Abstract
Background
Tremors are involuntary rhythmic movements commonly present in neurological diseases such as Parkinson's disease, essential tremor, and multiple sclerosis. Intention tremor is a subtype associated with lesions in the cerebellum and its connected pathways, and it is a common symptom in diseases associated with cerebellar pathology. While clinicians traditionally use tests to identify tremor type and severity, recent advancements in wearable technology have provided quantifiable ways to measure movement and tremor using motion capture systems, app-based tasks and tools, and physiology-based measurements. However, quantifying intention tremor remains challenging due to its changing nature.
Methodology & Results
This review examines the current state of upper limb tremor assessment technology and discusses potential directions to further develop new and existing algorithms and sensors to better quantify tremor, specifically intention tremor. A comprehensive search using PubMed and Scopus was performed using keywords related to technologies for tremor assessment. Afterward, screened results were filtered for relevance and eligibility and further classified into technology type. A total of 243 publications were selected for this review and classified according to their type: body function level: movement-based, activity level: task and tool-based, and physiology-based. Furthermore, each publication's methods, purpose, and technology are summarized in the appendix table.
Conclusions
Our survey suggests a need for more targeted tasks to evaluate intention tremors, including digitized tasks related to intentional movements, neurological and physiological measurements targeting the cerebellum and its pathways, and signal processing techniques that differentiate voluntary from involuntary movement in motion capture systems.
Similar content being viewed by others
Background
Introduction
Tremor is characterized as an involuntary, rhythmic, oscillatory movement of a body part [1], and it can manifest as a symptom of various neurological diseases, including essential tremor (ET), Parkinson’s disease (PD), and multiple sclerosis (MS). The categorization of tremors is based on clinical factors such as anatomical distribution, activation conditions, amplitude, frequency, and underlying etiology. Within the scope of this review, tremors will be classified according to their activation condition and corresponding neurological symptoms and diseases.
Tremor can be classified into two main categories: rest tremor [2], characterized by nonvoluntary activation that occurs when the individual is attempting to rest and is commonly observed in people with PD. In contrast, action tremor [1] involves voluntary movement. Action tremor can be further classified into two subtypes: postural tremor, which occurs when the subject maintains a position against gravity, and kinetic tremor, which is associated with any voluntary movement that can be constant (simple kinetic), specific to a particular activity, such as writing (task-specific), or that increases as the individual approaches a goal or visual target (intention tremor). Intention tremor refers to a rise in the amplitude of tremors when visually guided movements are made toward a target, especially when nearing it. This type of tremor can also be coupled with task-specific tremor as the individual performs targeted movements, for example, during drawing (Archimedes Spiral tests). Intention tremor is believed to be correlated with cerebellar pathology, its connected pathways, or both, and it is a common symptom in people with, for example, MS [3]. It is estimated that 25–60% of people with MS experience postural and intention tremor [4], which typically occurs in the upper limbs at a frequency of 3–4 Hz [3]. However, other types of tremors, such as rest, simple kinetic, and task-specific tremors, are not frequently observed in MS [5].
Assessing tremors in patients with neurological diseases is crucial for determining disease progression and the effectiveness of medical treatments. Traditionally, clinicians use various clinical tests to identify tremor type and severity in patients. However, with the advancement of wearable technologies, such as smartphones, smartwatches, and sophisticated muscle sensors, there are now quantifiable ways to measure movement and tremor. Although wearable technology is a promising approach for quantifying tremors, identifying relevant features for each type of tremor is necessary for practical use. Recent research has shown that analyzing tremor amplitude and frequency makes it possible to differentiate between different movement disorders such as ET and PD versus healthy controls, classify tremor severity, and correlate it with traditional qualitative-scored neurological tests [6]. However, the changing nature of intention tremors, whose amplitude depends on the movement intention of the patient, makes it difficult to quantify this type of tremor and extract valuable features using the current approaches.
Identifying and analyzing intention tremors can greatly aid disease progression monitoring and intervention efficacy assessment. This review examines the advancement of upper limb tremor assessment technology, methodology, and future directions for algorithm and sensor development to improve quantification of tremor in general and intention tremor specifically.
Neurological tests for tremor assessment correlation and comparison
Researchers evaluate tremor assessment technologies by performing specific tasks that amplify the targeted tremor type. These tasks are based on tests used in clinical practice to assess upper limb impairments. Table 1 displays the most common clinical tests used to correlate or as a reference for evaluating assessment technologies. The Fahn-Tolosa-Marin Tremor Scale (FTMRS) [7] and the Essential Tremor Rating Assessment Scale (TETRAS) [8] are frequently used to quantify rest, postural, and kinetic tremor, including tremor during activities of daily living (ADLs). When the technology is tailored for a single population, e.g., people with PD, a more disease-specific test such as the Movement Disorder Society Unified Parkinson’s Disease Rating Scale, Part III Motor Examination (UPDRS-III) [9] is used for correlation purposes.
Another example of a disease-specific test is the Scale for the Assessment and Rating of Ataxia (SARA) test [10], which focuses on cerebellar ataxia. SARA includes the finger to nose test (FTN) and the finger chase test, which specifically evaluates intention tremor.
In summary, clinical tests include different tasks assessing tremor severity depending on their type (see Fig. 1):
-
Rest tremor: Sitting with fully supported arms against gravity.
-
Postural tremor: Maintaining a specific posture against gravity, for example, stretching arms to the front so that the subject maintains their elbows stretched against gravity; or shoulder abduction with elbows flexed and hands held in a pronated position resembling a 'wing-beating' posture.
-
Kinetic tremor: Simple kinetic and task-specific tremors are evaluated using tasks such as handwriting, Archimedes spirals drawings, and finger tapping (FT), as well as ADLs involving whole-body movement, such as pouring drinks, eating, and dressing. Intention tremor severity can be measured using the finger to nose test (FTN). In this test, the subject touches their nose and then the examiner’s finger, with the tremor amplitude expected to increase as the hand approaches the finger. Intention tremor can also be assessed using the finger chase test, where the examiner performs sudden fast pointing movements in a frontal plane. At the same time, the subject follows with their finger as quickly and accurately as possible.
Literature search and data extraction
This review was primarily conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) scoping review checklist (see Additional file 2). In this review, we were interested in finding studies examining quantifiable upper limb tremor assessment strategies accessible to clinicians and patients without highly specialized equipment. To determine the criteria for inclusion and exclusion, we conducted a comprehensive search on PubMed and Scopus with the following title/abstract terms ("tremor") AND ("assessment" OR "measurement" OR "evaluation" OR "detection" OR "quantification" OR "monitoring" OR "correlation" OR "estimation" or "discrimination" OR "analysis" OR "differentiation" OR "classification") AND ("technology" OR "sensor" OR "device" OR "quantification") (last search date: 10 July 2023) (see Additional file 5 for the detailed search strings). Further publications were identified from the list of references of relevant papers and relevant review papers found in our search [6, 11, 12]. After screening the articles for relevance and eligibility, we excluded studies that (1) did not focus on upper limb impairment, (2) focused on upper limb symptoms that explicitly excluded tremor, (3) only used clinical tests and clinician evaluation without any sensor or any automated tool, (4) the type of technology is not portable or usable outside of specialized rooms (e.g., functional magnetic resonance (fMRI) or magnetoencephalography (MEG)) or are invasive, (5) only evaluated healthy subjects, (6) interventional studies using damping tools, such as exoskeletons or functional electrical stimulation (FES), (7) preprints, prospective studies, and not peer-reviewed, and (8) not written in English. The remaining studies, 243 publications (see the details on data extraction in Additional file 3), were analyzed to identify common themes and establish criteria based on the type of sensors, number of subjects, technology, methodology, purpose, and year of publication. According to our screened papers, tremor assessment technologies can be classified into three distinct types, as depicted in Fig. 2 and classified in the table of Additional file 1 and the database found in Additional file 4:
-
i.
Activity level: Based on tools and digitized tasks, using smartphones or tablets, assessment is made through manipulanda or touch-based games.
-
ii.
Based on physiological sensors, physiological measurements are used to detect and differentiate tremors using surface electromyography (EMG) sensors, muscle activation following motor unit recruitment, and electroencephalogram (EEG) measuring the brain's electrical activity from the scalp.
-
iii.
Body function level: Movement based on motion capture systems, the tremor and the posture of the subject’s upper limbs are captured using accelerometers, gyroscopes, inertial measurement units (IMUs), electromagnetic tracking, or camera systems, with or without markers.
Types of tremor assessment technologies include activity level tasks and tools such as tablets and smartphones for drawing, physiological technologies such as surface electromyography (EMG) and electroencephalogram (EEG), and body function level movement-based technologies such as inertial measurement units (IMUs) and camera systems for measuring upper limb pose and movement. Figures adapted from [13, 14] used under CC BY 4.0 and from [15] used under granted copyright by CCC RightsLink
Technologies for tremor assessment
The following sections will discuss the different assessment technologies and algorithms to quantify tremors. The studies in this section have been classified in detail according to sensor type, patient population, and tremor type in Additional files 1 and 4. We encourage the readers to consider this chapter together with those additional files. Table 2 presents an overview of the tools discussed in this chapter and the main type of tremor assessed with them.
Signal processing to quantify and analyze tremors
Tremor assessment technologies measure physical parameters and transform them into electronic signals. For instance, accelerometers placed on the subject’s hand analyze the frequency components of arm acceleration to detect tremors. Signal processing techniques are necessary to remove noise and measure various movement features. The publications in our review employ different algorithms and feature extraction methods based on signal processing techniques for tremor detection. To detect tremors, measurements are typically transformed from the time domain to the frequency domain, focusing on tremor frequencies (2–10 Hz) compared to regular movement. Fast Fourier transform (FFT) and power spectral distribution (PSD) analysis are commonly used. The FFT provides information about the amplitude and phase of individual frequency components in a signal, while the PSD offers insights into the power distribution across different frequency bands. The PSD is especially suitable for comparing signals of varying lengths because it focuses on the frequency distribution regardless of the signal length. In contrast, the FFT is dependent on the signal length.
In addition to the FFT and PSD, decomposing electronic signals in both time and frequency is advantageous, particularly for analyzing changes in frequency strength over time. The discrete wavelet transform (DWT) and Hilbert-Huang transform (HHT) [16] can be helpful for this. The DWT decomposes a signal into wavelets of different frequencies, scales, and orientations, making it more efficient to simultaneously analyze both frequency and time information, more robust to noise, and computationally efficient. On the other hand, the HHT decomposes a signal into its intrinsic mode functions (IMFs) using empirical mode decomposition (EMD) [17] and is better suited for analyzing nonstationary signals with precise time–frequency information. However, it may require more processing power. Thus, DWT and EMD are valuable tools to decompose voluntary and involuntary movement.
Manipulanda and technical tools to quantify tremors
One approach for assessing tremors involves using tools with embedded sensors that can measure the direction, speed, and force of movement [18,19,20,21,22,23,24]. Researchers have utilized tools such as pens [25,26,27,28,29,30] with embedded IMUs and load cells to quantify tremor amplitude while users hold it, attach it to their hands, or write with it. An advantage of embedded sensor tools is their ability to identify different features in virtual tasks [31,32,33]. For example, the Virtual Peg Insertion Test (VPIT), based on the 9HPT [34] test, employs a manipulandum with force sensors in a virtual game environment and serves as a digital health metric for predicting the response to neurorehabilitation interventions in neurological disorders.
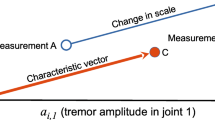
Kanzler et al. [13, 35] identified several features and studied their correlation to clinical tests. They found a high correlation between the SARA test and velocity and path length features in relation to intention tremor. Manipulanda have also been used to elicit intention tremor during goal-directed movements; for example, Feys et al. [36, 37] conducted studies involving people with MS (pwMS) and intention tremors, where they observed more significant target overshoot and unsteady eye fixation during goal-directed movement tasks.
Overall, pens with embedded IMUs have shown promise in measuring different types of tremors, particularly during task-specific movements such as writing or drawing [28]. However, wearable sensors may be more suitable and sensitive for measuring steady tremors than tools. On the other hand, analyzing digital features in addition to traditional completion time in tests such as the 9HPT could provide further insight into the characteristics of intention tremor. However, focused symptom testing is necessary to determine the effectiveness of these digital features in measuring intention tremor. Therefore, studies that specifically focus on it, using manipulanda in tasks similar to the finger chase test [36,37,38], would be advantageous; however, a quantification of intensity and its test correlation would still be required for future studies.
From measuring the duration of completion to quantifying the drawn lines
Digitized drawing tests, such as writing or drawing shapes on tablets or smartphones, offer advantages over traditional methods of assessing tremors. These tests allow for the quantification of drawn lines in terms of time and extraction of different features. The assessment of digitized drawings often involves calculating the power spectral density (PSD) of the drawing position, velocity, or acceleration to determine the frequency ranges of the movement. This can help distinguish subjects with tremors, who are expected to have distinguishable spectra at higher frequencies (> 2 Hz), from those without tremors. Digitizing tablets have been used to assess tremor by analyzing writing and drawing shapes and AS [39,40,41,42,43,44,45,46,47,48,49], as well as combining it with FT [50,51,52,53]. Studies have shown that the frequency spectrum of velocity profiles in digitized Archimedes spirals drawings is a reliable measure of tremor intensity and more accurate than traditional visual rating methods [54].
Smartphone apps offer greater accessibility and flexibility for at-home testing compared to tablets since individuals are more likely to possess a smartphone than a tablet. Furthermore, the choice between smartphones and tablets can affect the reproducibility and intravariability of results, and more straightforward tests may be preferred for smartphone-based MS assessment [55]. This could be advantageous, especially in using small screens where drawings are limited due to space. These approaches include drawing simpler shapes than Archimedes spirals [14, 56,57,58], tilting a smartphone to maintain an objective in position using the smartphone accelerometers [59,60,61], and finger tapping (FT) to assess upper limb impairment [62, 63].
Regarding intention tremor, Erasmus et al. [64] pioneered this method for quantification of ataxic symptoms in MS. They tested it in a large cohort of 342 pwMS where they drew an’8’ shape in a tablet. Consequently, Feys et al. [65] investigated the validity and reliability of drawing regular and squared Archimedes spirals on a tablet as a test for tremor severity. They successfully differentiate pwMS with intention tremor from pwMS with no tremor and healthy subjects (HS) by comparing the radial and tangential velocity PSD in the 3–5 Hz frequencies with FTMRS scores. Archimedes spirals drawings have also proven to be a good measure to identify the presence of intention tremor in pwMS by comparing it with FTN, 9HPT, and BBT [66]. Measuring the segment rate, i.e., the number of times the pen changes from the upward to the downward direction, is the feature that correlates more to visually inspected intention tremor. The advantage of this metric is probably related to the fact that the segment rate increases as the frequency of the movement increases, suggesting that intention tremor could also be detected by analyzing the PSD of the Archimedes spirals movement, as proven by Creagh et al. [56] during the DaS test.
In summary, digitized drawings and app-based games are accessible tools to quantify tremors that could be used in clinics and at home. Tasks such as Archimedes spirals are very effective in eliciting tremors in various neurological diseases. However, it is still unclear how this task is related to intention tremor. Further analysis and correlation to intention tremor tasks, for example, using it in combination with the SARA test, would provide a deeper understanding of its relation to intentional movements.
Physiological measurements: discriminating between different neurological diseases
Surface electromyography (EMG), measuring muscle electrical activity, and mechanomyography (MMG), measuring surface oscillations produced by motor units, are used to analyze muscle activation patterns in upper limb tremors. In the 80–90s, EMG was used to detect tremors using FFT and PSD in subjects with neurological disorders [67,68,69]. EMG has been used to distinguish muscle activation depending on the neurological disease [70,71,72]; for example, Nisticò and Vescio et al. [73, 74] showed that during rest tremor, the activation of antagonist muscles is synchronous in subjects with ET and alternating in those with PD. EMG and accelerometer/IMU combinations [75,76,77,78,79,80,81,82,83] have been extensively used to discriminate PD, ET [84,85,86,87,88,89], physiological tremor (PH) [90, 91], psychogenic tremor [92, 93], advanced ET [94], and MS [95] from each other by using ML techniques on DWT and HT signal decomposition during, in its majority, stretch and steady positions. MMG [96] was recently used with EMG, force sensors, and IMUs to detect tremor differences in PD after deep brain stimulation [97].
Electroencephalogram (EEG) measures the brain's electrical activity from the scalp, providing excellent temporal resolution. However, its low spatial resolution poses a challenge in precisely identifying activity in different brain structures. Despite this drawback, EEG is a valuable tool for evaluating motor tasks [98], as long as the influence of movement artifacts is carefully considered. EEG has been used to explore the involvement of the cerebellum in conditions such as spinocerebellar and cerebellar AT [99, 100], as well as ET in comparison with PD [101, 102], HS [103], and people with age-related tremors (ART) [104]. These studies consistently demonstrate a strong involvement and oscillations of cerebellar activity in ET and PD. Excessive oscillations in cerebellar EEG have been correlated with tremor intensity in ET [105, 106], while increased oscillations in the theta band of cerebellar EEG have been observed in PD [107]. EEG has also been employed to assess the effects of transcranial magnetic stimulation (TMS) therapy in individuals with multiple system atrophy cerebellar subtypes (MSA-C) [108], showing higher cerebello-frontal connectivity and a negative correlation to SARA.
EMG and MMG measurements have effectively been used to differentiate tremor pattern activations in different neurological conditions, even when the subjects perform the same type of activity. These results suggest that muscular activity could be a powerful tool to understand how tremor is propagated and where it is localized. On the other hand, the mentioned studies have emphasized the importance of EEG in studying the involvement of the cerebellum in movement disorders, which could provide valuable insights into the underlying pathophysiology of intention tremor and potential treatment strategies.
Inertial-based recordings using acceleration, orientation, and sensor fusion algorithms
Inertial measurement units (IMUs), consisting of accelerometers, gyroscopes, and magnetometers, measure linear acceleration, angular velocity, and magnetic field strength, respectively. As these signals vary depending on the orientation of the sensor, IMUs have become increasingly prevalent in modern technology applications. These sensors can be positioned on different parts of the limbs, such as the wrist, hand, or fingers, to analyze movement by measuring the acceleration, velocity, and orientation of the limbs. Furthermore, suppose multiple IMUs are used on each limb segment, i.e., hand, forearm, upper arm, and trunk. In that case, it is possible to extract the limb's position relative to the trunk and measure additional features such as range of motion and movement synergy.
In the past, accelerometers, gyroscopes, and magnetometers were available as separate components, and smartphones typically only included accelerometers due to cost considerations. At the end of the last century, accelerometers were used to detect tremors [109,110,111,112,113], quantify medication efficacy [114] in PD, and analyze intention tremors in patients with cerebellar pathology [115]. Accelerometers attached to the hands or wrist either in single form [116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144] or in the form of a smartwatch [145,146,147,148,149,150,151,152,153,154,155,156] or smartphone [157,158,159,160,161,162,163,164,165] have been extensively used to quantify tremors in different neurological diseases [166, 167], either by analyzing acceleration frequency [88, 168] and amplitude [169] or by using machine learning methods to classify measurements according to tremor type [170,171,172,173]. Gyroscopes can detect changes in angular velocity and measure the angular movement of a body part. Analogous to accelerometers, gyroscopes have also been used individually [174,175,176,177,178], in smartphones [179] and smartwatches [180,181,182] to decompose tremorous and voluntary movement using different signal processing techniques such as EMD, HHT [183, 184], WFLC, and EKF [185]. Other types of motion detection sensors, such as force transducers [186,187,188] or electromagnetic sensors [189,190,191,192,193,194], have been proposed to track tremors in ET, PD, and MS.
The miniaturization of IMUs has enabled the direct measurement of tremors on distal limbs using a single chip. Although some studies have utilized both accelerometers and gyroscopes [96, 97, 195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230] to gain insight into tremorous movements, only a portion of them have employed sensor fusion algorithms to integrate these data and improve measurement reliability [131, 231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255]. Sensor fusion filters are used in IMUs to combine data from multiple sensors and improve the accuracy and reliability of the measurements. Their output is no longer angular velocity or acceleration but the IMU orientation relative to a predefined reference. Popular filters include the Madgwick filter and extended Kalman filter (EKF). The Madgwick filter is computationally efficient, using quaternions to combine accelerometer, gyroscope, and magnetometer data for orientation estimation. In contrast, the EKF employs a mathematical model and Bayesian inference to estimate the system state by fusing data from multiple sensors.
Overall, measuring acceleration and angular velocity, using electromagnetic tracking to track upper limb movement, or using a combination of sensors embedded in IMUS has proven to be a popular and straightforward method for measuring tremors. To achieve a more accurate and comprehensive understanding of tremorous movement, future research should use sensor fusion algorithms, which are currently underutilized (less than 39% of the studies using IMUs). This approach would enable researchers to calculate limb position, velocity, and acceleration without the noise drawbacks from accelerometers and gyroscopes to characterize tremor movements. Additionally, this approach would benefit understanding movement synergies and tremor propagation.
Movement prediction with video recordings
Marker-based motion capture uses optical 3D motion analysis systems to track reflective markers placed strategically on the body during movement analysis. It uses infrared cameras to capture marker movement, which is then used to calculate various spatiotemporal, kinematic, and kinetic gait parameters through software calculations [256]. In particular, Deutschl et al. [257] used marker pose estimation to observe whether people with ET showed intention tremors by instructing the participants to grasp a target. The researchers identified the presence of intention tremors similar to that seen in MS and ataxia.
Leap motion systems use multiple cameras and infrared sensors to analyze hand motions within their field of view. While highly accurate, their range of motion is limited [258, 259]. Chen et al. [260] and Khwaounjoo et al. [261] used a leap motion sensor to quantify ET and PD postural tremor by measuring the finger tremor amplitude and frequency. Although their results were less accurate than using IMUs, they showed a strong correlation with respect to them; they localized the best positions for tremor identification and achieved high accuracy at lower frequencies.
Markerless pose estimation is a new technique used to estimate the position and movement of human body joints without using physical markers. Using standard video, it utilizes computer vision and machine learning algorithms to analyze movement in real-time. The technique involves detecting and recognizing key body landmarks, constructing a skeletal model, and estimating joint position and movement over time. Markerless pose estimation software is user-friendly and flexible. Still, it has limitations, including lower accuracy than marker-based systems, difficulty tracking occluded or partially visible body parts, and sensitivity to environmental factors. Nonetheless, ongoing advances in computer vision and machine learning are enhancing the accuracy and robustness of these techniques [262,263,264,265,266,267], making them potentially valuable for tremor characterization—for example, Park et al. [15] utilized Mediapipe [268] to analyze its feasibility in telemedicine for PD. Although the study involved healthy subjects, the findings suggested that movement tracking accuracy was hindered by poor video quality. Nevertheless, the researchers proposed that the software could be effectively utilized with better video setup and equipment. Furthermore, Pang et al. [269] used OpenPose [270], a real-time body pose estimation library using deep learning, to successfully track tremors and bradykinesia in PD using DWT to detect finger motion changes in the frequency domain.
In summary, marker-based estimation technologies capture tremors, but their setup and costs limit their evaluation in large patient cohorts or clinical practice. However, with advancements in computer vision based on deep learning algorithms, markerless pose estimators have the potential to become widely adopted for easy tremor analysis using simple setups such as phone cameras.
Conclusions: future avenues to assess intention tremor
Of all the collected studies, 52 (21% of the total) assessed intention tremor tasks. Furthermore, 37% of these studies [36, 37, 56, 65, 66, 71, 84, 115, 122, 124, 183, 193, 210, 241, 251,252,253, 257, 265] (less than 8% from all studies) focus on pwMS, ataxia, or cerebellar disease, who tend to exhibit intention tremor more clearly. The findings indicate that assessment technologies measuring intention tremor should design tasks that elicit intention tremor and involve individuals who exhibit relevant symptoms.
Although digitized drawings have been examined in people with intention tremor [14, 55, 56, 58, 65, 66], further comparison with other intention tremor tasks is needed, such as the SARA scale and the FTN or finger chase tasks. Moreover, the effectiveness of digitized drawings in eliciting intention tremor and their association with task-specific tremors require more investigation.
Regarding physiological sensors, EMG has been used in pwMS [84, 95]. Still, only one study has explored its application in intention tremor [84], yet their findings did not provide conclusive evidence concerning the relationship between accelerometry and EMG. The understanding of muscle activity in intention tremor remains incomplete, necessitating a more comprehensive analysis. For instance, conducting tasks specifically designed to elicit intention tremor in individuals with cerebellar pathology would facilitate an in-depth investigation of motor conduction times and activation patterns [62].
EEG could help to differentiate movement intention from tremor, as previously suggested by Gallego and Ibáñez et al. [98, 238] in their analysis of tremor in ET. Examining patients' brain activity with intention tremors may shed light on how cortical or cerebellar activities change during motor control tasks. From computational neuroanatomy and neuroimaging studies, the premotor, primary motor, parietal regions of the cortex, and cerebellum are believed to be involved in motor control [271] and tremorous movements [101, 272]. Assessing cerebellar activity during motor control and intention tremor tasks could be valuable, especially for patients with cerebellar pathology [107, 273, 274]. For example, recent studies observed heightened cerebellar activity through cerebellar EEG recordings of ET patients [105] with only one study, to the best of the authors’ knowledge, using an intention tremor task [106]. Additionally, the interaction between the motor, parietal, and cerebellar regions could be analyzed during motor execution and intention tremor tasks. A past study investigated the functional interaction (using EEG modular functional connectivity) of the somatomotor system and higher-order processing systems during a motor task [275].
Motion capture algorithms could be one of the best ways to assess intention tremors due to their easy integration with wearable technologies for intervention, such as tremor-damping exoskeletons. The valuable research conducted by Morgan et al. [115] and Deuschl et al. [257], investigating intention tremor during activities that induce this type of tremor, can now be easily replicated using markerless pose estimation software, as done by Pang et al. in PD [269]. On the other hand, IMU sensors have become practical and effective for tremor detection but require sensor fusion algorithms and signal processing techniques for reliable analysis [90, 183, 242]. Another study was performed by Carpinella et al. [183] effectively employed the combined capabilities of EMD and HHT to accurately detect minute variations in intention tremor tasks. They accomplished automatic classification and distinction between HS and pwMS and detected subtle tremors from voluntary movement in MS. Furthermore, Tran et al. [251, 252] used ballistic tracking (an intention tremor task analogous to the finger chase test) with an IMU and a Kinect camera to distinguish between ataxia and HS successfully. These outcomes present promising prospects for the automated detection and assessment of intention tremors. In addition to facilitating such analysis, this technique could also provide valuable insight into developing intention detection algorithms for individuals with neurological conditions such as pwMS, thereby enabling wearable technologies to function not only as assessment tools but also as sensors for interventions and assistive technologies in daily life.
This review examined the utilization of sensor technology in evaluating tremors across various neurological conditions. Some limitations of our review include manuscripts with unclear terminology related to tremor, e.g., studies not differentiating between the different types of kinetic tremor, and studies with imprecise methodology, especially on sensor fusion with IMUs. Nevertheless, in this review, we tried to the best of our abilities to systematically infer those missing fields using the information in other parts of the manuscripts, e.g., experimental protocol and patient population, to infer tremor type and results and conclusions to infer sensor fusion modalities.
While most research has focused on assessing tremor in PD and ET, intentional tremors observed in patients with lesions in the cerebellum could be better understood. This challenge can be approached by targeting intention tremors and leveraging existing technology (see Fig. 3). First and foremost, a technical contribution is needed to make better intention tremor assessments beyond the current tests. Furthermore, analyzing muscle activation and brain activity through EMG and EEG can provide insights into the underlying causes of intentional tremors. Regarding motion capture, it is crucial to optimize IMUs through sensor fusion algorithms that utilize the strengths of each sensor (accelerometer, gyroscope, magnetometer) to obtain an accurate limb position to extract tremorous movements using time–frequency analysis.
Intention tremor can be further studied through technology and specialized tasks, which isolate and amplify it. EMG and EEG provide insights into source localization and connectivity. Motion capture technologies and algorithms such as EMD reveal details about voluntary and involuntary actions. The figure is adapted from [276] and used under granted copyright by CCC RightsLink
Additionally, using markerless pose estimation would offer a more straightforward and flexible means of capturing data without requiring specialized equipment, enabling assessments to be conducted on more subjects exhibiting intention tremors, for example, at home. Distinguishing between voluntary and involuntary movement remains a challenge for the technologies discussed. Therefore, it is essential to use and further develop signal processing techniques that focus on separating different movement components, such as EMD or DWT, to enhance the detection of the distinct aspects of tremorous movements, their onset, and their differentiation from voluntary movements.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Abbreviations
- ET:
-
Essential tremor
- PD:
-
Parkinson’s disease
- MS:
-
Multiple sclerosis
- HS:
-
Healthy subjects
- ADLs:
-
Activities of daily living
- FTMRS:
-
Fahn-Tolosa-Marin Tremor Scale
- TETRAS:
-
Essential Tremor Rating Assessment Scale
- SARA:
-
Scale for the Assessment and Rating of Ataxia
- FTN:
-
Finger to nose test
- ARAT:
-
Action Research Arm Test
- 9HPT:
-
9 Hole Peg Test
- BBT:
-
Box and Blocks Test
- FT:
-
Finger tapping
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- fMRI:
-
Functional magnetic resonance
- MEG:
-
Magnetoencephalography
- FES:
-
Functional electrical stimulation
- EMG:
-
Electromyography
- EEG:
-
Electroencephalogram
- FFT:
-
Fast Fourier transform
- PSD:
-
Power spectral density
- DWT:
-
Discrete wavelet transform
- HHT:
-
Hilbert-Huang transform
- IMF:
-
Intrinsic model functions
- EMD:
-
Empirical mode decomposition
- VPIT:
-
Virtual Peg Insertion Test
- pwMS:
-
People with MS
- MMG:
-
Mechanomyography
- PH:
-
Physiological tremor
- ART:
-
Age-related tremors
- TMS:
-
Transcranial magnetic stimulation
- MSA-C:
-
Multiple system atrophy cerebellar subtype
- IMU:
-
Inertial measurement units
- EKF:
-
Extended Kalman filter
- IAS:
-
Institute for Advanced Study
- WFLC:
-
Weighted frequency Fourier linear combiner
- SVM:
-
Support vector machine
- CNN:
-
Convolutional neural network
References
Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, Raethjen J, Stamelou M, Testa CM, Deuschl G, the Tremor Task Force of the International Parkinson and Movement Disorder Society. Consensus Statement on the classification of tremors from the task force on tremor of the International Parkinson and Movement Disorder Society. Movement Disord. 2018;33:75–87.
Raethjen J, Austermann K, Witt K, Zeuner KE, Papengut F, Deuschl G. Provocation of Parkinsonian tremor. Mov Disord. 2008;23:1019–23.
Alusi SH, Worthington J, Glickman S, Bain PG. A study of tremor in multiple sclerosis. Brain. 2001;124:720–30.
Koch M, Mostert J, Heersema D, De Keyser J. Tremor in multiple sclerosis. J Neurol. 2007;254:133–45.
Labiano-Fontcuberta A, Benito-León J. Understanding tremor in multiple sclerosis: prevalence, pathological anatomy, and pharmacological and surgical approaches to treatment. Tremor Other Hyperkinet Mov. 2012;2:tre-02.
Vescio B, Quattrone A, Nisticò R, Crasà M, Quattrone A. Wearable devices for assessment of tremor. Front Neurol. 2021;12.
Fahn S, Tolosa E, Concepcion M. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. Baltimore, MD: Williams and Wilkins; 1993. p. 271–280.
Ondo WG, Pascual B, On behalf of the TR Group. Tremor research group essential tremor rating scale (TETRAS): assessing impact of different item instructions and procedures. Tremor Other Hyperkinet Mov. 2020;10:36.
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results: MDS-UPDRS: clinimetric assessment. Mov Disord. 2008;23:2129–70.
Weyer A, Abele M, Schmitz-Hübsch T, Schoch B, Frings M, Timmann D, Klockgether T. Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients. Mov Disord. 2007;22:1633–7.
De A, Bhatia KP, Volkmann J, Peach R, Schreglmann SR. Machine learning in tremor analysis: critique and directions. Mov Disord. 2023;38:717–31.
Lora-Millan JS, Delgado-Oleas G, Benito-León J, Rocon E. A review on wearable technologies for tremor suppression. Front Neurol. 2021;12: 700600.
Kanzler CM, Lessard I, Gassert R, Brais B, Gagnon C, Lambercy O. Reliability and validity of digital health metrics for assessing arm and hand impairments in an ataxic disorder. Ann Clin Transl Neurol. 2022;9:432–43.
Graves JS, Ganzetti M, Dondelinger F, Lipsmeier F, Belachew S, Bernasconi C, Montalban X, van Beek J, Baker M, Gossens C, Lindemann M. Preliminary validity of the Draw a Shape Test for upper extremity assessment in multiple sclerosis. Ann Clin Transl Neurol. 2023;10:166–80.
Park KW, Wu HJ, Yu T, Mahal R, Mirian MS, McKeown MJ. Potential pitfalls of remote and automated video assessments of movements disorders. Mov Disord. 2023;38:504–6.
Huang NE, Shen SSP. Hilbert-Huang transform and its applications. Singapore: World Scientific; 2014.
Huang NE, Shen Z, Long SR, Wu MC, Shih HH, Zheng Q, Yen N-C, Tung CC, Liu HH. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc R Soc Lond Ser A Math Phys Eng Sci. 1998;454:903–95.
Aisen ML, La Rocca NG. Quantitative assessment of tremor in multiple sclerosis patients: a new technique. Assist Technol. 1989;1:3–6.
Beuter A, De Geoffroy A, Cordo P. The measurement of tremor using simple laser systems. J Neurosci Methods. 1994;53:47–54.
Hacisalihzade SS, Albani C, Mansour M. Measuring parkinsonian symptoms with a tracking device. Comput Methods Programs Biomed. 1988;27:257–68.
Norman KE, Edwards R, Beuter A. The measurement of tremor using a velocity transducer: comparison to simultaneous recordings using transducers of displacement, acceleration and muscle activity. J Neurosci Methods. 1999;92:41–54.
Oliveira FHM, Rabelo AG, Luiz LMD, Pereira AA, Vieira MF, Andrade AO. On the use of non-contact capacitive sensors for the assessment of postural hand tremor of individuals with Parkinson’s disease. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Berlin, Germany: IEEE; 2019. p. 6591–6594. https://doi.org/10.1109/EMBC.2019.8856746.
Papapetropoulos S, Jagid JR, Sengun C, Singer C, Gallo BV. Objective monitoring of tremor and bradykinesia during DBS surgery for Parkinson disease. Neurology. 2008;70:1244–9.
Papapetropoulos S, Katzen HL, Scanlon BK, Guevara A, Singer C, Levin BE. Objective quantification of neuromotor symptoms in Parkinson’s disease: implementation of a portable. Comput Meas Tool Parkinson’s Dis. 2010;2010:1–6.
Júnior EP, Delmiro ILD, Magaia N, Maia FM, Hassan MM, Albuquerque VHC, Fortino G. Intelligent sensory pen for aiding in the diagnosis of Parkinson’s disease from dynamic handwriting analysis. Sensors. 2020;20:5840.
Maldonado-Naranjo A, Koop MM, Hogue O, Alberts J, Machado A. Kinematic metrics from a wireless stylus quantify tremor and bradykinesia in Parkinson’s disease. Parkinson’s Dis. 2019;2019:1–9.
Scanlon BK, Levin BE, Nation DA, Katzen HL, Guevara-Salcedo A, Singer C, Papapetropoulos S. An accelerometry-based study of lower and upper limb tremor in Parkinson’s disease. J Clin Neurosci. 2013;20:827–30.
Toffoli S, Lunardini F, Parati M, Gallotta M, De Maria B, Longoni L, Dell’Anna ME, Ferrante S. Spiral drawing analysis with a smart ink pen to identify Parkinson’s disease fine motor deficits. Front Neurol. 2023;14:1093690.
Zajki-Zechmeister T, Kögl M, Kalsberger K, Franthal S, Homayoon N, Katschnig-Winter P, et al. Quantification of tremor severity with a mobile tremor pen. Heliyon Cell. 2020;6(8):e04702.
Lunardini F, Febbo DD, Malavolti M, Cid M, Serra M, Piccini L, Pedrocchi ALG, Borghese NA, Ferrante S. A smart ink pen for the ecological assessment of age-related changes in writing and tremor features. IEEE Trans Instrum Meas. 2021;70:1–13.
Ferenčík N, Jaščur M, Bundzel M, Cavallo F. The rehapiano—detecting, measuring, and analyzing action tremor using strain gauges. Sensors. 2020;20:663.
Goetz CG, Stebbins GT, Wolff D, DeLeeuw W, Bronte-Stewart H, Elble R, Hallett M, Nutt J, Ramig L, Sanger T, Wu AD, Kraus PH, Blasucci LM, Shamim EA, Sethi KD, Spielman J, Kubota K, Grove AS, Dishman E, Taylor CB. Testing objective measures of motor impairment in early Parkinson’s disease: feasibility study of an at-home testing device. Mov Disord. 2009;24:551–6.
Kim J, Wichmann T, Inan OT, DeWeerth SP. Fitts’ law based performance metrics to quantify tremor in individuals with essential tremor. IEEE J Biomed Health Inform. 2022;26:2169–79.
Kellor M, Frost J, Silberberg N, Iversen I, Cummings R. Hand strength and dexterity. Am J Occup Ther. 1971;25:77–83.
Kanzler CM, Lamers I, Feys P, Gassert R, Lambercy O. Personalized prediction of rehabilitation outcomes in multiple sclerosis: a proof-of-concept using clinical data, digital health metrics, and machine learning. Med Biol Eng Comput. 2022;60:249–61.
Feys P, Helsen WF, Lavrysen A, Nuttin B, Ketelaer P. Intention tremor during manual aiming: a study of eye and hand movements. Mult Scler. 2003;9(1):44–54.
Feys P, Helsen WF, Liu X, Lavrysen A, Loontjens V, Nuttin B, Ketelaer P. Effect of visual information on step-tracking movements in patients with intention tremor due to multiple sclerosis. Mult Scler. 2003;9:492–502.
Aisen ML, Arnold A, Baiges I, Maxwell S, Rosen M. The effect of mechanical damping loads on disabling action tremor. Neurology. 1993;43:1346–1346.
Elble RJ, Brilliant M, Leffler K, Higgins C. Quantification of essential tremor in writing and drawing. Mov Disord. 1996;11:70–8.
Elble RJ, Ellenbogen A. Digitizing tablet and Fahn–Tolosa–Marín Ratings of Archimedes spirals have comparable minimum detectable change in essential tremor. Tremor Other Hyperkinet Mov. 2017;7:481.
Elble RJ, Sinha R, Higgins C. Quantification of tremor with a digitizing tablet. J Neurosci Methods. 1990;32:193–8.
Ferleger BI, Sonnet KS, Morriss TH, Ko AL, Chizeck HJ, Herron JA. A tablet- and mobile-based application for remote diagnosis and analysis of movement disorder symptoms. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). Montreal, QC, Canada: IEEE; 2020. p. 5588–5591. https://doi.org/10.1109/EMBC44109.2020.9176044.
Legrand AP, Rivals I, Richard A, Apartis E, Roze E, Vidailhet M, Meunier S, Hainque E. New insight in spiral drawing analysis methods—application to action tremor quantification. Clin Neurophysiol. 2017;128:1823–34.
Lipsmeier F, Simillion C, Bamdadian A, Tortelli R, Byrne LM, Zhang Y-P, Wolf D, Smith AV, Czech C, Gossens C, Weydt P, Schobel SA, Rodrigues FB, Wild EJ, Lindemann M. A remote digital monitoring platform to assess cognitive and motor symptoms in Huntington disease: cross-sectional validation study. J Med Internet Res. 2022;24: e32997.
Pullman SL. Spiral analysis: a new technique for measuring tremor with a digitizing tablet. Mov Disord. 1998;13:85–9.
Riviere CN, Reich SG, Thakor NV. Adaptive Fourier modeling for quantification of tremor. J Neurosci Methods. 1997;74:77–87.
Sisti JA, Christophe B, Seville AR, Garton ALA, Gupta VP, Bandin AJ, Yu Q, Pullman SL. Computerized spiral analysis using the iPad. J Neurosci Methods. 2017;275:50–4.
Stanley K, Hagenah J, Brüggemann N, Reetz K, Severt L, Klein C, Yu Q, Derby C, Pullman S, Saunders-Pullman R. Digitized spiral analysis is a promising early motor marker for Parkinson disease. Parkinsonism Relat Disord. 2010;16:233–4.
Wang Y, Yang J, Cai M, Liu X, Lu K, Lou Y, Li Z. Application of optimized convolutional neural networks for early aided diagnosis of essential tremor: automatic handwriting recognition and feature analysis. Med Eng Phys. 2023;113: 103962.
Aghanavesi S, Nyholm D, Senek M, Bergquist F, Memedi M. A smartphone-based system to quantify dexterity in Parkinson’s disease patients. Inform Med Unlocked. 2017;9:11–7.
Szumilas M, Lewenstein K, Ślubowska E, Szlufik S, Koziorowski D. A multimodal approach to the quantification of kinetic tremor in Parkinson’s disease. Sensors. 2019;20:184.
Wilkins KB, Petrucci MN, Kehnemouyi Y, Velisar A, Han K, Orthlieb G, Trager MH, O’Day JJ, Aditham S, Bronte-Stewart H. Quantitative digitography measures motor symptoms and disease progression in Parkinson’s disease. JPD. 2022;12:1979–90.
Zham P, Arjunan SP, Raghav S, Kumar DK. Efficacy of guided spiral drawing in the classification of Parkinson’s disease. IEEE J Biomed Health Inform. 2018;22:1648–52.
Haubenberger D, Kalowitz D, Nahab FB, Toro C, Ippolito D, Luckenbaugh DA, Wittevrongel L, Hallett M. Validation of digital spiral analysis as outcome parameter for clinical trials in essential tremor. Mov Disord. 2011;26:2073–80.
Messan KS, Pham L, Harris T, Kim Y, Morgan V, Kosa P, Bielekova B. Assessment of smartphone-based spiral tracing in multiple sclerosis reveals intra-individual reproducibility as a major determinant of the clinical utility of the digital test. Front Med Technol. 2022;3: 714682.
Creagh AP, Simillion C, Scotland A, Lipsmeier F, Bernasconi C, Belachew S, van Beek J, Baker M, Gossens C, Lindemann M, Vos MD. Smartphone-based remote assessment of upper extremity function for multiple sclerosis using the Draw a Shape Test. Physiol Meas. 2020;41: 054002.
Lipsmeier F, Taylor KI, Postuma RB, Volkova-Volkmar E, Kilchenmann T, Mollenhauer B, Bamdadian A, Popp WL, Cheng W-Y, Zhang Y-P, Wolf D, Schjodt-Eriksen J, Boulay A, Svoboda H, Zago W, Pagano G, Lindemann M. Reliability and validity of the Roche PD mobile application for remote monitoring of early Parkinson’s disease. Sci Rep. 2022;12:12081.
Montalban X, Graves J, Midaglia L, Mulero P, Julian L, Baker M, Schadrack J, Gossens C, Ganzetti M, Scotland A, Lipsmeier F, van Beek J, Bernasconi C, Belachew S, Lindemann M, Hauser SL. A smartphone sensor-based digital outcome assessment of multiple sclerosis. Mult Scler. 2022;28:654–64.
Boukhvalova AK, Fan O, Weideman AM, Harris T, Kowalczyk E, Pham L, Kosa P, Bielekova B. Smartphone level test measures disability in several neurological domains for patients with multiple sclerosis. Front Neurol. 2019;10:358.
Kuosmanen E, Kan V, Visuri A, Vega J, Nishiyama Y, Dey AK, Harper S, Ferreira D. Mobile-based monitoring of Parkinson’s disease. In: Proceedings of the 17th International Conference on Mobile and Ubiquitous Multimedia. New York, NY, USA: Association for Computing Machinery; 2018. p. 441–448. (MUM ’18). https://doi.org/10.1145/3282894.3289737.
Kuosmanen E, Wolling F, Vega J, Kan V, Nishiyama Y, Harper S, Laerhoven KV, Hosio S, Ferreira D. Smartphone-based monitoring of Parkinson disease: quasi-experimental study to quantify hand tremor severity and medication effectiveness. JMIR Mhealth Uhealth. 2020;8: e21543.
Gulde P, Cetin M, Hermsdörfer J, Rieckmann P. Changes in thumb tapping rates and central motor conduction times are associated in persons with multiple sclerosis. Neurol Sci. 2022;43:4945–51.
Zhan A, Mohan S, Tarolli C, Schneider RB, Adams JL, Sharma S, Elson MJ, Spear KL, Glidden AM, Little MA, Terzis A, Dorsey ER, Saria S. using smartphones and machine learning to quantify Parkinson disease severity: the mobile Parkinson disease score. JAMA Neurol. 2018;75:876–80.
Erasmus L-P, Sarno S, Albrecht H, Schwecht M, Pöllmann W, König N. Measurement of ataxic symptoms with a graphic tablet: standard values in controls and validity in multiple sclerosis patients. J Neurosci Methods. 2001;108:25–37.
Feys P, Helsen W, Prinsmel A, Ilsbroukx S, Wang S, Liu X. Digitised spirography as an evaluation tool for intention tremor in multiple sclerosis. J Neurosci Methods. 2007;160:309–16.
DelMastro HM, Ruiz JA, Gromisch ES, Garbalosa JC, Triche EW, Olson KM, Lo AC. Quantification characteristics of digital spiral analysis for understanding the relationship among tremor and clinical measures in persons with multiple sclerosis. J Neurosci Methods. 2018;307:254–9.
Bacher M, Scholz E, Diener HC. 24 Hour continuous tremor quantification based on EMG recording. Electroencephalogr Clin Neurophysiol. 1989;72:176–83.
Deuschl G, Blumberg H, Lücking CH. Tremor in reflex sympathetic dystrophy. Arch Neurol. 1991;48:1247–52.
Timmer J, Lauk M, Deuschl G. Quantitative analysis of tremor time series. Electroencephalogr Clin Neurophysiol/Electromyogr Motor Control. 1996;101:461–8.
Lin F, Wang Z, Zhao H, Qiu S, Liu R, Shi X, Wang C, Yin W. Hand movement recognition and salient tremor feature extraction with wearable devices in Parkinson’s patients. IEEE Trans Cogn Dev Syst. 2023. https://doi.org/10.1109/TCDS.2023.3266812.
Milanov I. Electromyographic differentiation of tremors. Clin Neurophysiol. 2001;112:1626–32.
Wang S-Y, Aziz TZ, Stein JF, Liu X. Time–frequency analysis of transient neuromuscular events: dynamic changes in activity of the subthalamic nucleus and forearm muscles related to the intermittent resting tremor. J Neurosci Methods. 2005;145:151–8.
Nisticò R, Pirritano D, Salsone M, Novellino F, Giudice FD, Morelli M, Trotta M, Bilotti G, Condino F, Cherubini A, Valentino P, Quattrone A. Synchronous pattern distinguishes resting tremor associated with essential tremor from rest tremor of Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:30–3.
Vescio B, Nisticò R, Augimeri A, Quattrone A, Crasà M, Quattrone A. Development and validation of a new wearable mobile device for the automated detection of resting tremor in Parkinson’s disease and essential tremor. Diagnostics. 2021;11:200.
Basu I, Graupe D, Tuninetti D, Shukla P, Slavin KV, Metman LV, Corcos DM. Pathological tremor prediction using surface electromyogram and acceleration: potential use in ‘ON–OFF’ demand driven deep brain stimulator design. J Neural Eng. 2013;10: 036019.
Boroojerdi B, Ghaffari R, Mahadevan N, Markowitz M, Melton K, Morey B, Otoul C, Patel S, Phillips J, Sen-Gupta E, Stumpp O, Tatla D, Terricabras D, Claes K, Wright JA, Sheth N. Clinical feasibility of a wearable, conformable sensor patch to monitor motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2019;61:70–6.
Brennan KC, Jurewicz EC, Ford B, Pullman SL, Louis ED. Is essential tremor predominantly a kinetic or a postural tremor? A clinical and electrophysiological study. Mov Disord. 2002;17:313–6.
Cohen O, Pullman S, Jurewicz E, Watner D, Louis ED. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol. 2003;60:405–10.
Cole BT, Roy SH, De Luca CJ, Nawab SH. Dynamical learning and tracking of tremor and dyskinesia from wearable sensors. IEEE Trans Neural Syst Rehabil Eng. 2014;22:982–91.
Foerster F, Smeja M. Joint amplitude and frequency analysis of tremor activity. Electromyogr Clin Neurophysiol. 1999;39:11–9.
Roy SH, Cole BT, Gilmore LD, De Luca CJ, Thomas CA, Saint-Hilaire MM, Nawab SH. High-resolution tracking of motor disorders in Parkinson’s disease during unconstrained activity. Mov Disord. 2013;28:1080–7.
Spieker S, Ströle V, Sailer A, Boose A, Dichgans J. Validity of long-term electromyography in the quantification of tremor. Mov Disord. 1997;12:985–91.
Spieker S, Boose A, Breit S, Dichgans J. Long-term measurement of tremor. Mov Disord. 1998;13:81–4.
Ayache SS, Chalah MA, Al-Ani T, Farhat WH, Zouari HG, Créange A, Lefaucheur J-P. Tremor in multiple sclerosis: the intriguing role of the cerebellum. J Neurol Sci. 2015;358:351–6.
Breit S, Spieker S, Schulz JB, Gasser T. Long-term EMG recordings differentiate between parkinsonian and essential tremor. J Neurol. 2008;255:103–11.
Ghassemi NH, Marxreiter F, Pasluosta CF, Kugler P, Schlachetzki J, Schramm A, Eskofier BM, Klucken J. Combined accelerometer and EMG analysis to differentiate essential tremor from Parkinson’s disease. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2016. p. 672–675. https://doi.org/10.1109/EMBC.2016.7590791.
Hossen A, Al-Hakim Z, Muthuraman M, Raethjen J, Deuschl G, Heute U. Discrimination of Parkinsonian tremor from essential tremor by voting between different EMG signal processing techniques. J Eng Res. 2014;11:11–22.
Hossen A, Muthuraman M, Al-Hakim Z, Raethjen J, Deuschl G, Heute U. Discrimination of Parkinsonian tremor from essential tremor using statistical signal characterization of the spectrum of accelerometer signal. Bio-Med Mater Eng. 2013;23:513–31.
Hossen A, Muthuraman M, Raethjen J, Deuschl G, Heute U. Discrimination of Parkinsonian tremor from essential tremor by implementation of a wavelet-based soft-decision technique on EMG and accelerometer signals. Biomed Signal Process Control. 2010;5:181–8.
Ayache SS, Al-ani T, Lefaucheur J-P. Distinction between essential and physiological tremor using Hilbert-Huang transform. Neurophysiol Clin. 2014;44:203–12.
Hossen A, Deuschl G, Groppa S, Heute U, Muthuraman M. Discrimination of physiological tremor from pathological tremor using accelerometer and surface EMG signals. Technol Health Care. 2020;28:461–76.
Piboolnurak P, Rothey N, Ahmed A, Ford B, Yu Q, Xu D, Pullman SL. Psychogenic tremor disorders identified using tree-based statistical algorithms and quantitative tremor analysis. Mov Disord. 2005;20:1543–9.
Zeuner KE, Shoge RO, Goldstein SR, Dambrosia JM, Hallett M. Accelerometry to distinguish psychogenic from essential or parkinsonian tremor. Neurology. 2003;61:548–50.
Nisticò R, Quattrone A, Crasà M, De Maria M, Vescio B, Quattrone A. Evaluation of rest tremor in different positions in Parkinson’s disease and essential tremor plus. Neurol Sci. 2022;43:3621–7.
Hossen A, Anwar AR, Koirala N, Ding H, Budker D, Wickenbrock A, Heute U, Deuschl G, Groppa S, Muthuraman M. Machine learning aided classification of tremor in multiple sclerosis. EBioMedicine. 2022;82: 104152.
Huo W, Angeles P, Tai YF, Pavese N, Wilson S, Hu MT, Vaidyanathan R. A heterogeneous sensing suite for multisymptom quantification of Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng. 2020;28:1397–406.
Angeles P, Tai Y, Pavese N, Wilson S, Vaidyanathan R. Automated assessment of symptom severity changes during deep brain stimulation (DBS) therapy for Parkinson’s disease. In: 2017 International Conference on Rehabilitation Robotics (ICORR). 2017. p. 1512–1517.
Ibáñez J, Serrano JI, Del Castillo MD, Gallego JA, Rocon E. Online detector of movement intention based on EEG—application in tremor patients. Biomed Signal Process Control. 2013;8:822–9.
Aoh Y, Hsiao H-J, Lu M-K, Macerollo A, Huang H-C, Hamada M, Tsai C-H, Chen J-C. Event-related desynchronization/synchronization in spinocerebellar ataxia type 3. Front Neurol. 2019;10:822.
Verleger R, Wascher E, Wauschkuhn B, Jas’kowski P, Allouni B, Trillenberg P, Wessel K. Consequences of altered cerebellar input for the cortical regulation of motor coordination, as reflected in EEG potentials. Exp Brain Res. 1999;127:409–22.
Muthuraman M, Heute U, Arning K, Anwar AR, Elble R, Deuschl G, Raethjen J. Oscillating central motor networks in pathological tremors and voluntary movements. What makes the difference? Neuroimage. 2012;60:1331–9.
Muthuraman M, Raethjen J, Koirala N, Anwar AR, Mideksa KG, Elble R, Groppa S, Deuschl G. Cerebello-cortical network fingerprints differ between essential, Parkinson’s and mimicked tremors. Brain. 2018;141:1770–81.
Pedrosa DJ, Nelles C, Brown P, Volz LJ, Pelzer EA, Tittgemeyer M, Brittain J-S, Timmermann L. The differentiated networks related to essential tremor onset and its amplitude modulation after alcohol intake. Exp Neurol. 2017;297:50–61.
Muthuraman M, Deuschl G, Anwar AR, Mideksa KG, von Helmolt F, Schneider SA. Essential and aging-related tremor: differences of central control. Mov Disord. 2015;30:1673–80.
Pan M-K, Li Y-S, Wong S-B, Ni C-L, Wang Y-M, Liu W-C, Lu L-Y, Lee J-C, Cortes EP, Vonsattel J-PG, Sun Q, Louis ED, Faust PL, Kuo S-H. Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci Transl Med. 2020;12:eaay1769.
Wong S-B, Wang Y-M, Lin C-C, Geng SK, Vanegas-Arroyave N, Pullman SL, Kuo S-H, Pan M-K. Cerebellar oscillations in familial and sporadic essential tremor. Cerebellum. 2022;21:425–31.
Bosch TJ, Groth C, Singh A. Resting-state low-frequency cerebellar oscillations can be abnormal in Parkinson’s disease. Cerebellum. 2022;21:1139–43.
Song P, Li S, Wang S, Wei H, Lin H, Wang Y. Repetitive transcranial magnetic stimulation of the cerebellum improves ataxia and cerebello-fronto plasticity in multiple system atrophy: a randomized, double-blind, sham-controlled and TMS-EEG study. Aging. 2020;12:20611–22.
Cleeves L, Findley LJ. Variability in amplitude of untreated essential tremor. J Neurol Neurosurg Psychiatry. 1987;50:704–8.
Jankovic J, Schwartz KS, Ondo W. Re-emergent tremor of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:646–50.
Matsumoto JY, Dodick DW, Stevens LN, Newman RC, Caskey PE, Fjerstad W. Three-dimensional measurement of essential tremor. Mov Disord. 1999;14:288–94.
Van Someren EJW, Van Gool WA, Vonk BFM, Mirmiran M, Speelman JD, Bosch DA, Swaab DF. Ambulatory monitoring of tremor and other movements before and after thalamotomy: a new quantitative technique. J Neurol Sci. 1993;117:16–23.
Van Someren EJW, Vonk BFM, Thijssen WA, Speelman JD, Schuurman PR, Mirmiran M, Swaab DF. A new actigraph for long-term registration of the duration and intensity of tremor and movement. IEEE Trans Biomed Eng. 1998;45:386–95.
Kulisevsky J, Avila A, Barbanoj M, Antonijoan R, Torres J, Arcelus R. Levodopa does not aggravate postural tremor in Parkinson’s disease. Clin Neuropharmacol. 1995;18:435.
Morgan MH, Hewer RL, Cooper R. Intention tremor—a method of measurement. J Neurol Neurosurg Psychiatry. 1975;38:253–8.
Adam V, Havlík J. Parameterization of the tremor signal from accelerometers in multiple sclerosis. In: 2021 International Conference on Applied Electronics (AE). 2021. p. 1–4.
Ali SM, Arjunan SP, Peters J, Perju-Dumbrava L, Ding C, Eller M, Raghav S, Kempster P, Motin MA, Radcliffe PJ, Kumar DK. Wearable sensors during drawing tasks to measure the severity of essential tremor. Sci Rep. 2022;12:5242.
Bravo M, Bermeo A, Huerta M, Llumiguano C, Bermeo J, Clotet R, Soto A. A system for finger tremor quantification in patients with Parkinson’s disease. 2017. https://doi.org/10.1109/EMBC.2017.8037623.
Caligiuri M, Tripp R. A portable hand-held device for quantifying and standardizing tremor assessment. J Med Eng Technol. 2004;28:254–62.
Gauthier-Lafreniere E, Aljassar M, Rymar VV, Milton J, Sadikot AF. A standardized accelerometry method for characterizing tremor: application and validation in an ageing population with postural and action tremor. Front Neuroinform. 2022;16: 878279.
Gorbunov A, Gromov Y, Dolgov E, Tugolukov E, Neprokin A. Accelerometric studies of night-time motor activity with essential tremor. In: 2020 2nd International Conference on Control Systems, Mathematical Modeling, Automation and Energy Efficiency (SUMMA). 2020. p. 642–645. https://doi.org/10.1109/SUMMA50634.2020.9280755.
Havlík J, Szentpétery T, Němečková M, Vávrová D, Řasová K, Zeman J, Sovka P. Design and realization of measuring device for tremor evaluation. In: 2015 International Workshop on Computational Intelligence for Multimedia Understanding (IWCIM). 2015. p. 1–4. https://doi.org/10.1109/IWCIM.2015.7347079.
Hoff JI, van der Meer V, van Hilten JJ. Accuracy of objective ambulatory accelerometry in detecting motor complications in patients with Parkinson disease. Clin Neuropharmacol. 2004;27:53–7.
Iwasaki Y, Hirotomi T, Oguro H, Nakamura M. Preliminary study on using accelerometers to measure involuntary movements for the assessment of neurological motor impairments. In: 2013 Second IIAI International Conference on Advanced Applied Informatics. 2013. p. 32–38. https://doi.org/10.1109/IIAI-AAI.2013.46.
Kavindya P, Awantha WVI, Wanasinghe AT, Kulasekera AL, Chathuranga DS, Senanayake B. Evaluation of hand tremor frequency among patients in Sri Lanka using a soft glove. In: 2020 Moratuwa Engineering Research Conference (MERCon). 2020. p. 301–306. https://doi.org/10.1109/MERCon50084.2020.9185382.
Keijsers NLW, Horstink MWIM, Gielen SCAM. Ambulatory motor assessment in Parkinson’s disease. Mov Disord. 2006;21:34–44.
Khodakarami H, Farzanehfar P, Horne M. The use of data from the Parkinson’s KinetiGraph to identify potential candidates for device assisted therapies. Sensors. 2019;19:2241.
Koçer A, Oktay AB. Nintendo Wii assessment of Hoehn and Yahr score with Parkinson’s disease tremor. THC. 2016;24:185–91.
Lee HJ, Lee WW, Kim SK, Park H, Jeon HS, Kim HB, Jeon BS, Park KS. Tremor frequency characteristics in Parkinson’s disease under resting-state and stress-state conditions. J Neurol Sci. 2016;362:272–7.
Li Y, Wang Z, Dai H. Improved Parkinsonian tremor quantification based on automatic label modification and SVM with RBF kernel. Physiol Meas. 2023;44: 025003.
Li Y, Yin J, Liu S, Xue B, Shokoohi C, Ge G, Hu M, Li T, Tao X, Rao Z, Meng F, Shi H, Ji X, Servati P, Xiao X, Chen J. Learning hand kinematics for Parkinson’s disease assessment using a multimodal sensor glove. Adv Sci. 2023. https://doi.org/10.1002/advs.202206982.
Liu S, Yuan H, Liu J, Lin H, Yang C, Cai X. Comprehensive analysis of resting tremor based on acceleration signals of patients with Parkinson’s disease. THC. 2022;30:895–907.
Niazmand K, Tonn K, Kalaras A, Fietzek UM, Mehrkens JH, Lueth TC. Quantitative evaluation of Parkinson’s disease using sensor based smart glove. In: 2011 24th International Symposium on Computer-Based Medical Systems (CBMS). 2011. p. 1–8. https://doi.org/10.1109/CBMS.2011.5999113.
Rabelo A, Folador JP, Bittar AP, Maire L, Costa S, Rueda A, Krishnan S, Lima V, Almeida RMA, Andrade AO. Low amplitude hand rest tremor assessment in Parkinson’s disease based on linear and nonlinear. In: XXVII Brazilian Congress on Biomedical Engineering. Cham: Springer International Publishing; 2022. p. 301–306. https://doi.org/10.1007/978-3-030-70601-2_46.
Rahimi F, Bee C, Debicki D, Roberts AC, Bapat P, Jog M. Effectiveness of BoNT A in Parkinson’s disease upper limb tremor management. Can J Neurol Sci. 2013;40:663–9.
Rigas G, Tzallas AT, Tsipouras MG, Bougia P, Tripoliti EE, Baga D, Fotiadis DI, Tsouli SG, Konitsiotis S. Assessment of tremor activity in the Parkinson’s disease using a set of wearable sensors. IEEE Trans Inf Technol Biomed. 2012;16:478–87.
Santiago A, Langston JW, Gandhy R, Dhall R, Brillman S, Rees L, Barlow C. Qualitative evaluation of the personal KinetiGraphTM movement recording system in a Parkinson’s clinic. J Parkinsons Dis. 2019;9:207–19.
Shaikh AG, Jinnah HA, Tripp RM, Optican LM, Ramat S, Lenz FA, Zee DS. Irregularity distinguishes limb tremor in cervical dystonia from essential tremor. J Neurol Neurosurg Psychiatry. 2008;79:187–9.
Smeja M, Foerster F, Fuchs G, Emmans D, Hornig A, Fahrenberg J. 24-h Assessment of tremor activity and posture in Parkinson’s disease by multi-channel accelerometry. J Psychophysiol. 2006. https://doi.org/10.1027//0269-8803.13.4.245.
Smid A, Elting JWJ, Van Dijk JMC, Otten B, Oterdoom DLM, Tamasi K, Heida T, Van Laar T, Drost G. Intraoperative quantification of MDS-UPDRS tremor measurements using 3D accelerometry: a pilot study. JCM. 2022;11:2275.
Synnott J, Chen L, Nugent CD, Moore G. WiiPD—objective home assessment of Parkinson’s disease using the Nintendo Wii Remote. IEEE Trans Inform Technol Biomed. 2012;16:1304–12.
Thielgen T, Foerster F, Fuchs G, Hornig A, Fahrenberg J. Tremor in Parkinson’s disease: 24-hr monitoring with calibrated accelerometry. Electromyogr Clin Neurophysiol. 2004;44:137–46.
Yuan H, Liu S, Liu J, Lin H, Yang C, Cai X, Zeng L, Li S. Detection and quantification of resting tremor in Parkinson’s disease using long-term acceleration data. Math Probl Eng. 2021;2021: e5669932.
Zhang A, San-Segundo R, Panev S, Tabor G, Stebbins K, Whitford A, De la Torre F, Hodgins J. Automated tremor detection in Parkinson’s disease using accelerometer signals. In: 2018 IEEE/ACM International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE). 2018. p. 13–14. https://doi.org/10.1145/3278576.3278582.
Battista L, Romaniello A. A novel device for continuous monitoring of tremor and other motor symptoms. Neurol Sci. 2018;39:1333–43.
Battista L, Romaniello A. A wearable tool for selective and continuous monitoring of tremor and dyskinesia in Parkinsonian patients. Parkinsonism Relat Disord. 2020;77:43–7.
van Brummelen EMJ, Ziagkos D, de Boon WMI, Hart EP, Doll RJ, Huttunen T, Kolehmainen P, Groeneveld GJ. Quantification of tremor using consumer product accelerometry is feasible in patients with essential tremor and Parkinson’s disease: a comparative study. J Clin Mov Disord. 2020;7:4.
Burq M, Rainaldi E, Ho KC, Chen C, Bloem BR, Evers LJW, Helmich RC, Myers L, Marks WJ, Kapur R. Virtual exam for Parkinson’s disease enables frequent and reliable remote measurements of motor function. NPJ Digit Med. 2022;5:65.
Elm JJ, Daeschler M, Bataille L, Schneider R, Amara A, Espay AJ, Afek M, Admati C, Teklehaimanot A, Simuni T. Feasibility and utility of a clinician dashboard from wearable and mobile application Parkinson’s disease data. NPJ Digit Med. 2019;2:1–6.
Lima ALSd, Hahn T, Evers LJW, de Vries NM, Cohen E, Afek M, Bataille L, Daeschler M, Claes K, Boroojerdi B, Terricabras D, Little MA, Baldus H, Bloem BR, Faber MJ. Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS ONE. 2017;12: e0189161.
Pahwa R, Bergquist F, Horne M, Minshall ME. Objective measurement in Parkinson’s disease: a descriptive analysis of Parkinson’s symptom scores from a large population of patients across the world using the Personal KinetiGraph®. J Clin Mov Disord. 2020;7:5.
Sigcha L, Pavón I, Costa N, Costa S, Gago M, Arezes P, López JM, De Arcas G. Automatic resting tremor assessment in Parkinson’s disease using smartwatches and multitask convolutional neural networks. Sensors. 2021;21:291.
Varghese J, van Alen CM, Fujarski M, Schlake GS, Sucker J, Warnecke T, Thomas C. Sensor validation and diagnostic potential of smartwatches in movement disorders. Sensors. 2021;21:3139.
Wile DJ, Ranawaya R, Kiss ZHT. Smart watch accelerometry for analysis and diagnosis of tremor. J Neurosci Methods. 2014;230:1–4.
Zheng X, Vieira A, Marcos SL, Aladro Y, Ordieres-Meré J. Activity-aware essential tremor evaluation using deep learning method based on acceleration data. Parkinsonism Relat Disord. 2019;58:17–22.
Zheng X, Vieira Campos A, Ordieres-Meré J, Balseiro J, Labrador Marcos S, Aladro Y. Continuous monitoring of essential tremor using a portable system based on smartwatch. Front Neurol. 2017;8:96.
Barrantes S, Egea AJS, Rojas HAG, Martí MJ, Compta Y, Valldeoriola F, Mezquita ES, Tolosa E, Valls-Solè J. Differential diagnosis between Parkinson’s disease and essential tremor using the smartphone’s accelerometer. PLoS ONE. 2017;12: e0183843.
Bazgir O, Habibi SAH, Palma L, Pierleoni P, Nafees S. A classification system for assessment and home monitoring of tremor in patients with Parkinson’s disease. J Med Signals Sens. 2018;8:65–72.
Daneault J-F, Carignan B, Codère CÉ, Sadikot A, Duval C. Using a smart phone as a standalone platform for detection and monitoring of pathological tremors. Front Hum Neurosci. 2013;6:357.
Fraiwan L, Khnouf R, Mashagbeh AR. Parkinson’s disease hand tremor detection system for mobile application. J Med Eng Technol. 2016;40:127–34.
Joundi RA, Brittain J-S, Jenkinson N, Green AL, Aziz T. Rapid tremor frequency assessment with the iPhone accelerometer. Parkinsonism Relat Disord. 2011;17:288–90.
LeMoyne R, Mastroianni T, Cozza M, Coroian C, Grundfest W. Implementation of an iPhone for characterizing Parkinson’s disease tremor through a wireless accelerometer application. In: 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology. 2010. p. 4954–4958. https://doi.org/10.1109/IEMBS.2010.5627240.
Molparia B, Schrader B, Cohen E, Wagner J, Gupta S, Gould S, Hwynn N, Spencer E, Torkamani A. Combined accelerometer and genetic analysis to differentiate essential tremor from Parkinson’s disease. PeerJ. 2018;6: e5308.
Pan D, Dhall R, Lieberman A, Petitti DB. A mobile cloud-based Parkinson’s disease assessment system for home-based monitoring. JMIR mHealth uHealth. 2015;3: e29.
Woods AM, Nowostawski M, Franz EA, Purvis M. Parkinson’s disease and essential tremor classification on mobile device. Pervasive Mob Comput. 2014;13:1–12.
Atashzar SF, Shahbazi M, Samotus O, Tavakoli M, Jog MS, Patel RV. Characterization of upper-limb pathological tremors: application to design of an augmented haptic rehabilitation system. IEEE J Sel Top Signal Process. 2016;10:888–903.
Zhang B, Huang F, Liu J, Zhang D. A novel posture for better differentiation between Parkinson’s tremor and essential tremor. Front Neurosci. 2018;12.
Teufl S, Preston J, van Wijck F, Stansfield B. Quantifying upper limb tremor in people with multiple sclerosis using Fast Fourier Transform based analysis of wrist accelerometer signals. J Rehabil Assist Technol Eng. 2021;8:2055668320966955.
Marino S, Cartella E, Donato N, Muscarà N, Sorbera C, Cimino V, De Salvo S, Micchìa K, Silvestri G, Bramanti A, Di Lorenzo G. Quantitative assessment of Parkinsonian tremor by using biosensor device. Medicine. 2019;98: e17897.
Bazgir O, Frounchi J, Habibi SAH, Palma L, Pierleoni P. A neural network system for diagnosis and assessment of tremor in Parkinson disease patients. In: 2015 22nd Iranian Conference on Biomedical Engineering (ICBME). 2015. p. 1–5. https://doi.org/10.1109/ICBME.2015.7404105.
Loaiza Duque JD, González-Vargas AM, Sánchez Egea AJ, González Rojas HA. Using machine learning and accelerometry data for differential diagnosis of Parkinson’s disease and essential tremor. In: Applied Computer Sciences in Engineering. Cham: Springer International Publishing; 2019. p. 368–378. https://doi.org/10.1007/978-3-030-31019-6_32.
Mahadevan N, Demanuele C, Zhang H, Volfson D, Ho B, Erb MK, Patel S. Development of digital biomarkers for resting tremor and bradykinesia using a wrist-worn wearable device. NPJ Digit Med. 2020;3:1–12.
Patel S, Lorincz K, Hughes R, Huggins N, Growdon J, Standaert D, Akay M, Dy J, Welsh M, Bonato P. Monitoring motor fluctuations in patients with Parkinson’s disease using wearable sensors. IEEE Trans Inf Technol Biomed. 2009;13:864–73.
Kwon D-Y, Kwon Y-R, Choi Y-H, Eom G-M, Ko J, Kim J-W. Quantitative measures of postural tremor at the upper limb joints in patients with essential tremor. THC. 2020;28:499–507.
Kwon D-Y, Kwon Y-R, Ko J, Kim J-W. Comparison of resting tremor at the upper limb joints between patients with Parkinson’s disease and scans without evidence of dopaminergic deficit. THC. 2023;31:515–23.
Kwon Y-R, Eom G-M, Ko J, Kim J-W. Quantitative analysis of essential tremor during clinical spiral drawing task using gyro sensors. J Mech Med Biol. 2021;21:2140050.
Salarian A, Russmann H, Wider C, Burkhard PR, Vingerhoets FJG, Aminian K. Quantification of tremor and bradykinesia in Parkinson’s disease using a novel ambulatory monitoring system. IEEE Trans Biomed Eng. 2007;54:313–22.
Surangsrirat D, Thanawattano C, Pongthornseri R, Dumnin S, Anan C, Bhidayasiri R. Support vector machine classification of Parkinson’s disease and essential tremor subjects based on temporal fluctuation. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2016. p. 6389–6392. https://doi.org/10.1109/EMBC.2016.7592190.
Bermeo A, Bravo M, Huerta M, Soto A. A system to monitor tremors in patients with Parkinson’s disease. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2016. p. 5007–5010. https://doi.org/10.1109/EMBC.2016.7591852.
Hssayeni MD, Jimenez-Shahed J, Burack MA, Ghoraani B. Wearable sensors for estimation of parkinsonian tremor severity during free body movements. Sensors. 2019;19:4215.
López-Blanco R, Velasco MA, Méndez-Guerrero A, Romero JP, del Castillo MD, Serrano JI, Benito-León J, Bermejo-Pareja F, Rocon E. Essential tremor quantification based on the combined use of a smartphone and a smartwatch: the NetMD study. J Neurosci Methods. 2018;303:95–102.
López-Blanco R, Velasco MA, Méndez-Guerrero A, Romero JP, del Castillo MD, Serrano JI, Rocon E, Benito-León J. Smartwatch for the analysis of rest tremor in patients with Parkinson’s disease. J Neurol Sci. 2019;401:37–42.
Carpinella I, Cattaneo D, Ferrarin M. Hilbert-Huang transform based instrumental assessment of intention tremor in multiple sclerosis. J Neural Eng. 2015;12: 046011.
de Lima ER, Andrade AO, Pons JL, Kyberd P, Nasuto SJ. Empirical mode decomposition: a novel technique for the study of tremor time series. Med Bio Eng Comput. 2006;44:569–82.
Gallego JA, Rocon E, Roa JO, Moreno JC, Pons JL. Real-time estimation of pathological tremor parameters from gyroscope data. Sensors. 2010;10:2129–49.
van den Noort JC, Verhagen R, van Dijk KJ, Veltink PH, Vos MCPM, de Bie RMA, Bour LJ, Heida CT. Quantification of hand motor symptoms in Parkinson’s disease: a proof-of-principle study using inertial and force sensors. Ann Biomed Eng. 2017;45:2423–36.
Pradhan SD, Brewer BR, Carvell GE, Sparto PJ, Delitto A, Matsuoka Y. Assessment of fine motor control in individuals with Parkinson’s disease using force tracking with a secondary cognitive task. J Neurol Phys Ther. 2010;34:32–40.
Rozman J, Bartolić A, Ribarič S. A new method for selective measurement of joint movement in hand tremor in Parkinson’s disease patients. J Med Eng Technol. 2007;31:305–11.
Charles SK, Geiger DW, Davidson AD, Pigg AC, Curtis CP, Allen BC. Toward quantitative characterization of essential tremor for future tremor suppression. In: 2017 International Conference on Rehabilitation Robotics (ICORR). 2017. p. 175–180. https://doi.org/10.1109/ICORR.2017.8009242.
Dai H, Cai G, Lin Z, Wang Z, Ye Q. Validation of inertial sensing-based wearable device for tremor and bradykinesia quantification. IEEE J Biomed Health Inform. 2021;25:997–1005.
O’Suilleabhain PE, Dewey RB Jr. Validation for tremor quantification of an electromagnetic tracking device. Mov Disord. 2001;16:265–71.
Patel V, Burns M, Pourfar M, Mogilner A, Kondziolka D, Vinjamuri R. QAPD: an integrated system to quantify symptoms of Parkinson’s disease. 2016. https://doi.org/10.1109/EMBC.2016.7591073.
Perera T, Lee W-L, Yohanandan SAC, Nguyen A-L, Cruse B, Boonstra FMC, Noffs G, Vogel AP, Kolbe SC, Butzkueven H, Evans A, Van Der Walt A. Validation of a precision tremor measurement system for multiple sclerosis. J Neurosci Methods. 2019;311:377–84.
Yu Su, Allen CR, Geng D, Burn D, Brechany U, Bell GD, Rowland R. 3-D motion system (“data-gloves”): application for Parkinson’s disease. IEEE Trans Instrum Meas. 2003;52:662–74.
Bai Q, Shen T, Xu B, Yu Q, Zhang H, Mao C, Liu C, Wang S. Quantification of the motor symptoms of Parkinson’s disease. In: 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER). 2017. p. 82–85.
Bhidayasiri R, Petchrutchatachart S, Pongthornseri R, Anan C, Dumnin S, Thanawattano C. Low-cost, 3-dimension, office-based inertial sensors for automated tremor assessment: technical development and experimental verification. J Parkinson’s Dis. 2014;4:273–82.
Channa A, Ifrim R-C, Popescu D, Popescu N. A-WEAR bracelet for detection of hand tremor and Bradykinesia in Parkinson’s patients. Sensors. 2021;21:981.
Contreras R, Huerta M, Sagbay G, LLumiguano C, Bravo M, Bermeo A, Clotet R, Soto A. Tremors quantification in parkinson patients using smartwatches. In: 2016 IEEE Ecuador Technical Chapters Meeting (ETCM). 2016. p. 1–6. https://doi.org/10.1109/ETCM.2016.7750866.
Dai H, Zhang P, Lueth TC. Quantitative assessment of Parkinsonian tremor based on an inertial measurement unit. Sensors. 2015;15:25055–71.
Ferreira JJ, Godinho C, Santos AT, Domingos J, Abreu D, Lobo R, Gonçalves N, Barra M, Larsen F, Fagerbakke Ø, Akeren I, Wangen H, Serrano JA, Weber P, Thoms A, Meckler S, Sollinger S, van Uem J, Hobert MA, Maier KS, Matthew H, Isaacs T, Duffen J, Graessner H, Maetzler W. Quantitative home-based assessment of Parkinson’s symptoms: The SENSE-PARK feasibility and usability study. BMC Neurol. 2015;15:89.
Fuchs C, Nobile MS, Zamora G, Degeneffe A, Kubben P, Kaymak U. Tremor assessment using smartphone sensor data and fuzzy reasoning. BMC Bioinform. 2021;22:57.
Giuffrida JP, Riley DE, Maddux BN, Heldman DA. Clinically deployable Kinesia™ technology for automated tremor assessment. Mov Disord. 2009;24:723–30.
Hadley AJ, Riley DE, Heldman DA. Real-world evidence for a smartwatch-based Parkinson’s motor assessment app for patients undergoing therapy changes. Digit Biomark. 2021;5:206–15.
Heijmans M, Habets J, Kuijf M, Kubben P, Herff C. Evaluation of Parkinson’s disease at home: predicting tremor from wearable sensors. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2019. p. 584–587. https://doi.org/10.1109/EMBC.2019.8857717.
Heldman DA, Harris DA, Felong T, Andrzejewski KL, Dorsey ER, Giuffrida JP, Goldberg B, Burack MA. Telehealth management of Parkinson’s disease using wearable sensors: an exploratory study. Digit Biomark. 2017;1:43–51.
Heldman DA, Jankovic J, Vaillancourt DE, Prodoehl J, Elble RJ, Giuffrida JP. Essential tremor quantification during activities of daily living. Parkinsonism Relat Disord. 2011;17:537–42.
Jeon H, Lee W, Park H, Lee HJ, Kim SK, Kim HB, Jeon B, Park KS. Automatic classification of tremor severity in Parkinson’s DISEASE USING A WEARABLE DEVICE. Sensors. 2017;17:2067.
Kim HB, Lee WW, Kim A, Lee HJ, Park HY, Jeon HS, Kim SK, Jeon B, Park KS. Wrist sensor-based tremor severity quantification in Parkinson’s disease using convolutional neural network. Comput Biol Med. 2018;95:140–6.
Kostikis N, Hristu-Varsakelis D, Arnaoutoglou M, Kotsavasiloglou C. A smartphone-based tool for assessing Parkinsonian hand tremor. IEEE J Biomed Health Inform. 2015;19:1835–42.
Krishna R, Pathirana PN, Horne M, Power L, Szmulewicz DJ. Quantitative assessment of cerebellar ataxia, through automated limb functional tests. J Neuroeng Rehabil. 2019;16:31.
Kubben PL, Kuijf ML, Ackermans LPCM, Leentjes AFG, Temel Y. TREMOR12: an open-source mobile app for tremor quantification. SFN. 2016;94:182–6.
Lipsmeier F, Taylor KI, Kilchenmann T, Wolf D, Scotland A, Schjodt-Eriksen J, Cheng W-Y, Fernandez-Garcia I, Siebourg-Polster J, Jin L, Soto J, Verselis L, Boess F, Koller M, Grundman M, Monsch AU, Postuma RB, Ghosh A, Kremer T, Czech C, Gossens C, Lindemann M. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov Disord. 2018;33:1287–97.
Lonini L, Dai A, Shawen N, Simuni T, Poon C, Shimanovich L, Daeschler M, Ghaffari R, Rogers JA, Jayaraman A. Wearable sensors for Parkinson’s disease: which data are worth collecting for training symptom detection models. NPJ Digital Med. 2018;1:1–8.
Mcgurrin P, Mcnames J, Wu T, Hallett M, Haubenberger D. Quantifying tremor in essential tremor using inertial sensors—validation of an algorithm. IEEE J Transl Eng Health Med. 2021;9:1–10.
Mera TO, Burack MA, Giuffrida JP. Quantitative assessment of levodopa-induced dyskinesia using automated motion sensing technology. In: 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2012. p. 154–157. https://doi.org/10.1109/EMBC.2012.6345894.
Mostile G, Giuffrida JP, Adam OR, Davidson A, Jankovic J. Correlation between Kinesia system assessments and clinical tremor scores in patients with essential tremor. Mov Disord. 2010;25:1938–43.
Musab R, As’arry A, Rezali KAM, Jalil NAA, Ahmad RMKR, Zain MZM. Tremor quantification and its measurements using shimmer. J Phys Conf Ser. 2019;1262: 012024.
Oyama G, Burq M, Hatano T, Marks WJ, Kapur R, Fernandez J, Fujikawa K, Furusawa Y, Nakatome K, Rainaldi E, Chen C, Ho KC, Ogawa T, Kamo H, Oji Y, Takeshige-Amano H, Taniguchi D, Nakamura R, Sasaki F, Ueno S, Shiina K, Hattori A, Nishikawa N, Ishiguro M, Saiki S, Hayashi A, Motohashi M, Hattori N. Analytical and clinical validity of wearable, multi-sensor technology for assessment of motor function in patients with Parkinson’s disease in Japan. Sci Rep. 2023;13:3600.
Peres LB, Calil BC, Da Silva APSPB, Dionísio VC, Vieira MF, De OliveiraAndrade A, Pereira AA. Discrimination between healthy and patients with Parkinson’s disease from hand resting activity using inertial measurement unit. BioMed Eng OnLine. 2021;20:50.
Powers R, Etezadi-Amoli M, Arnold EM, Kianian S, Mance I, Gibiansky M, Trietsch D, Alvarado AS, Kretlow JD, Herrington TM, Brillman S, Huang N, Lin PT, Pham HA, Ullal AV. Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson’s disease. Sci Transl Med. 2021;13:7865.
Pulliam CL, Heldman DA, Brokaw EB, Mera TO, Mari ZK, Burack MA. Continuous assessment of levodopa response in Parkinson’s disease using wearable motion sensors. IEEE Trans Biomed Eng. 2018;65:159–64.
Rigas G, Gatsios D, Fotiadis DI, Chondrogiorgi M, Tsironis C, Konitsiotis S, Gentile G, Marcante A, Antonini A. Tremor UPDRS estimation in home environment. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2016. p. 3642–3645. https://doi.org/10.1109/EMBC.2016.7591517.
Sahin G, Halje P, Uzun S, Jakobsson A, Petersson P. Tremor evaluation using smartphone accelerometry in standardized settings. Front Neurosci. 2022;16: 861668.
Senova S, Querlioz D, Thiriez C, Jedynak P, Jarraya B, Palfi S. Using the accelerometers integrated in smartphones to evaluate essential tremor. Stereotact Funct Neurosurg. 2015;93:94–101.
Shawen N, O’Brien MK, Venkatesan S, Lonini L, Simuni T, Hamilton JL, Ghaffari R, Rogers JA, Jayaraman A. Role of data measurement characteristics in the accurate detection of Parkinson’s disease symptoms using wearable sensors. J Neuroeng Rehabil. 2020;17:52.
Sun M, Watson A, Blackwell G, Jung W, Wang S, Koltermann K, Helm N, Zhou G, Cloud L, Pretzer-Aboff I. TremorSense: tremor detection for Parkinson’s disease using convolutional neural network. In: 2021 IEEE/ACM Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE). 2021. p. 1–10. https://doi.org/10.1109/CHASE52844.2021.00009.
Teskey WJE, Elhabiby M, El-Sheimy N. Inertial sensing to determine movement disorder motion present before and after treatment. Sensors. 2012;12:3512–27.
Thanawattano C, Pongthornseri R, Anan C, Dumnin S, Bhidayasiri R. Temporal fluctuations of tremor signals from inertial sensor: a preliminary study in differentiating Parkinson’s disease from essential tremor. BioMed Eng OnLine. 2015;14:101.
Tsiouris KM, Gatsios D, Rigas G, Miljkovic D, Koroušić Seljak B, Bohanec M, Arredondo MT, Antonini A, Konitsiotis S, Koutsouris DD, Fotiadis DI. PD_Manager: an mHealth platform for Parkinson’s disease patient management. Healthc Technol Lett. 2017;4:102–8.
Tzallas AT, Tsipouras MG, Rigas G, Tsalikakis DG, Karvounis EC, Chondrogiorgi M, Psomadellis F, Cancela J, Pastorino M, Waldmeyer MTA, Konitsiotis S, Fotiadis DI. PERFORM: a system for monitoring, assessment and management of patients with Parkinson’s disease. Sensors. 2014;14:21329–57.
Cai G, Lin Z, Dai H, Xia X, Xiong Y, Horng S-J, Lueth TC. Quantitative assessment of parkinsonian tremor based on a linear acceleration extraction algorithm. Biomed Signal Process Control. 2018;42:53–62.
Carpinella I, Cattaneo D, Ferrarin M. Quantitative assessment of upper limb motor function in multiple sclerosis using an instrumented action research arm test. J Neuroeng Rehabil. 2014;11:67.
Chan PY, Ripin ZM, Halim SA, Tharakan J, Muzaimi M, Ng KS, Kamarudin MI, Eow GB, Hor JY, Tan K, Cheah CF, Soong N, Then L, Yahya AS. An in–laboratory validity and reliability tested system for quantifying hand-arm tremor in motions. IEEE Trans Neural Syst Rehabil Eng. 2018;26:460–7.
Channa A, Ruggeri G, Mammone N, Ifrim R-C, Iera A, Popescu N. Parkinson’s disease severity estimation using deep learning and cloud technology. In: 2022 IEEE International Conference on Omni-layer Intelligent Systems (COINS). 2022. p. 1–7. https://doi.org/10.1109/COINS54846.2022.9854945.
Delrobaei M, Memar S, Pieterman M, Stratton TW, McIsaac K, Jog M. Towards remote monitoring of Parkinson’s disease tremor using wearable motion capture systems. J Neurol Sci. 2018;384:38–45.
Di Lazzaro G, Ricci M, Al-Wardat M, Schirinzi T, Scalise S, Giannini F, Mercuri NB, Saggio G, Pisani A. Technology-based objective measures detect subclinical axial signs in untreated, de novo Parkinson’s disease. JPD. 2020;10:113–22.
Erb MK, Karlin DR, Ho BK, Thomas KC, Parisi F, Vergara-Diaz GP, Daneault J-F, Wacnik PW, Zhang H, Kangarloo T, Demanuele C, Brooks CR, Detheridge CN, Shaafi Kabiri N, Bhangu JS, Bonato P. mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinson’s disease. NPJ Digit Med. 2020;3:1–10.
Gallego JA, Rocon E, Ibañez J, Dideriksen JL, Koutsou AD, Paradiso R, Popovic M. B, Belda-Lois M, Gianfelici F, Farina D, Popovic B. B, Manto M, D’Alessio T, Pons J. L. A soft wearable robot for tremor assessment and suppression. In: 2011 IEEE International Conference on Robotics and Automation. 2011. p. 2249–2254. https://doi.org/10.1109/ICRA.2011.5979639.
Heldman DA, Espay AJ, LeWitt PA, Giuffrida JP. Clinician versus machine: reliability and responsiveness of motor endpoints in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:590–5.
Isaacson SH, et al. Prospective home-use study on non-invasive neuromodulation therapy for essential tremor. Tremor Other Hyperkinetic Mov. 2020;10:29.
Kashyap B, Phan D, Pathirana PN, Horne M, Power L, Szmulewicz D. Objective assessment of cerebellar ataxia: a comprehensive and refined approach. Sci Rep. 2020;10:9493.
Ketteringham LP, Neild SA, Hyde RA, Jones RJS, Smith AMD. Measuring intention tremor in multiple sclerosis using inertial measurement unit (IMU) devices. In: International Conference on Biomedical Electronics and Devices. 2011.
Lambrecht S, Gallego JA, Rocon E, Pons JL. Automatic real-time monitoring and assessment of tremor parameters in the upper limb from orientation data. Front Neurosci. 2014;8:221.
Locatelli P, Alimonti D. Differentiating essential tremor and Parkinson’s disease using a wearable sensor—a pilot study. In: 2017 7th IEEE International Workshop on Advances in Sensors and Interfaces (IWASI). 2017. p. 213–218. https://doi.org/10.1109/IWASI.2017.7974254.
Locatelli P, Alimonti D, Traversi G, Re V. Classification of essential tremor and Parkinson’s tremor based on a low-power wearable device. Electronics. 2020;9:1695.
Ma C, Li D, Pan L, Li X, Yin C, Li A, Zhang Z, Zong R. Quantitative assessment of essential tremor based on machine learning methods using wearable device. Biomed Signal Process Control. 2022;71: 103244.
Ma C, Zhang P, Wang J, Zhang J, Pan L, Li X, Yin C, Li A, Zong R, Zhang Z. Objective quantification of the severity of postural tremor based on kinematic parameters: a multi-sensory fusion study. Comput Methods Programs Biomed. 2022;219: 106741.
Pulliam CL, Eichenseer SR, Goetz CG, Waln O, Hunter CB, Jankovic J, Vaillancourt DE, Giuffrida JP, Heldman DA. Continuous in-home monitoring of essential tremor. Parkinsonism Relat Disord. 2014;20:37–40.
Ricci M, Lazzaro GD, Errico V, Pisani A, Giannini F, Saggio G. The impact of wearable electronics in assessing the effectiveness of levodopa treatment in Parkinson’s disease. IEEE J Biomed Health Inform. 2022;26:2920–8.
Sanchez-Perez LA, Sanchez-Fernandez LP, Shaout A, Martinez-Hernandez JM, Alvarez-Noriega MJ. Rest tremor quantification based on fuzzy inference systems and wearable sensors. Int J Med Informatics. 2018;114:6–17.
Tran H, Nguyen KD, Pathirana PN, Horne M, Power L, Szmulewicz DJ. Multimodal data acquisition for the assessment of cerebellar ataxia via ballistic tracking. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). 2020. p. 859–862. https://doi.org/10.1109/EMBC44109.2020.9176379.
Tran H, Pathirana PN, Horne M, Power L, Szmulewicz DJ. Automated evaluation of upper limb motor impairment of patient with cerebellar ataxia. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2019. p. 6846–6849. https://doi.org/10.1109/EMBC.2019.8856330.
Western DG, Neild SA, Jones R, Davies-Smith A. Personalised profiling to identify clinically relevant changes in tremor due to multiple sclerosis. BMC Med Inform Decis Mak. 2019;19:162.
Zhou Y, Jenkins ME, Naish MD, Trejos AL. Development of a wearable tremor suppression glove. In: 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob). 2018. p. 640–645. https://doi.org/10.1109/BIOROB.2018.8487197.
Zwartjes DGM, Heida T, van Vugt JPP, Geelen JAG, Veltink PH. Ambulatory monitoring of activities and motor symptoms in Parkinson’s disease. IEEE Trans Biomed Eng. 2010;57:2778–86.
Das S, Trutoiu L, Murai A, Alcindor D, Oh M, De la Torre F, Hodgins J. Quantitative measurement of motor symptoms in Parkinson’s disease: A study with full-body motion capture data. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2011. p. 6789–6792. https://doi.org/10.1109/IEMBS.2011.6091674.
Deuschl G, Wenzelburger R, Löffler K, Raethjen J, Stolze H. Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain. 2000;123:1568–80.
Kim MJ, Naydanova E, Hwang BY, Mills KA, Anderson WS, Salimpour Y. Quantification of Parkinson’s disease motor symptoms: a wireless motion sensing approach. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). Montreal, QC, Canada: IEEE; 2020. p. 3658–3661. https://doi.org/10.1109/EMBC44109.2020.9175616.
Lugo G, Ibarra-Manzano M, Ba F, Cheng I. Virtual reality and hand tracking system as a medical tool to evaluate patients with Parkinson’s. In: Proceedings of the 11th EAI International Conference on Pervasive Computing Technologies for Healthcare. New York, NY, USA: Association for Computing Machinery; 2017. p. 405–408. https://doi.org/10.1145/3154862.3154924.
Chen K-H, Lin P-C, Chen Y-J, Yang B-S, Lin C-H. Development of method for quantifying essential tremor using a small optical device. J Neurosci Methods. 2016;266:78–83.
Khwaounjoo P, Singh G, Grenfell S, Özsoy B, MacAskill MR, Anderson TJ, Çakmak YO. Non-contact hand movement analysis for optimal configuration of smart sensors to capture Parkinson’s disease hand tremor. Sensors. 2022;22:4613.
Casacanditella L, Cosoli G, Ceravolo MG, Tomasini EP. Non-contact measurement of tremor for the characterisation of Parkinsonian individuals: comparison between Kinect and Laser Doppler vibrometer. J Phys Conf Ser. 2017;882: 012002.
Ismail II, Kamel WA, Al-Hashel JY. Assessing the usability of an Instagram filter in monitoring essential tremor: a proof-of-concept study. Movement Disord Clin Pract. 2023;10:274–8.
Li MH, Mestre TA, Fox SH, Taati B. Vision-based assessment of parkinsonism and levodopa-induced dyskinesia with pose estimation. J Neuroeng Rehabil. 2018;15:97.
Mitsui Y, Zin TT, Ishii N, Mochizuki H. Imaging tremor quantification for neurological disease diagnosis. Sensors. 2020;20:6684.
Saraguro W, Barzallo B, Guillermo J, García-Cedeño A, Soto A, Rivas D, Clotet R, Huerta M. Analysis of hand movements in patients with Parkinson’s Disease using Kinect. In: 2019 IEEE International Conference on E-health Networking, Application & Services (HealthCom). 2019. p. 1–6. https://doi.org/10.1109/HealthCom46333.2019.9009589.
Williams S, Fang H, Relton SD, Wong DC, Alam T, Alty JE. Accuracy of smartphone video for contactless measurement of hand tremor frequency. Mov Dis Clin Pract. 2021;8:69–75.
Lugaresi C, Tang J, Nash H, McClanahan C, Uboweja E, Hays M, Zhang F, Chang C-L, Yong M, Lee J, Chang W-T, Hua W, Georg M, Grundmann M. MediaPipe: a framework for perceiving and processing reality. 2019. https://mixedreality.cs.cornell.edu/s/NewTitle_May1_MediaPipe_CVPR_CV4ARVR_Workshop_2019.pdf.
Pang Y, Christenson J, Jiang F, Lei T, Rhoades R, Kern D, Thompson JA, Liu C. Automatic detection and quantification of hand movements toward development of an objective assessment of tremor and bradykinesia in Parkinson’s disease. J Neurosci Methods. 2020;333: 108576.
Cao Z, Hidalgo G, Simon T, Wei S-E, Sheikh Y. OpenPose: realtime multi-person 2D pose estimation using part affinity fields. IEEE Trans Pattern Anal Mach Intell. 2021;43:172–86.
Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–81.
Pollok B, Makhloufi H, Butz M, Gross J, Timmermann L, Wojtecki L, Schnitzler A. Levodopa affects functional brain networks in parkinsonian resting tremor. Mov Disord. 2009;24:91–8.
Andersen LM, Jerbi K, Dalal SS. Can EEG and MEG detect signals from the human cerebellum? Neuroimage. 2020;215: 116817.
Kumar A, Lin C-C, Kuo S-H, Pan M-K. Physiological recordings of the cerebellum in movement disorders. Cerebellum. 2022. https://doi.org/10.1007/s12311-022-01473-6.
Ding K, Chen Y, Bose R, Osborn LE, Dragomir A, Thakor NV. Sensory stimulation for upper limb amputations modulates adaptability of cortical large-scale systems and combination of somatosensory and visual inputs. Sci Rep. 2022;12:20467.
Ding K, Dragomir A, Bose R, Osborn LE, Seet MS, Bezerianos A, Thakor NV. Towards machine to brain interfaces: sensory stimulation enhances sensorimotor dynamic functional connectivity in upper limb amputees. J Neural Eng. 2020;17: 035002.
Acknowledgements
We gratefully acknowledge the funding and support from the Institute for Advanced Study (IAS)—Technical University of Munich.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Hans Fischer Senior Fellowship from the Institute for Advanced Study (TUM-IAS).
Author information
Authors and Affiliations
Contributions
NP was involved in the conception, organization, and execution of the research project and the design, execution, review, and critique of the statistical analysis. NP also played a role in writing the first draft of the manuscript and provided input during its review and critique. DU participated in the statistical analysis, provided feedback during the manuscript preparation, and contributed to its review and critique. KD contributed to the manuscript's preparation and writing during the review and critique process. NT was involved in organizing the research project and the review and critique of the manuscript. GC was involved in the research project's conception and organization, took part in the design, execution, and review of the statistical analysis, and contributed to the review and critique of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors confirm that the approval of an institutional review board or ethics committee was not required for this work. Informed patient consent was not necessary for this work. We confirm that this manuscript aligns with the guidelines of the Journal's stance on ethical publication matters.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. Author’s financial disclosures for the previous 12 months: NP is supported by the Institute for Cognitive Systems (TUM-ICS) and the Institute for Advance Studies from the Technical University of Munich (TUM-IAS). DU is supported by the Department of Neurology, Klinikum rechts der Isar of the Technical University of Munich. KD is supported by the U.S. Department of Energy, Office of Science, Office of Advanced Scientific Computing Research under award number DE-SC0022150. NT is a co-founder of Infinite Biomedical Technologies and Vigilant Medical Technologies and serves on their board as a scientific advisor. His intellectual property has been licensed to Vasopatic Medical and Phantom Robotics, although he has not received any royalties. GC is a shareholder of intouch-robotics GmbH. This study is not related to the company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Studies using tremor assessment technologies classified according to its type. This additional file is a table including all studies considered in this review. The table categorizes the studies into assessment type, number and type of patients, technology used, method, purpose, and year.
Additional file 2.
PRISMA checklist for scoping reviews. This checklist structures the reporting items of our scoping review by providing the page number where each section can be found.
Additional file 3.
Results of literature search and data extraction. This flow diagram shows the number of sources of evidence screened and assessed for eligibility and the number of studies excluded at each stage of the data extraction process.
Additional file 4.
Database of selected studies. This table shows the database of the selected studies. It provides additional information than Additional file 1, such as the type of IMU sensors, and disseminates the data to automatically analyze it.
Additional file 5.
Database search strings. This text document contains the search strings used in PubMed and Scopus for data retrieval.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Paredes-Acuna, N., Utpadel-Fischler, D., Ding, K. et al. Upper limb intention tremor assessment: opportunities and challenges in wearable technology. J NeuroEngineering Rehabil 21, 8 (2024). https://doi.org/10.1186/s12984-023-01302-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-023-01302-9