore, in regions where HPV vaccination programs are not yet established, conducting screening and HPV genotyping can bolster public health efforts by enabling early detection and prevention of HPV-related cancers. It’s crucial to understand the prevalence of HPV types in each country to devise effective vaccines and national vaccination strategies. Regional HPV genotype data is vital for shaping public health policies and evaluating the impact of current vaccines or the necessity for tailored vaccine formulations in specific areas.
The primary aim of this research is to assess the prevalence of various HPV genotypes, categorized into HR and LR, across different age groups in females who visited the laboratory in Karaj, Iran. These results could aid in guiding future research endeavors, shaping public health strategies, and informing the development of vaccines and national vaccination programs.
Material and methods
Study population and sample collection
This investigation, conducted between March 2022 and February 2024, involved cooperation with the Clinical Virology Research Center (RCCV) at Tehran University of Medical Sciences. It encompassed a total of 2109 genital samples, all exclusively collected from female individuals. Samples were collected from genital sites of individuals. These samples collected from females visiting laboratories in Karaj city, who either had recent high-risk sexual behavior, had an abnormal Pap smear result and were referred by their doctor for further testing, having genital warts or had a sexual partner infected with HPV, prompting them to seek HPV testing for health reassurance. Participants who agreed to join the study were asked to complete a written consent form.
HPV tests are conducted in the laboratory three days a week, on alternating days. Samples received on testing days are processed immediately, while those received on non-testing days are stored in a refrigerator at 4 °C and tested on the next scheduled day. After testing, each sample is kept at 4 °C for up to six months. The sample collection containers, which contain LBC medium with IVD, are used by gynecologists and urologists to collect samples using a silicone brush. For women, samples are collected from the vaginal area and secretions, and if warts are present, samples are also taken from the warts. The criteria were expanded to include individuals advised by their healthcare provider to undergo testing due to abnormal cytological results, as well as women engaging in high-risk sexual behavior, with a sexual partner infected with HPV, showing suspicious symptoms, or having genital warts, who sought HPV testing for reassurance about their health status.
DNA extraction and PCR
Sample pre-amplification, DNA extraction, and HPV genotyping were performed in the Molecular Genetics Department of the laboratory, following protocols set by the quality control supervisor and overseen by RCCV. HPV-DNA extraction utilized the DNA/RNA extraction AmpliSense kit (AmpliSens, Moscow, Russia), following the manufacturer’s instructions. PCR analysis of the extracted genome was performed using an HPV detection kit, with genotyping conducted using GenoFlow HPV Array test kit (GenoFlow; DiagCor Bioscience, Hong Kong) HPV genotyping and homemade HPV genotyping kit. For the early detection of HPV genotypes, we initially used the GenoFlow HPV Array test kit. This kit is capable of identifying 17 HR-HPV genotypes, including 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82, as well as 15 LR-HPV genotypes, including 6, 11, 40, 42, 43, 44, 54, 55, 57, 61, 70, 71, 72, 81, and 84. For validation and to detect additional HPV genotypes, we subsequently employed a homemade HPV genotyping kit, which has the capability to identify 32 HR and LR HPV viral genotypes, including 6, 11, 16, 18, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 68, 69, 70, 71, 74, 81, 82, 83, and 90. The homemade assay utilized in this study is a real-time PCR based method designed for the detection and quantification of DNA. To ensure the accuracy and reliability of the assay, an internal control gene, beta-globulin, is included.
Statistical analysis
Data were characterized using measures such as frequency, median, mean, and standard deviation. To assess the comparability of HPV-positive and HPV-negative cases across various age groups, a chi-square test was conducted, with a significance level set at 5%. Analysis was conducted utilizing the statistical software IBM SPSS-27.
Results
Demographic data
A total of 2109 female subjects were analyzed, with 367 (17.4%) testing positive for HPV. Thus, the prevalence of HPV infection among the referred females was approximately 17%. Since, the exact age of subjects was recorded only for HPV positive subjects, the mean and standard deviation of age only computed for this group. Amongst HPV positives, the youngest and the oldest subjects were 16 and 68 years old, respectively. The mean of their age was 36 with a standard deviation of 9.6. For HPV negative subjects, age was recorded in some categories. Therefore, all ages were categorized for the subsequent analyses. The contributors were categorized into four age groups for examination. Table 1 presents the frequency and percentage of infection in each age group. The highest proportion of positive test results was observed in individuals under 30 years old (35%) and those aged 40–50 (18%). Conversely, the age group between 30 and 40 had a higher number of HPV-positive cases (in terms of the actual number).
Age mean rank in HPV negative subjects was higher than in HPV positives (1076.0 vs. 955.1 respectively) and the difference was significant (Mann–whitney U = 283,001, Z = -− 3848, p-value < 0.001). According to the mann whitney test results, subjects in HPV-negative group were older than in HPV-positives, in average.
Distribution of HPV genotypes
Infected subjects were investigated according to genotype groups. The total number of genotype groups detected was 474. From which, 219 (46.2%) detected genotypes were low-risk, 44(9.3%) were potentially high-risk, and 211 (44.5%) were high-risk. In our subjects, 335 (91.3%) individuals had just one group of genotypes (high risk, low risk, or potentially high risk), 30 (8.2%) had a combination of two genotype groups, and only 2 (0.5%) subjects had all three genotype groups. 282 (76.8%) individuals were single-type infected (only one genotype was detected). 85 (23.2%) subjects were multi-type infected (more than one genotype was detected); among them, 69 had two genotypes, 11 had three and 2 had four genotypes. The highest number of genotypes in one subject was 5. Two individuals had 5 different genotypes. Table 2 displays the frequency of HPV genotype groups across different age groups. Our study found that individuals in the < 30 age group were mainly infected with HR genotypes. In other age groups, the prevalence of low-risk and high-risk genotypes does not show significant differences.
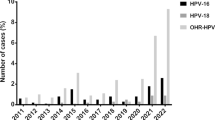
The most frequent genotypes are 16, 54, 56, 40, 42, 59, 66, 6, 44, and 81 respectively. Type 16 had the highest occurrence among HR genotypes, with 43 cases (11.7%), followed by type 56 with 31 cases (8.4%). Among LR genotypes, type 54 had the highest frequency, accounting for 38 cases (10.3%), followed by type 40 with 30 cases (8.1%). The frequency of observed genotypes is shown in Fig. 1.
Prevalence of genotypes. HPV-16 was the most prevalent within the high-risk category, representing 11.7% of cases, succeeded by genotype 56 at 8.4%, with 43 and 31 cases, respectively. Within the low-risk group, genotype 54 exhibited the highest occurrence, comprising 10.3% of cases, trailed by genotype 40 at 8.1%, with 38 and 30 cases, respectively
Discussion
Rates of HPV-related diseases vary depending on factors such as the type of HPV, geographical location, and the specific part of the body sampled [16]. Thus, having epidemiological information on HPV genotypes in each country could prove highly beneficial in devising effective strategies to prevent HPV-related illnesses. This research provides a current examination of HPV genotype distribution among women in Karaj, Iran, employing an accurate molecular method. It should be explicitly stated that the women included in this study were not a random sample of the population. The study population consisted of females visiting laboratories in Karaj city. These individuals were include based on the following criteria: recent high-risk sexual behavior, abnormal Pap smear results leading to a referral by their healthcare provider for further testing, or having a sexual partner infected with HPV, which prompted them to seek HPV testing for reassurance about their health status. These findings may assist in directing future research efforts, influencing public health policies, and contributing to the design of vaccines and national vaccination initiatives.
This research unveiled that 17.4% of the 2109 females tested positive for HPV. Among HPV-positive individuals, our findings indicate that 44.5% harbored high-risk genotypes, 9.3% harbored potentially high-risk genotypes, and 46.2% carried low-risk HPV genotypes. The most frequent genotype was HPV-16. HPV-54 and HPV-56 were the second and third most frequent genotypes, respectively. Even though all existing HPV vaccines safeguard against HPV-16, this high-risk genotype remained the most frequently detected HPV strain among HPV-positive cases in our study (11.7%). This could be attributed to several factors. In countries like Iran, vaccines are supplied by private entities, and insurance companies do not cover the costs, either in full or partially. Consequently, the HPV vaccine remains out of reach and unaffordable for many individuals [17, 18]. Additionally, in Iran, the vaccine is not included in the national immunization schedule [19]. Another contributing factor is the absence of evidence regarding the cost-effectiveness of HPV vaccines in Iran [20, 21]. A study by Khatibi et al. assessed the cost-effectiveness of the quadrivalent HPV vaccine (Gardasil) in Iran found that, the Gardasil is not cost-effective in the country [22]. Moreover, other obstacles such as the high price of vaccines, economic challenges, and insufficient awareness significantly hinder HPV vaccination efforts within the Iranian population [23, 24]. According to an analysis of data from the Iranian Ministry of Health, Mohammadpour et al. found that, the use of Gardasil has seen a significant rise in recent years [20]. However, the current practices of healthcare providers in administering Gardasil are inadequate, resulting in considerable financial strain on both the community and the government. Thus, there is a pressing need for effective national-level interventions and immediate oversight to manage HPV vaccine usage [20].
In line with findings from other research studies, this study identified HPV-16 as the most prevalent HR -HPV type among the study population [25,26,27,28,29,30]. For instance, our results are consistent with the Hassani et al. study, which conducted a community-based survey to determine the prevalence of HPV and the distribution of its genotypes among the general female population in 11 provinces of Iran. The study included 2,562 women aged 15–59 from different regions of the country. This report highlighted that genotype 16 had the highest prevalence among their study population [26]. In another study conducted by Chalabiani, 2969 outpatient and suspected women referred to the Noor pathobiology laboratory from 24 provinces of Iran were examined. The results showed that HPV-16 was the most common high-risk HPV type, representing 179 out of 585 (30.5%) cases [31]. HPV-16 poses the highest risk for cancer development and is a primary target for routine HPV vaccination. While some studies in Iran have identified HPV-52 or HPV-18 as the second most common HR genotype after HPV-16, this study observed HPV-56 as the second most common HR genotype [25, 30, 32,33,34,35]. These disparities in HPV genotype distribution highlight variations in HPV prevalence across different regions.
In our study, among LR genotypes, HPV-54 is the most commonly encountered, followed by HPV-40. However, in several studies carried out across various regions of Iran and globally, the most prevalent LR genotypes is HPV-6 [30, 35,36,37,38]. As well, our analysis demonstrates that the majority of females are infected with LR-HPV genotypes rather than HR HPVs. In line with our findings, the study by Olia et al., which aimed to identify the HPV genotypes causing vaginal infections in women in Urmia, revealed that the majority of infected individuals, 16 out of 30 (53.4%), were associated with low-risk (LR) genotypes [39]. Additionally, a cross-sectional study conducted in Southern Khorasan, eastern Iran, examined 253 randomized pap smear samples from women referred to gynecologist clinics. The study found an HPV prevalence of 18.5% among the participants, with the majority of infected females carrying low-risk HPVs [37]. However, some investigations present contrasting results, such as the Hashemnejad et al. study in Karaj. This cross-sectional research involved 503 Iranian women who were referred to the gynecology clinic at Kamali Hospital in Karaj for routine cervical cancer screening. The study found that approximately 23% (116/503) of the female patients were infected with high-risk HPV genotypes, 9.7% (49/503) had low-risk HPV genotypes, and 7.2% (36/503) had both types [40]. Additionally, the Chalabiani study found that 29.3% (871 out of 2969) of females tested positive for HPV, with approximately 67.2% of these positive cases being infected with high-risk HPVs [31].
In a study conducted in Rasht involving 301 women who visited the medical institute in Rasht, Iran, with an average age of 33.4 ± 6.5 years (ranging from 18 to 61 years), the prevalence of HPV was found to be 36.5% [41]. In a separate investigation involving 12,076 Iranian women who underwent regular examinations from November 2016 to November 2018, it was found that the overall prevalence of HPV was 38.6%. HPV 16 emerged as the most prevailing high-risk genotype among all participants [30]. Additionally, the study demonstrated that in Southern Khorasan, eastern Iran, the prevalence of HPV among a total of 253 women aged 18–65 years old was 18.57% [37]. Another study involving 851 women aged 18–65 years, attending routine gynecological appointments, revealed the detection of nineteen distinct types of human papillomavirus in 31.1% (265 out of 851) of patients [42]. Furthermore, research conducted on Iranian female sex workers suggested that the prevalence of the HPV virus was estimated to be 49.1% [43].
This survey also examined HPV frequency among different age groups and discovered that the highest rate of infection was found in individuals under 30 years old (35%), followed by the 40–50 age group, which had a prevalence of 18%. The data presented here are derived from women who have visited the laboratory. Consistent with our findings, the study by Hamkar et al. similarly demonstrates a higher prevalence of HPV among individuals under the age of 30 [34]. Furthermore, in a cross-sectional study comprising 2453 healthy Iranian women for HPV DNA typing, the highest prevalence was observed among individuals under the age of 25 (15.6%) [44]. These findings underscore the critical importance of developing comprehensive educational programs tailored for high schools to effectively raise knowledge about HPV. However, contrary to this trend, several other studies have indicated a different pattern, noting that the highest prevalence (7.3%) was spotted in the age group of 40–49 [45].
HPV infection rates and their spread varied worldwide depending on age, location, and cytology results. A comprehensive analysis demonstrated that globally, the prevalence of HPV is estimated to be 11.7%. Particularly high rates were found in sub-Saharan Africa (24%), Eastern Europe (21%), and Latin America (16%), whereas West Asia exhibited the lowest prevalence at 2% [46]. According to a systematic review and meta-analysis aimed at estimating the overall prevalence of HPV among Iranian women, an assessment of 26 eligible studies with a total sample size of 5560 revealed an overall HPV prevalence of 23%. The highest prevalence was found in Tehran at 97%, while the lowest was in Isfahan at 2.2% [47]. Based on the Wu EQ et al. survey, HPV prevalence among Chinese women was 14.3% [48]. McQuillan and colleagues found that among American women aged 18–59 years, the prevalence rates were 39.9% for any type of HPV and 20.4% for high-risk HPV strains [49]. The prevalence of HPV infection among young European women is documented at 46% [47]. This variation in HPV prevalence can be explained by multiple factors. Different elements related to lifestyle such as sexual practices, financial situation, knowledge about disease prevention and screening measures, along with the effectiveness of laboratory techniques and the sensitivity of HPV diagnostic methods, can influence the rate of HPV prevalence in different regions [25]. Moreover, factors like insufficient understanding, lack of awareness about how the infection spreads, and doubts regarding the effectiveness of vaccination methods all play a role in elevating prevalence rates [50].
Studies suggest that screening for HR-HPV genotypes can be an efficient first step in detecting cervical cancer, with a sensitivity of 93.9% [51]. Consequently, we investigated the prevalence and occurrence of various HPV genotypes. The most dominant HR genotypes were HPV-16 (11.7%), HPV-56 (8.4%), HPV-59 (6.8%). On a global scale, findings show that HPV-16 is the most commonly detected high-risk strain in cancer, aligning with our own observations [52]. Nevertheless, a study conducted on samples from 1214 women in Mashhad, who underwent cervical cancer screening between 2015 and 2020, revealed that HPV-31 was the most frequent HR-HPV genotype detected [36]. In an analysis done across various provinces in Iran, HPV-52 was identified as the second most prevalent HR genotype, which contrasts with our findings [29, 30]. As we discussed, in our study, HPV-56 is ranked as the second most common high-risk genotype. Yet, in another specific area, a separate genotype is becoming prominent as the second most prevalent HR type, including HPV-68 in Tehran, Iran [28], and HPV-31 in Hungary [53].
The findings of our study emphasize a critical aspect: beyond HPV-16, the genotypes HPV-54, HPV-56, HPV-40, HPV-42, and HPV-59 emerged as the most common strains identified. Importantly, with the exception of HPV-16, current vaccines do not offer protection against these particular genotypes. This gap in coverage underscores the urgent need for the development and implementation of more extensive preventive measures and enhanced screening programs. Expanding our preventive and screening efforts is essential to address these uncovered genotypes effectively and reduce the overall burden of HPV-related diseases.
It's important to recognize certain limitations in our current study. First, all of our participants were females, which may not precisely reflect the definite epidemiology of HPV. As such, these results cannot be attributed to the male population, indicating a need for further research targeting the male population in this region. It is important to highlight that, our results cannot be generalized to the broader population of women. The samples were collected from individuals who specifically visited the laboratory for HPV testing, likely due to significant symptoms or high-risk behaviors. Therefore, the high infection rate observed in this some subgroups reflects the specific context of these sampled individuals rather than the general population. Second, despite our study having a large sample size, additional studies with an even larger total number of participants are necessary to further substantiate our findings. Thirdly, it should be noted that the samples were not randomly selected. In fact, no HPV screening was conducted, and the samples were not obtained through random screening of the general population of Karaj.
Conclusion
This study revealed that 17.4% of the 2109 women tested were positive for HPV in Karaj. These females visited laboratories in Karaj city due to recent high-risk sexual behavior, abnormal Pap smear results prompting further testing by their doctor, the presence of genital warts, or having a sexual partner infected with HPV, leading them to seek HPV testing for health reassurance. Among those with HPV, our results show that 44.5% had high-risk genotypes, 9.3% had potentially high-risk genotypes, and 46.2% had low-risk HPV genotypes. The elevated prevalence of HR HPV subtypes, notably HPV-16, highlights the urgent requirement for proactive interventions, endorsing the incorporation of the HPV vaccine in Iran's national immunization program. Furthermore, following HPV-16, HPV-54, HPV-56, HPV-40, HPV-42, and HPV-59 were the predominant genotypes identified in our study, respectively. These genotypes are not targeted by current vaccines, highlighting the necessity for broader and more comprehensive preventive and screening strategies. Introducing the HPV vaccination alongside an awareness campaign, with a specific focus on females under 30 years old, identified with a notable prevalence of HPV infection in our research, stands as a crucial preventive approach. This tailored intervention holds promise in alleviating the impact of HPV-associated ailments like cancer, consequently fostering enhanced public health outcomes within the area.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Luria L, Cardoza-Favarato G. Human papillomavirus. 2017.
Park IU, Introcaso C, Dunne EF. Human papillomavirus and genital warts: a review of the evidence for the 2015 centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Diseases. 2015;61(suppl_8):S849–55.
Haręża DA, Wilczyński JR, Paradowska E. Human papillomaviruses as infectious agents in gynecological cancers. Oncogenic properties of viral proteins. Int J Mol Sci. 2022;23(3):1818.
Shanmugasundaram S, You J. Targeting persistent human papillomavirus infection. Viruses. 2017;9(8):229.
Alhamlan FS, Alfageeh MB, Al Mushait MA, Al-Badawi IA, Al-Ahdal MN. Human papillomavirus-associated cancers. Microb Pathog Infect Immun. 2021. https://doi.org/10.1007/978-3-030-67452-6_1.
de Villiers E-M. Cross-roads in the classification of papillomaviruses. Virology. 2013;445(1–2):2–10.
Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25:2–23.
Correa RM, Baena A, Valls J, Colucci MC, Mendoza L, Rol M, et al. Distribution of human papillomavirus genotypes by severity of cervical lesions in HPV screened positive women from the ESTAMPA study in Latin America. PLoS ONE. 2022;17(7):e0272205.
De Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56.
Guan P, Howell-Jones R, Li N, Bruni L, De Sanjosé S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131(10):2349–59.
Pimple S, Mishra G. Cancer cervix: epidemiology and disease burden. Cytojournal. 2022. https://doi.org/10.25259/CMAS_03_02_2021.
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–89.
Casey RM, Akaba H, Hyde TB, Bloem P. Covid-19 pandemic and equity of global human papillomavirus vaccination: descriptive study of World Health Organization-Unicef vaccination coverage estimates. BMJ Med. 2024. https://doi.org/10.1136/bmjmed-2023-000726.
Kamolratanakul S, Pitisuttithum P. Human papillomavirus vaccine efficacy and effectiveness against cancer. Vaccines. 2021;9(12):1413.
Kumar S, Biswas M, Jose T. HPV vaccine: current status and future directions. Med J Armed Forces India. 2015;71(2):171–7.
Soheili M, Keyvani H, Soheili M, Nasseri S. Human papilloma virus: a review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers. Med J Islam Repub Iran. 2021;35:65.
Hakimi S, Lami F, Allahqoli L, Alkatout I. Barriers to the HPV vaccination program in the Eastern Mediterranean region: a narrative review. J Turk German Gynecol Assoc. 2023;24(1):48.
Ong SK, Abe SK, Thilagaratnam S, Haruyama R, Pathak R, Jayasekara H, et al. Towards elimination of cervical cancer–human papillomavirus (HPV) vaccination and cervical cancer screening in Asian National Cancer Centers Alliance (ANCCA) member countries. Lancet Reg Health-Western Pac. 2023. https://doi.org/10.1016/j.lanwpc.2023.100860.
Hazar N, Mousavi SA, Hosseini S. Recommendations on human papilloma virus vaccination to reduce the incidence of cervical cancer: yes or no in the current situation. Iran J Blood Cancer. 2021;13(3):102–4.
Mohammadpour F, Mansouri A, Hadjibabaie M. Utilization evaluation of human papilloma virus vaccine (GARDASIL®) in Iran; a cross-sectional study. Iran J Pharm Res IJPR. 2020;19(1):68.
Yaghoubi M, Nojomi M, Vaezi A, Erfani V, Mahmoudi S, Ezoji K, et al. Cost-effectiveness analysis of the introduction of HPV vaccination of 9-year-old-girls in Iran. Value Health Reg Issues. 2018;15:112–9.
Khatibi M, Rasekh HR, Shahverdi Z. Cost-effectiveness evaluation of quadrivalent human papilloma virus vaccine for HPV-related disease in Iran. Iran J Pharm Res IJPR. 2014;13(Suppl):225.
Azh N, Hosseinzadeh K, Javadi A, Gholami-Toranposhti S. Factors predicting mothers’ intention toward human papilloma virus vaccination of adolescents: a cross-sectional study among Iranian Families. Iran J Nurs Midwifery Res. 2021;26(6):495–9.
Taebi M, Riazi H, Keshavarz Z, Afrakhteh M. Knowledge and attitude toward human papillomavirus and HPV vaccination in Iranian population: a systematic review. Asian Pac J Cancer Prevent APJCP. 2019;20(7):1945.
Haddadi M, Atefmehr L, Motlaghzadeh S, Hejami F, Elyasi FS, Zafarian N, et al. Prevailing of HPV-16 and 52 genotype in 2022–2023 in Sanandaj. Iran Virol J. 2024;21(1):1–8.
Hassani S, Nadji PS, Mohseni A, Rahnamaye Farzami M, Mirab Samiee S, Sadr M, et al. Evaluation frequency of human papillomavirus and its related genotypes in women of the General Population living in 11 provinces of Iran. Can J Infect Diseases Med Microbiol. 2022;2022:8668557.
Rezaee Azhar I, Yaghoobi M, Mossalaeie MM, Kollaee Darabi A, Nejadeh AH, Jamshidi M, et al. Prevalence of human papilloma virus (HPV) genotypes between outpatients males and females referred to seven laboratories in Tehran. Iran Infect Agents Cancer. 2022;17(1):7.
Shalchimanesh Z, Ghane M, Kalantar E. Prevalence of human papillomavirus genotypes in Tehran. Iran J Res Health Sci. 2022. https://doi.org/10.34172/jrhs.2022.88.
Kesheh MM, Keyvani H. The prevalence of HPV genotypes in Iranian population: an update. Iran J Pathol. 2019;14(3):197.
Bitarafan F, Hekmat MR, Khodaeian M, Razmara E, Ashrafganjoei T, Gilani MM, et al. Prevalence and genotype distribution of human papillomavirus infection among 12 076 Iranian women. Int J Infect Dis. 2021;111:295–302.
Chalabiani S, Nazari MK, Shabani M, Davoodi NR, Sarafnejad A, Amirzargar AA. Retrospective analysis of prevalence of high-risk and low-risk Human Papillomavirus (HPV) genotypes in iranian women during 2013–2016. Asian Pac J Cancer Biol. 2017;2(4):85–90.
Khorasanizadeh F, Hassanloo J, Khaksar N, Taheri SM, Marzaban M, Rashidi BH, et al. Epidemiology of cervical cancer and human papilloma virus infection among Iranian women—analyses of national data and systematic review of the literature. Gynecol Oncol. 2013;128(2):277–81.
Ghaffari SR, Sabokbar T, Mollahajian H, Dastan J, Ramezanzadeh F, Ensani F, et al. Prevalence of human papillomavirus genotypes in women with normal and abnormal cervical cytology in Iran. Asian Pac J Cancer Prev. 2006;7(4):529–32.
Hamkar R, Shoja Z, Ghavami N, Heydari N, Farahmand M, Jalilvand S. Type-specific human papillomavirus prevalence in Iranian women with normal cervical cytology: the impact of current HPV vaccines. Intervirology. 2018;60(4):125–30.
Taghizadeh E, Taheri F, Abdolkarimi H, Ghorbani Renani P, Gheibi Hayat SM. Distribution of human papillomavirus genotypes among women in Mashhad, Iran. Intervirol. 2017;60(1–2):38–42.
Bakhshani A, Ganjali R, Tabatabaeizadeh S-E. Prevalence of human papillomavirus (HPV) genotypes among women during 2015–2020 in Mashhad, Iran. Arch Iran Med. 2023;26(8):419.
Javanmard D, Namaei MH, Haghighi F, Ziaee M, Behravan M, Mirzaei J, et al. The frequency and typing of human Papilloma virus among women with normal and abnormal cytology in Southern Khorasan. Eastern Iran Jundishapur J Microbiol. 2017. https://doi.org/10.5812/jjm.43213.
Sabet F, Mosavat A, Ghezeldasht SA, Basharkhah S, Shamsian SAA, Abbasnia S, et al. Prevalence, genotypes and phylogenetic analysis of human papillomaviruses (HPV) in northeast Iran. Int J Infect Dis. 2021;103:480–8.
Olia J, Ansari M, Yaghmaei P, Ayatollahi H, Khalkhali H. Genotyping the human papillomavirus Infection in Iranian women referred to Shahid Motahari Hospital, in Urmia, with real-time polymerase chain reaction techniques. Ann Trop Med Public Health. 2018;11(3):78–82.
Hashemnejad M, Mirmajidi R, Rahimzadeh M, Ataei M. The prevalence of high-risk human papillomavirus genotypes and related risk factors among Iranian women. J Med Life. 2022;15(11):1340.
Pourseify GR, Mehrafza M, Raoufi A, Nikpouri Z, Askari M, Hosseinzadeh E, et al. The prevalence of high-risk human papillomavirus type 16 and 18 in women in Rasht-Iran. J Midwifery Reprod Health. 2021. https://doi.org/10.22038/jmrh.2021.56804.1691.
Shafaghi B, Jarollahi A, Yousefzadeh B, Ameri A, Moghadam S, Mostafavi M. Human papilloma virus prevalence and types among Iranian women attending regular gynecological visits. Rep Radiother Oncol. 2013;1(2):73–9.
Shahesmaeili A, Karamouzian M, Shokoohi M, Kamali K, Fahimfar N, Nadji SA, et al. Symptom-based versus laboratory-based diagnosis of five sexually transmitted infections in female sex workers in Iran. AIDS Behav. 2018;22:19–25.
Jamdar F, Farzaneh F, Navidpour F, Younesi S, Balvayeh P, Hosseini M, et al. Prevalence of human papillomavirus infection among Iranian women using COBAS HPV DNA testing. Infect Agents Cancer. 2018;13:1–5.
Huang W, Xu H, Hu H, Zhang D, Liu Y, Guo Y, et al. The prevalence of human papillomavirus among women in northern Guangdong Province of China. Sci Rep. 2022;12(1):13353.
Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–23.
Hojjati M, Reshadati M, Rashidi M, Moghadam AG, Salari N, Abdolmaleki A, et al. The prevalence of human papillomavirus in iranian women’s: a comprehensive systematic review and meta-analysis. Indian J Gynecol Oncol. 2024;22(1):8.
Wu E-Q, Liu B, Cui J-F, Chen W, Wang J-B, Lu L, et al. Prevalence of type-specific human papillomavirus and pap results in Chinese women: a multi-center, population-based cross-sectional study. Cancer Causes Control. 2013;24:795–803.
McQuillan GM, Kruszon-Moran D, Markowitz LE, Unger ER, Paulose-Ram R. Prevalence of HPV in adults aged 18–69: United States, 2011–2014. 2017.
Krokidi E, Rao AP, Ambrosino E, Thomas PP. The impact of health education interventions on HPV vaccination uptake, awareness, and acceptance among people under 30 years old in India: a literature review with systematic search. Front Reproduct Health. 2023;5:1151179.
Ruan Y, Li H, Liu M, Cao G, Xu X, Han L, et al. A retrospective analysis of human papillomavirus (HPV) prevalence and genotype distribution among 25,238 women in Shanghai, China revealed the limitations of current HPV-based screening and HPV vaccine. Cancer Epidemiol. 2023;84:102372.
Xia C, Li S, Long T, Chen Z, Chan PK, Boon SS. Current updates on cancer-causing types of human papillomaviruses (HPVs) in East, Southeast, and South Asia. Cancers. 2021;13(11):2691.
Fogarasi AI, Benczik M, Moravcsik-Kornyicki Á, Kocsis A, Gyulai A, Kósa Z. The prevalence of high-risk human papillomavirus in hungary—a geographically representative, cross-sectional study. Pathol Oncol Res. 2022;28:1610424.
Funding
None.
Author information
Authors and Affiliations
Contributions
A.L: Supervision, Review and editing, Investigation, Validation, Methodology. S.M, O.SA, B.M, S.S, M.B, A.VF, F.T: Methodology, Investigation, Writing original draft. A.K, S.S: Statistical analysis. A.AV, M.P, Y.B: Investigation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research adhered to the principles of the Declaration of Helsinki and received approval from the Ethics Committee of Tehran University of Medical Sciences in Tehran, Iran.
Consent for publication
Informed consent for publication of identifiable information/ images in open access journal was obtained from all study participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Letafati, A., Motlaghzadeh, S., Ardekani, O.S. et al. Uncommon high distribution of HPV-16, HPV-54, and HPV-56 in female referred to a laboratory in Karaj, Iran: indications of a paradigm shift in HPV genotypes?. Virol J 21, 182 (2024). https://doi.org/10.1186/s12985-024-02457-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02457-0