Abstract
Background
Virtual reality (VR) is a promising solution for individuals with Parkinson’s disease (PD) who experience symptoms that affect their daily activities and independence. Through VR-based rehabilitation, patients can improve their motor skills in a safe and stress-free environment, making it an attractive alternative to traditional in-person rehabilitation during the COVID-19 pandemic. This study aimed to provide the most recent and convincing evidence on the rehabilitative effects of VR technology compared with conventional treatments.
Methods
Two investigators systematically searched Embase, MEDLINE, CINAHL, PEDro, and the Cochrane Library from their inception until May 31, 2022, to identify randomized controlled trials (RCTs) comparing the effectiveness of VR training with that of conventional treatment for patients with PD. Studies were selected based on the patient, intervention, comparator, and outcome criteria and assessed for the risk of bias using the Cochrane tool. Meta-analysis was conducted by pooling mean differences with 95% confidence intervals.
Results
A total of 14 RCTs, involving 524 participants, were included in the meta-analysis. The results indicated that VR-based rehabilitation significantly improved balance function, as measured using the Berg balance scale (BBS) and activities-specific balance confidence. However, no statistically significant differences in gait ability, activities of daily living, motor function, and quality of life were observed between the experimental and control groups. Subgroup analysis revealed that combination therapy affected heterogeneity in the BBS analysis. Meta-regression analysis demonstrated a significant positive relationship, indicating that more recent studies have shown greater improvements in balance function.
Conclusion
This study’s findings suggest that VR-based rehabilitation is a promising intervention for improving balance function in patients for PD compared with conventional treatment, and recent research supports its efficacy. However, future research should focus on conducting long-term follow-up studies and developing standardized protocols to comprehensively establish this intervention’s potential benefits.
Similar content being viewed by others
Background
Patients with Parkinson’s disease (PD) often experience tremors, balance problems, and decreased motor abilities, which can significantly affect their daily activities [1]. These symptoms can negatively impact their quality of life and independence [2]. One potential solution to these challenges is virtual reality (VR) [3].
VR technology is a computer technology that simulates an environment similar to that of the real world. This technology allows users to interact in a three-dimensional (3D) virtual environment and experience various situations. Users can easily experience difficult situations in the real world using VR technology [4]. Depending on the degree of immersion, there are three types VR: non-immersive, semi-immersive, and fully immersive VR. Non-immersive reality is a type of VR where you can interact with the virtual environment through a computer screen, possibly a video game console, or an interface device. Semi-immersive VR primarily involves the use of large screens or projections to visually experience virtual 3D spaces. Fully-immersive VR involves the use of advanced wearable devices, such as head-mounted displays (HMDs), to enable users to actively participate in movement and interaction within virtual spaces, thereby experiencing a high level of presence and realism. All types of VR enable users to experience physically challenging situations in a safe environment, which can be particularly helpful in the rehabilitation of neurological conditions such as PD [5].
VR-based rehabilitation typically involves moving and performing actions in a virtual environment. These actions are implemented through computer simulations, and patients can improve their motor skills by identifying targets, following paths, and performing daily life movements in 3D virtual spaces [6]. Compared with previous rehabilitation methods, VR technology has several advantages. First, it allows users to safely and effectively experience dangerous or impossible situations in real environments. Second, in a virtual environment, patients can exercise at their own pace and difficulty level without experiencing stress. Third, VR technology can help patients build confidence in dealing with difficult real-world situations.
The COVID-19 pandemic has caused significant disruptions in healthcare delivery, including rehabilitation services [7]. Patients with PD are particularly vulnerable to the negative effect of social distancing and isolation, which can lead to decreased physical activity and worsening symptoms [8]. Home-based VR refers to the utilization of VR technology in the comfort and convenience of patients’ own home for rehabilitation. It allows patients to engage in therapy and exercises remotely, eliminating the need for frequent visits to hospitals or rehabilitation facilities. This approach enables patients to rehabilitate remotely, from the comfort and safety of their homes, guided by medical professionals and therapists [9, 10].
VR-based rehabilitation has shown promising results in improving motor function, balance, gait, and ADL in patients with neurological and musculoskeletal disorders. It is an effective treatment option for stroke, multiple sclerosis, and traumatic brain injury [11,12,13]. Several recent studies have shown that VR-based rehabilitation can also effectively improve balance and gait of patients with PD [14]. Furthermore, some interventions, including customized VR, have demonstrated efficacy in enhancing cognitive abilities such as attention and memory [15,16,17]. This can be a beneficial intervention for improving non-motor symptoms in patients with PD, such as PD-mild cognitive impairment or PD dementia.
Nonetheless, from a comprehensive perspective, the RCT results were inconsistent, with some showing significant improvement and others showing no difference compared with traditional therapy. We therefore conduct a systematic review of this field to present the latest trends and prospects. This systematic review and meta-analysis aimed to provide the most recent and convincing evidence on the rehabilitative effects of VR technology compared with conventional treatments.
Methods
Registration and search strategy
This study was conducted in compliance with the PRISMA guidelines [18]. The study protocol was registered under number CRD42022310868 in PROSPERO. A thorough search of the Embase, MEDLINE, CINAHL, PEDro, and Cochrane Library databases from their inception to May 31, 2022, was performed. The main search terms include Parkinson’s disease (“Parkinson’s disease” or “Parkinson*”) and virtual reality (“virtual reality,” “VR,” “game,” “gaming,” or “exergam*”). Boolean operators were used for combining the search terms. The literature search strategy in each database is presented in Additional file 1: Table S1.
Study selection
The selection was performed independently by two reviewers (KSH and PJK) based on predetermined criteria. Disagreements were resolved through discussion or arbitration involving a third author (KYH) until a consensus was obtained. In accordance with the patient, intervention, comparator, and outcome (PICO) criteria, studies were included if they met the following inclusion criteria: (1) patients clinically diagnosed with PD without any limitations on sex, age, and disease duration or severity, (2) interventions including VR training compared with conventional treatment; and (3) randomized controlled trials (RCTs). VR training was defined as an intervention that uses specially programmed computers, visual immersion devices, and simulated experiences in artificially created environments to help improve or maintain patients’ physical and cognitive functions [5]. Conventional treatments, which are used as a control group in studies for comparison with VR training, include various physical therapies such as muscle strengthening and flexibility exercises, balance training, treadmill training, and functional training [19]. There were no limitations on the type or strategy of intervention in the experimental and control groups. The inclusion and exclusion criteria are detailed in Additional file 2: Table S2.
Data extraction and quality assessment
The following data were extracted by two of the authors (KSH and PJK): (1) study characteristics: author, publication year and country; (2) sample characteristics for each study: sex, age, Hoehn and Yahr stage, intervention, protocol, supervision, related outcome and P value; (3) primary outcomes: balance function and gait ability; and (4) secondary outcomes: activities of daily living (ADL), motor function, and quality of life. Among the studies published by the same research team, those with overlapping research periods and participants were excluded.
Two authors (KSH and PJK) independently assessed the risk of bias in the included studies. We used version 2 of the Cochrane risk of bias tool for randomized trials (RoB2) to assess the risk of bias in the RCTs included in this systematic review [20]. The criteria included the following: (1) randomization process, (2) deviations from the intended intervention, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of reported results. Each criterion was judged as ‘low risk of bias,’ ‘some concerns,’ or ‘high risk of bias.’
Statistical analysis
Meta-analyses were conducted using the Review Manager 5.4 software (Cochrane Collaboration, Oxford, UK). For continuous variables, the inverse variance method was used to pool mean differences (MDs) with 95% confidence intervals (CIs). If the median and range were reported instead of mean and standard deviation (SD), these values were estimated using the methods developed by Luo et al. and Wan et al. [21, 22]. Heterogeneity among studies was assessed using the Q test and I2 statistic. I2 statistic was interpreted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. If the Cochrane Q statistical P value was < 0.10 and the I2 statistic was > 50%, significant heterogeneity was considered present, and a random-effects model was used. If otherwise, a fixed effects model was used [23]. A meta-regression analysis was performed using the publication year as the predictor variable to investigate whether the effect size of the studies included in the present meta-analysis varied as a function of the publication year. A mixed-effects model with publication year as the only covariate was fitted using the R package meta. To assess publication bias for asymmetry in the funnel plot, we performed Egger’s regression test. The intercept and slope of the regression line were estimated, and statistical significance was set at P < 0.1.
Results
Study characteristics
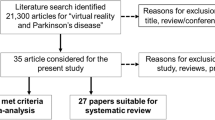
A total of 2219 studies were identified through the literature search (Fig. 1). After excluding 658 duplicate articles, 1367 articles were excluded based on language and article type after reviewing the abstracts. The remaining articles underwent full-text screening to ensure that they met the inclusion criteria, which resulted in the exclusion of additional 180 articles. Finally, 14 RCTs met all the inclusion criteria [24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Participants’ demographics, interventions, and related outcome measures are presented in Table 1. The characteristics of intervention are detailed in Additional file 3: Table S3. The criteria used to assess the risk of bias in the included studies were based on RoB2 (Fig. 2). In summary, three studies were assessed as having a low risk of bias, eight studies were judged to raise some concerns, and three studies were deemed to have a high risk of bias.
PRISMA diagram of the search strategy. A systematic search was performed across multiple databases including Embase, MEDLINE, CINAHL, PEDro, and Cochrane Library. After removing duplicate articles, excluding articles based on language and article type after reviewing abstracts, and conducting full-text screening, a final set of 14 randomized controlled trials (RCTs) met all the inclusion criteria
Balance function
12 studies comprising 388 patients assessed balance ability using the Berg balance scale (BBS) scores (Fig. 3a) [24,25,26,27, 29,30,31,32,33, 35, 37]. An analysis of these studies revealed significantly higher BBS scores in the experimental group compared with the control group (MD = 2.71, 95% CI = 1.45 to 3.96, P < 0.001), and moderate heterogeneity was observed (I2 = 50%, P = 0.02). To further explore this heterogeneity, subgroup analysis was performed based on whether or not combination therapy was administered, dividing the total group into VR and VR+PT subgroups. Heterogeneity was no longer significant in the VR subgroup (I2 = 32%, P = 0.15), whereas heterogeneity remained significant in the VR+PT subgroup (I2 = 68%, P = 0.08).
Three studies, involving 154 patients, evaluated balance confidence by measuring scores on the activities-specific balance confidence (ABC) scale (Fig. 3b) [24, 30, 31]. A significant increase in the ABC scale score was observed in the VR-based rehabilitation group compared with the control group (MD = 9.43, 95% CI = 5.67 to 13.19, P < 0.001). No heterogeneity was detected among the included studies (I2 = 8%, P = 0.34).
The balance performance during gait was measured using the dynamic gait index (DGI) in five studies involving 191 patients (Fig. 3c) [25, 26, 30, 32]. The pooled analysis revealed no statistically significant difference between the two groups (MD = 0.61, 95% CI = − 0.19 to 1.41, P = 0.14), and no heterogeneity was detected among the included studies (I2 = 0%, P = 0.56).
Finally, one study involving 28 patients investigated the effects of VR-based rehabilitation on postural stability and balance using the functional gait assessment (FGA) [27]. The results showed a significant improvement in the experimental group (P < 0.05).
Gait ability
Six studies involving 150 patients examined functional mobility using the time up and go test (TUGT) (Fig. 4a) [26, 27, 31, 33, 34]. The difference between the experimental and control groups was not statistically significant (MD = − 0.83, 95% CI = − 2.80 to 1.14, P = 0.41); however, heterogeneity was observed (I2 = 75%, P = 0.001).
The 10-m walk test (10MWT) was evaluated in five studies involving 206 patients (Fig. 4b) [28, 30, 31, 35], and the 6-min walk test (6MWT) was reported in three studies involving 82 patients (Fig. 4c) [28, 29]. The results showed a significant improvement in gait speed, as measured using the 10MWT, in the experimental group (MD = 0.09, 95% CI = 0.01 to 0.18, P = 0.04), whereas no change in walking capacity was observed using the 6MWT (MD = − 18.84, 95% CI = − 53.56 to 15.89, P = 0.29). The heterogeneity was insignificant (respectively, I2 = 43%, P = 0.14; I2 = 0%, P = 0.45).
Freezing severity was evaluated in one study involving 37 patients using the freezing of gait questionnaire (FOGQ) [31], and there was no statistically significant difference between the experimental and control groups (P = 0.695, Cohen’s d = − 0.13).
Activities of daily living/motor function
Two studies involving 73 patients assessed the ADL using the Unified Parkinson’s disease rating scale (UPDRS) II (Fig. 5a) [24, 37]. The results showed no statistically significant difference between the two groups (MD = − 2.18, 95% CI = − 7.50 to 3.14, P = 0.42). High statistical heterogeneity was observed among the studies (I2 = 90%, P = 0.002).
Forest plot for meta-analysis of secondary outcomes related to activities of daily living and motor function. Mean difference (95% CI) of the effect of VR-based rehabilitation (experimental group) compared with conventional treatment (control group) on a UPDRS II and b UPDRS III. CI confidence interval, SD standard deviation
Five studies involving 159 patients evaluated motor function using UPDRS III (Fig. 5b) [24, 27, 31, 32, 35]. The results revealed that the UPDRS III scores were equivalent between the two groups (MD = − 3.68, 95% CI = − 9.31 to 1.95, P = 0.20), and a substantial heterogeneity was observed (I2 = 73%, P = 0.006).
Quality of life
Nine studies involving 146 patients investigated the effect of VR-based rehabilitation on the quality of life using the Parkinson’s disease questionnaire-39 (PDQ-39) (Fig. 6) [26, 28, 31, 32, 34,35,36]. The meta-analysis demonstrated no significant difference between the two groups (MD = − 1.92, 95% CI = − 6.55 to 2.71, P = 0.42), and no heterogeneity was observed among the included studies (I2 = 0%, P = 0.55).
Meta-regression
A meta-regression analysis was performed to examine the association between the publication year and the MD in BBS scores (Fig. 7) [24,25,26,27, 29,30,31,32,33, 35, 37]. The results indicated a significant positive relationship (β = 0.42, 95% CI = 0.06 to 0.79, P < 0.05), suggesting that the improvement in balance function varies depending on the publication year, with more recent studies showing greater improvement. Heterogeneity among the studies was low (I2 = 21.4%) after including the predictor, indicating no significant unexplained variability. The Q test for heterogeneity was insignificant (Q = 12.13, df = 10, P = 0.28), suggesting that the observed heterogeneity was likely due to chance.
Publication bias
Visual assessment of the funnel plot showed that the studies were distributed symmetrically regarding BBS [24,25,26,27, 29,30,31,32,33, 35, 37], suggesting no publication bias in the present meta-analysis. Egger’s regression test (P = 0.99) confirmed the absence of potential publication bias. In addition, funnel plot analysis was performed for other functional outcomes such as balance function, gait ability, activities of daily living, motor function, and quality of life. However, it should be noted that the number of papers included in our study for these outcomes was less than 10, and as a result, publication bias cannot be reliably analyzed using the funnel plot analysis or Egger’s test. The results are presented in Additional file 4: Figs. S1, S2, S3, and S4.
Discussion
This systematic review included 14 RCTs with 524 patients, comparing the effectiveness of VR-based rehabilitation to conventional interventions in patients with PD. The results provided valuable insights into the benefits of VR-based rehabilitation in improving balance function in patients with PD. Notably, meta-regression analysis revealed a positive relationship between the publication year and improvement in balance function, suggesting that more recent studies showed greater advancements in this field. On the other hand, in the meta-analysis, there was no statistically significant differences in gait ability, ADL, motor function, and quality of life. This indicates that current VR-based interventions are partially effective in improving symptoms, highlights the need for further technological advances focusing on gait and motor function to enhance patients’ overall quality of life.
One of the reasons why VR-based rehabilitation is particularly effective in improving balance function in patients with PD is because it challenges the patient’s balance through game-based VR program that incorporate activities requiring weight shifting and balance maintenance [24, 26, 29, 30, 37]. Incorporating game-like elements and other interactive features can increase patient enjoyment and willingness to participate in training sessions. This increased motivation can lead to better training outcomes and an overall improvement in balance. Another advantage of VR-based rehabilitation is that it can be tailored to individual patient needs. Customized training can help patients progress faster and more effectively than conventional treatments [31, 33, 35]. In addition, patients can observe their performance in real-time and receive immediate feedback regarding movement and balance control. This allows them to coordinate their movements and immediately improve their balance, leading to faster improvements in overall balance [38]. Therefore, VR-based rehabilitation effectively improves balance in patients with PD because it provides a high level of immersion, customized training, immediate feedback, and motivation through a game-like experience. These advantages make it a powerful tool for rehabilitation and a promising avenue for future research on PD treatment.
The significant association between the publication year and improvement in balance indicates that recent studies have developed more effective rehabilitation methods and measurement techniques than previous studies, which can increase the practical value of rehabilitation therapy. With advances in VR technology, it has become possible to create more realistic virtual environments, making VR a more suitable tool for rehabilitation. This technological development has enabled patients to experience virtual environments more realistically, thereby providing more effective rehabilitation therapy [39]. In addition, the increase in the accessibility of VR technology may be associated with the recent trend of its increased effectiveness [40, 41]. Previously, VR-based rehabilitation was limited because expensive equipment and specialized skills were needed. However, with recent technological advancements, VR-based rehabilitation equipment has become cheaper and easier to use, making it available to more patients [42]. This can increase patient engagement and participation, leading to more effective rehabilitation therapies. Therefore, VR-based rehabilitation treatments have recently shown more effective results.
VR-based rehabilitation has shown promising results in terms of clinical outcomes in patients with PD. However, much remains to be explored regarding the potential benefits of this therapy in different patient subgroups. For example, it may be valuable to investigate the effectiveness of VR-based rehabilitation in patients with varying disease severities, ages, and cognitive functions. Furthermore, future studies should focus on comparing the efficacy of VR-based rehabilitation with that of other treatment types, such as combination therapy with existing treatments or emerging technologies. Despite including some studies that investigated the combined use of VR-based rehabilitation and conventional treatment for PD, the interpretation of the results remains challenging owing to the limited number of studies available and the heterogeneity of the intervention protocols. Therefore, further studies with larger sample sizes and standardized protocols are warranted to fully elucidate the potential benefits of combining VR-based technologies with conventional treatments.
Regarding the safety of VR-based rehabilitation for patients with PD, the literature indicates that adverse events associated with VR are generally rare and mild. However, as the level of immersion in VR increases, there are additional considerations for ensuring safe rehabilitation, such as regular rest periods, securing a safe surrounding environment, and emotional stability. Particularly in rehabilitation, it is essential for instructors to educate users on proper usage techniques and safety procedures. The majority of studies analyzed in this systematic review utilized non-immersive VR technology (including one study using semi-immersive VR [25]) under the supervision of physical therapist during training sessions, and no significant adverse effects were reported or mentioned in each study. Additionally, two of the studies demonstrated the safety and effectiveness of remote rehabilitation by conducting home-based VR under supervision [30, 32]. It has been reported that even fully immersive VR can be considered safe for walking on the elderly and patients with PD [43]. Nevertheless, caution should be exercised regarding potential adverse effects such as cybersickness or falls that may occur when using HMDs [44,45,46].
RCTs are considered the gold standard for evaluating the efficacy of interventions. However, several limitations of RCTs on VR-based rehabilitation for patients with PD need to be addressed. First, blinding participants and therapists to treatment allocation is challenging in VR studies because it is difficult to blind participants to the type of therapy they receive. This can lead to a bias in the results because the participants may have had different expectations or preferences for each treatment arm. Second, conventional clinical outcome measures in VR studies may not accurately capture the specific changes in motor and non-motor symptoms that can be achieved with VR-based rehabilitation. Therefore, there is a need to develop new and specific outcome measures for VR-based rehabilitation for patients with PD. Finally, long-term follow-up assessments are needed in VR-based rehabilitation studies. Notably, some studies have reported positive outcomes immediately after VR-based rehabilitation; however, it is unclear whether these outcomes are maintained in the long term. Therefore, long-term follow-up assessments are required to determine the sustainability of VR-based rehabilitation.
Conclusion
The results of this meta-analysis suggest that VR-based rehabilitation can effectively improve balance in patients with PD. Specifically, the experimental group demonstrated a significantly higher BBS and ABC scale scores than the control group. In addition, walking speed measured using 10MWT was significantly improved in the experimental group. However, no significant differences were observed in DGI, 6MWT, or UPDRS II and III scores. Meta-regression analysis suggested that the improvement in balance function varies depending on the publication year, with more recent studies showing greater improvement. Overall, these results indicate that VR-based rehabilitation programs may have a positive effect on balance function in patients with PD, and further studies are needed to determine the optimal VR intervention for these patients.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- PD:
-
Parkinson’s disease
- VR:
-
Virtual reality
- 3D:
-
Three-dimensional
- RCTs:
-
Randomized controlled trials
- BBS:
-
Berg balance scale
- ABC:
-
Activities-specific balance confidence
- DGI:
-
Dynamic gait index
- FGA:
-
Functional gait assessment
- 6MWT:
-
6-Minute walk test
- 10MWT:
-
10-Meter walk test
- TUGT:
-
Time up and go test
- FOGQ:
-
Freezing of gait questionnaire
- ADL:
-
Activities of daily living
- UPDRS:
-
Unified Parkinson’s disease rating scale
- PDQ-39:
-
Parkinson’s disease questionnaire-39
- RoB2:
-
Version 2 of the Cochrane risk of bias tool for randomized trials
- MD:
-
Mean difference
- CI:
-
Confidence interval
References
Mazzoni P, Shabbott B, Cortés JC. Motor control abnormalities in Parkinson’s disease. Cold Spring Harbor Perspect Med. 2012;2(6): a009282.
Anette S, Marjan J, Niall Q. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69(3):308.
Rose T, Nam CS, Chen KB. Immersion of virtual reality for rehabilitation—review. Appl Ergon. 2018;69:153–61.
Cipresso P, Giglioli IAC, Raya MA, Riva G. The past, present, and future of virtual and augmented reality research: a network and cluster analysis of the literature. Front Psychol. 2018;9:2086.
Canning CG, Allen NE, Nackaerts E, Paul SS, Nieuwboer A, Gilat M. Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat Rev Neurol. 2020;16(8):409–25.
Levac DE, Huber ME, Sternad D. Learning and transfer of complex motor skills in virtual reality: a perspective review. J Neuroeng Rehabil. 2019;16(1):121.
Ray M, Sharon S, Zoe AM, Anna Mae S, Justin C, Emma JT, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021;11(3): e045343.
Leta V, Boura I, van Wamelen DJ, Rodriguez-Violante M, Antonini A, Chaudhuri KR. Chapter four—Covid-19 and Parkinson’s disease: acute clinical implications, long-COVID and post-COVID-19 parkinsonism. Int Rev Neurobiol. 2022;165:64–89.
Merlijn S, Staal JB, van Harry G. Could virtual reality play a role in the rehabilitation after COVID-19 infection? BMJ Open Sport Exerc Med. 2020;6(1): e000943.
Derick TW. Rehabilitation after COVID-19: an evidence-based approach. Clin Med. 2020;20(4):359.
Lee HS, Park YJ, Park SW. The effects of virtual reality training on function in chronic stroke patients: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:7595639.
Maggio MG, Russo M, Cuzzola MF, Destro M, La Rosa G, Molonia F, et al. Virtual reality in multiple sclerosis rehabilitation: a review on cognitive and motor outcomes. J Clin Neurosci. 2019;65:106–11.
Zanier ER, Zoerle T, Di Lernia D, Riva G. Virtual reality for traumatic brain injury. Front Neurol. 2018;9:345.
Dockx K, Bekkers EMJ, Van den Bergh V, Ginis P, Rochester L, Hausdorff JM, et al. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst Rev. 2016;12:CD010760.
Garcia-Agundez A, Folkerts AK, Konrad R, Caserman P, Tregel T, Goosses M, et al. Recent advances in rehabilitation for Parkinson’s disease with exergames: a systematic review. J Neuroeng Rehabil. 2019;16(1):17.
Marotta N, Calafiore D, Curci C, Lippi L, Ammendolia V, Ferraro F, et al. Integrating virtual reality and exergaming in cognitive rehabilitation of patients with Parkinson disease: a systematic review of randomized controlled trials. Eur J Phys Rehabil Med. 2022;58(6):818–26.
Triegaardt J, Han TS, Sada C, Sharma S, Sharma P. The role of virtual reality on outcomes in rehabilitation of Parkinson’s disease: meta-analysis and systematic review in 1031 participants. Neurol Sci. 2020;41(3):529–36.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2008;23(5):631–40.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2016;27(6):1785–805.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557.
Kashif M, Ahmad A, Bandpei MAM, Gilani SA, Hanif A, Iram H. Combined effects of virtual reality techniques and motor imagery on balance, motor function and activities of daily living in patients with Parkinson’s disease: a randomized controlled trial. BMC Geriatr. 2022;22(1):381.
Pazzaglia C, Imbimbo I, Tranchita E, Minganti C, Ricciardi D, Lo Monaco R, et al. Comparison of virtual reality rehabilitation and conventional rehabilitation in Parkinson’s disease: a randomised controlled trial. Physiotherapy. 2020;106:36–42.
Santos P, Machado T, Santos L, Ribeiro N, Melo A. Efficacy of the Nintendo Wii combination with conventional exercises in the rehabilitation of individuals with Parkinson’s disease: a randomized clinical trial. NeuroRehabilitation. 2019;45:255–63.
Feng H, Li C, Liu J, Wang L, Ma J, Li G, et al. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson’s disease patients: a randomized controlled trial. Med Sci Monit. 2019;25:4186–92.
Ferraz DD, Trippo KV, Duarte GP, Neto MG, Bernardes Santos KO, Filho JO. The effects of functional training, bicycle exercise, and exergaming on walking capacity of elderly patients with Parkinson disease: a pilot randomized controlled single-blinded trial. Arch Phys Med Rehabil. 2018;99(5):826–33.
Ribas CG, Alves da Silva L, Corrêa MR, Teive HG, Valderramas S. Effectiveness of exergaming in improving functional balance, fatigue and quality of life in Parkinson’s disease: a pilot randomized controlled trial. Parkinsonism Relat Disord. 2017;38:13–8.
Gandolfi M, Geroin C, Dimitrova E, Boldrini P, Waldner A, Bonadiman S, et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: a multicenter, single-blind, randomized, controlled trial. Biomed Res Int. 2017;2017:7962826.
Carpinella I, Cattaneo D, Bonora G, Bowman T, Martina L, Montesano A, et al. Wearable sensor-based biofeedback training for balance and gait in Parkinson disease: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98(4):622-30.e3.
Yang W-C, Wang H-K, Wu R-M, Lo C-S, Lin K-H. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: a randomized controlled trial. J Formos Med Assoc. 2016;115(9):734–43.
Shih MC, Wang RY, Cheng SJ, Yang YR. Effects of a balance-based exergaming intervention using the Kinect sensor on posture stability in individuals with Parkinson’s disease: a single-blinded randomized controlled trial. J Neuroeng Rehabil. 2016;13(1):78.
Liao Y-Y, Yang Y-R, Cheng S-J, Wu Y-R, Fuh J-L, Wang R-Y. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Randomized controlled trial. Neurorehabil Neural Repair. 2014;29(7):658–67.
van den Heuvel MR, Kwakkel G, Beek PJ, Berendse HW, Daffertshofer A, van Wegen EE. Effects of augmented visual feedback during balance training in Parkinson’s disease: a pilot randomized clinical trial. Parkinsonism Relat Disord. 2014;20(12):1352–8.
Pedreira G, Prazeres A, Cruz D, Gomes IN, Monteiro L, Melo A. Virtual games and quality of life in Parkinson’s disease: a randomised controlled trial. Adv Parkinsons Dis. 2013;02(4):5.
Pompeu JE, Mendes FA, Silva KG, Lobo AM, Oliveira Tde P, Zomignani AP, et al. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy. 2012;98(3):196–204.
Tieri G, Morone G, Paolucci S, Iosa M. Virtual reality in cognitive and motor rehabilitation: facts, fiction and fallacies. Expert Rev Med Dev. 2018;15(2):107–17.
Guo Q-F, He L, Su W, Tan H-X, Han L-Y, Gui C-F, et al. Virtual reality for neurorehabilitation: a bibliometric analysis of knowledge structure and theme trends. Front Public Health. 2022;10:1042618.
Xie B, Liu H, Alghofaili R, Zhang Y, Jiang Y, Lobo FD, et al. A review on virtual reality skill training applications. Front Virtual Real. 2021;2: 645153.
Plow M, Finlayson M. A qualitative study exploring the usability of Nintendo Wii Fit among persons with multiple sclerosis. Occup Ther Int. 2014;21(1):21–32.
Keshner EA. Virtual reality and physical rehabilitation: a new toy or a new research and rehabilitation tool? J Neuroeng Rehabil. 2004;1(1):8.
Kim A, Darakjian N, Finley JM. Walking in fully immersive virtual environments: an evaluation of potential adverse effects in older adults and individuals with Parkinson’s disease. J Neuroeng Rehabil. 2017;14(1):16.
Anthes C, García-Hernández RJ, Wiedemann M, Kranzlmüller D, editors. State of the art of virtual reality technology. In: IEEE aerospace conference. 2016. https://doi.org/10.1109/AERO.2016.7500674.
Saredakis D, Szpak A, Birckhead B, Keage HAD, Rizzo A, Loetscher T. Factors associated with virtual reality sickness in head-mounted displays: a systematic review and meta-analysis. Front Hum Neurosci. 2020;14:96.
Weech S, Kenny S, Barnett-Cowan M. Presence and cybersickness in virtual reality are negatively related: a review. Front Psychol. 2019;10:158.
Acknowledgements
Not applicable.
Funding
This research was supported by a fund (2021-NI-020-02) by Research of Korea Disease Control and Prevention Agency.
Author information
Authors and Affiliations
Contributions
KSH and PJK both contributed equally to the study design, literature search, and data analysis. KSH conducted data extraction, and PJK provided critical feedback on data analysis. KSH drafted the manuscript, and PJK provided critical revisions to the manuscript for important intellectual content. KYH provided guidance on all aspects of the study. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved this manuscript for publication. This manuscript has not previously been published and is nor pending publication elsewhere.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Literature search strategy in each databases.
Additional file 2: Table S2.
Details on inclusion and exclusion criteria.
Additional file 3: Table S3.
Intervention characteristics of included studies.
Additional file 4: Figure S1.
Funnel plots for meta-analysis of balance function. Figure S2. Funnel plots for meta-analysis of gait ability. Figure S3. Funnel plots for meta-analysis of activities of daily living and motor function. Figure S4. Funnel plot for meta-analysis of quality of life.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kwon, SH., Park, J.K. & Koh, Y.H. A systematic review and meta-analysis on the effect of virtual reality-based rehabilitation for people with Parkinson’s disease. J NeuroEngineering Rehabil 20, 94 (2023). https://doi.org/10.1186/s12984-023-01219-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-023-01219-3