Abstract
Objectives
The primary objective was to evaluate the safety of the anti-PD-1 antibody camrelizumab in people living with HIV (PLWH); the secondary objective was to evaluate tumor response.
Methods
From May 8, 2018, to December 10, 2021, twenty-four patients with HIV and advanced cancer as well as a CD4+ T-cell count greater than or equal to 100 cells/µL were treated with camrelizumab in daily practice. We describe the demographic characteristics, safety, and clinical course of these 24 PLWH with cancer treated with camrelizumab. Safety was assessed using the current Common Terminology Criteria for Adverse Events (CTCAE). The tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1).
Results
The median number of cycles was 8 (4–26). Only two grade 3 adverse reactions were reported (no toxic deaths or immune-related deaths). Among the 24 patients, 2 (8%) complete responses and 6 (25%) partial responses were observed. 7 patients (29%) were at stable tumor status and others progressed.
Conclusions
Data from the present study strongly support the use of camrelizumab (monoclonal antibodies targeting the PD-1 pathway) in this population, as it appears to be a feasible approach with no deleterious effects on PLWH and tolerability and acceptable efficacy. In addition, these findings further support the inclusion of PLWH with cancer in clinical trials evaluating the safety and efficacy of ICIs on cancer.
Highlights
Camrelizumab had an acceptable safety profile in 24 participants with HIV, a CD4+ T-cell count of greater than 100 cells/μL, and advanced cancer. This study increases confidence in immunotherapy for HIV.
Similar content being viewed by others
Background
With the advent of highly active antiretroviral therapy in recent years, people living with HIV (PLWH) have become less likely to die of AIDS-related illnesses. However, non-AIDS-related malignancies have been identified as the leading cause of death in PLWH. Compared with the general population, PLWH are at a higher risk of cancer [1,2,3]. The treatment regimens and outcomes in select patients with several cancers are like those in the general population [4, 5]. Currently, there is no standard therapy for PLWH with metastatic or locally advanced cancers, those with previous therapy failure due to disease progression or relapse, or those ineligibles to receive standard therapy [1].
Immune checkpoint inhibitors (ICIs) have emerged as a powerful new tool in cancer treatment. Immunotherapy has received considerable critical attention; however, current research and guidelines lack information on immunotherapy in PLWH advanced cancers [6]. Immunotherapy in PLWH may have beneficial treatment outcomes. As the indications for ICIs expand to various cancers, PLWH have been excluded from clinical immunotherapy studies. However, several case reports have reported on the immunotherapy treatment of PLWH with different types of advanced cancer. Although treatment with ICIs appears to be generally well tolerated in PLWH in these studies [6,7,8,9]. Lack of knowledge about using cancer therapies in PLWH, health care disparities, and HIV-associated immunosuppression may affect outcomes [10]. Camrelizumab, a highly-affinity, fully humanized, selective IgG4 monoclonal antibody against PD-1, has shown activity across a wide range of solid tumors [11, 12]. Yet, anti-PD-1 safety data are needed to guide the treatment of patients with HIV and to inform HIV-related eligibility criteria for future immune therapy.
The adverse event (AE) profile of checkpoint inhibitors targeting PD-1、PD-L1 has been tested in the general population with cancer and the immune-related AEs related to anti-PD-1 therapy occur in fewer than 30% of patients with cancer. The most common AEs are with skin, musculoskeletal, gastrointestinal, and endocrine, being generally mild to moderate [13]. Safety and efficacy data are lacking for ICIs in PLWH, because these patients have been systematically excluded from clinical trials. So, we determined to evaluate the safety and tumor response of camrelizumab in PLWH with advanced cancers.
Methods
Study design
This retrospective observational study included patients diagnosed with locally advanced or metastatic cancers who received treatment in our hospital from 8th May 2018 and 10th July 2021. Included cases were followed up by telephone until June 2022. Patients ’clinical data were taken from electronic health records in the hospital information system. The clinical and laboratory data were retrospectively retrieved by telephone and hospital medical case records. This study was subject to approval by the Ethics Review Committee of our hospital.
Patient selection
Inclusion criteria were the following: (1) with controlled HIV viral load; (2) the baseline CD4 + T-cell counts > 100 cells/µL; (3) with no central nervous system metastases, (4) with complete data, including general laboratory and radiological data, related oncology treatment data ; (5) with an Eastern Cooperative Oncology Group (ECOG) performance score of ≤ 1;(6) not AIDS-defining cancers.
Data collecting
A clinical physician collected data available on the case report form, including characteristics, baseline CD4+ T-cells count, HIV viral load before immunotherapy, and the number of cycles received. Camrelizumab (200 mg) wea administered intravenously every 2 weeks until death or intolerability. Treatment continued until disease progression, unacceptable toxicity, patient withdrawal, or investigator decision. The tumor response outcome was assessed by the radiographic data according to Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). The adverse events were collected by the follow-up according to the Common Terminology Criteria for Adverse Events, version 4.03.
Changes in CD4 + T-cell count from baseline to the time of last treatment cycle were evaluated by Wilcoxon signed rank test. The cut-off time for follow-up was until May 2022, after the completing the last cycle or death. SPSS (version 26) were used for statistical analysis.
Endpoints
The primary objective was to assess the safety and tolerability of camrelizumab in patients with HIV on ART with locally advanced or metastatic cancer. Immune-related AEs of grade 2 or higher were considered immune-related events of clinical interest. The secondary objective was to produce preliminary insights into clinical benefits. Tumor response was assessed by clinical trial group criteria, and clinical benefit was defined as complete response (CR) and partial response (PR).
Results
Patient characteristics
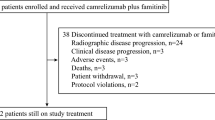
From May 8, 2018, to December 10, 2021, 314 PLWH with cancer have been presented. Thirty-six patients were proposed for Anti-PD-1 therapy, 2 selected other anti-PD-1 medicine, 2 with a low CD4+ T-cell count (< 100 cells/µL), and 8 were in other clinical trials, were excluded. A total of 24 patients were included in this study. (Table 1) The median age (range) was 59 (41–75) years. 21 (87.5%) participants were men and 3 (12%) were women. They are all Chinese, twenty-two of them Han, 1 Mongolian, and 1 Uygur. The median CD4+ T-cell count was 528/µL (range, 125–1309 cells/µL). Twenty-three (96%) received ART before the Anti-PD-1 therapy. No change in HIV viral load was changed during follow-up. CD4+ T-cell counts were monitored during treatment in 13 patients. Figure 1 showed the evolution of CD4+ T-cell counts in 13 patients who had value under camrelizumab. There is no significant change in CD4+ T-cell count between the baseline and the time of the last treatment (p = 0.786). Overall, there was no obvious change.
Safety outcomes
Safety was noted over the course of 207 cycles in 24 participants. The median number of cycles was 8 (range, 4–26). At the time of analyses, Five participants continued to receive Anti-PD-1 therapy. Treatment-emergent AE s at least possibly attributed to Camrelizumab that occurred in participants are presented in Table 2. Most AEs were grade 1 or 2 (n = 22), with 2 (8%) being grade 3. Reactive cutaneous capillary endothelial (RCCEP) was the most frequent AE and Grade 3 of RCCEP occurred in 1 patient with non-Hodgkin lymphoma. And 1 patient’s grade 3 of lymphocyte count decreased. No toxic deaths have been recorded.
Tumor type and response
The tumor types and response were presented in Table 3. The swimmer plot of duration response was shown in Fig. 2. Eighteen (75%) patients were treated with monotherapy. The most tumor type in this study was bladder cancer (6%). Unfortunately, nine had progressed and a total of 4 patients in the study died at the time of analysis. Defined clinical benefit (CR and PR) was noted in 2 (8%) with bladder cancer and 6 (25%) participants, respectively. The median duration of response in these 8 responding patients was 9 weeks. Seven patients had stable tumor status, and others progressed (Fig. 2). A total of 7 patients underwent genetic testing, where PD-L1 expression was > 1% in 4 patients and < 1% in the remaining 3 patients. Six patients received the combined treatment with camrelizumab and chemotherapy. The progression-free survival (PFS) for all cohorts is shown in Fig. 3.
Discussion
In the last few years, cancer immunotherapy has significantly advanced. Immune checkpoint proteins, like PD-1, have taken an active role in mediating T-cell exhaustion both in cancer and chronic infections. Upon interaction with their ligands, for example, PD-L1, a negative signaling cascade is activated within the T cell that blocks T cell receptor-mediated activation. Consequently, the T cell loses its effector function, and thus, its ability to destroy cancer cells or provide cytokines for proper immune homeostasis. Once the interaction between the checkpoint protein and its ligand is interrupted, for example, by a blocking antibody, T-cells can regain effector functions and participate again in cancer surveillance [14]. Anti-PD1 therapy has been approved for a variety of cancers that increased incidence in PLWH, including squamous cell skin cancer [15], cervical cancer [16], lung cancer [17, 18], Hodgkin lymphoma [19, 20] and hepatocellular carcinoma [21].
In this study, CD4 T-cell count levels were found to fluctuate, slightly increasing or decreasing, and at the last therapy cycle, there were 3 patients with levels that were lower than the baseline level. Our results showed that camrelizumab had no detrimental effect on CD4 T-cell counts. The previous study [6]. found CD4 T-cell had a median increase of 19 cells/µL in HIV patients treated with pembrolizumab. Spano’s study of 23 HIV patients treated with anti-PD1 therapy reported a slight decrease in CD4 T-cell count [22]. Potential concerns have included the administration of increased expression of PD-1 in HIV infection, which is inversely correlated with CD4 T-cell count. In a case report of an HIV-positive patient with advanced lung cancer, nivolumab injections increased HIV-specific CD8 T-cells and a drastic and transient diminution of the HIV reservoir [23]. However, our retrospective study did not allow us to assess the impact on the HIV reservoir or HIV-specific CD8+ T-cell.
The most common AE was reactive capillary endothelial proliferation (RCEP) (238 [79.9%]. vs. 32 [10.8%].) in the previous study, this adverse event mainly occurred at grade 1 or 2 [24]. Most patients with RCEP did not require special treatment, and it spontaneously regressed after discontinuing camrelizumab. RCEP is considered an immune response of capillary endothelial cells. Previous studies have been reported that its occurrence is positively associated with tumor response [25]. The proportion of grade 3 and 4 AEs was similar to that previously reported in general patients receiving anti-PD-1 therapy [17, 18, 24]. The outcomes in this research are consistent with previous ones. However, the safety of this therapy in PLWH has not been previously explored prospectively. This study confirms the primary safety of camrelizumab in PLWH with cancers.
Data on the tolerance and efficacy of Anti-PD-1 in PLWH are scarce. To the best of our knowledge, this is the first study that evaluated camrelizumab in PLWH with advanced cancers. Previous studies assessed the safety of pembrolizumab in PLWH and advanced cancers, and tumor regression in participants with a range of tumor types confirmed the activity of anti-PD-1 therapy in PLWH [6]. Spano et al. [22]. reported the efficacy and tolerance of nivolumab or pembrolizumab in PLWH with cancers. The observed clinical benefit rate in this study was 33.3% (PR + CR), which was very impressive. Immune checkpoint inhibitors provide an important treatment option for PLWH with cancer [7, 26]. Unfortunately, anti-PD1-related data on the tolerance and efficacy of ICIs in PLWH is scarce, as these patients are usually excluded from clinical trials [24, 27]. Expanding clinical trial eligibility to include patients with HIV is justified in most cases and may accelerate the development of effective therapies in this area of unmet clinical need. Currently, there are several ongoing clinical trials to evaluate immune checkpoint inhibitors in PLWH, like DURVAST, NCT03094286, and NCT02869789. Results of these ongoing clinical trials are awaited to determine and hopefully address the safety and efficacy of anti-PD-1 therapy in PLWH.
Study limitation
The retrospective study design and sample size did not allow for a formal comparison of rates of specific AEs in the general population. In addition, this study was not randomized. Although this study demonstrated camrelizumab had a clinical benefit in PLWH with several cancers, this study did not have enough participants with a tumor to evaluate the tumor response accurately.
Conclusion
Data from the present study strongly support the use of camrelizumab (monoclonal antibodies targeting the PD-1 pathway) in this population, as it appears to be a feasible approach with no deleterious effects on PLWH and tolerability and acceptable efficacy. In addition, these findings further support the inclusion of PLWH with cancer in clinical trials evaluating the safety and efficacy of ICIs on cancer.
Availability of data and materials
All generated and available data for this paper has been included.
References
Reid E, Suneja G, Ambinder RF, Ard K, Baiocchi R, Barta SK, et al. Cancer in people living with HIV, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(8):986–1017.
Ji Y, Lu H. Malignancies in HIV-infected and AIDS patients. Adv Exp Med Biol. 2017;1018:167–79.
Morlat P, Roussillon C, Henard S, Salmon D, Bonnet F, Cacoub P, et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS. 2014;28(8):1181–91.
Mosam A, Shaik F, Uldrick TS, Esterhuizen T, Friedland GH, Scadden DT, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr. 2012;60(2):150–7.
Dunleavy K, Little RF, Pittaluga S, Grant N, Wayne AS, Carrasquillo JA, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115(15):3017–24.
Uldrick TS, Gonçalves PH, Abdul-Hay M, Claeys AJ, Emu B, Ernstoff MS, et al. Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer-a phase 1 study. JAMA Oncol. 2019;5(9):1332–9.
Cook MR, Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: a systematic review. JAMA Oncol. 2019;5(7):1049–54.
Luo L, Xu Y, Li T. Immune checkpoint inhibitor therapy for cancer patients infected with HIV: a systematic review. Asia-Pac J Clin Oncol. 2022;18(2):e17–e22.
Gonzalez-Cao M, Martinez-Picado J, Karachaliou N, Rosell R, Meyerhans A. Cancer immunotherapy of patients with HIV infection. Clin Transl Oncol. 2019;21(6):713–20.
Suneja G, Shiels MS, Angulo R, Copeland GE, Gonsalves L, Hakenewerth AM, et al. Cancer treatment disparities in HIV-infected individuals in the United States. J Clin Oncol. 2014;32(22):2344–50.
Markham A, Keam SJ. Camrelizumab: first global approval. Drugs. 2019;79(12):1355–61.
Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–42.
Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, et al. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. Oncologist. 2021;26(7):e1110-24.
Lan C, Shen J, Wang Y, Li J, Liu Z, He M, et al. Camrelizumab plus apatinib in patients with advanced cervical cancer (CLAP): a multicenter, open-label, single-arm, phase II trial. J Clin Oncology. 2020;38(34):4095–106.
Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305–14.
Zhou C, Wang Y, Zhao J, Chen G, Liu Z, Gu K, et al. Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced nonsquamous NSCLC previously treated with chemotherapy. Clin Cancer Res. 2021;27(5):1296–304.
Nie J, Wang C, Liu Y, Yang Q, Mei Q, Dong L, et al. Addition of low-dose decitabine to Anti-PD-1 antibody camrelizumab in relapsed/refractory classical hodgkin lymphoma. J Clin Oncology: Official J Am Soc Clin Oncol. 2019;37(17):1479–89.
Liu Y, Wang C, Li X, Dong L, Yang Q, Chen M, et al. Improved clinical outcome in a randomized phase II study of anti-PD-1 camrelizumab plus decitabine in relapsed/refractory Hodgkin lymphoma. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2021-002347.
Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–11.
Spano J-P, Veyri M, Gobert A, Guihot A, Perré P, Kerjouan M, et al. Immunotherapy for cancer in people living with HIV: safety with an efficacy signal from the series in real life experience. AIDS. 2019;33(11):F13–9.
Guihot A, Marcelin AG, Massiani MA, Samri A, Soulié C, Autran B, et al. Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Ann Oncol. 2018;29(2):517–8.
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free Survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–25.
Wang F, Qin S, Sun X, Ren Z, Meng Z, Chen Z, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13(1):47.
Gobert A, Spano J-P, Cadranel J. HIV-associated cancers: the role of a unique multidisciplinary board to optimize patient’s care behalf the CANCERVIH Group. Med Oncol. 2018;36(1):13.
Killock D. Camrelizumab efficacious in NPC. Nat Rev Clin Oncol. 2021;18(9):542.
Acknowledgements
The authors express their gratitude to all the patients who participated in the study.
Funding
No funding was secured for this study.
Author information
Authors and Affiliations
Contributions
MW collected the data and wrote the manuscript. MW, XZ, and YZ finished the tables and figures. JS and JZ reviewed the article. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was received from the Ethical committee of Beijing Youan Hospital (BYH2019043) and all 24 patients voluntarily consented to participate in the study.
Consent for publication
Not applicable.
Competing interests
None declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, M., Zheng, X., Zhang, Y. et al. Camrelizumab for cancers in patients living with HIV: one-single center experience. AIDS Res Ther 20, 23 (2023). https://doi.org/10.1186/s12981-023-00518-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12981-023-00518-y