Abstract
Suckermouth armoured catfish (Loricariidae) are a highly speciose and diverse freshwater fish family, which bear upper and lower lips forming an oral disc. Its hierarchical organisation allows the attachment to various natural surfaces. The discs can possess papillae of different shapes, which are supplemented, in many taxa, by small horny projections, i.e. unculi. Although these attachment structures and their working mechanisms, which include adhesion and interlocking, are rather well investigated in some selected species, the loricariid oral disc is unfortunately understudied in the majority of species, especially with regard to comparative aspects of the diverse oral structures and their relationship to the ecology of different species. In the present paper, we investigated the papilla and unculi morphologies in 67 loricariid species, which inhabit different currents and substrates. We determined four papilla types and eight unculi types differing by forms and sizes. Ancestral state reconstructions strongly suggest convergent evolution of traits. There is no obvious correlation between habitat shifts and the evolution of specific character states. From handling the structures and from drying artefacts we could infer some information about their material properties. This, together with their shape, enabled us to carefully propose hypotheses about mechanisms of interactions of oral disc structures with natural substrates typical for respective fish species.
Similar content being viewed by others
Background
The suckermouth armored catfishes (Loricariidae, Siluriformes, Teleostei) are among the most interesting animal groups, because this family is, with about 1000 species [1], highly specious as a result of an evolutionary radiation, which included convergent evolution of traits [2, 3]. These neotropical freshwater fish are particularly diverse with regard to body colorations and shapes, reflecting their high degree of specialisation to different habitats and speciation.
Loricariidae are characterized by a depressed body shape covered by bony plates and the modification of the mouth to an oral sucker disc, which is used, besides for feeding, for the attachment to various substrates [2, 4,5,6,7]. In specialized species, it also facilitates climbing [8]. This oral disc, which can only be found in three more catfish families (Astroblepidae, some genera in Mochokidae and in Sisoridae), is an adaptation to fast flowing water bodies and is used to generate negative pressure to allow surface attachment [6]. In fish taxa, such as Balitoridae, Gobiesocidae, Cyclopteridae, Liparidae, Echeneidae, and Cyprinidae, attachment structures and attachment mechanisms have been previously investigated [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23].
In general, attachment mechanisms are omnipresent in animals, including insects [24,25,26,27,28,29,30,31,32], molluscs [33, 34], reptiles [35, 36], amphibians [37, 38], mammals [39, 40] and, as mentioned above, fishes [17, 41, 42].
These mechanisms are diverse and can include interlocking structures, such as hooks, locks, clamps or spacers [26], wet and dry adhesion [43, 44], and/or suction cups [15, 17]. Depending on the attachment system, physical effects as friction, mechanical interlocking, muscular force, viscous forces, chemical bonding, capillary effects, van der Waals forces, and electrostatic forces are involved and can lead to permanent, transitory and temporary attachment time to the substrate [26, 45, 46].
With regard to the aquatic environment, two main attachment strategies, bioadhesive secretion or suction attachment, seem to be present as adaptation to the specific physical conditions [see reviews 46, 47]. Glue-like bioadhesive secretions include complex mixtures of proteins, lipids and sugars and can be found in echnioderms, mussels, or barnacles. Suction attachment involves muscular contraction to generate pressure differences and can be found in cephalopods, some insect taxa, and fish. In some species, both mechanisms can be found, as in lottiid limpets or fish. Depending on the taxa, attachment is achieved by multiple points of interaction, as in Echniodermata or Cephalopoda, or by one single attachment point, as in limpets or fish [see review 47].
In fish, the attachment structure, i.e., the suction disc, represents a chamber of subatmospheric pressure to create adhesion by suction to various substrates [7, 9, 14, 16, 17, 41, 42, 48, 49], which can even enable climbing vertical surfaces outside the water column [8, 11, 50,51,52].
Performance of the fish sucker depends on many different factors of the attachment structure itself, such as muscle contraction, kinematics, material properties, size, and shape [9, 15, 16, 22, 42, 53,54,55,56,57,58]. Additionally, the fish attachment ability is affected by the intensity of the water stream [59, 60] and the substrate curvature and its surface properties [13, 16, 17, 22, 48, 59, 61,62,63,64,65,66]. During attachment, the animals maintain their grip by friction with structures at micro- and nanoscale, i.e. papillae or microvilli [16, 19, 41, 42, 48, 67,68,69,70,71, 20, 21, 23]. Additionally, the mucus between and on these structures provides strong contribution to the attachment strength [72].
The whole body of the fish can be modified as ventral sucker (Balitoridae, Gastromyzontidae), or the fins have convergently evolved to attachment structures (Oxudercidae, Gobiesocidae, Cyclopteridae, Liparidae, Echeneidae, Cyprinidae) [9,10,11,12,13,14,15,16,17,18,19, 66]. In Gyrinocheilidae, some Gobiesocidae, and suckermouth catfishes of Africa (Mochokidae) and South America (Loricariidae, Astroblepidae), the mouth structures are transformed to an oral sucker, allowing animals to adhere to surfaces while simultaneously foraging and performing respiration [6, 7, 11, 15, 48, 61, 73,74,75]. This specialized suckermouth is similar to attachment structures of other fish, highly textured with papillae bearing small keratinized outgrowths of single epithelia cells, i.e., unculi [67,68,69, 74, 76,77,78,79].
The papillae are probably used for foraging and increasing the attachment capability by friction to reinforce the seal of the disc [48, 73, 74, 76, 80]. The unculi can be found on top of the papillae, which are highly diverse in morphology among fish [67]. In Loricariidae, they are potentially involved in feeding [73, 76], but probably increase the friction during attachment as well [6, 48, 67,68,69, 74, 81].
In general, attachment structures in fish have received attention in the last decades, which even enabled the development of adhesive materials or gripping and adhesive devices [42, 56, 58, 65, 72, 82,83,84,85,86,87, 20,21,22,23, 88].
However, in contrast to remoras, gobies, hillstream loaches, darters, or clingfish, which were experimentally studied with regard to attachment performance and mechanisms [9, 16, 17, 41, 42, 49, 60, 63,64,65,66, 70, 89], to date only a few experiments [48, 59] addresses the real attachment performance of the loricariid suckermouth fish.
Besides of the above-mentioned references, little is known about the structures of the suckermouth and oral papillae, even though they are highly diverse [2, 3, 7, 67, 90]. We here study the morphology of papillae and unculi of 67 species and undescribed taxa from all kinds of habitats to pave the way for more research addressing the diversity of oral discs. According to their shape, the structures were sorted to different categories. Although, comparative studies on the attachment performance of these fish taxa are lacking, we here present hypotheses on the interaction between mouth and substrate based on the diverse literature on attachment structures on different animal taxa.
Results
Morphology of papillae
On the lower lips (Fig. 1A–D), we could differentiate four papilla types (Fig. 1E–H):
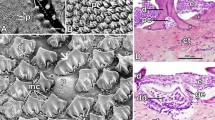
A–D Suckermouths of selected taxa. A Ancistrus sp. L464. B Baryancistrus xanthellus. C Panaque nigrolineatus. D Chaetostoma formosae. The highlighted region was documented by SEM to study the papillae and unculi. E–H Types of papillae. E Flat type, Pseudohemiodon almendarizi. F Short type, Ancistrus sp. L464. G Medium type, Acanthicus adonis. H Long type, Hypancistrus sp. L333. The sizes of the insets relate the sizes of the papillae to another. Scale bars: A 6 mm; B 6.5 mm; C 10.5 mm; D 9 mm; E, F 100 µm; G, H 250 µm
-
1.
Flat papillae
This papilla is broad with a flattened tip (Fig. 1E). Each papilla measures between 100–200 μm in diameter and has a height of approximately 50 μm. Up to 20 papillae are found on each studied area, depending on the size of the lip. When this papilla type is manipulated, the bases seem relatively flexible and the tip rather stiff. This pattern is detected in Ancistrus ranunculus, Pseudacanthicus pitanga, Crossoloricaria cephalaspis, Loricaria luciae, Loricaria simillima, Pseudohemiodon almendarizi (Fig. 1E), Pterosturisoma microps, and Spatuloricaria puganensis.
-
2.
Short papillae
Papillae are rather roundish in profile (Fig. 1F). They are of 100–200 μm diameter and 70–100 μm in height. When the papillae are manipulated, the tip seems rather flexible whereas the base seems rather stiff. This kind is determined for Hypoptopoma inexspectatum, Ancistrus sp. L464 (Fig. 1F), Chaetostoma formosae, Scobinancistrus aureatus, and Rineloricaria melini.
-
3.
Medium papillae
These papillae are roundish and of medium height (300–400 μm) and of 100–300 μm diameter (Fig. 1G). The tip seems rather flexible, but the base rather stiff. We observed them in Otocinclus cocama, Rhinotocinclus isabelae, Acanthicus adonis (Fig. 1G), Peckoltia sabaji, Ancistomus spilomma, Ancistrus cirrhosus, Ancistrus dolichopterus, Ancistrus luzia, Ancistrus sp. L107, Ancistrus sp. L519, Baryancistrus niveatus, Baryancistrus xanthellus, Chaetostoma dorsale, Chaetostoma lineopunctatum, Dekeyseria picta, Guyanancistrus brevispinis, Hypancistrus contradens, Hypancistrus inspector, Hypancistrus sp. L174, Hypancistrus zebra, Hypostomus cochliodon, Leporacanthicus joselimai, Leporacanthicus cf. galaxias, ‘Spectracanthicus’ immaculatus, Panaqolus sp. L271, Panaqolus sp. L351, Panaque nigrolineatus, Parancistrus nudiventris, Pseudacanthicus sp. L97, Pseudacanthicus sp. L185, Pseudacanthicus sp. L273, Pseudacanthicus sp. L65, Pseudacanthicus spinosus, Pseudolithoxus dumus, Scobinancistrus pariolispos, Cteniloricaria platystoma, Farlowella oxyrryncha, Farlowella platorynchus, Hemiodontichthys acipenserinus, Lamontichthys filamentosus, Lamontichthys stibaros, Sturisomatichthys aureus, and Sturisomatichthys festivus.
-
4.
Long papillae
The papillae are roundish and long (500–550 μm) and of 80–100 μm diameter (Fig. 1H). The tip seems rather flexible, but the base rather stiff. We detect long papillae in Ancistrus claro, Ancistrus megalostomus, Aphanotorulus horridus, Hypancistrus sp. L333 (Fig. 1H), Hypostomus bolivianus, Hypostomus laplatae, Parancistrus aurantiacus, Peckoltia sp. L76, Scobinancistrus raonii, Pseudorinelepis genibarbis, and Rhinelepis aspera.
Morphology of unculi
We could not detect a high degree of intraspecific variability in species with more than one examined specimen (Additional file 1: Figure S1). Overall, we can differentiate eight types related to the unculi on the papillae surfaces (see below) (Fig. 2).
Types of unculi. A None is present, Pseudohemiodon almendarizi. B Suction cup, Acanthicus adonis. C Hooks, Ancistrus sp. L464. D Mushrooms, Chaetostoma formosae. E Small mushrooms, Spatuloricaria puganensis. F Honey-combed, Ancistrus megalostomus. G Long filaments, Pseudacanthicus pitanga. H Folds, Hypostomus bolivianus. Scale bars: A, H, E 100 µm; B 150 µm; C, D 15 µm; F 50 µm; G 1 mm
-
1.
No unculi
Here, no small projections but a rather bulky surface of the papilla is found (Fig. 2A). The surface seems rather stiff. We observed this in Rhinotocinclus isabelae, Ancistrus ranunculus, Dekeyseria picta, Hypancistrus sp. L174, Hypancistrus zebra, Leporacanthicus joselimai, Leporacanthicus cf. galaxias, ‘Spectracanthicus’ immaculatus, Parancistrus aurantiacus, Parancistrus nudiventris, Crossoloricaria cephalaspis, Loricaria luciae, Loricaria simillima, and Pseudohemiodon almendarizi (Fig. 2A).
-
2.
Suction cups (no unculi)
On the central surface of the papilla, a round to oval structure with a thick outer bulge and an inner depression is determined (Fig. 2B). The papillae themselves vary from 150 to 200 μm in diameter and the structure is of about 50 μm in diameter. The tip of each papilla seems flexible and the base rather stiff. This surface structure is detected in Acanthicus adonis (Fig. 2B).
-
3.
Elongated unculi with hook-like tips
Long projections of 15–20 μm length are situated in the centre of each papilla (Fig. 2C). Each papilla hosts 20–40 single unculi, depending on the papilla size. The tips of the unculi are formed like a hook and most of them are pointed into the same direction. Tips of the hooks seem rather stiff, whereas the bases seem rather flexible. We document these unculi in Ancistrus sp. L464 (Fig. 2C) and Lamontichthys stibaros.
-
4.
Mushroom-like unculi
Here, elongated unculi of 15–25 μm with flattened tips are found (Fig. 2D). The papillae are entirely covered with 25–40 unculi, depending on the size of the papillae. The tips seem rather stiff, whereas the bases seem rather flexible. This is observed in Otocinclus cocama, Ancistrus cirrhosus, Ancistrus claro, Ancistrus luzia, Ancistrus sp. L107, Ancistrus sp. L519, Chaetostoma dorsale, Chaetostoma formosae (Fig. 2D), Chaetostoma lineopunctatum, Scobinancistrus aureatus, Scobinancistrus raonii, Cteniloricaria platystoma, Farlowella oxyrryncha, Farlowella platorynchus, Lamontichthys filamentosus, Sturisomatichthys aureus, and Pseudorinelepis genibarbis.
-
5.
Small mushroom-like unculi
In some species, unculi of 10–15 μm length with flattened tips are found (Fig. 2E). The papillae are covered with 10–15 unculi. The tips seem rather stiff, whereas the bases seem rather flexible. We document this type of unculi for Hypancistrus contradens, Rineloricaria melini, Hemiodontichthys acipenserinus, and Spatuloricaria puganensis (Fig. 2E).
-
6.
Honey-combed pattern
Here, the whole papilla surface (150–200 μm diameter) pattern is reticulated (Fig. 2F). The parts of the structure interacting with the target surface seem rather flexible and the bases rather stiff. This type is determined for Ancistrus megalostomus (Fig. 2F).
-
7.
Long filamentous unculi
Unculi are thin (~ 1 µm thick) and of 10 μm length (Fig. 2G). On each papilla, 30–40 single filaments are found. They seem very flexible. This surface pattern is detected in Hypancistrus sp. L333, Pseudacanthicus pitanga (Fig. 2G), Pseudacanthicus sp. L97, Pseudacanthicus sp. L185, Pseudacanthicus sp. L273, Pseudacanthicus sp. L65, Pseudacanthicus spinosus, Sturisomatichthys festivus, and Rhinelepis aspera.
-
8.
Folds
Unculi are of 800–1000 μm length and of 90–100 μm width (Fig. 2H). On each side of the papilla, the unculi are arranged inversely. At the very tip of papilla, the unculi form a fold. The unculi seem rather flexible on their bases and stiff at their tips. This pattern is detected in Aphanotorulus horridus, Baryancistrus niveatus, Baryancistrus xanthellus, Hypostomus bolivianus (Fig. 2H), Hypostomus laplatae, Panaqolus sp. L271, Panaqolus sp. L351, Panaque nigrolineatus, Peckoltia sp. L76, and Scobinancistrus pariolispos.
Relationship between ecology, morphology, and systematic position
Most of the studied taxa either inhabit rivers with strong or medium current (see Additional file 1: Table S1). In strong current, most species could be found on stone, followed by wood, and finally sand. Most of the species inhabiting streams with medium current inhabited wood, followed by stone, sand, and both wood and stone. Only one species was found in slow flowing water; here the studied species adhered to wood.
In strong currents, most species possessed medium papillae, followed by long, flat, and short papillae (see Additional file 1: Table S1). Most taxa bore either mushrooms or no unculi, followed by long filaments, folds, honey-combed and hooks. In medium currents, most species possessed medium-sized papillae, followed by long, short and flat papillae. Here, most species bore mushrooms or folds, followed by no unculi, small mushrooms, long filaments, hooks and suction cups. The two species inhabiting slow streams possessed long papillae with mushroom-shaped unculi; these species can be found on wood.
When the substrate is on the focus, we find that most species could be found on stone, followed by wood, sand, and finally both wood and stone (see Additional file 1: Table S1). Most species adhering to stone were found in rivers with strong current, followed by medium current. Wood as preferred substrate seemed to be mostly inhabited in medium currents, followed by strong and slow current. Most species inhabiting sand were found in streams with medium, followed by strong current. Both wood and stone were inhabited in medium currents.
Most stone-inhabitants possessed medium papillae, followed by long, short and flat papillae (see Additional file 1: Table S1). Here, most taxa bore no unculi, followed by long filaments and mushrooms, folds, and finally honey-combed papillae and small mushrooms. When wood was preferred, most taxa possessed medium papillae, followed by long, short, and finally flat ones. Most species bore mushrooms, followed by folds, hooks, and finally long filaments, no unculi or suction cups. Sand-inhabitants mostly possessed flat papillae, followed by medium and short ones. Here, taxa bore mostly no unculi or small mushrooms. The two species adhering to both wood and stones possessed medium papillae with either long filaments or mushrooms.
With regard to the phylogenetic position (Fig. 3), we found that species inhabiting streams with strong current belonged to Hypostominae, Loricariinae, and Rhinelepinae. Rivers with medium current were colonized by Hypoptopomatinae, Hypostominae, and Loricariinae. The one species living in slow flowing waters belonged to the Rhinelepinae. In most cases, when more than one species of a genus was studied (Leporacanthicus, Panaqolus, Scobinancistrus, Parancistrus, Baryancistrus, Chaetostoma, Hypostomus, Loricaria, Lamontichthys, Sturisomatichthys, Farlowella), we found that the taxa of the same genus preferred the same current type. In some cases (Pseudacanthicus, Hypancistrus, Ancistrus, Peckoltia), species of the same genus inhabited different current types (Fig. 3).
Summary of ecological data and results from morphological analyses, visualized on a cladogram. From left to right: current type, preferred substrate, papilla type, unculi type, proposed type of interaction between substrate and organism – for each species studied. When the field is empty, no data was available
Preference of stone could be determined in some taxa of Hypostominae, Loricariinae, and Rhinelepinae (Fig. 3). Wood-preference was found in Hypoptopomatinae, Hypostominae, Loricariinae, and Rhinelepinae. Sand-dwelling was observed in Dekeyseria (Hypostominae) and some Loricariinae taxa. Both wood and stones were only inhabited by Sturisomatichthys, belonging to Loricariinae. In most cases, when more than one species of a genus was studied (Pseudacanthicus, Leporacanthicus, Panaqolus, Scobinancistrus, Parancistrus, Hypancistrus, Peckoltia, Baryancistrus, Chaetostoma, Hypostomus, Loricaria, Lamontichthys, Sturisomatichthys, Farlowella), we found that the taxa of the same genus preferred the same substrate. Only in Ancistrus, species adhere to different substrates (Fig. 3).
Flat papillae were detected in Hypostominae and Loricariinae (Fig. 3). The short type was determined for Hypoptopomatinae, Hypostominae, and Loricariinae. Medium-sized ones were identified for Hypoptopomatinae, Hypostominae, and Loricariinae. Large ones were found in Hypostominae and Rhinelepinae. In some cases, when more than one species of a genus was studied (Leporacanthicus, Panaqolus, Baryancistrus, Loricaria, Lamontichthys, Sturisomatichthys, Farlowella), we found that the taxa of the same genus possessed the same papilla type. In most cases (Pseudacanthicus, Scobinancistrus, Hypancistrus, Ancistrus, Chaetostoma, Hypostomus, Parancistrus, Peckoltia), species of the same genus showed different types (Fig. 3).
Unculi of the fold type were determined in Hypostominae (Fig. 3). The suction cups were only found in Acanthicus adonis (Hypostominae) and the honey-combed type in Ancistrus megalostomus (Hypostominae). Hooks were detected in Ancistrus sp. L464 (Hypostominae) and Lamontichthys stibaros (Loricariinae). Mushrooms were determined in Hypoptopomatinae, Hypostominae, Loricariinae, and Rhinelepinae. Small mushrooms were found in Hypancistrus contradens (Hypostominae) and some Loricariinae. Long filaments were detected in taxa of Hypostominae, Sturisomatichthys festivus (Loricariinae), and Rhinelepis aspera (Rhinelepinae). No unculi were found in some species of Hypoptopomatinae, Hypostominae, and Loricariinae. In most cases, when more than one species of a genus was studied (Pseudacanthicus, Leporacanthicus, Panaqolus, Parancistrus, Baryancistrus, Chaetostoma, Hypostomus, Loricaria, Farlowella), we found that the taxa of the same genus possessed the same unculi type. In some cases (Scobinancistrus, Hypancistrus, Ancistrus, Lamontichthys, Sturisomatichthys), species of the same genus showed different types (Fig. 3).
Proposed interaction between organisms and substrate
As comparative experimental data on species with different mouthpart morphologies is lacking, we can only hypothesize about their attachment capabilities. However, attachment structures were well investigated in various animal phyla and basic principles of adhesion and interlocking are known, we can infer some functionality based on the morphology analysis together with material property estimations and propose hypotheses about the interaction of papillae and unculi with some different kinds of substrate that can be found in nature (Fig. 4). These substrate types include stiff and smooth (stones or wood that were smoothened by the stream), stiff and rough (stones or wood), or soft substrate (plant covers on stone or wood).
Proposed interaction between some papillae and unculi types and some substrate occurring naturally in the habitat (stiff and smooth as e.g. rounded stone or wood; stiff and rough as e.g. wood or stone; soft substrate as e.g. plant covers on stone or wood). The grey color gradients in the structures relate to the mechanical properties (black = stiff; white = flexible), which are inferred from manipulation of structures and by documenting the artefacts caused by drying and shrinking. Purple boxes identify the structures, which probably facilitate interlocking (interlocking type) and the blue boxes identify the structures, which probably support adhesion to the target surface (adhesion type). Hexagons show, how presumably well the specific structures enable attachment with the target surface (orange = not well, yellow = medium, green = well). A Flat papillae with no unculi but a bulky surface. B Medium papillae with suction cups. C Medium papillae with hooks. D Medium papillae with mushrooms. E Medium papillae with small mushrooms. F Medium papillae with honey-combed unculi. G Medium papillae with long filaments. H Medium papillae with folds
The flat papillae (detected in Pseudacanthicus pitanga, Ancistrus ranunculus, Crossoloricaria cephalaspis, Pseudohemiodon almendarizi, Loricaria luciae, Loricaria simillima, Spatuloricaria puganensis, and Pterosturisoma microps) seem to be composed of rather rigid material, which is embedded in the softer and more flexible lip (Fig. 4A). In some species (Ancistrus ranunculus, Crossoloricaria cephalaspis, Pseudohemiodon almendarizi, Loricaria luciae, and Loricaria simillima), no unculi were detected and the papilla surface seems to be rather bulky. During attachment, the papillae are probably capable of interlocking with rather stiff and rough substrate (Ancistrus ranunculus), which is facilitated by the soft embedment. We, however, expect these species to underperform on stiff and smooth substrates and to hardly attach to soft substrates (plant covers), since contact areas are reduced (Fig. 4A). This could potentially explain, why this pattern is mostly found in species living on sand or mud (Crossoloricaria cephalaspis, Pseudohemiodon almendarizi, Loricaria luciae, and Loricaria simillima), where attachment will probably not play such an important role. Here, the species probably only temporarily attach to substrate (e.g., to rough substrate or wood) and might not need a tight and more continuous attachment. However, thick mucus, covering the bulky surface, might compensate these shortcomings, which should be investigated in the future. However, in some species (Pseudacanthicus pitanga, and Spatuloricaria puganensis), these papillae are covered by flexible long and thin filaments or by small mushrooms. Here, we expect the unculi to adapt to the substrate increasing either adhesion (by filaments) or interlocking (by mushrooms) (Fig. 4E,G).
The short papillae were detected in Hypoptopoma inexspectatum, Scobinancistrus aureatus, Ancistrus sp. L464, Chaetostoma formosae, and Rineloricaria melini. These papillae seem to have limited range of motion as well, but could potentially function as bolsters, when the unculi interact with the substrate, or support rearrangement during attachment, because the papillae tips seem to be flexible. They were either covered by unculi of the mushroom type (Scobinancistrus aureatus, Chaetostoma formosae), hooks (Ancistrus sp. L464), or short mushrooms (Rineloricaria melini). Here, unculi together with the flexible papillae could enable a tight interaction with the stiff and rough or with the soft substrate by interlocking and with the stiff and smooth one by adhesion (Fig. 4C–E). However, mucus could also be potentially distributed between the unculi and additionally support adhesion under water.
The medium-sized papillae were detected in most studied species. Due to the length of the papillae we expect this type to have a higher range of motion, which presumably enables them to adapt to rather challenging surfaces. The bases of the papillae seem to be stiffer and the tips more flexible, and therefore we expect high attachment forces, as the flexible papilla tips (which make unculi bases flexible) can easily adapt to corrugated substrates and to interact with them. They were usually covered with unculi, either with mushrooms (Otocinclus cocama, Ancistrus sp. L519, Ancistrus sp. L107, Ancistrus cirrhosus, Ancistrus luzia, Chaetostoma dorsale, Chaetostoma lineopunctatum, Lamontichthys filamentosus, Sturisomatichthys aureus, Farlowella platorynchus, Farlowella oxyrryncha, and Cteniloricaria platystoma), hooks (Lamontichthys stibaros), folds (Panaqolus sp. L271, Panaqolus sp. L351, Panaque nigrolineatus, Scobinancistrus pariolispos, Baryancistrus xanthellus, and Baryancistrus niveatus), long filaments (Pseudacanthicus spinosus, Pseudacanthicus sp. L97, Pseudacanthicus sp. L65, Pseudacanthicus sp. L185, Pseudacanthicus sp. L273, and Sturisomatichthys festivus) or short mushrooms (Hypancistrus contradens and Hemiodontichthys acipenserinus). The species bearing mushrooms, hooks, or short mushrooms can probably interact with the stiff and rough substrates and the soft substrate (plants, biofilm, etc.) by interlocking and with the stiff and smooth substrate by adhesion (Fig. 4C–E). However, on stiff and smooth substrates, contact points might be reduced, since less unculi are in contact. For the species bearing long filaments, we expect a high degree of interlocking on stiff and rough or soft substrates and of adhesion on stiff and smooth substrate, as these soft structures seem flexible enough to establish contact on most surfaces (Fig. 4G). The unculi of the fold type are potentially rather used for establishing contact by interlocking, since their tips seem to be rather stiff (Fig. 4H). We expect this type to underperform on soft substrates (i.e., plant covers); however, mucus between these structures might increase their attachment ability. In one species (Acanthicus adonis), we determined medium papillae with a suction cups surface pattern. Due to morphology and material property estimation, we expect this type to adhere tightly with stiffer surfaces (both smooth and rough), but underperform on soft substrates (Fig. 4B). Only a few species (Rhinotocinclus isabelae, Leporacanthicus joselimai, Leporacanthicus cf. galaxias, ‘Spectracanthicus’ immaculatus, Parancistrus nudiventris, Hypancistrus sp. L174, Hypancistrus zebra, and Dekeyseria picta) did not have unculi but rather a bulky surface (Fig. 4A). For these species, we expect an interlocking mechanism to be present. In this case, the relatively flexible papilla bases probably adapt to the target surface and the stiffer bulky surface enables interlocking with the rough and stiff substrate.
Long papillae were detected in some species (Scobinancistrus raonii, Peckoltia sp. L76, Parancistrus aurantiacus, Hypancistrus sp. L333, Ancistrus claro, Ancistrus megalostomus, Aphanotorulus horridus, Hypostomus bolivianus, Hypostomus laplatae, Pseudorinelepis genibarbis, and Rhinelepis aspera). Because of their length, we expect these papillae to adapt best to very rough surfaces, due to an increased range of motion. On top of these papillae, we detected either mushrooms (Scobinancistrus raonii, Ancistrus claro, and Pseudorinelepis genibarbis), folds (Peckoltia sp. L76, Aphanotorulus horridus, Hypostomus bolivianus, and Hypostomus laplatae), bulky surface without unculi (Parancistrus aurantiacus), long filaments (Hypancistrus sp. L333 and Rhinelepis aspera) or honey-combs (Ancistrus megalostomus). Since the mushrooms, long filaments and honey-combs seem to be flexible, we expect these structures to adhere to any surface either by interlocking or by adhesion (Fig. 4D,F,G). For the folds, which seem to be stiffer at their tips, we expect an underperformance on soft plant surfaces (Fig. 4H) due to their limited ability to adapt their shape to the target substrate, which would hinder the oral disc to form an effective seal. The bulky surfaces (Fig. 4A) potentially also underperform on soft plant surfaces, due to the limited flexibility, and on stiff and smooth surfaces, because interlocking is here not facilitated.
Ancestral state reconstruction
Ancestral state reconstruction (Additional file 1: Figure S3) suggested that Loricariidae lived initially lived in medium or strong currents. Only Pseudorinelepis genibarbis (Rhinelepinae) colonized slow-flowing rivers. Based on the tree, 19 shifts between streams with different velocities were reconstructed.
With regard to the substrate, stone seemed to be ancestral (Additional file 1: Figure S4). There were at least 11 shifts between substrate types, mostly from stony substrates to wood. Only few species live in streams with sandy substrates, but even this habitat type has been colonized twice, in a larger clade of the Loricariinae and in Dekeyseria picta (Hypostominae).
With regard to the papillae (see Additional file 1: Figure S5), either the medium or long papilla was ancestral. In the group containing Loricariinae, Hypostominae, and Hypoptopomatinae the medium-sized papillae evolved and were kept in most lineages. Long papillae are found in all examined Rhinelepinae, but convergently evolved from medium papillae at least seven times convergently within Hypostominae. The flat papilla evolved at least four times convergently from the medium-sized type (within Loricariinae: in Pterosturisoma microps, Spatuloricaria puganensis, and the group containing Crossoloricaria and Loricaria highlighted in Additional file 1: Figure S5 by a blue box; within Hypostominae: in Ancistrus ranunculus and Pseudacanthicus pitanga). Medium papilla evolved at least five times into short papillae.
With regard to the unculi, the ancestral state reconstruction suggested that mushrooms were ancestral (Additional file 1: Figure S6). Mushrooms were replaced by long filaments at least two times convergently (in Rhinelepinae: in Rhinelepis aspera; in Loricariinae: in Sturisomatichthys festivus). Evolution from mushrooms to hooks convergently happened in Lamontichthys stibaros and in Ancistrus sp. L464. Mushrooms convergently evolved into small mushrooms in Hypancistrus contradens and ‘Spectracanthicus’ immaculatus in Hypostominae and in the group of Loricariinae highlighted in Additional file 1: Figure S6 by a blue box. In the latter, small mushrooms were lost in the clade containing Crossoloricaria, Pseudohemiodon, and Loricaria. In Hypostominae, mushrooms were lost several times, but apparently were regained in Scobinancistrus aureatus and Scobinancistrus raonii. Mushrooms evolved into honey-combs in Ancistrus megalostomus. In the group highlighted in Additional file 1: Figure S6 by a red box, folds evolved, which were lost convergently again. Long filaments convergently evolved at least four times, whereas suction cusps evolved only in Acanthicus adonis. In Hypoptopomatinae, the mushrooms were lost in Rhinotocinclus isabelae.
With regard to the interaction type, ancestral state reconstruction suggested that both adhesion and interlocking occurred in the ancestral state (Additional file 1: Figure S7). Within Rhinelepinae, Loricariinae, and Hypostominae, adhesion-dominated interaction convergently evolved at least four times (in Rhinelepis aspersa, Ancistrus megalostomus, Sturisomatichthys festivus, the group containing Acanthicus, Leporacanthicus, and Pseudacanthicus). Within the latter group, this shifted to interlocking in Leporacanthicus. Using both adhesion and interlocking was convergently replaced by interlocking-dominated attachment at least five times (in Loricariinae: in the group containing Crossoloricaria, Pseudohemiodon, and Loricaria; in Hypoptopomatinae: in Rhinotocinclus isabelae; in Hypostominae: in Dekeyseria, in Ancistrus ranunculus, in the group containing Panaque and Ancistomus highlighted in Additional file 1: Figure S7 by a red box). In the latter group, interlocking changed to adhesion-dominated attachment in Hypancistrus sp. L333 and to both types of interaction convergently in Hypancistrus contradens, Scobinancistrus aureatus, and Scobinancistrus raonii.
Discussion
We here aim at presenting the structural diversity of papillae and unculi types in Loricariidae. As here only 67 species were studied, we expect that more diversity can be potentially discovered, when more species are included in such a study.
The loricariid oral disc is composed of upper and lower jaw and is surrounded by a softer outer rim, which was found to make tight contact with the substrates during attachment [6]. This seems similar to the soft tissues surrounding the suckers of remoras, which conform to the local roughness and curvature of the substrate [91], or to the outer papillae, setae or microvilli (which are of smaller diameter and densely packed) of the clingfish and loaches [16, 17, 41, 42, 70, 71].
The fleshy lips of Loricariidae are highly variable with regard to morphology, size and the content of collagen, which was previously found to relate to the substrate and the flow [92]. The collagen probably reinforces the oral suction cups and reduces slipping, failure or buckling in streams with high flow velocities [92], while being manipulated and bolstered by the jaws and maxillary barbels [73]. The lips are covered ventrally by uniculiferous papillae [73, 74, 76, 80], which probably increase wet friction and hydrodynamic adhesion to reinforce the seal of the oral disc [48, 80]. The geometry and arrangement of papillae in other fish taxa were previously found to support the resistance to shear forces and to arrests cracks at the interface between suction cups and substrate, which would compromise the subambient pressure in the mouth chamber [16, 17, 41, 70, 71]. This mechanism is partially similar to segmented adhesive pads of insects [27, 32, 93].
The unculi, which can be found on top of the papillae, are potentially involved in feeding [73, 76, 81]. But additionally, they probably increase the friction/interlocking during attachment on rough substrates [6, 48, 67, 74, 81]. This, together with the mucus, increases attachment strength as in other fish taxa [9, 72, 94].
With regard to interspecific variation of papilla size and morphology in Loricariidae, there is a huge lack of knowledge. In the here examined species, we were able to recognize four papilla types (Fig. 1), which differ in their height and range of motion, and eight unculi types (Fig. 2).
The reconstructions of ancestral habitats and character states (Additional file 1: Figures S3-S7) suggested that the different habitat types were colonized repeatedly and the states of all studied traits convergently evolved multiple times in Loricariidae. High levels of convergent evolution were previously also proposed for mandibles and body shapes in suckermouth armoured catfishes [2, 3] and for foot adhesive pads in animals [32].
With regard to the ecological data collected in the field, the current (slow, medium, strong) and the preferred substrate (stone, wood, sand) type, we could detect that medium and long papillae were often found in species inhabiting strong and medium currents. The length of the structure probably relates positively to the attachment ability on most types of substrate, except surely for sand. This is likely important in strong currents, since with higher current velocities higher forces act on the fish and thus a higher attachment is needed. With regard to the unculi we found that folds, mushrooms, as well as no unculi related to medium and strong currents. Here, we propose that the presence of unculi and the surface pattern of the papillae increase the attachment performance by interlocking, which is especially necessary in strong currents. This however awaits further investigation in e.g. the course of controlled experiments on model species with different unculi and papilla types. The comparison of the ancestral state reconstructions (Additional file 1: Figures S3-S7) revealed no obvious correlations between habitat shifts and the evolution of specific character states.
Since comparative experiments on species with different mouthpart morphologies have not been performed, we could only hypothesize about their attachment capabilities to some natural surfaces that are omnipresent in the fish habitat (stiff and smooth as e.g. rounded stones and wood; stiff and rough as e.g. rough stones or wood; soft as e.g. plants covering stones or wood) in this manuscript. However, our hypotheses were based on the knowledge about attachment structures in other animal phyla and the basic principles of adhesion and interlocking. With this we could infer some functionality based on the morphology analysis together with material property estimations and propose hypotheses about the interaction of papillae and unculi with different kinds of substrate (Fig. 4). Since attachment in gobies was previously found to rely on the ability to form an effective seal and to underperform on rougher surfaces [13, 49], we expect therefore that the suction cusp unculi type underperforms on stiff and rough surfaces as well.
We, however, do not know the position of the unculi and the papillae during attachment, which hopefully can be tested experimentally in the future. In addition to the micro- and nanostructures, the mucus covering the mouth apparatus is on expect to contribute to the contact formation and adhesion as well and therefore should be investigated deeply in the future.
Conclusion
The oral discs of suckermouth armoured catfish (Loricariidae), which enable attachment and interlocking to various natural surfaces, are highly diverse with regard to the morphology of the papillae and of the unculi, small horny projections. Here, we studied 67 taxa and determined four papilla types and eight unculi types. From handling the structures and from drying artefacts we could infer some information about their material properties. This, together with their shape, enabled us to carefully propose hypotheses about mechanisms of interactions of oral disc structures with natural substrates typical for respective fish species. Reconstructions of ancestral habitats and states indicated frequent habitat shifts and a highly convergent evolution of most character states in Loricariidae. There is no obvious correlation between habitat shifts and the evolution of specific character states.
Methods
Specimens and preparation
In this project, 67 species and undescribed taxa (here named according to their L numbers) were studied (see Additional file 1: Table S1). For each species, between one and five specimens were examined, depending on the availability of material: some species are quite rare and only one specimen could be gathered, whereas for other taxa more individuals could be used (see Additional file 1: Table S1). From the species with a higher quantity of individuals, we were able to study the intraspecific variability with regard to the morphology of the adhesive suckermouth papilla structures. Since this was not high, we decided to include the species with only one specimen in this study as well. As papillae and unculi are regularly shed, we investigated every papilla on the studied lip part to obtain information about unculi types.
We did not experiment with living fish or kill fish for this study; instead we used the network of German suckermouth catfish owners (they were kept either directly by DKV or other hobbyists) which provided us with specimens that died naturally in the aquariums (fish died between 1995 and 2022). All animals were wild caught, imported to Germany by the ornamental fish trade, and then kept by hobbyists. Fish were, directly after death, fixed and stored in 70% EtOH.
In general, suckermouths are very variable between species. To illustrate this diversity, we have included some images of alive fish in the Additional file 1: Figures S8-S23. For this study, we studied the lower left lip of the specimens, which were carefully dissected using scalpel and forceps. Samples were stored in 70% EtOH and cleaned from mucus by a short ultrasonic bath for 2 min. The samples were dried in a critical point dryer (Betta-Tech-Controls, Blakelands, UK). For this method, samples were transferred to 100% EtOH first, then to the liquid CO2 and then slowly critical point dried from the CO2.
Scanning electron microscopy
Lips were attached to scanning-electron-microscopy (SEM) sample holders by double-sided adhesive carbon tapes. Samples were then sputter-coated with gold–palladium (layer of 10 nm) employing a Leica EM SCD400 (Leica Microsystems, Wetzlar, DE). All samples were documented with a Scanning Electron TM3000 Tabletop Microscope (Hitachi, Tokyo, JP). All images were taken with different magnifications (30x – 2500x) at 5 kV.
Some lips from species with multiple animals at hand were investigated more detailed employing a cryo-SEM S4800 (Hitachi, Tokyo, JP) equipped with the Gatan ALTO-2500 cryo-preparation system (Gatan, Abingdon, UK). For this purpose, the lips were carefully attached to SEM sample holders and then frozen in liquid nitrogen at – 210 °C, to avoid the formation of ice crystals, in the cryo-preparation chamber. Then, the temperature was raised to − 95 °C, initiating the process of sublimation (freezing-drying). After sublimation for 5–7 min., the temperature was reduced to − 140 °C and the sample was sputter-coated by gold–palladium (layer of 3–4 nm) directly in the cryo-preparation chamber. Afterwards the samples were transferred into the SEM and observed at – 120 °C at 3 kV accelerating voltage.
We concentrated on two different hierarchical levels of the oral disc morphology: the labial papillae and the unculi on the papillae. In few species, the unculi type could not be determined due to high content of mucus. In these cases, we were only able to collect data on papilla morphology.
Material property estimation
We did not perform any material property tests for this manuscript. However, from the manipulation of papillae before critical point drying by forceps and from the observation of artefacts resulting from drying and shrinking of the unculi and the papillae, we can propose some hypotheses about the relative stiffness and flexibility of different sites of samples. We hope that the characterization of material properties of different parts of the sucker can be addressed by using micro- and nanoindentation in the future.
Data on ecology
Since data on the precise microhabitat of each species are lacking and await future investigations, we here rather chose rather broad categories (see Additional file 1: Table S1). The current or stream (macro habitat) was categorized in slow, medium and strong. We use this term according to its meaning as a flowing body of water or a ‘continuous flow of a liquid’. It can either be a small creek with slow current, a broad river with fast current, or a stream in between. The preferred substrate (micro habitat) was wood, stone, wood and stone, or sand. This data was obtained from the personal observation of the collectors, mainly DKV, in the field (see Additional file 1: Figure S2 and Table S1).
Systematization
We studied 67 species belonging to four subfamilies of Loricariidae: Hypoptopomatinae, Loricariinae, Rhinelepinae and Hypostominae (see Additional file 1: Table S1). To gain insight, whether the specific morphology of the suckermouth structures relates to phylogeny, we plotted the data obtained on a cladogram based on recent phylogenies [95,96,97,98]; personal comment from Jon Armbruster, Auburn University]. Within each genus, we sorted the species in alphabetical order, since there is, to the best of our knowledge, no phylogeny which includes all of the species studied.
Character state evolution
Ancestral states of habitats and characters at internal nodes were reconstructed under maximum parsimony assumptions using the Trace Character History command in Mesquite [99]. Current velocity (slow, medium, strong) and interaction states (adhesion, both, interlocking) were treated as ordered; substrate states (wood, stone, sand) were treated as unordered. Transitions between flat, short and medium papillae as well as between medium and long papillae were counted as one step; transitions between flat or short and long papillae were counted as two steps. Unculi were classified into two categories, one including folds, suction cups and honey combed unculi, the other including hooks, mushrooms, small mushrooms and long filaments. Transitions within a category were counted as one step; transitions between the two categories were counted as two steps. Transitions between any state of unculi and no unculi were counted as one step.
Availability of data and materials
The datasets are included in the supplementary.
References
Eschmeyer WN, Fricke R, Van der Laan. Catalog of fishes: genera, species, references. California Academy of Sciences. 2023. http://www.researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
Lujan NK, Armbruster JW. Morphological and functional diversity of the mandible in suckermouth armored catfishes (Siluriformes: Loricariidae). J Morphol. 2012;273:24–39.
Black CR, Armbruster JW. Evolutionary integration and modularity in the diversity of the suckermouth armoured catfishes. R Soc Open Sci. 2022;9:220713.
Schaefer SA, Lauder GV. Historical transformation of functional design: evolutionary morphology of feeding mechanisms in loricarioid catfishes. Syst Biol. 1986;35(4):489–508.
Adriaens D, Geerinckx T, Vlassenbroeck J, Van Hoorebeke L, Herrel A. Extensive jaw mobility in suckermouth armored catfishes (Loricariidae): a morphological and kinematic analysis of substrate scraping mode of feeding. Physiol Biochem Zool. 2009;82(1):51–62.
Geerinckx T, Herrel A, Adriaens D. Suckermouth armored catfish resolve the paradox of simultaneous respiration and suction attachment: a kinematic study of Pterygoplichthys disjunctivus. J Exp Zool A Ecol Genet Physiol Mar. 2011;315(3):121–31.
Geerinckx T, De Kegel B. Functional and evolutionary anatomy of the African suckermouth catfishes (Siluriformes: Mochokidae): convergent evolution in Afrotropical and Neotropical faunas. J Anat. 2014;225:197–208.
Hoese G, Addison A, Toulkeridis T, Toomey R III. Observation of the catfish Chaetostoma microps climbing in a cave in Tena. Ecuador Subterr Biol. 2015;15:29–35.
Arita GS. A comparative study of the structure and function of the adhesive apparatus of the Cyclopteridae and Gobiesocidae. 1962. M.Sc. thesis, University of British Columbia, Vancouver, BC, Canada.
Davenport J, Thorsteinsson V. Sucker action in the lumpsucker Cyclopterus lumpus L. Sarsia. 1990;75(1):33–42.
Schoenfuss HL, Blob RW. Kinematics of waterfall climbing in Hawaiian freshwater fishes (Gobiidae): vertical propulsion at the aquaticterrestrial interface. J Zool. 2003;261:191–205.
Blob RW, Lagarde R, Diamond KM, Keeffe RM, Bertram RS, Ponton D, Schoenfuss HL. Functional diversity of evolutionary novelties: insights from waterfall-climbing kinematics and performance of juvenile gobiid fishes. Integr Org Biol. 2019;1:obz029.
Blob RW, Rai R, Julius ML, Schoenfuss HL. Functional diversity in extreme environments: effects of locomotor style and substrate texture on the waterfall-climbing performance of Hawaiian gobiid fishes. J Zool. 2006;268:315–24.
Fulcher BA, Motta PJ. Suction disk performance of echeneid fishes. Can J Zool. 2006;84:42–50.
Maie T, Schoenfuss HL, Blob RW. Performance and scaling of a novel locomotor structure: adhesive capacity of climbing gobiid fishes. J Exp Biol. 2012;215:3925–36.
Wainwright DK, Kleinteich T, Kleinteich A, Gorb SN, Summers AP. Stick tight: suction adhesion on irregular surfaces in the northern clingfish. Biol Lett. 2013;9:1–5.
Ditsche P, Wainwright DK, Summers AP. Attachment to challenging substrates – fouling, roughness and limits of adhesion in the northern clingfish (Gobiesox maeandricus). J Exp Biol. 2014;217:2548–54.
De Meyer J, Geerinckx T. Using the whole body as a sucker: combining respiration and feeding with an attached lifestyle in hill stream loaches (Balitoridae, Cypriniformes). J Morphol. 2014;275:1066–79.
Beckert M, Flammang BE, Nadler JH. Remora fish suction pad attachment is enhanced by spinule friction. J Exp Biol. 2015;218:3551–8.
Chen Y, Meng J, Gu Z, Wan X, Jiang L, Wang S. Bioinspired multiscale wet adhesive surfaces: structures and controlled adhesion. Adv Funct Mater. 2020;30:1905287.
Arzt E, Quan H, McMeeking RM, Hensel R. Functional surface microstructures inspired by nature – From adhesion and wetting principles to sustainable new devices. Prog Mater Sci. 2021;120:100823.
Huie JM, Summers AP. The effects of soft and rough substrates on suction-based adhesion. J Exp Biol. 2022;225:jeb243773.
Zhang D, Xu J, Liu X, Zhang Q, Cong Q, Chen T, Liu C. Advanced bionic attachment equipment inspired by the attachment performance of aquatic organisms: a review. Biomimetics. 2023;8:85.
Gorb SN. Attachment pads. In: Attachment devices of insect cuticle. Springer, Dordrecht; 2002. p.135–75.
Gorb SN. Uncovering insect stickiness: structure and properties of hairy attachment devices. Am Entomol. 2005;51(1):31–5.
Gorb SN. Biological attachment devices: exploring nature’s diversity for biomimetics. Phil Trans R Soc A. 2008;366:1557–74.
Gorb SN, Sinha M, Peressadko A, Daltorio KA, Quinn RD. Insects did it first: a micropatterned adhesive tape for robotic applications. Bioinspir Biomim. 2007;2:117–25.
Chen Y, Shih M-C, Wu M-H, Yang E-C, Chi K-J. Underwater attachment using hairs: the functioning of spatula and sucker setae from male diving beetles. J R Soc Interface. 2014;11:20140273.
Zhou Y, Robinson A, Steiner U, Federle W. Insect adhesion on rough surfaces: analysis of adhesive contact of smooth and hairy pads on transparent microstructured substrates. J R Soc Interface. 2014;11:20140499.
Song Y, Dai Z, Wang Z, Ji A, Gorb SN. The synergy between the insect-inspired claws and adhesive pads increases the attachment ability on various rough surfaces. Sci Rep. 2016;6:26219.
Heepe L, Höft S, Michels J, Gorb SN. Material gradients in fibrillar insect attachment systems: the role of joint-like elements. Soft Matter. 2018;14:7026–33.
Büscher TH, Gorb SN. Physical constraints lead to parallel evolution of micro- and nanostructures of animal adhesive pads: a review. Beilstein J Nanotechnol. 2021;12:725–43.
Kier WM, Smith AM. The structure and adhesive mechanism of octopus suckers. Integr Comp Biol. 2006;42:1146–53.
Li J, Ma C, Liu J, Dong X, Liu J. The co-effect of microstructures and mucus on the adhesion of abalone from a mechanical perspective. Biosurf Biotribol. 2021;7(4):180–6.
Autumn K, Liang YA, Hsieh ST, Zesch W, Chan WP, Kenny T, Fearing R, Full RJ. Adhesive force of a single gecko foot-hair. Nature. 2000;405:671–702.
Russell AP. The structure of anoline (Reptilia: Dactyloidae: Anolis) toe pads in relation to substratum conformity. Acta Zool. 2017;98:300–9.
Endlein T, Barnes WJP, Samuel DS, Crawford NA, Biaw AB, Grafe U. Sticking under wet conditions: The remarkable attachment abilities of the torrent frog, Staurois guttatus. PLoS ONE. 2013;8:e73810.
Kappl M, Kaveh F, Barnes WJP. Nanoscale friction and adhesion of tree frog toe pads. Bioinspir Biomim. 2016;11:035003.
Thewissen AJGM, Etnier SA. Adhesive devices on the thumb of vespertilionoid bats (Chiroptera). J Mammal. 1995;76:925–36.
Riskin DK, Fenton MB. Sticking ability in Spix’s disk-winged bat, Thyroptera tricolor (Microchiroptera: Thyropteridae). Can J Zool. 2001;79:2261–7.
Chuang Y, Chang H, Liu G, Chen P. Climbing upstream: multiscale structural characterization and underwater adhesion of the Pulin river loach (Sinogastromyzon puliensis). J Mech Behav Biomed Mater. 2017;73:76–85.
Ditsche P, Summers A. Learning from Northern clingfish (Gobiesox maeandricus): bioinspired suction cups attach to rough surfaces. Phil Trans R Soc B. 2019;374:20190204.
Federle W, Barnes WJP, Baumgartner W, Drechsler P, Smith JM. Wet but not slippery: boundary friction in tree frog adhesive toe pads. J R Soc Interface. 2006;3:689–97.
Wicaksono A, Hidayat S, Damayanti Y, Jin DSM, Sintya E, Retnoaji B, Alam P. The significance of pelvic fin flexibility for tree climbing fish. Zoology. 2016;119:511–7.
Heepe L, Gorb SN. Biologically inspired mushroom-shaped adhesive microstructures. Annu Rev Mater Res. 2014;44(1):173–203.
Ditsche P, Summers AP. Aquatic versus terrestrial attachment: water makes a difference. Beilstein J Nanotechnol. 2014;5:2424–39.
Delroisse J, Kang V, Gouveneaux A, Santos R, Flammang P. Convergent evolution of attachment mechanisms in aquatic animals. In: Bels VL, Russell AP, editors. Convergent evolution. Fascinating life sciences. Cham: Springer; 2023. p. 523–57.
Gerstner CL. Effect of oral suction and other friction-enhancing behaviors on the station-holding performance of suckermouth catfish (Hypostomus spp.). Can J Zool. 2007;85(1):133–40.
Palecek AM, Schoenfuss HL, Blob RW. Sticking to it: testing passive pull-off forces in waterfall-climbing fishes across challenging substrates. J Exp Biol. 2021;224(2):jeb228718.
Evermann BW, Kendall WC. An interesting species of fish from the high Andes of Central Ecuador. Proc Biol Soc Wash. 1905;18:91–106.
Johnson RDO. Notes on the habits of a climbing catfish (Arges marmoratus) from the Republic of Colombia. N Y Acad Sci. 1912;22:327–33.
Carvajal-Quintero JD, Maldonado-Ocampo JA, Urbano-Bonilla A. Climbing behavior of Cordylancistrus sp. in the Colombian Andes. Universitas Scientiarum. 2015;20:209–15.
Maie T, Schoenfuss HL, Blob RW. Musculoskeletal determinants of pelvic sucker function in hawaiian stream gobiid fishes: interspecific comparisons and allometric scaling. J Morphol. 2013;274:733–42.
Schoenfuss HL, Maie T, Moody KN, Lesteberg KE, Blob RW, Schoenfuss TC. Stairway to heaven: evaluating levels of biological organization correlated with the successful ascent of natural waterfalls in the hawaiian stream goby Sicyopterus stimpsoni. PLoS ONE. 2013;8:1–14.
Taft NK, Taft BN, Henck H, Diamond KM, Schoenfuss HL, Blob RW. Comparative morphology and mechanical properties of the lepidotrichia of climbing and non-climbing Hawaiian gobioid fishes. Cybium. 2017;41:107–15.
Sandoval JA, Jadhav S, Quan H, Deheyn DD, Tolley MT. Reversible adhesion to rough surfaces both in and out of water, inspired by the clingfish suction disc. Bioinspir Biomim. 2019;14:066016.
Griner J, Palecek A, Diamond K, Schoenfuss H, Blob R. Geometric morphometrics of climbing kinematics in waterfall climbing goby fishes. In: Proceedings of the Society for Integrative and Comparative Biology. Washington, DC; 2021.
Palecek AM, Schoenfuss HL, Blob RW. Sucker shapes, skeletons and bioinspiration: how hard and soft tissue morphology generates adhesive performance in waterfall climbing goby fishes. Integr Comp Biol. 2022;62:934–44.
Blake RW. Biomechanics of rheotaxis in six teleost genera. Can J Zool. 2006;84(8):1173–86.
Huie JM, Wainwright DK, Summers AP, Cohen KE. Sticky, stickier and stickiest—a comparison of adhesive performance in clingfish, lumpsuckers and snailfish. J Exp Biol. 2022;225(22):jeb244821.
MacDonnell AJ, Blake RW. Rheotaxis in Otocinclus sp. (Teleostei: Loricariidae). Can J Zool. 1990;68:599–601.
Witten PE, Hall BK. Teleost skeletal plasticity: modulation, adaptation, and remodeling. Copeia. 2015;103(4):727–39.
Carlson RL, Lauder GV. Living on the bottom: kinematics of benthic station-holding in darter fishes (Percidae: Etheostomatinae). J Morphol. 2010;271:25–35.
Carlson RL, Lauder GV. Escaping the flow: boundary layer use by the darter Etheostoma tetrazonum (Percidae) during benthic station holding. J Exp Biol. 2011;214(7):1181–93.
Wang J, Ji C, Wang W, Zou J, Yang H, Pan M. An adhesive locomotion model for the rock-climbing fish, Beaufortia kweichowensis. Sci Rep. 2019;9(1):16571.
Blob RW, Diamond KM, Lagarde R, Maie T, Moody KN, Palecek AM, Ward JL, Schoenfuss HL. Integrating biomechanics in evolutionary studies, with examples from the amphidromous goby model system. J Exp Biol. 2023;226(Suppl 1):jeb244942.
Roberts TR. Unculi (horny projections arising from single cells), an adaptive feature of the epidermis of ostariophysan fishes. Zool Scr. 1982;11:55–76.
Hora LS. Structural modifications in the fish of mountain torrents. Rec Zool Surv India. 1922;24(1):31–61.
Saxena SC. Adhesive apparatus of a hill stream cyprinid fish Garra mullya (Sykes). Proc Natl Inst Sci India. 1959;25:205–14.
Zou J, Wang J, Ji C. The adhesive system and anisotropic shear force of Guizhou Gastromyzontidae. Sci Rep. 2016;6:37221.
Sandoval JA, Sommers J, Peddireddy KR, Robertson-Anderson RM, Tolley MT, Deheyn DD. Toward bioinspired wet adhesives: lessons from assessing surface structures of the suction disc of intertidal clingfish. ACS Appl Mater Interfaces. 2020;12(40):45460–75.
Tsujioka K, Matsuo Y, Shimomura M, Hirai Y. A new concept for an adhesive material inspired by clingfish sucker nanofilaments. Langmuir. 2022;38(3):1215–22.
Geerinckx T, Brunain M, Herrel A, Aerts P, Adriaens D. A head with a suckermouth: a functional-morphological study of the head of the suckermouth armoured catfish Ancistrus cf. triradiatus (Loricariidae, Siluriformes). Belg J Zool. 2007;137:47–66.
Geerinckx T, De Poorter J, Adriaens D. Morphology and development of teeth and epidermal brushes in loricariid catfishes. J Morphol. 2007;268:805–14.
De Crop W, Pauwels E, Van Hoorebeke L, Geerinckx T. Functional morphology of the Andean climbing catfishes (Astroblepidae, Siluriformes): alternative ways of respiration, adhesion, and locomotion using the mouth. J Morphol. 2013;274(10):1164–79.
Ono RD. Fine structure and distribution of epidermal projections associated with taste buds on the oral papillae in some loricariid catfishes (Siluroidei: Loricariidae). J Morphol. 1980;165:139–59.
Teimori A, Esmaeili H, Ansari M. Micro-structure consideration of the adhesive organ in doctor fish, Garra rufa (Teleostei; Cyprinidae) from the Persian Gulf Basin. Turkish J Fish. 2011;11:407–11.
Conway KW, Lujan NK, Lundberg JG, Mayden RL, Siegel DS. Microanatomy of the paired-fin pads of ostariophysan fishes (Teleostei: Ostariophysi). J Morphol. 2012;273:1127–49.
Hussain JF, Bordoloi S. Adaptive modifications in four fish species of the genus Garra (Teleostei; Cyprinidae) in Basistha River, Assam. India Microsc Microanal. 2018;24(3):310–7.
Rauther M. Beiträge zur Kenntnis der Panzerwelse. Zool Jb Anat. 1911;31:497–528.
Abd-Elhafeez HH, Mokhtar DM. Comparative morphological study of lips and associated structures of two algal grazer fish. J Adv Microsc Res. 2014;9:275.
Beckert M, Flammang B, Nadler J. A model of interfacial permeability for soft seals in marine-organism, suction-based adhesion. MRS Adv. 2016;1(36):2531–43.
Wang S, Li L, Sun W, Wainwright D, Wang H, Zhao W, Chen B, Chen Y, Wen L. Detachment of the remora suckerfish disc: kinematics and a bio-inspired robotic model. Bioinspir Biomim. 2020;15:056018.
Wang Y, Li Z, Elhebeary M, Hensel R, Arzt E, Saif MTA. Water as a “glue”: elasticity-enhanced wet attachment of biomimetic microcup structures. Sci Adv. 2022;8:eabm9341.
Wang Y, Yang X, Chen Y, Wainwright DK, Kenaley CP, Gong Z, Liu Z, Liu H, Guan J, Wang T, Weaver JC, Wood RJ, Wen L. A biorobotic adhesive disc for underwater hitchhiking inspired by the remora suckerfish. Sci Robot. 2017;2:eann8072.
Rao P, Sun TL, Chen L, Takahashi R, Shinohara G, Guo H, King DR, Kurokawa T, Gong JP. Tough hydrogels with fast, strong, and reversible underwater adhesion based on a multiscale design. Adv Mater. 2018;30:1801884.
Sandoval JA, Xu T, Adibnazari I, Deheyn DD, Tolley MT. Combining suction and friction to stabilize a soft gripper to shear and normal forces, for manipulation of soft objects in wet environments. IEEE Robot. 2022;7(2):4134–41.
Zhou W, Wu X. Enhanced adhesion of synthetic discs with micro-patterned margins. Biomimetics. 2022;7:202.
Willis J, de Perera TB, Newport C, Poncelet G, Sturrock CJ, Thomas A. The structure and function of the sucker systems of hill stream loaches. bioRxiv. 2019. https://doi.org/10.1101/851592v1.
Lujan NK, Winemiller KO, Armbruster JW. Trophic diversity in the evolution and community assembly of loricariid catfishes. BMC Evol Biol. 2012;12:124.
Cohen KE, Crawford CH, Hernandez LP, Beckert M, Nadler JH, Flammang BE. Sucker with a fat lip: the soft tissues underlying the viscoelastic grip of remora adhesion. J Anat. 2020;237:643–54.
Bressman NR, Armbruster JW, Lujan NK, Udoh I, Ashley-Ross MA. Evolutionary optimization of an anatomical suction cup: lip collagen content and its correlation with flow and substrate in neotropical suckermouth catfishes (Loricarioidei). J Morphol. 2020;281:676–87.
Chung JY, Chaudhury MK. Roles of discontinuities in bio-inspired adhesive pads. J R Soc Interface. 2005;2(2):55–61.
Green DM, Barber DL. The ventral adhesive disc of the clingfish Gobiesox maeandricus: integumental structure and adhesive mechanisms. Can J Zool. 1988;66:1610–9.
Covain R, Fisch-Muller S, Oliveira C, Mol JH, Montoya-Burgos JI, Dray S. Molecular phylogeny of the highly diversified catfish subfamily Loricariinae (Siluriformes, Loricariidae) reveals incongruences with morphological classification. Mol Phylogenet Evol. 2016;94(Pt B):492–517.
Lujan NK, Cramer CA, Covain R, Fisch-Muller S, López-Fernández H. Multilocus molecular phylogeny of the ornamental wood-eating catfishes (Siluriformes, Loricariidae, Panaqolus and Panaque) reveals undescribed diversity and parapatric clades. Mol Phylogenet Evol. 2017;109:321–36.
Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC, Melo BF, Albert JS, Chang J, Foresti F, Alfaro ME, Oliveira C. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phylogenet Evol. 2019;135:148–65.
Londoño-Burbano A, Reis RE. A combined molecular and morphological phylogeny of the Loricariinae (Siluriformes: Loricariidae), with emphasis on the Harttiini and Farlowellini. PLoS ONE. 2021;16(3):e0247747.
Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 3.81. 2023. http://www.mesquiteproject.org.
Acknowledgements
We would like to thank the collectors in the field for providing us data on species ecology, the hobbyists for supplying us with dead specimens, Jon Armbruster, from Auburn University, and the anonymous reviewers for their very helpful comments.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was financed by the Deutsche Forschungsgemeinschaft (DFG) grant 470833544 to WK. We acknowledge financial support from the Open Access Publication Fund of Universität Hamburg.
Author information
Authors and Affiliations
Contributions
DKV and SG initiated the study. DKV, WK, and SG performed SEM analyses. DKV provided specimens and data on the ecology of species. WK collated the data and wrote the first draft of the manuscript. BH performed the ancestral state reconstruction. All authors contributed to and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary material including list of species with localities, images of the localities, and the intraspecific variability of unculi.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Krings, W., Konn-Vetterlein, D., Hausdorf, B. et al. Holding in the stream: convergent evolution of suckermouth structures in Loricariidae (Siluriformes). Front Zool 20, 37 (2023). https://doi.org/10.1186/s12983-023-00516-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12983-023-00516-w