Abstract
About four years have passed since the detection of the first cases of COVID-19 in China. During this lethal pandemic, millions of people have lost their lives around the world. Since the first waves of COVID-19 infection, various pharmacotherapeutic agents have been examined in the management of COVID-19. Despite all these efforts in pharmacotherapy, drug repurposing, and design and development of new drugs, multiple organ involvement and various complications occurred during COVID-19. Some of these complications became chronic and long-lasting which led to the “long COVID” syndrome appearance. Therefore, the best way to eradicate this pandemic is prophylaxis through mass vaccination. In this regard, various vaccine platforms including inactivated vaccines, nucleic acid-based vaccines (mRNA and DNA vaccines), adenovirus-vectored vaccines, and protein-based subunit vaccines have been designed and developed to prevent or reduce COVID-19 infection, hospitalization, and mortality rates. In this focused review, at first, the most commonly reported clinical presentations of COVID-19 during these four years have been summarized. In addition, different therapeutic regimens and their latest status in COVID-19 management have been listed. Furthermore, the “long COVID” and related signs, symptoms, and complications have been mentioned. At the end, the effectiveness of available COVID-19 vaccines with different platforms against early SARS-CoV-2 variants and currently circulating variants of interest (VOI) and the necessity of booster vaccine shots have been summarized and discussed in more detail.
Similar content being viewed by others
Background
Approximately four years have passed since the first cases of COVID-19 were detected in Wuhan city of China. During these challenging years, various therapeutic options, prophylactic actions, social distancing, and vaccine development have been considered. Despite these efforts especially in the field of patient care and pharmacotherapy, unfortunately, to date (04-05-2024) more than 775 million people have been infected with SARS-CoV-2 and more than 7 million of them died due to COVID-19 infection around the world. Also, according to the epidemiological mortality rate estimation studies, the mortality rate exceeded over 18.2 million till now and many people will lose their lives during this lethal pandemic [1].
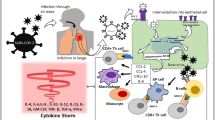
SARS-CoV-2 is an enveloped and single-stranded RNA (ssRNA) virus that can bind to its target sites through the angiotensin converting enzyme II (ACE2) receptors. The SARS-CoV-2 recognition, viral fusion, and finally viral entry through the cell membrane can take place via the spike glycoproteins of the virus that call S proteins. These proteins are located on the surface of SARS-CoV-2 that bind to the receptor binding domain (RBD) of ACE2. ACE2 receptors are commonly available in various pulmonary and extra-pulmonary regions including the kidneys, heart, endothelium, and small intestine. Therefore, viral invasion and tissue damage to the lung and all of these organs would be possible during COVID-19 infection. Binding of the SARS-CoV-2 S proteins to ACE2 receptors can lead to cytokine storm and pro-inflammatory processes which in turn can induce tissue damage [2]. Various complications including cardiovascular [3], thrombotic [4], pulmonary [5], and neurological complications [6] have been reported following COVID-19 post infection.
Antiviral drugs that specifically target SARS-CoV-2 can be classified as fusion inhibitors, protease inhibitors, transcription inhibitors, nucleoside reverse transcriptase inhibitors, and those that target the M2 channel protein. Various antiviral agents have been considered for COVID-19 management through the drug repurposing process [7] including remdesivir, favipiravir, ribavirin, umifenovir, lopinavir, ritonavir, molnupiravir, etc [8].. In this regard, remdesivir, an adenosine analog antiviral agent, interferes with RNA-dependent RNA-polymerase (RdRp) which in turn interferes with the viral replication process and has been approved by FDA for COVID-19 management. In addition, molnupiravir, a synthetic nucleoside analog has received FDA approval as an anti-COVID-19 agent in mild to moderate COVID-19 infection [8]. Nirmatrelvir/ritonavir (Paxlovid®) is the first FDA approved oral treatment for COVID-19 which received the emergency use authorization on December 2021 and final approval on approval on May 2023. Paxlovid® tablets (nirmatrelvir 300 mg/ritonavir 100 mg) can be administered orally in non-hospitalized COVID-19 patients with mild to moderate infection who are at high risk of disease progression. Paxlovid® should be initiated within the 5 days of symptom presentation. Non-hospitalized adults and adolescents aged ≥ 12 years and weighting ≥ 40 kg are eligible to receive Paxlovid® during their mild to moderate COVID-19 infection [9].
During the COVID-19 pandemic, we focused on various aspects of COVID-19 pharmacotherapy and different therapeutic options, pharmacokinetic aspects of considered drugs, potential drug-drug interactions (PDDI) among anti-COVID-19 drugs with others, and adverse drug reactions (ADRs) managements in our previous studies [10,11,12,13,14,15]. In addition, we published our personal experience during the COVID-19 infection and emphasized on the necessity of early initiation of anti-inflammatory agents in patients with mild-to-moderate COVID-19 [16]. In this focused review, at first, the most commonly reported clinical presentations of COVID-19 during these four years have been summarized. In addition, pharmacotherapy aspects and different therapeutic regimens considered in COVID-19 treatment and the latest NIH recommendations on each one have been discussed. Furthermore, the “long COVID” term and its related signs and symptoms have been described. At the end, the effectiveness of available COVID-19 vaccines with different platforms against various SARS-CoV-2 variants and the latest recommendations regarding the necessity of booster dose administration have been summarized and discussed in more detail.
Clinical presentation
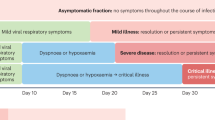
The most commonly reported signs and symptoms during the COVID-19 pandemic with different SARS-CoV-2 variants are summarized in Table 1.
Results of a comparative study revealed that patients who were infected with the Omicron SARS-CoV-2 variant were younger, had a lower rate of dyspnea, and had a lower rate of hospitalization due to COVID-19 in comparison to those who were infected with the Delta variant. In addition, patients with the Omicron variant infection were more vaccinated and had a lower rate of underlying diseases including obesity. Furthermore, the rate of ICU admission, mechanical ventilation, and in-hospital mortality was significantly higher among patients with Delta variant infection [31].
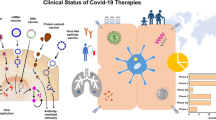
Pharmacotherapy
Among all considered therapeutic options, the NIH guideline only recommended remdesivir and ritonavir-boosted nirmatrelvir (Paxlovid®) as preferred agents in non-hospitalized COVID-19 patients. Paxlovid has received FDA approval on 25th May 2023 in non-hospitalized patients with mild to moderate COVID-19, especially those who are prone to develop severe COVID-19 disease. The recommended dose of Paxlovid is 300 mg from nirmatrelvir and 100 mg from ritonavir that should be administered orally twice daily for 5 days [9]. Other therapeutic regimens in this group of patients would be molnupiravir and bebtelovimab. According to NIH COVID-19 Treatment Guidelines, in all non-hospitalized patients, symptom therapy should be considered. In addition, this guideline recommends against the use of dexamethasone or other systemic corticosteroids in non-hospitalized COVID-19 patients. However, in those patients who are at high risk of developing severe COVID-19 infection, Paxlovid and Remdesivir are preferred therapeutic agents, while, bebtelovimab and molnupiravir are considered as alternative therapies when the preferred therapeutic agents are not available [32]. In hospitalized COVID-19 patients who do not require supplemental oxygen therapy dexamethasone or other systemic corticosteroids should not be administered, however in those who are at high risk of progressing to severe COVID-19 infection, remdesivir can be administered. In addition, prophylactic doses of heparin are recommended in these patients [33]. In hospitalized COVID-19 patients who require supplemental oxygen, remdesivir plus dexamethasone can be administered. In addition, in this group of patients therapeutic or prophylactic doses of anticoagulation with heparin should be considered based on the patients’ special condition. In patients who are receiving systemic corticosteroids and still require rapidly enhancing oxygen supply and those with systemic inflammation, baricitinib or tocilizumab can be added to the therapeutic regimen [33]. Finally, in hospitalized COVID-19 patients who require mechanical ventilation or extracorporeal membrane oxygenation (ECMO), dexamethasone plus tocilizumab or dexamethasone plus baricitinib can be administered along with a prophylactic dose of anticoagulation with heparin [34]. In patients who are receiving a therapeutic dose of anticoagulation in the non-ICU setting and then transferred to the ICU, it has been recommended that the therapeutic dose of heparin switch to its prophylactic dose [35]. A summary of the latest recommendations on the most commonly considered therapeutic agents in COVID-19 treatment is summarized in Table 2.
Long COVID
Long COVID, also known as “post-acute sequelae of COVID-19” (PASC), could be attributed to the persistent sign and symptoms of COVID-19 or organ failure after acute COVID-19 infection. The incidence of long COVID can vary from 10 to 87% in different studies. Both multisystem inflammatory syndrome in children (MIS-C) and multisystem inflammatory syndrome in adults (MIS-A) which are delayed COVID-19 syndromes can also be considered as long COVID [34]. The common symptoms of long COVID and its effect on various organs are summarized in Table 3. Among the listed signs and symptoms in Table 3, headache, fatigue, hair loss, attention deficit, and dyspnea were the most commonly reported ones.
Currently circulating SARS-CoV-2 variants
Currently circulating variants of concern (VOCs)
Fortunately, there is no currently circulating SARS-CoV-2 variant that fulfill the VOC criteria.
Currently circulating variants of interest (VOIs)
Some of the Omicron lineages including XBB.1.5, XBB.1.16, and EG.5 are considered as currently circulating VOIs.
XBB.1.5
XBB.1.5 lineage was first detected in the United States of America (USA) and have the spike mutations of N460K, S486P, and F490S. The transmissibility, immunity, and disease severity of this lineage is similar to the baseline [90]. Therefore, XBB.1.5 has no additional public health risk in comparison to other currently circulating Omicron variants and showed a low level of risk of growth rate and disease severity with a moderate risk of antibody escape [91]. The neutralizing activity of the available mRNA vaccines against XBB.1.5 lineage has been reduced in comparison to the early Omicron lineages [92].
XBB.1.16
XBB.1.16 is an Omicron lineage that has a genetic profile similar to XBB.1.5 lineage. XBB.1.16 has no additional public health risk in comparison to other currently circulating SARS-CoV-2 variants and lineages. The level of risk of growth rate and antibody escape of XBB.1.16 lineage is moderate. Also, the level of risk of disease severity of this lineage is low [93].
EG.5
EG.5 is another Omicron lineage which is a currently circulating VOI. It has shown F456L, N460K, S486P, and F490S spike mutations with similar transmissibility to the early Omicron sublineages. EG.5 has a moderate level of risk of growth rate and antibody escape with a low risk of disease severity. Although the prevalence, growth rate, and antibody escape potential of EG.5 lineage have been enhanced in comparison to the other currently circulating Omicron sublinages, however, the COVID-19 disease severity has not increased significantly. To date, EG.5 Omicron lineage is the most prevalent SARS-CoV-2 variant worldwide [94].
BA.2.86
BA.2.86 is a linage of BA.2 Omicron which was first detected in Israel and Denmark on 24th July 2023. This lineage is considered as currently circulating VOI with low public health risk at the global level. BA.2.86 has numerous mutations in the spike protein. Although the BA.2.86 lineage is potential to induce a sudden increase in the number of COVID-19 cases, however, the severity of disease would not be higher. According to the recent studies, convalescent plasma of those who have XBB infection, showed sufficient neutralizing activity against BA.2.86, therefore, it seems that XBB.1.5 monovalent COVID-19 vaccines can be effective against the BA.2.86 lineage. To date, no changes in clinical presentation or the severity of COVID-19 have been reported with BA.2.86 infection [95].
JN.1
JN.1 is a sublineage of BA.2.86 with high transmissibility which was first detected on August 2023 and is currently considered as VOI. Nowadays, JN.1 is the most prevalent SARS-CoV-2 sublineage in the world, however, it has low public health risk and no changes in disease severity or hospitalization rate have been reported yet with JN.1 sublineage. Although an immune escape has been reported with JN.1 in comparison to other co-circulating SARS-CoV-2 lineages, however, it seems that the monovalent XBB.1.5 booster vaccine would be still effective against JN.1 sublineage [95].
Currently circulating variants under monitoring (VUMs)
Some of the Omicron lineages including XBB, XBB.1.9.1, and XBB.2.3 are considered as currently circulating VUMs. There are limited data available regarding the transmissibility, growth rate, immune escape of these new Omicron lineages. The main spike mutations that have been occurred in these lineages are K444T, L452R, E180V, T478R, F486P, I332V, D339H, R403K, V445H, G446S, N450D, L452W, N481K, 483del, and E484K. However, the epidemiological and phenotypic impact of these mutations and related lineages are still remaining unclear and further studies and monitoring are required [90].
De-escalated SARS-CoV-2 variants
Alpha
Alpha variant, also known as B.1.1.7, was first detected on September 2020 in the United Kingdom. Alpha variant showed 30% increase in viral transmissibility and infectivity and also could induce severe COVID-19 disease. The key mutations of the Alpha variant were N501Y, H69/V70, P681H, and Y144. Due to these mutations, the effectiveness of vaccines and monoclonal antibodies were reduced against this variant in comparison to wild type SARS-CoV-2. Moreover, the ACE2 binding potential was increased by 10-fold [96, 97].
Beta
Beta variant, also known as B.1.351, was first detected on October 2020 in the South Africa. Beta variant showed 50% increase in viral transmissibility and infectivity and also resulted in severe COVID-19 disease. The key mutations of the Beta variant were N501Y, E484K, K417N, L18F, D80A, D215G, and A701V. Due to these mutations, the effectiveness of vaccines and monoclonal antibodies were reduced against this variant in comparison to wild type SARS-CoV-2. Moreover, the ACE2 binding potential was increased by two-fold [96, 97].
Gamma
Gamma variant, also known as P.1 lineage, was first detected on November 2020 in the Japan and Brazil. Gamma variant was accompanied by 30–40% increase in viral transmissibility and infectivity and also resulted in severe COVID-19 disease. The key mutations of the Gamma variant were K417T, E484K, AND N501Y. Due to these mutations, the effectiveness of vaccines and monoclonal antibodies were reduced against this variant in comparison to wild type SARS-CoV-2. Moreover, the ACE2 binding potential was increased by five-fold [96, 97].
Delta
Delta variant, also known as B.1.617, was first detected on December 2020 in the India. Delta variant was accompanied by > 90% increase in viral transmissibility and infectivity and induced severe COVID-19 disease. Furthermore, the infected patients had much higher viral RNA load in comparison to the previous variants. The key mutations of the Delta variant were R158G, L452R, T478K, D614G P681R, and D950N. Due to these mutations, vaccine effectiveness was reduced, however, the effectiveness was 80% remained against severe COVID-19 disease and hospitalization. Administration of monoclonal antibodies including casirivimab, imdevimab and sotrovimab was associated with reduced hospitalization and mortality rate due to the Delta variant infection. Moreover, the ACE2 binding potential was increased by two-fold [96, 97].
COVID-19 vaccines effectiveness against early SARS-CoV-2 variants
Due to the several irretrievable complications of COVID-19 and the high mortality rates around the world, the best way to eradicate or to silence this pandemic is prophylaxis through mass vaccination. In this regard, soon after the COVID-19 pandemic occurrence, many scientists around the world focused on vaccine design and development to overcome this pandemic and its related morbidities and mortalities. Therefore, various vaccine platforms including inactivated vaccines, nucleic acid-based vaccines (mRNA and DNA vaccines), adenovirus-vectored vaccines, and protein-based subunit vaccines have been developed [98]. Available COVID-19 vaccines, their effectiveness, and coverage against early SARS-CoV-2 variants are summarized in Table 4. Through the worldwide mass vaccination, the COVID-19 pandemic has been relatively controlled and both the mortality and infection rates have been reduced significantly worldwide which emphasizes on vaccine effectiveness in COVID-19 prevention or reduce its severity. Only in moderately or severely immunocompromised patients who cannot be fully vaccinated, tixagevimab/cilgavimab (300 mg/300 mg) (Evusheld®) can be administered as an IM injection in two consecutive doses for the purpose of SARS-CoV-2 pre-expose prophylaxis [34]. A summary of available COVID-19 vaccines, different vaccine platforms, their safety and effectiveness have been discussed in detail in our previous review [98]. According to the early circulating SARS-CoV-2 variants of concerns (VOCs) especially the Omicron variant, administration of booster doses of vaccines would be essential to protect against severe infection and enhance vaccine effectiveness against both hospitalization and mortality due to COVID-19. Results of recent studies revealed that the efficacy of different COVID-19 vaccines has been diminished against the early Omicron VOCs. The results of recent studies emphasized the necessity of booster dose administration to enhance vaccine protection against the newly emerging Omicron variant and its sublineages. In this regard, results of a recent study performed in England revealed that among individuals who were vaccinated with either Pfizer-BioNTech, Moderna, or Oxford-AstraZeneca vaccines as their first and second vaccine shots and those who received either Pfizer-BioNTech or Moderna vaccine as their booster shots, the vaccine effectiveness was significantly increased after booster dose administration against the BA.1 and BA.2 Omicron sublineages. Furthermore, the results of this study revealed the highest effectiveness of a full-dose Pfizer-BioNTech or a half-dose Moderna vaccine as booster dose options to protect against the Omicron variant infection and its related morbidities and mortalities [99]. In addition, it seems that mRNA-based vaccines can be adjusted more feasibly by inducing some changes in their encapsulated S-protein to enhance their effectiveness against the early circulating BA.4 and BA.5 Omicron VOCs to avoid the occurrence of further COVID-19 waves with these sublineages predominance around the world. The BA.2.12.1, BA.4, and BA.5 Omicron sublineages showed higher transmissibility over the BA.1 and BA.2 Omicron sublineages. In addition, higher neutralization evasion was reported with BA.2.12.1 BA.4/BA.5 sublineage in comparison to BA.2 after 3-dose COVID-19 vaccination. Furthermore, it seems that the BA.1-derived booster vaccines might not induce broad-spectrum protection against the newly emerging BA.2.12.1 and BA.4/BA.5 Omicron sublineages [100]. It has been reported that the BA.4/BA.5 subvariants were 4.2 folds more resistant against boosted vaccinated individuals in comparison to the BA.2 subvariant [101]. The BA.4/BA.5 neutralizations against the sera of triple-dose vaccinated individuals with either Pfizer-BioNTech or Oxford-AstraZeneca vaccine were reduced significantly in comparison to the previous BA.1 and BA.2 subvariants. The higher viral escape of BA.4/BA.5 can be attributed to the L452R and F486V mutations [102]. Therefore, design and development of some modified COVID-19 vaccines, especially mRNA-based vaccines, with higher vaccine effectiveness and higher protection against these new subvariants would be crucial to avoid further large COVID-19 waves around the world. In this regard, new bivalent mRNA vaccines (Moderna and Pfizer-BioNTech bivalent COVID-19 vaccines), which are containing two mRNA components of ancestral and BA4/BA5 sublinages, have been designed and developed to enhance vaccine effectiveness against the recent SARS-CoV-2 lineages [103].
COVID-19 vaccines effectiveness against currently circulating SARS-CoV-2 variants
About one year ago on 31st August 2022, FDA approved the emergency use of bivalent mRNA vaccines with enhanced effectiveness against the Omicron variant and its lineages. These bivalent vaccines are composed of two mRNA components of SARS-CoV-2 virus (ancestral and BA.4/5) and therefore can be more effective against the new emerging Omicron lineages including BA4/BA5 [124].
Results of a recent study revealed that the mRNA vaccine effectiveness against the XBB.1.5 Omicron lineage was considerably lower than the wild type or BA.2 Omicron lineage. Therefore, XBB.1.5 could significantly evade the vaccine-induced humoral immunity and administration of bivalent vaccines can improve humoral immune response against the XBB.1.5. lineage as a currently circulating SARS-CoV-2 VOI [125]. Results of another study revealed that administration of a single dose of bivalent mRNA vaccine as a booster shot in those who have received 2 to 4 doses of monovalent mRNA vaccines can significantly enhance effectiveness against XBB.1.5. Omicron lineage [126]. In addition, recently a monovalent XBB.1.5 BNT162b2 COVID-19 vaccine has been approved by CDC as a single-dose vaccine for all individuals ages 6 months and older [127].
There are no sufficient data available regarding the vaccine effectiveness against XBB.1.16 and EG.5 Omicron lineages that are considered as currently circulating SARS-CoV-2 VOIs [128]. However, since only minor changes have been occurred in XBB.1.16 and EG.5 lineages in comparison to XBB.1.5 lineage, it seems that monovalent XBB.1.5 BNT162b2 COVID-19 vaccine would still be effective against these newly emerging VOIs [129].
Potential drug-vaccine interactions
Among the discussed COVID-19 vaccines and therapeutic options, potential interaction between prednisone and Moderna vaccine (or other equivalent vaccines) has been reported. In this regard, it has been mentioned that in patients who are receiving prednisone, based on the dose and duration of prednisone therapy, response to Moderna vaccine can be diminished. In addition, it has been suggested that the initiation of prednisone therapy in those who are currently vaccinated with Moderna vaccine can be postponed to the next couple of weeks or more. Based on CDC recommendation, prophylactic therapy with analgesics and antipyretics including acetaminophen, aspirin, and other NSAIDs before COVID-19 vaccination should be avoided. However, if pain or fever occurred after vaccination, analgesics and/or antipyretics can be administered when appropriate. In patients who are receiving regular regimens of these agents including daily aspirin intake due to myocardial infarction or stroke, the drug should be continued regardless of vaccine injection. Prophylactic antihistamine therapy prior to COVID-19 vaccination should be avoided. Since antihistamines can’t prevent the potential occurrence of mRNA vaccine-induced anaphylactic reactions and they also may mask the crucial signs of allergic reactions related to the vaccine injection. However, for those who are receiving typical antihistamines for other indications, holding the medication prior to COVID-19 vaccination would not be necessary. Since the approved COVID-19 vaccines are not live vaccines, therefore, there is no potential interaction between antiviral drugs and /or antibiotics with COVID-19 vaccines and vaccine effectiveness cannot be affected by these agents. However, in patients with sign and symptoms of viral and/or bacterial infection, COVID-19 vaccination should be delayed until the patient recovery [130].
Conclusions
Almost four years have passed since the first cases of COVID-19 in Wuhan city of China and to date (04-05-2024) more than 775 million people have been infected with SARS-CoV-2 and more than 7 million of them died due to COVID-19 infection around the world. During these challenging years, various therapeutic options and prophylactic approaches including COVID-19 vaccination were considered to reduce COVID-19 related mortality and morbidities. COVID-19 infection was associated with various organ involvements including respiratory, cardiovascular, renal, gastrointestinal, hematological, neuropsychological, and dermatological complications. Unfortunately, in many cases some of these complications can be prolonged and may lead to post-acute COVID-19 syndrome or “long COVID” syndrome. Therefore, till now, numerous therapeutic agents have been tried as prophylactic and/or therapeutic agents in COVID-19 management as summarized in Table 2 in order to enhance patients’ survival. Optimum therapeutic regimens should be considered individually based on the specific patients’ condition, underlying diseases, and COVID-19 severity. Although many efforts have been made in pharmacotherapy and disease management, millions of people have lost their lives due to COVID-19. Therefore, prevention through the recruitment of COVID-19 vaccines would be the best way to get rid of the COVID-19 pandemic and its complications. According to Table 4, the vaccine effectiveness has diminished during the previous emergence of SARS-CoV-2 variants. However, among different COVID-19 vaccine platforms, the mRNA-based vaccines including Pfizer-BioNTech and Modernavaccines maintained the highest effectiveness against these variants. Vaccine effectiveness against the currently circulating VOIs, including XBB.1.5, XBB.1.16, and EG.5 Omicron lineages, has also been reduced significantly. However, new bivalent and monovalent mRNA-based vaccines including COMIRNATY Original/Omicron BA.4/5 COVID-19 vaccine and monovalent XBB.1.5 BNT162b2 COVID-19 vaccine have been designed and showed enhanced effectiveness against these newly emerging COVID-19 lineages.
In conclusion, although four years have been passed from the initiation of COVID-19 pandemic, it has not been eradicated completely. Therefore, a comprehensive and updated knowledge regarding various COVID-19 signs and symptoms, differential diagnosis, selection and administration of suitable pharmacotherapy regimen based on disease severity and various stages would be crucial. In addition, detection and differentiation of “long COVID” and its related complications is essential. Fortunately, nowadays COVID-19 has relatively become under-control that is result of the massive worldwide COVID-19 vaccination. Due to the newly emerging SARS-CoV-2 variants around the world especially the Omicron variant and its EG.5, BA.2.86, and JN.1 lineages, the necessity of boosted immunity and administration of newly developed bivalent or monovalent mRNA-based COVID-19 vaccines as booster shots should be highlighted to avoid further new COIVD-19 waves occurrence.
Data availability
No datasets were generated or analysed during the current study.
References
Wang H, Paulson KR, Pease SA, Watson S, Comfort H, Zheng P, et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399(10334):1513–36.
Mostafa-Hedeab G. ACE2 as drug target of COVID-19 virus treatment, simplified updated review. Rep Biochem Mol Biology. 2020;9(1):97.
Yu W-L, Toh HS, Liao C-T, Chang W-T. Cardiovascular complications of COVID-19 and associated concerns: a review. Acta Cardiol Sinica. 2021;37(1):9.
Page EM, Ariëns RA. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res. 2021;200:1–8.
Liu L, Jing H, Wu X, Xiang M, Novakovic VA, Wang S et al. The cross-talk of lung and heart complications in COVID-19: endothelial cells dysfunction, thrombosis, and treatment. Front Cardiovasc Med. 2022;9.
Shehata GA, Lord KC, Grudzinski MC, Elsayed M, Abdelnaby R, Elshabrawy HA. Neurological complications of COVID-19: underlying mechanisms and management. Int J Mol Sci. 2021;22(8):4081.
Al-Karmalawy AA, Soltane R, Abo Elmaaty A, Tantawy MA, Antar SA, Yahya G, et al. Coronavirus disease (COVID-19) control between drug repurposing and vaccination: a comprehensive overview. Vaccines. 2021;9(11):1317.
Ashour NA, Abo Elmaaty A, Sarhan AA, Elkaeed EB, Moussa AM, Erfan IA et al. A systematic review of the global intervention for SARS-CoV-2 combating: from drugs repurposing to molnupiravir approval. Drug design, development and therapy. 2022:685–715.
Ritonavir-Boosted Nirmatrelvir. (Paxlovid) [ https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir--paxlovid-/.
Ghasemiyeh P, Mohammadi-Samani S. COVID-19 outbreak: challenges in pharmacotherapy based on pharmacokinetic and pharmacodynamic aspects of drug therapy in patients with moderate to severe infection. Heart Lung. 2020;49(6):763–73.
Ghasemiyeh P, Borhani-Haghighi A, Karimzadeh I, Mohammadi-Samani S, Vazin A, Safari A, et al. Major neurologic adverse drug reactions, potential drug–drug interactions and pharmacokinetic aspects of drugs used in covid-19 patients with stroke: a narrative review. Ther Clin Risk Manag. 2020;16:595.
Ghasemiyeh P, Mortazavi N, Karimzadeh I, Vazin A, Mahmoudi L, Moghimi-Sarani E, et al. Psychiatric adverse drug reactions and potential Anti-COVID-19 drug interactions with psychotropic medications. Iran J Pharm Research: IJPR. 2021;20(3):66.
Ghasemiyeh P, Mohammadi-Samani S. Iron chelating agents: promising supportive therapies in severe cases of COVID-19? Trends Pharm Sci. 2020;6(2):65–6.
Ghasemiyeh P, Mohammadi-Samani S, Vazin A. Micronutrients supplementation in pregnant women during COVID-19 pandemy: pros and cons. Trends Pharm Sci. 2021;7(3):153–60.
Zarkesh K, Entezar-Almahdi E, Ghasemiyeh P, Akbarian M, Bahmani M, Roudaki S, et al. Drug-based therapeutic strategies for COVID-19-infected patients and their challenges. Future Microbiol. 2021;16(18):1415–51.
Ghasemiyeh P, Mohammadi-Samani S. The necessity of early anti-inflammatory therapy initiation in cases with mild-to-moderate COVID-19: a personal experience from an attending pharmacist and his resident. Acta Bio-medica: Atenei Parmensis. 2021;92(3):e2021250–e.
Vetter P, Vu DL, L’Huillier AG, Schibler M, Kaiser L, Jacquerioz F. Clinical features of covid-19. British Medical Journal Publishing Group; 2020.
Pelaia C, Tinello C, Vatrella A, De Sarro G, Pelaia G. Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications. Ther Adv Respir Dis. 2020;14:1753466620933508.
Bazdyrev E, Rusina P, Panova M, Novikov F, Grishagin I, Nebolsin V. Lung fibrosis after COVID-19: treatment prospects. Pharmaceuticals. 2021;14(8):807.
Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Translational Res. 2020;226:57–69.
Shih AR, Misdraji J. COVID-19: gastrointestinal and hepatobiliary manifestations. Hum Pathol. 2023;132:39–55.
Kunutsor SK, Laukkanen JA. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. 2020;52(7):345–53.
Gjonbalaj N, Uka S, Olluri E, Sulovari A, Vishaj M, Kamberi L, et al. Renal artery thrombosis as a long-term complication of COVID-19. Radiol Case Rep. 2023;18(1):260–5.
Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E, et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141(23):1930–6.
Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38(7):1504–7.
Sheraton M, Deo N, Kashyap R, Surani S. A review of neurological complications of COVID-19. Cureus. 2020;12(5).
Nagu P, Parashar A, Behl T, Mehta V. CNS implications of COVID-19: a comprehensive review. Rev Neurosci. 2021;32(2):219–34.
Guck AJ, Buck K, Lehockey K. Psychological complications of COVID-19 following hospitalization and ICU discharge: recommendations for treatment. Professional Psychology: Research and Practice; 2021.
Alahyari S, Moradi M, Rajaeinejad M, Jalaeikhoo H. Post-COVID-19 hematologic complications: a systematic review. Expert Rev Hematol. 2022(just-accepted).
Fanaroff AC, Lopes RD. COVID-19 thrombotic complications and therapeutic strategies. Annu Rev Med. 2023;74:15–30.
Bouzid D, Visseaux B, Kassasseya C, Daoud A, Fémy F, Hermand C, et al. Comparison of patients infected with Delta versus Omicron COVID-19 variants presenting to Paris emergency departments: a retrospective cohort study. Ann Intern Med. 2022;175(6):831–7.
Therapeutic Management of Nonhospitalized Adults With COVID. -19 [ https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults--therapeutic-management/#:~:text=The%20Panel%20recommends%20using%20nirmatrelvir,of%20symptom%20onset%20(%20AIIa%20).
Therapeutic Management of Hospitalized Adults With COVID. -19 [ https://www.covid19treatmentguidelines.nih.gov/tables/therapeutic-management-of-hospitalized-adults/.
Coronavirus Disease. 2019 (COVID-19) Treatment Guidelines [ https://www.covid19treatmentguidelines.nih.gov/.
Spyropoulos AC, Goldin M, Giannis D, Diab W, Wang J, Khanijo S, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612–20.
Thiruchelvam K, Kow CS, Hadi MA, Hasan SS. The use of remdesivir for the management of patients with moderate-to-severe COVID-19: a systematic review. Expert Rev anti-infective Therapy. 2022;20(2):211–29.
Gupte V, Hegde R, Sawant S, Kalathingal K, Jadhav S, Malabade R, et al. Safety and clinical outcomes of remdesivir in hospitalised COVID-19 patients: a retrospective analysis of active surveillance database. BMC Infect Dis. 2022;22(1):1–8.
Remdesivir [Available from. https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/remdesivir/.
Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54(1):516–23.
Tanne JH. Covid-19: FDA authorises pharmacists to prescribe Paxlovid. British Medical Journal Publishing Group; 2022.
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20.
McCreary EK, Kip KE, Collins K, Minnier TE, Snyder GM, Steiner A, et al. editors. Evaluation of Bebtelovimab for treatment of COVID-19 during the SARS-CoV-2 Omicron variant era. Open Forum Infectious Diseases; 2022.
Kozani PS, Sheikhi M, Baharifar N, Shokoohi SD, Sheikhi S, Mirarefin SMJ et al. Bebtelovimab: the FDA-approved monoclonal antibody for treating patients with mild-to-moderate COVID-19. Trends Med Sci. 2022;2(3).
Vilobelimab [Available from. https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/.
Kuritzkes DR. Bamlanivimab for Prevention of COVID-19. JAMA. 2021;326(1):31–2.
Herman GA, O’Brien MP, Forleo-Neto E, Sarkar N, Isa F, Hou P, et al. Efficacy and safety of a single dose of casirivimab and imdevimab for the prevention of COVID-19 over an 8-month period: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2022;22(10):1444–54.
Ao G, Li A, Wang Y, Tran C, Qi X. Lack of efficacy for sotrovimab use in patients with COVID-19: a meta-analysis. J Infect. 2022.
Jorda A, Kussmann M, Kolenchery N, Siller-Matula JM, Zeitlinger M, Jilma B, et al. Convalescent plasma treatment in patients with Covid-19: a systematic review and Meta-analysis. Front Immunol. 2022;13:817829.
Troxel AB, Petkova E, Goldfeld K, Liu M, Tarpey T, Wu Y, et al. Association of convalescent plasma treatment with clinical status in patients hospitalized with COVID-19: a meta-analysis. JAMA Netw open. 2022;5(1):e2147331–e.
Kirkham AM, Bailey AJ, Monaghan M, Shorr R, Lalu MM, Fergusson DA, et al. Updated Living Systematic Review and Meta-analysis of controlled trials of mesenchymal stromal cells to treat COVID-19: a Framework for Accelerated Synthesis of Trial evidence for Rapid Approval—FASTER approval. Stem Cells Translational Med. 2022;11(7):675–87.
Dorward J, Yu L-M, Hayward G, Saville BR, Gbinigie O, Van Hecke O, et al. Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial. Br J Gen Pract. 2022;72(720):e446–55.
Ghaith HS, Gabra MD, Nafady MH, Elshawah HE, Negida A, Mushtaq G et al. A review of the rational and current evidence on colchicine for COVID-19. Curr Pharm Design. 2022.
Griesel M, Wagner C, Mikolajewska A, Stegemann M, Fichtner F, Metzendorf M-I et al. Inhaled corticosteroids for the treatment of COVID-19. Cochrane Database Syst Reviews. 2022(3).
Clemency BM, Varughese R, Gonzalez-Rojas Y, Morse CG, Phipatanakul W, Koster DJ, et al. Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial. JAMA Intern Med. 2022;182(1):42–9.
Nyirenda JL, Sofroniou M, Toews I, Mikolajewska A, Lehane C, Monsef I et al. Fluvoxamine for the treatment of COVID-19. Cochrane Database Syst Reviews. 2022(9).
Mehta P, Chambers RC, Dagna L. Granulocyte-macrophage colony stimulating factor in COVID-19: friend or foe? Lancet Rheumatol. 2021;3(6):e394–5.
Lee TC, McDonald EG, Butler-Laporte G, Harrison LB, Cheng MP, Brophy JM. Remdesivir and systemic corticosteroids for the treatment of COVID-19: a bayesian re-analysis. Int J Infect Dis. 2021;104:671–6.
Noreen S, Maqbool I, Madni A, Dexamethasone. Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur J Pharmacol. 2021;894:173854.
Martinez-Guerra BA, Gonzalez-Lara MF, Roman-Montes CM, Tamez-Torres KM, Dardón-Fierro FE, Rajme-Lopez S, et al. Outcomes of patients with severe and critical COVID-19 treated with dexamethasone: a prospective cohort study. Emerg Microbes Infections. 2022;11(1):50–9.
Inhaled C. [ https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/inhaled-corticosteroids/.
Khani E, Shahrabi M, Rezaei H, Pourkarim F, Afsharirad H, Solduzian M. Current evidence on the use of anakinra in COVID-19. Int Immunopharmacol. 2022:109075.
Rezaei Tolzali MM, Noori M, Shokri P, Rahmani S, Khanzadeh S, Nejadghaderi SA et al. Efficacy of tocilizumab in the treatment of COVID-19: an umbrella review. Rev Med Virol. 2022:e2388.
Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41–51.
Guimarães PO, Quirk D, Furtado RH, Maia LN, Saraiva JF, Antunes MO, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385(5):406–15.
Ely EW, Ramanan AV, Kartman CE, de Bono S, Liao R, Piruzeli MLB, et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial. Lancet Respiratory Med. 2022;10(4):327–36.
Kifle ZD. Bruton tyrosine kinase inhibitors as potential therapeutic agents for COVID-19: a review. Metabolism Open. 2021;11:100116.
Khan FA, Stewart I, Fabbri L, Moss S, Robinson K, Smyth AR, et al. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax. 2021;76(9):907–19.
Caricchio R, Abbate A, Gordeev I, Meng J, Hsue PY, Neogi T, et al. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA. 2021;326(3):230–9.
Pilia E, Belletti A, Fresilli S, Finco G, Landoni G. Efficacy and safety of heparin full-dose anticoagulation in hospitalized non-critically ill COVID-19 patients: a meta-analysis of multicenter randomized controlled trials. J Thromb Thrombolysis. 2022:1–11.
Hoogenboom WS, Lu JQ, Musheyev B, Borg L, Janowicz R, Pamlayne S, et al. Prophylactic versus therapeutic dose anticoagulation effects on survival among critically ill patients with COVID-19. PLoS ONE. 2022;17(1):e0262811.
Antithrombotic Therapy in Patients With COVID. -19 [ https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/.
Spaetgens B, Nagy M, Ten Cate H. Antiplatelet therapy in patients with COVID-19—more is less? JAMA. 2022;327(3):223–4.
Florescu S, Stanciu D, Zaharia M, Kosa A, Codreanu D, Kidwai A, et al. Effect of antiplatelet therapy on survival and organ support–free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2022;327(13):1247–59.
Olczak-Pruc M, Swieczkowski D, Ladny JR, Pruc M, Juarez-Vela R, Rafique Z, et al. Vitamin C supplementation for the treatment of COVID-19: a systematic review and Meta-analysis. Nutrients. 2022;14(19):4217.
Firouzi S, Pahlavani N, Navashenaq JG, Clayton ZS, Beigmohammadi MT, Malekahmadi M. The effect of vitamin C and zn supplementation on the immune system and clinical outcomes in COVID-19 patients. Clin Nutr Open Sci. 2022.
Balboni E, Zagnoli F, Filippini T, Fairweather-Tait SJ, Vinceti M. Zinc and selenium supplementation in COVID-19 prevention and treatment: a systematic review of the experimental studies. J Trace Elem Med Biol. 2022:126956.
Feiner Solís Á, Avedillo Salas A, Luesma Bartolomé MJ, Santander Ballestín S. The effects of vitamin D supplementation in COVID-19 patients: a systematic review. Int J Mol Sci. 2022;23(20):12424.
Tentolouris N, Samakidou G, Eleftheriadou I, Tentolouris A, Jude EB. The effect of vitamin D supplementation on mortality and intensive care unit admission of COVID-19 patients. A systematic review, meta‐analysis and meta‐regression. Diab/Metab Res Rev. 2022;38(4):e3517.
Silva Andrade B, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, dos Santos Freitas A, et al. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4):700.
Al-Jahdhami I, Al-Naamani K, Al-Mawali A, Bennji SM. Respiratory complications after COVID-19. Oman Med J. 2022;37(1):e343.
Yan Z, Yang M, Lai C-L. Long COVID-19 syndrome: a comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines. 2021;9(8):966.
Cooper SL, Boyle E, Jefferson SR, Heslop CR, Mohan P, Mohanraj GG, et al. Role of the renin–angiotensin–aldosterone and kinin–kallikrein systems in the cardiovascular complications of COVID-19 and long COVID. Int J Mol Sci. 2021;22(15):8255.
Srinivasan A, Wong F, Couch LS, Wang BX. Cardiac complications of COVID-19 in low-risk patients. Viruses. 2022;14(6):1322.
Elseidy SA, Awad AK, Vorla M, Fatima A, Elbadawy MA, Mandal D, et al. Cardiovascular complications in the Post-acute COVID-19 syndrome (PACS). IJC Heart Vasculature. 2022;40:101012.
Korompoki E, Gavriatopoulou M, Fotiou D, Ntanasis-Stathopoulos I, Dimopoulos MA, Terpos E. Late‐onset hematological complications post COVID‐19: an emerging medical problem for the hematologist. Am J Hematol. 2022;97(1):119–28.
Theofilis P, Vordoni A, Kalaitzidis RG. COVID-19 and kidney disease: a clinical perspective. Curr Vasc Pharmacol. 2022.
Desai AD, Lavelle M, Boursiquot BC, Wan EY. Long-term complications of COVID-19. Am J Physiology-Cell Physiol. 2022;322(1):C1–11.
de Francisco ÁM, Fresnedo GF. Long COVID-19 renal disease: A present medical need for nephrology. Nefrología (English Edition). 2023.
Raveendran A, Misra A. Post COVID-19 syndrome (long COVID) and diabetes: challenges in diagnosis and management. Diabetes Metabolic Syndrome: Clin Res Reviews. 2021;15(5):102235.
SARS-CoV-2. variants of concern as of 21 September 2023 [ https://www.ecdc.europa.eu/en/covid-19/variants-concern.
XBB.1.5. Updated Risk Assessment, 20 June 2023 [ https://www.who.int/docs/default-source/coronaviruse/20230620xbb.1.5.pdf?sfvrsn=fff6f686_3.
Davis-Gardner ME, Lai L, Wali B, Samaha H, Solis D, Lee M, et al. Neutralization against BA. 2.75. 2, BQ. 1.1, and XBB from mRNA Bivalent Booster. N Engl J Med. 2023;388(2):183–5.
XBB.1.16. Updated Risk Assessment, 05 June 2023 [ https://www.who.int/docs/default-source/coronaviruse/05062023xbb.1.16.pdf?sfvrsn=f1845468_3.
EG.5 Updated Risk Evaluation, 21. September 2023 [ https://www.who.int/docs/default-source/coronaviruse/eg5-risk-evaluation.pdf?sfvrsn=6e9690e0_6.
Tracking SARS-. CoV-2 variants [ https://www.who.int/activities/tracking-SARS-CoV-2-variants.
Hoteit R, Yassine HM. Biological properties of SARS-CoV-2 variants: epidemiological impact and clinical consequences. Vaccines. 2022;10(6):919.
Andre M, Lau L-S, Pokharel MD, Ramelow J, Owens F, Souchak J, et al. From alpha to omicron: how different variants of concern of the SARS-Coronavirus-2 impacted the world. Biology. 2023;12(9):1267.
Ghasemiyeh P, Mohammadi-Samani S, Firouzabadi N, Dehshahri A, Vazin A. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. Int Immunopharmacol. 2021;100:108162.
Kirsebom FC, Andrews N, Stowe J, Toffa S, Sachdeva R, Gallagher E, et al. COVID-19 vaccine effectiveness against the omicron (BA. 2) variant in England. The Lancet Infectious Diseases; 2022.
Cao Y, Yisimayi A, Jian F, Song W, Xiao T, Wang L et al. BA. 2.12. 1, BA. 4 and BA. 5 escape antibodies elicited by Omicron infection. Nature. 2022:1–3.
Wang Q, Guo Y, Iketani S, Nair MS, Li Z, Mohri H et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA. 2.12. 1, BA. 4, & BA 5 Nat. 2022:1–3.
Tuekprakhon A, Huo J, Nutalai R, Dijokaite-Guraliuc A, Zhou D, Ginn HM et al. Antibody escape of SARS-CoV-2 Omicron BA. 4 and BA. 5 from vaccine and BA. 1 serum. Cell. 2022.
Coronavirus (COVID-19) Update. FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose [ https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use.
Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81(4):495–501.
Thiagarajan K. What do we know about India’s Covaxin vaccine? BMJ. Br Med J (Online). 2021;373.
Sánchez-Sendra B, Albert E, Zulaica J, Torres I, Giménez E, Botija P, et al. Neutralizing antibodies against SARS-CoV-2 variants of concern elicited by the comirnaty COVID-19 vaccine in nursing home residents. Sci Rep. 2022;12(1):1–8.
Wilhelm A, Toptan T, Pallas C, Wolf T, Goetsch U, Gottschalk R, et al. Antibody-mediated neutralization of authentic SARS-CoV-2 B. 1.617 variants harboring L452R and T478K/E484Q. Viruses. 2021;13(9):1693.
Tada T, Zhou H, Samanovic MI, Dcosta BM, Cornelius A, Mulligan MJ et al. Comparison of neutralizing antibody titers elicited by mRNA and adenoviral vector vaccine against SARS-CoV-2 variants. BioRxiv. 2021.
Bassi J, Giannini O, Silacci-Fregni C, Pertusini L, Hitz P, Terrot T, et al. Poor neutralization and rapid decay of antibodies to SARS-CoV-2 variants in vaccinated dialysis patients. PLoS ONE. 2022;17(2):e0263328.
Del Rio C, Malani PN, Omer SB. Confronting the delta variant of SARS-CoV-2, summer 2021. JAMA. 2021;326(11):1001–2.
Sheikh A, Robertson C, Taylor B. BNT162b2 and ChAdOx1 nCoV-19 vaccine effectiveness against death from the delta variant. N Engl J Med. 2021;385(23):2195–7.
Chagla Z. In high-risk adults, the Moderna vaccine had 94% efficacy against COVID-19 ≥ 14 d after the 2nd dose. Ann Intern Med. 2021;174(3):JC28.
Grannis SJ, Rowley EA, Ong TC, Stenehjem E, Klein NP, DeSilva MB, et al. Interim estimates of COVID-19 vaccine effectiveness against COVID-19–associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B. 1.617. 2 (Delta) variant predominance—nine states, June–August 2021. Morb Mortal Wkly Rep. 2021;70(37):1291.
Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Aghayari Sheikh Neshin S, Khatami A, et al. Effectiveness of COVID-19 vaccines against Delta (B. 1.617. 2) variant: a systematic review and meta-analysis of clinical studies. Vaccines. 2021;10(1):23.
Griffin S. Covid-19: AstraZeneca vaccine prevents 79% of symptomatic disease and 100% of severe disease, US study finds. British Medical Journal Publishing Group; 2021.
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–201.
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Final analysis of efficacy and safety of single-dose Ad26. S New Engl J Med. 2022;COV2(9):847–60.
Fiolet T, Kherabi Y, MacDonald C-J, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2021.
Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172–83.
Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20(1):1–15.
Organization WH. Background document on the inactivated vaccine Sinovac-CoronaVac against COVID-19: background document to the WHO interim recommendations for use of the inactivated COVID-19 vaccine, CoronaVac, developed by Sinovac, 24 May 2021. World Health Organization; 2021.
Ranzani OT, Hitchings MD, Dorion M, D’Agostini TL, de Paula RC, de Paula OFP et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;374.
Ahamed F, Ganesan S, James A, Zaher WA. Understanding perception and acceptance of Sinopharm vaccine and vaccination against COVID–19 in the UAE. BMC Public Health. 2021;21(1):1–11.
Firouzabadi N, Ghasemiyeh P, Moradishooli F, Mohammadi-Samani S. Update on the effectiveness of COVID-19 vaccines on different variants of SARS-CoV-2. Int Immunopharmacol. 2023:109968.
Uraki R, Ito M, Kiso M, Yamayoshi S, Iwatsuki-Horimoto K, Furusawa Y, et al. Antiviral and bivalent vaccine efficacy against an omicron XBB. 1.5 isolate. Lancet Infect Dis. 2023;23(4):402–3.
Link-Gelles R, Ciesla AA, Roper LE, Scobie HM, Ali AR, Miller JD, et al. Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to Omicron BA. 5–and XBB/XBB. 1.5–related sublineages among immunocompetent adults—increasing Community Access to Testing Program, United States, December 2022–January 2023. Morb Mortal Wkly Rep. 2023;72(5):119.
Monovalent XBB. 1.5 BNT162b2 COVID-19 Vaccine [ https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/10-covid-modjarrad-508.pdf.
Looi M-K. What do we know about the Arcturus XBB. 1.16 subvariant? bmj. 2023;381.
Abbasi J. What to know about EG. 5, the latest SARS-CoV-2 variant of interest. JAMA. 2023.
Feature Article. Medications and COVID-19 Vaccines: What You Should Know [ https://www.chop.edu/news/feature-article-medications-and-covid-19-vaccines-what-you-should-know.
Acknowledgements
We appreciate Iran’s National Elites Foundation for their support.
Funding
This study was supported by Iran’s National Elites Foundation.
Author information
Authors and Affiliations
Contributions
P.G. contributed to study design, data gathering, and writing-original draft, writing-reviewing and revising. S.M-S. contributed to conceptualization, study design, supervision, project administration, and writing-original draft, writing-reviewing and revising. Both authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghasemiyeh, P., Mohammadi-Samani, S. Lessons we learned during the past four challenging years in the COVID-19 era: pharmacotherapy, long COVID complications, and vaccine development. Virol J 21, 98 (2024). https://doi.org/10.1186/s12985-024-02370-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02370-6