Abstract
Background
The causal role of high-risk Human papillomavirus (HR-HPV) in the pathogenesis of anogenital cancers is well established. In contrast, information on HR-HPV distribution of continuous anatomic sites within the female genital tract is limited, and the impact of sample type on the clinical performance in HPV-based cervical cancer screening warrants investigation.
Methods
A total of 2,646 Chinese women were enrolled in the study from May 2006 to April 2007. We analyzed the infection features by infection status and pathological diagnoses of 489 women with complete HR-HPV type and viral load data on the cervix, upper vagina, lower vagina, and perineum samples. Additionally, we assessed the clinical performance for detecting high-grade cervical intraepithelial neoplasia of grade two or worse (≥ CIN2) among these four types of samples.
Results
HR-HPV positivity rate was lower in the cervix (51.53%) and perineum (55.83%), higher in the upper (65.64%) and lower vagina (64.42%), and increased with the severity of cervical histological lesions (all P<0.001). Single infection was more dominant than multiple infections at each anatomic site of the female genital tract. The proportion of single HR-HPV infection decreased successively from the cervix (67.05%) to the perineum (50.00%) (Ptrend=0.019) in cervical intraepithelial neoplasia grade 1 (CIN1) and was higher in samples of the cervix (85.11%) and perineum (72.34%) in ≥ CIN2. In addition, the highest viral load was observed in the cervix compared to the other three sites. The overall agreement of the cervical and perineum samples was 79.35% and increased continuously from normal (76.55%) to ≥ CIN2 (91.49%). As for the detection of ≥ CIN2, the sensitivity was 100.00%, 97.87%, 95.74%, and 91.49% for the cervix, upper vagina, lower vagina, and perineum samples, respectively.
Conclusions
Single HR-HPV infection predominated throughout the female genital tract, but the viral load was lower compared to multiple HR-HPV infections. Despite the decreasing viral load from cervix to perineum, the clinical performance for detecting ≥ CIN2 of the perineum sample was comparable to that of the cervix.
Similar content being viewed by others
Introduction
Genital Human papillomavirus (HPV) infection causes almost all cervical cancer and is associated with other anogenital cancers. Nearly 4.5% of all cancers worldwide (630,000 new cancer cases per year) are mainly due to HPV infection: 8.6% in women and 0.8% in men [1]. Of the over 200 types of HPV, about 40 types can infect the epithelium of the anogenital tract or other mucosae [2, 3], and at least 13 types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) are highly carcinogenic to humans [4]. These carcinogenic (or high-risk [HR]) HPV infections are frequent after sexual contact. Although most HPV infections are asymptomatic and generally cleared within two years after sexual transmission, persistent high-risk HPV infections are at the greatest risk for starting the oncogenic process from a preneoplastic lesion to invasive cancer [5]. HPV testing has become a crucial part of cervical cancer screening and is an effective primary screening method in cervical cancer prevention strategies [6].
Within the past two decades, there has been an increased interest in the clinical validity of various sampling methods in cervical cancer screening, such as urine and vaginal HPV self-sampling [7, 8]. These sampling methods can increase the screening coverage of a targeted population [9] and maintain good clinical performance for detecting cervical lesions [10]. Due to their low cost, non-invasiveness, and acceptability, vaginal and urine self-collected samples provide an alternative way to solve the problem of poor screening uptake. However, further evaluation on these sampling methods and the comparison with the “gold standard” cervical sample is necessary [11].
The distal vagina, clitoris, and urethra are integrated entities covered superficially by the vulval skin and its epithelial features [12]. As a result, urine HPV might be affected by the HPV viral load in the perineum or genital tract. The infection features at other anatomic sites of the female genital tract beyond the cervix (e.g., the vagina and perineum) may explain the natural history of HR-HPV infection in the female reproductive tract, help control the HR-HPV infection, and provide improved HPV detection in urine.
In a previous study of HR-HPV genotype distribution in the female genital tract, we found concordance between the cervix and other genital sites [13]. However, infection features of HR-HPV throughout the female genital tract, including viral load and status of single/multiple infections, are poorly understood, especially for the perineum.
Here, we describe the infection status and viral load of HR-HPV in the cervix, upper vagina, lower vagina, and perineum to provide more evidence for the natural history of HR-HPV infection throughout the female genital tract. In addition, we evaluate the concordance of HPV detection for cervical intraepithelial neoplasia of grade two or worse (≥ CIN2) using different samples.
Materials and methods
Study population
This research was part of a multi-center, population-based study of cervical cancer screening in China’s rural areas (Shanxi Province Cervical Cancer Screening Study III, SPOCCS III). A total of 2,646 women were enrolled in the study from May 2006 to April 2007. The inclusion and exclusion criteria and study procedures have been described previously [13, 14]. Briefly, specimens were collected by local gynecologists sequentially from 4 anatomic sites of the female genital tract: perineum, lower vagina, upper vagina, and cervix. Hybrid Capture2 High-Risk HPV DNA (HC2 HR-HPV) test and Linear Array were adopted as the HPV detection methods. Women with cervical HR-HPV positive tested by HC2 HR-HPV test or abnormal cytology received a colposcopy test, and the samples from the other three sites were further tested by HC2 HR-HPV test and Linear Array. Approximately 10% of screen-negative women were randomly selected and completed the screening workflow. Ultimately, 489 women with complete HPV type and viral load data on the cervix, upper vagina, lower vagina, and perineum samples were included in the final analysis (Fig. 1). This study was approved by the institutional review board (IRB) of the Cleveland Clinic and the Cancer Institute/Hospital of the Chinese Academy of Medical Sciences (CICAMS).
Pathology diagnosis
Histological slides were diagnosed by local pathologists, with a quality control sample of 35 slides (15 CIN1, 10 CIN2, and 10 CIN3) reread by a panel of three pathologists. The original histological interpretation by the local pathologists was used for data analysis, and screen-negative women were defined as pathology-negative.
Detection of HPV viral load and genotyping
HR-HPV viral load was estimated using the signal strength of relative light unit /cutoff ratio (RLU/CO) detected by Hybrid Capture2 High-Risk HPV DNA test, and genotypes of HPV were further identified by Linear Array (Roche, Pleasanton, CA) assay. The HC2 HR-HPV test was a signal-amplified hybridization microplate-based assay and can detect 13 high-risk genotypes, including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 semi-quantitatively. RLU/CO ≥ 1 was defined as positive for the HC2 HR-HPV test [13].
Linear Array is a HPV genotyping test and can detect up to 37 individual HPV genotypes simultaneously (i.e., genotypes 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39, and CP6108) [15]. Linear Array results were considered HR-HPV positive only if one of the 13 HR-HPV types targeted by HC2 was detected. All detections were performed according to the manufacturer’s instructions.
Statistical analysis
Specimens with RLU/CO of one by HC2 HR-HPV test were supposed to contain one pg/ml of viral load. Therefore, the signal strength of HC2 HR-HPV was used to describe the viral load of each sample. Infection rate of HR-HPV was calculated based on the Linear Array test result. The single infection was defined as an infection of only one of the 13 HR-HPV types, whereas multiple infections were defined as co-infections with two or more of the 13 HR-HPV types. Comparison of infection rates of HR-HPV for different anatomic sites of the female genital tract was analyzed using Chi-square tests. Chi-square trend tests were used to investigate the HR-HPV infection variation tendency from the cervix to the perineum and from normal to high-grade cervical lesions. ANOVA tests were conducted to estimate the viral load variation in different anatomic sites of the female genital tract and pathological diagnoses. Data were analyzed using R software (V4.0.3), and P < 0.05 (two-sided) was considered statistically significant.
Results
HR-HPV positivity rate by infection status and pathological diagnoses
Among the 489 subjects, there were 47 ≥ CIN2, 88 CIN1, and 354 with negative pathology. The overall HR-HPV positivity rate was significantly different among female genital sites (P < 0.001, Fig. 2A), with lower percentage in cervix (n = 252, 51.53%) and perineum (n = 273, 55.83%) compared to upper (n = 321, 65.64%) and lower vagina (n = 315, 64.42%). The positivity rate of HR-HPV single infection showed no significance among different anatomic genital sites (P = 0.303, Fig. 2A). However, the prevalence of multiple infections demonstrated significant differences among anatomic genital sites (P < 0.001), with increasing tendency from the cervix (n = 36, 7.36%) to the perineum (n = 76, 15.54%) (Ptrend<0.001, Fig. 2A). In addition, at each anatomic site of the female genital tract, the prevalence of single infection was higher than that of multiple infections (Fig. 2A). The positivity rates of HR-HPV increased with the elevation of cervical histological lesions at each site of the female genital tract (Fig. 2B). Positivity rates of HR-HPV showed different tendencies from the cervix to perineum in women with different histological diagnoses. In women diagnosed with ≥ CIN2, the positivity rate decreased successively from the cervix (n = 47, 100%) to the perineum (n = 43, 91.48%) (Ptrend=0.026), while in women diagnosed with CIN1, higher positivity rates were found in the upper (n = 81, 92.04%) and lower vagina (n = 80, 90.91%) compared to cervix (n = 75, 85.23%) and perineum (n = 71, 80.68%) (Fig. 2B).
Trends in single or multiple infections stratified by pathological diagnoses
There were significant differences in the positivity rate of HPV when focusing on single and multiple infection rates in different anatomic sites by pathological diagnoses (Fig. 3). The positivity rate of single HR-HPV infection decreased successively from the cervix (n = 59, 67.05%) to the perineum (n = 44, 50.00%) (Ptrend=0.019) in women with CIN1 (Fig. 3A). In women with ≥ CIN2, the single HR-HPV infection rate was higher in samples from the cervix (n = 40, 85.11%) and perineum (n = 34, 72.34%); however, in women with a normal cervix, higher positivity rates were found in samples from the upper (n = 145, 40.96%) and lower vagina (n = 134, 37.85%) (Fig. 3A). As for multiple infections (Fig. 3B), the HR-HPV positivity rate increased successively from the cervix to the perineum, which was statistically different among the four sites of the female genital tract for women with normal or CIN1 cervix (P < 0.001). In women with ≥ CIN2, the multiple infection rates differed by anatomic sites, but no statistical difference was found (P = 0.156, Fig. 3B). In addition, in different cervical pathological diagnoses, the single HR-HPV infection was more dominant than multiple infections at each anatomic site of the female genital tract (Fig. 3).
Variation of HR-HPV viral load by infection status and pathological diagnoses
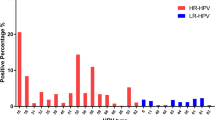
Viral load in the cervix was higher than the other three anatomical sites, regardless of the infection status or pathological diagnoses (Fig. 4), with viral load in the perineum being the lowest. At each site of the female genital tract, the viral load of the single infection was lower, on average, than that of multiple infections (Figure S1). In the upper vagina, the viral load difference between single and multiple infections was significant in women with ≥ CIN2 (P = 0.007) or normal cervix (P = 0.040) but not in women with CIN1 (P = 0.891). In the lower vagina, the viral load of different infection statuses was statistically different in the normal cervix (P<0.001) but not statistically significant in CIN1(P = 0.491) or ≥ CIN2 (P = 0.144). At the perineum, single infection viral load was significantly lower than multiple infections across all pathological diagnoses (P<0.05, respectively).
Agreement of HR-HPV between cervix and perineum sample detected by Linear array
Table 1 shows the agreement of HR-HPV between the cervix and perineum samples detected by Linear Array. Overall, the positive agreement rate was 67.73% (95%CI: 63.59–71.87%), and the overall agreement rate was 79.35% (95%CI: 75.76–82.94%). When considering pathological diagnoses, positive agreement and overall agreement increased with the elevation of cervical lesions. From normal cervix to ≥ CIN2, positive agreement increased from 55.38% (95%CI: 50.20-60.56%) to 91.49% (95%CI: 83.51–99.47%) (Ptrend<0.001), and overall agreement increased from 76.55% (95%CI: 72.14–80.96%) to 91.49% (95%CI: 83.51–99.47%) (Ptrend=0.026).
Clinical performance for the detection of ≥ CIN2 by different samples
Clinical performance for detecting ≥ CIN2 using different samples is depicted in Table 2. Detection sensitivity was not statistically different (P = 0.158), but showed decreasing trend from cervix to perineum (Ptrend=0.026), which was 100.00% (95%CI: 98.40–100.00%) in cervix samples, 97.87% (95%CI: 88.89–99.62%) in upper vagina samples, 95.74% ( 95%CI: 85.75–98.83) in lower vagina samples, and 91.49% in perineum samples (95%CI: 80.07–96.64%). In addition, the specificity of upper (37.78%, 95%CI: 33.39–42.39%) and lower vagina (38.91%, 95%CI: 34.48–43.54%) samples were silghtly lower compared to cervical sample (P < 0.05). The area under the curve (AUC) of cervix samples and perineum samples were 0.768 (95%CI: 0.720–0.817) and 0.697 (95%CI: 0.632–0.763), respectively.
Discussion
Cervical cancer screening strategies should consider accuracy, accessibility, and acceptability, especially for women living in low- and middle-income areas [16]. Studying the features of HR-HPV infection throughout the female genital tract will provide an epidemiological basis for seeking the most cost-effective strategies to prevent and control HR-HPV infection [17]. In this research, we analyzed the infection status and viral load of HR-HPV and evaluated the clinical performance for detecting ≥ CIN2 at four anatomic sites of the female genital tract in a population of Chinese women. To our knowledge, this is the first study that simultaneously explores HR-HPV prevalence and viral load variation encompassing everything from the cervix to the perineum.
Our data indicated that the overall HR-HPV prevalence in the perineum is comparable to that of the cervix, while higher infection rates were found in the upper and lower vagina, perhaps due to the high prevalence of multiple HR-HPV infections. As for infection status, previous studies showed that single HR-HPV infection predominated in the HPV-positive population [18,19,20], and a similar phenomenon was observed in this current study. Additionally, although the prevalence of single HR-HPV infection was decreasing from the cervix to the perineum (not statistically significant), for the upper vagina, lower vagina, and perineum, single HR-HPV infection was also the dominant status, regardless of the pathological diagnoses. On the other hand, the infection rate for multiple HR-HPV infections increased from the cervix to the perineum.
The high prevalence of multiple HR-HPV infections might contribute to higher viral load [21], one of the major determinants of HPV persistence [2]. Therefore, despite the predominant prevalence of single HR-HPV infection at all sites of the genital tract, its viral load was lower than that of multiple HR-HPV infections. In addition, since the multiple infections were more common in the upper and lower vagina, compared with the cervix or the perineum, this might indicate a higher possibility of HPV persistence in these sites, but how it affects cervical infections requires further study. Moreover, the viral load of HR-HPV increased sequentially from the perineum to the cervix in both single and multiple-infection status, which might explain the higher risk of HPV infection resulting in developing cervical cancer than vaginal or vulvar cancer. The low viral load of the perineum also suggested that the limit of detection (LOD) of an HPV detection technology should be taken into consideration, and polymerase chain reaction (PCR)-based techniques seem to be more appropriate for HPV detection of perineum or urine samples, given the ability to identify viral DNA at low levels.
Similar to the cervix [22, 23], HR-HPV positivity rates in the upper vagina, lower vagina, or perineum increased with the severity of cervical lesions. However, the variation of HR-HPV viral load was different. Only in the cervix was the HR-HPV viral load positively-changed with the cervical lesions; in the upper vagina, lower vagina, or perineum, higher HR-HPV viral load was found in CIN1. Since high viral loads are associated with infection persistence [24], more prospective studies are needed to determine whether the cervix HPV infection status will be affected by the viral load in the upper and lower vagina or not.
The agreement of HR-HPV between the cervix and the perineum samples was good and increased with the elevation of cervical lesions, which suggests that HPV in the perineum is a good reflection of the infection status of the cervix, especially in women with ≥ CIN2. Although the sensitivity of the perineum sample was lower than that of the cervix, the upper vagina, or the lower vagina, it was still as high as 91%. At the same time, the specificity and PPV of the perineum sample were comparable to the cervix sample and higher than that of the upper and lower vagina sample. In this study, specificity was lower compared to other studies [25], which might be due to the small number of negative tests in the population. However, the PCR-based HPV detection using perineum samples proved consistent with cervical samples, supporting the feasibility of HPV detection in urine.
Several limitations should be mentioned in this study. Firstly, HPV viral load was defined as the signal strength tested by the HC2 HR-HPV test, which might be affected by the number of heavily-infected cells sampled. This study could not measure the bias in sampling collection. However, viral load measured by HC2 RLU/CO was found to correlate well with that by real-time polymerase chain reaction [26]. In addition, this study lacked prospective follow-up, and information on the correlation between persistent HR-HPV infection status in different genital sites and the risk of cervical or genital lesions is limited.
Conclusions
This study updated epidemiologic evidence on HR-HPV infection and viral load variation among the cervix, the upper vagina, the lower vagina, and the perineum. We observed that the single HR-HPV infection predominated throughout the female genital tract, but the viral load of single HR-HPV infection was lower than multiple infections at any site of the female genital tract. Then, we noted that despite the variation in viral loads, the clinical performance of the perineum sample was comparable to that of the cervix sample, and PCR-based techniques were recommended for HPV detection of perineum or urine samples. Lastly, more in-depth studies will need to be conducted to determine whether the high viral load of the upper and lower vagina in CIN1 affects the persistent HR-HPV infection of the cervix.
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HR-HPV:
-
High risk Human papillomavirus
- ≥CIN2:
-
High-grade cervical intraepithelial neoplasia of grade two or worse
- CIN1:
-
Cervical intraepithelial neoplasia grade 1
- HC2 HR-HPV:
-
Hybrid Capture2 High-Risk HPV DNA test
- IRB:
-
Institutional review board
- CICAMS:
-
Cancer Institute/Hospital of the Chinese Academy of Medical Sciences
- RLU/CO:
-
Relative light unit /cutoff ratio
- AUC:
-
The area under the curve
- CI:
-
Confidence interval
- LOD:
-
Limit of detection
- PCR:
-
Polymerase chain reaction
- PPV:
-
Positive predictive value
References
de Martel C, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70. https://doi.org/10.1002/ijc.30716
de Sanjosé S, Brotons M, Pavón MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–13. https://doi.org/10.1016/j.bpobgyn.2017.08.015
Steben M, Duarte-Franco E. Human papillomavirus infection: epidemiology and pathophysiology. Gynecol Oncol. 2007;107(2 Suppl 1):S2–5. https://doi.org/10.1016/j.ygyno.2007.07.067
Mirabello L, et al. HPV16 Sublineage Associations with Histology-Specific Cancer Risk using HPV whole-genome sequences in 3200 women. J Natl Cancer Inst. 2016;108(9). https://doi.org/10.1093/jnci/djw100
Schiffman M, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. https://doi.org/10.1016/s0140-6736(07)61416-0
Zhang J, et al. Effectiveness of high-risk human papillomavirus testing for Cervical Cancer Screening in China: a Multicenter, Open-label, Randomized Clinical Trial. JAMA Oncol. 2021;7(2):263–70. https://doi.org/10.1001/jamaoncol.2020.6575
Daponte A, et al. Urine HPV in the Context of Genital and Cervical Cancer Screening-An Update of current literature. Cancers (Basel). 2021;13(7). https://doi.org/10.3390/cancers13071640
Xu HF, et al. [Acceptance evaluation of urine self-sampling, vaginal self-sampling and physician sampling in cervical cancer screening]. Zhonghua Zhong Liu Za Zhi. 2021;43(12):1282–6. https://doi.org/10.3760/cma.j.cn112152-20190419-00252
Madzima TR, Vahabi M, Lofters A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women: focused literature review. Can Fam Physician. 2017;63(8):597–601.
Zhao FH, et al. Pooled analysis of a self-sampling HPV DNA test as a cervical cancer primary screening method. J Natl Cancer Inst. 2012;104(3):178–88. https://doi.org/10.1093/jnci/djr532
Kitchener HC, Owens GL. Urine testing for HPV. BMJ. 2014;349:g5542. https://doi.org/10.1136/bmj.g5542
O’Connell HE, et al. The anatomy of the distal vagina: towards unity. J Sex Med. 2008;5(8):1883–91. https://doi.org/10.1111/j.1743-6109.2008.00875.x
Zhang SK, et al. Comparison of HPV genotypes and viral load between different sites of genital tract: the significance for cervical cancer screening. Cancer Epidemiol. 2014;38(2):168–73. https://doi.org/10.1016/j.canep.2014.01.004
Belinson JL, et al. Prevalence of type-specific human papillomavirus in endocervical, upper and lower vaginal, perineal and vaginal self-collected specimens: implications for vaginal self-collection. Int J Cancer. 2010;127(5):1151–7. https://doi.org/10.1002/ijc.25144
Stevens MP, et al. Comparison of the Digene Hybrid capture 2 assay and Roche AMPLICOR and LINEAR ARRAY human papillomavirus (HPV) tests in detecting high-risk HPV genotypes in specimens from women with previous abnormal pap smear results. J Clin Microbiol. 2007;45(7):2130–7. https://doi.org/10.1128/jcm.02438-06
Valdez M, et al. Effectiveness of novel, lower cost molecular human papillomavirus-based tests for cervical cancer screening in rural china. Int J Cancer. 2016;138(6):1453–61. https://doi.org/10.1002/ijc.29877
Wei F, et al. The prevalence and concordance of human papillomavirus infection in different anogenital sites among men and women in Liuzhou, China: a population-based study. Int J Cancer. 2018;142(6):1244–51. https://doi.org/10.1002/ijc.31128
Ge Y, et al. Prevalence of human papillomavirus infection of 65,613 women in East China. BMC Public Health. 2019;19(1):178. https://doi.org/10.1186/s12889-019-6487-9
Li M, et al. Prevalence characteristics of single and multiple HPV infections in women with cervical cancer and precancerous lesions in Beijing, China. J Med Virol. 2019;91(3):473–81. https://doi.org/10.1002/jmv.25331
Chaturvedi AK, et al. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect Dis. 2011;203(7):910–20. https://doi.org/10.1093/infdis/jiq139
Schmitt M, et al. Multiple human papillomavirus infections with high viral loads are associated with cervical lesions but do not differentiate grades of cervical abnormalities. J Clin Microbiol. 2013;51(5):1458–64. https://doi.org/10.1128/jcm.00087-13
Zhang L, et al. Human papillomavirus infections among women with cervical lesions and cervical cancer in Eastern China: genotype-specific prevalence and attribution. BMC Infect Dis. 2017;17(1):107. https://doi.org/10.1186/s12879-017-2223-1
Zhang J, Cheng K, Wang Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: a meta-analysis. Arch Gynecol Obstet. 2020;302(6):1329–37. https://doi.org/10.1007/s00404-020-05787-w
Oyervides-Muñoz MA, et al. Multiple HPV infections and viral load Association in Persistent Cervical Lesions in Mexican Women. Viruses. 2020;12(4). https://doi.org/10.3390/v12040380
Arbyn M, et al. Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin Microbiol Infect. 2015;21(9):817–26. https://doi.org/10.1016/j.cmi.2015.04.015
Gravitt PE, et al. A comparison between real-time polymerase chain reaction and hybrid capture 2 for human papillomavirus DNA quantitation. Cancer Epidemiol Biomarkers Prev. 2003;12(6):477–84.
Acknowledgements
The authors would like to acknowledge DCEG Fellows Editorial Board in NCI for assisting in revising the manuscript.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant No. 2021-I2M-1-004); and the National Natural Science Foundation of China (Grant No. 819731136).
Author information
Authors and Affiliations
Contributions
TYL and SMC wrote the main manuscript text and contributed equally to this work. XYL, ZNW, YQZ, JFC, and BL collected and checked the data. FC performed HC2 tests. XZ gave pathological diagnosis. YLQ guided the research. WC guided the study and edited the draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board (IRB) of the Cleveland Clinic and the Cancer Institute/Hospital of the Chinese Academy of Medical Sciences (CICAMS). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, T., Chen, S., Li, X. et al. The features of high-risk human papillomavirus infection in different female genital sites and impacts on HPV-based cervical cancer screening. Virol J 20, 116 (2023). https://doi.org/10.1186/s12985-023-02073-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-023-02073-4