Abstract
Parechovirus A (PeV-A, Parechovirus, Picornaviridae) are human pathogens associated with mild to severe gastrointestinal and respiratory diseases in young children. While several studies have investigated the association of PeV-A with human disease, little is known about its epidemiology or detection in Latin America. Between the years 2014 and 2015, a total of 200 samples were collected from Panamanian pediatric patients aged < 16 years old exhibiting symptoms associated with respiratory (n = 64), gastrointestinal (n = 68), or neurological (n = 68) diseases. These samples were gathered from patients who had previously received negative diagnoses for the main respiratory viruses, rotavirus, and neurological viruses like herpes virus, enterovirus, and cytomegalovirus. The presence of PeV-A was analyzed by real time RT-PCR.
Eight positive PeV-A infections (4.0%, 95% CI: 1.7 to 7.7) were detected: two in respiratory samples (3.0%, 95% CI: 0.3 to 10.8), five in gastrointestinal samples (7.3%, 95% CI: 2.4 to 16.3), and one in cerebrospinal fluid (1.5%, 95% CI: 1.4 to 7.9). The study provides evidence of PeV-A circulation in Panama and the data collectively, remarked on the importance of considering PeV-A in the Panamanian pediatric diagnostic landscape, especially when conventional testing for more common viruses yields negative results.
Impact statement
The presence of Parechovirus A (PeV-A) in Latin America has been an unexplored area of research, although it has been associated with gastrointestinal and respiratory diseases in young children. The study was conducted between 2014 and 2015. Two hundred samples were collected from pediatric patients with symptoms related to respiratory, gastrointestinal, or neurological illnesses, with a negative diagnosis of common viruses.
The findings revealed the presence of PeV-A in Panama with varying prevalence among the sample types: two in respiratory samples, five in fecal samples, and one in cerebrospinal fluid.
Understanding the epidemiology and detection of PeV-A in Latin America can lead to better diagnosis and management of pediatric diseases and enhance public health efforts in this region.
Similar content being viewed by others
Introduction
Parechovirus A (HPe-A; Parechovirus, Picornaviridae) are non-enveloped, single-stranded, positive-sense RNA viruses with icosahedral capsids [21]. The first strain was identified in 1956 [22]. Parechoviruses have been divided into six species, of which Parechovirus A (formerly named human parechovirus HpeV) is the only one infecting humans [20]. Other species are zoonotic and important in veterinary medicine: Parechovirus B (formerly named the Ljungan virus)(Lindberg & Johansson, [9], Parechovirus C (Sebokele virus) [6], Parechovirus D (ferret parechovirus) [19], Parechovirus E (falcon parechovirus) [14], Parechovirus F (gecko parechovirus) [17]. PeV-A has a genome of approximately 7,000 nucleotides that encode structural proteins, non-structural enzymatic genes, and conserved untranslated regions [5].
The clinical manifestations of PeV-A range from asymptomatic to severe gastrointestinal or respiratory disease in children, which can be associated with long-term neurodevelopmental sequelae. Encephalitis, meningitis, myocarditis, and sepsis have also been associated with PeV-A infection, and evidence suggests that disease severity depends on the genotype, PEV-A being subdivided to date into 19 genotypes, and the age of patients [4]. Transmission mechanisms of PeV-A occurs through fecal–oral or respiratory routes [21].
Although PeV-A cases have been reported worldwide, most of these cases are concentrated in studies undertaken in Europe, Asia, and North America [3]. Evidence of PeV-A circulation in the Latin American region is limited to a few studies on retrospective surveillance, clinical reports, and analysis of urban streams in Chile, Argentina, and Ecuador [4]. Clinical suspicion of PeV-A circulation in Panama has been suggested to be linked to febrile diseases in neonates; however, laboratory confirmation of these cases is not available [11]. Here, we aimed to detect the circulation of PeV-A in Panama by analyzing clinical samples collected during 2014 and 2015 from pediatric patients with gastrointestinal, neurologic or acute respiratory infections.
Materials and methods
The study protocol was approved by the Gorgas Memorial Institute of Health Studies (ICGES) Bioethics Committee (no. 981/CBI/ICGES/16). From January 2014 to December 2015, nasopharyngeal (NP) and oropharyngeal (OP) swabs, cerebrospinal fluid (CSF), fecal, serum, and ocular samples were collected from pediatric patients aged < 16 years who presented with gastroenteritis, respiratory, and/or neurologic symptoms from different regions from Panama and were sent in cold chain (40C) to the ICGES in Panama City, Panama. Samples were initially screened for Enteroviruses, Cytomegalovirus (CMV), Varicella-zoster virus (VZV), Herpes simplex virus (HSV), Rotavirus, Adenovirus, Influenza A, Influenza B, Human Metapneumovirus, Parainfluenza 1, 2, and 3, Rhinovirus, and Respiratory Syncytial Virus (RSV). After the first screening, only the negative samples from 200 patients were used to investigate the presence of PeV-A infections.
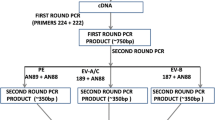
Viral RNA was extracted using the QIAamp® Viral Mini Kit (Qiagen, Valencia, CA, USA). Samples were tested to detect PeV-A viral RNA using a specific single-target qualitative Real Time RT-PCR with the primers ParechoF31 (5’ CTGGGGCCAAAAGCCA-3’) and K30 (5’-GGTACCTTCTGGGCATCCTTC-3’), and the probe FAM-HPeV-MGB (5’-AACACTAGTTGTA(A/T)GGCCC-3’) described by [2] with AgPath-ID ™ One-Step RT-PCR Reagents (Applied Biosystems Life Technologies) and the Applied Biosystems® 7500 Fast Real-Time PCR System. Any sample with a CT < 37 was considered positive. A sequenced-confimed positive sample was used as control.
Results are presented as proportions with bimonial 95% confidence intervals.
Results
Of the 200 clinical samples, 68 neurological samples (CSF and serum) (34%), 68 gastrointestinal samples (fecal and eyes/mouth/anus swaps) (34%), and 64 respiratory samples (nasopharyngeal swab) (32%) were utilized for the study. Eight positive PeV-A infections (4.0%, 95% CI: 1.7 to 7.7; n = 8/200) were detected: two in respiratory samples (3.0%, 95% CI: 0.3 to 10.8; n = 2/64), five in fecal samples (7.3%, 95% CI: 2.4 to 16.3; n = 5/68), and one in cerebrospinal fluid (1.5%, 95% CI: 1.4 to 7.9; n = 1/68) (Table 1). Of these eight positive samples, 3 (three) were from females, 3 (three) from male and 2 (two) were of unidentified sex.
The analyzed samples came from all over the country. The largest number of cases came from the Hospital del Niño Dr. Jose Renan Esquivel in Panama City (128, 64%), which is the main tertiary pediatric public hospital serving patients from throughout the country (Table 1). Over 50% of the samples came from children living in the metropolitan area of the province of Panama, whereas only 1 positive sample was detected in Chiriqui, East Panama and Ngobe-Bugle native reserve (Fig. 1).
The two PeV-A-confirmed respiratory cases had rhinorrhea, cough, and respiratory distress, which began on the day of the initial evaluation. The five confirmed cases of gastrointestinal disease presented with vomiting and diarrhea, one of them also had pneumonia, and the one neurological case had encephalitis (Table 1). Other clinical symptoms included abdominal pain (50%, n = 4/8 positive cases, 80%, n = 4/5 gastrointestinal samples) and fever and respiratory distress to a lesser extent (12.5%, n = 1/8 positive cases).
A total of 119 patients (59.5%, n = 119/200) were younger than two years of age with a median age of 5 months. Of these, 5 were positive for PeV-A (4.2%, n = 5/119 of children under 2 years), all of them being less than one year old (Table 1). The 3 positive cases that were not part of this age group corresponded to gastrointestinal samples. Two of these positive patient samples had no age information, and the other positive case came from a patient of 4 years old (3.7%, N = 3/81) (Table 1).
Discussion
While clinical suspicions have hinted at the circulation of Parechovirus A (PeV-A) in Panama [11], this study explicates compelling laboratory evidence substantiation for the actual presence of PeV-A in the country. Our findings showed that PeV-A is associated with acute gastrointestinal, respiratory, and neurological pediatric infections. Moreover, a notable trend emerged wherein the highest incidence of positive PeV-A cases was observed in gastrointestinal samples, exhibiting a frequency of 4%. This aligns with the previously documented prevalence range from 2 to 16.3% of PeV-A positivity among children presenting with diarrhea [1, 23]. This consistency lends additional weight to the findings in terms of PeV-A’s potential association with gastrointestinal manifestations [1]. This proportion of 4% was similar to that in a Nigerian study of children with a similar age range [13], but lower than that reported in studies from Chile, Spain, and Malawi [3]. Although a higher prevalence of PeV-A was obtained from fecal samples, the clinical symptoms of acute infection could not be directly related to PeV-A, as it has been shown that the duration of the virus in fecal samples can last several months [7].
The second most common symptoms in positive PeV-A samples were respiratory, with a frequency of 3%, similarly to a study carried out in China (3.43%) from 2009 to 2015, and a 10 years follow-up study (2006 to 2016) from Norway, which reported a positivity of 8.8% [18, 24]This follow-up study described that patients positive with PeV-A had mainly low respiratory tract infections [18] similarly, our positive patients reported higher and lower respiratory tract infections.
In this study, we described a PeV-A confirmed case of CNS infection in a newborn in Panama, which is consistent with previous studies showing that CNS infections are detected mainly in newborns and infants under 6 months of age [4, 10].
PeV-A infections were more common in infants under one year of age, regardless of sex, and these had more severe symptoms, these observations are similar to the results obtained in Missouri in 2019 [16]. The observed detection rate among children under 2 years of age during the analyzed years (2014–2015) stood at 2.5%. In contrast, results from Germany displayed rates exceeding 10% [1].
Our study had some limitations. A general caveat is the potential introduction of bias in the epidemiological surveillance, arised from the limited number of clinically suspected samples received in reference laboratories, primarily from hospitalized patients rather than from the outpatient setting. Another caveat, is that, even if we observed that most positive cases are infants and young children, we did not have enough samples for statistical correlation between age and severity [8] future studies with a higher number of samples encompassing more years are needed. Our monoinfection model did not encompass an assessment of coinfection, an aspect that warrants attention in future investigations due to the noteworthy coinfection rates recorded in Japan. There, 59.2% of PeV-A-positive samples exhibited coinfection with other enteric viruses [15]. It is important to note that our results do not imply the absence of co-infection within Panama. Finally, this study did not genotype the confirmed PeV-A cases, which is an important information for ensuring better medical care and general epidemiological surveillance. Indeed, some genotypes have been associated with specific types of symptoms and severity; the PeV-A1 genotype has been more frequently associated with gastrointestinal and respiratory diseases, whereas the PeV-A3 genotype is the main genotype causing CNS and severe diseases in newborns and infants [4, 10, 12] There is a need for future development in the detection and genotyping capabilities within Panama and Central America.
In summary, our findings highlight the detectability of PeV-A in acute samples collected from pediatric hospitalized patients who were initially classified as having associated infections with known etiological agents. This underscores the challenges encountered in executing a differential diagnosis when symptom profiles exhibit substantial overlap with other viral infections, including but not limited to Herpes Virus, Enterovirus, RSV, and rotavirus. In addtion, this study underscores the need for continued surveillance and research to elucidate the full scope of PeV-A’s impact on human health, specially in children, in the Latin American context.
Data availability
N/A
References
Baumgarte S, de Souza Luna LK, Grywna K, Panning M, Drexler JF, Karsten C, Huppertz HI, Drosten C. Prevalence, types, and RNA concentrations of human parechoviruses, including a sixth parechovirus type, in stool samples from patients with acute enteritis. J Clin Microbiol. 2008;46(1):242–8. https://doi.org/10.1128/JCM.01468-07.
Benschop K, Minnaar R, Koen G, van Eijk H, Dijkman K, Westerhuis B, Molenkamp R, Wolthers K. Detection of human enterovirus and human parechovirus (HPeV) genotypes from clinical stool samples: polymerase chain reaction and direct molecular typing, culture characteristics, and serotyping. Diagn Microbiol Infect Dis. 2010;68(2):166–73. https://doi.org/10.1016/j.diagmicrobio.2010.05.016.
Brouwer L, Karelehto E, Han AX, Thomas XV, Bruning AHL, Calis JCJ, van Hensbroek MB, Westerhuis BM, Amarthalingam D, Koekkoek SM, Rebers SPH, Phiri KS, Wolthers KC, Pajkrt D. High frequency and diversity of parechovirus A in a cohort of Malawian children. Arch Virol. 2019;164(3):799–806. https://doi.org/10.1007/s00705-018-04131-7.
Fox B, Paz VS, Incardona MA, Elisiri ME, Fraga SG, Solana CL, Fernández-Canigia L. (2021). Rapid syndromic molecular testing and human parechovirus Infection in children: a report of three cases in Argentina. Revista Argentina de Microbiologia. https://doi.org/10.1016/j.ram.2021.02.003.
Hyypiä T, Horsnell C, Maaronen M, Khan M, Kalkkinen N, Auvinen P, Kinnunen L, Stanway G. A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci USA. 1992;89(18):8847–51. https://doi.org/10.1073/pnas.89.18.8847.
Joffret ML, Bouchier C, Grandadam M, Zeller H, Maufrais C, Bourhy H, Despres P, Delpeyroux F, Dacheux L. Genomic characterization of sebokele virus 1 (SEBV1) reveals a new candidate species among the genus Parechovirus. J Gen Virol. 2013;94(PART7):1547–53. https://doi.org/10.1099/vir.0.053157-0.
Kapusinszky B, Minor P, Delwart E. Nearly constant shedding of diverse enteric viruses by two healthy infants. J Clin Microbiol. 2012;50(11):3427–34. https://doi.org/10.1128/JCM.01589-12.
Khatami A, Burrell R, McMullan BJ, Rawlinson W, Givney RC, Kok J, Alexandersen S, Jones CA, Macartney KK, Britton PN. Epidemic and inter-epidemic Burden of Pediatric Human Parechovirus Infection in New South Wales, Australia, 2017–2018. Pediatr Infect Disease J. 2020;39(6):507–11. https://doi.org/10.1097/INF.0000000000002615.
Lindberg AM, Johansson S. Phylogenetic analysis of Ljungan virus and A-2 plaque virus, new members of the Picornaviridae. Virus Res. 2002;85(1):61–70. https://doi.org/10.1016/S0168-1702(02)00018-7.
Martín del Valle F, Calvo C, Martinez-Rienda I, Cilla A, Romero MP, Menasalvas AI, Reis-Iglesias L, Roda D, Pena MJ, Rabella N, Portugués de la Red MdelM, Megías G, Moreno-Docón A, Otero A, Cabrerizo M. Epidemiological and clinical characteristics of infants admitted to hospital due to human parechovirus Infections: a prospective study in Spain. An Pediatr. 2018;88(2):82–8. https://doi.org/10.1016/j.anpedi.2017.02.009.
Norero X. Infecciones virales en neonatos febriles con sepsis clínica. Hospital Del Niño. Panamá Diciembre 2013- Junio 2014. Pediátr Panamá. 2015;44(2):12–22. https://docs.bvsalud.org/biblioref/2017/11/848726/201544212-22.pdf.
Olijve L, Jennings L, Walls T. Human parechovirus: an increasingly recognized cause of Sepsis-Like Illness in Young infants. Clin Microbiol Rev. 2018;31(1). https://doi.org/10.1128/CMR.00047-17.
Osundare FA, Opaleye OO, Akindele AA, Adedokun SA, Akanbi OA, Bock C-T, Diedrich S, Böttcher S. Detection and characterization of human enteroviruses, human cosaviruses, and a New Human parechovirus type in healthy individuals in Osun State, Nigeria, 2016/2017. Viruses. 2019;11(11). https://doi.org/10.3390/v11111037.
Pankovics P, Boros Á, Mátics R, Kapusinszky B, Delwart E, Reuter G. Ljungan/Sebokele-like picornavirus in birds of prey, common kestrel (Falco tinnunculus) and red-footed falcon (F. Vespertinus). Infection, Genetics and Evolution. J Mol Epidemiol Evolutionary Genet Infect Dis. 2017;55:14–9. https://doi.org/10.1016/j.meegid.2017.08.024.
Pham NTK, Thongprachum A, Shimizu Y, Shiota I, Hoque SA, Khamrin P, Takano C, Trinh QD, Okitsu S, Komine-Aizawa S, Shimizu H, Maneekarn N, Hayakawa S, Ushijima H. Genetic diversity of Parechovirus A in infants and children with acute gastroenteritis in Japan during 2016–2018. Infection, Genetics and Evolution. J Mol Epidemiol Evolutionary Genet Infect Dis. 2021;90:104776. https://doi.org/10.1016/j.meegid.2021.104776.
Sasidharan A, Harrison CJ, Banerjee D, Selvarangan R. Emergence of Parechovirus A4 Central Nervous System Infections among infants in Kansas City, Missouri, USA. J Clin Microbiol. 2019;57(5). https://doi.org/10.1128/JCM.01698-18.
Shi M, Lin XD, Chen X, Tian JH, Chen LJ, Li K, Wang W, Eden JS, Shen JJ, Liu L, Holmes EC, Zhang YZ. The evolutionary history of vertebrate RNA viruses. Nature. 2018;556(7700):197–202. https://doi.org/10.1038/s41586-018-0012-7.
Skanke LH, Lysvand H, Heimdal I, Moe N, Krokstad S, Christensen A, Risnes K, Nordbø SA, Døllner H. Parechovirus A in Hospitalized Children with respiratory tract Infections: a 10-Year-long study from Norway. J Pediatr Infect Dis Soc. 2021;10(6):722–9. https://doi.org/10.1093/jpids/piab009.
Smits SL, Raj VS, Oduber MD, Schapendonk CME, Bodewes R, Provacia L, Stittelaar KJ, Osterhaus AD, M. E., Haagmans BL. Metagenomic analysis of the ferret fecal viral flora. PLoS ONE. 2013;8(8):e71595. https://doi.org/10.1371/journal.pone.0071595.
Sridhar A, Karelehto E, Brouwer L, Pajkrt D, Wolthers KC. Parechovirus a pathogenesis and the enigma of genotype A-3. Viruses. 2019;11(11):1–18. https://doi.org/10.3390/v11111062.
Westerhuis B, Kolehmainen P, Benschop K, Nurminen N, Koen G, Koskiniemi M, Simell O, Knip M, Hyöty H, Wolthers K, Tauriainen S. Human parechovirus seroprevalence in Finland and the Netherlands. J Clin Virol. 2013;58(1):211–5. https://doi.org/10.1016/j.jcv.2013.06.036.
Wigand R, Sabin AB. Properties of ECHO types 22, 23 and 24 viruses. Archiv Fur Die Gesamte Virusforschung. 1961;11:224–47. https://doi.org/10.1007/BF01241688.
Yip CCY, Lo K-L, Que T-L, Lee RA, Chan K-H, Yuen K-Y, Woo PCY, Lau SK P. Epidemiology of human parechovirus, Aichi virus and salivirus in fecal samples from hospitalized children with gastroenteritis in Hong Kong. Virol J. 2014;11:182. https://doi.org/10.1186/1743-422X-11-182.
Zhang XA, Zhao RQ, Chen JJ, Yuan Y, Tang X, Zhou ZW, Ren L, Lu Q, Bin, Wang YN, Zhang HY, Zhang PH, Fang LQ, Zhou HS, Liu EM, Xu HM, Liu W. The identification and genetic characterization of parechovirus Infection among Pediatric patients with wide clinical spectrum in Chongqing, China. Front Microbiol. 2021;12. https://doi.org/10.3389/fmicb.2021.709849.
Acknowledgements
We thank all members of the Department of Research in Virology and Biotechnology and the Clinical Research Unit of the ICGES. SLV and LA are members of the Sistema Nacional de Investigación (SNI) of SENACYT, Panama.
Funding
This work was supported by the National Institute on Minority Health and Health Disparities (NIMHD) [grant number Nº 5T37MD001376-2012].
Author information
Authors and Affiliations
Contributions
[Mandatory for Access Microbiology, encouraged for all other journals. A section describing each author?s contribution to the research, using the CRediT taxonomy from CASRAI: https://casrai.org/credit/] Conceptualization: LA, NS, BM Methodology: LA, VS, LG, LFR Validation: LA, ZCB, JMP, JPC, LFR, JPC, JC Formal analysis: EF, LFR, JPC, JC Investigation: LA, SLV, NS Resources: DF, MC, BM writing—original draft preparation: EF, LFR, JPC, JC, ZCB writing—review and editing: LA, SLV, LFR, JPC, JC ZCB supervision: LA, NS, JPC, JMP project administration: LA, ZCB funding acquisition: LA, JC, NS.
Corresponding author
Ethics declarations
Ethical approval
The research protocol was approved by ICGES IRB, Note N ° 981 / CBI / ICGES / 16 on September 16, 2016.
Consent for publication
N/A.
Conflict of interest
We confirm that neither the manuscript nor any parts of its content are currently under consideration or published in another journal. All authors have approved the manuscript and agree with its submission. Authors have disclosed any conflicts of interest related to this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gutierrez, L., Sáenz, V., Franco, D. et al. Detection of parechovirus A in respiratory, gastrointestinal, and neurological clinical samples of pediatric patients from Panama (2014–2015). Virol J 20, 302 (2023). https://doi.org/10.1186/s12985-023-02268-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-023-02268-9