Abstract
Thailand has the highest prevalence of HIV among countries in Asia but has also been a pioneer in HIV prevention and treatment efforts in the region, reducing the incidence of new infections significantly over the last two decades. Building upon this remarkable history, Thailand has set an ambitious goal to stop the AIDS epidemic in the country by 2030. A key component of the strategy to achieve this goal includes scale-up of HIV screening programs to facilitate early HIV diagnosis and investment in mechanisms to support immediate initiation of antiretroviral therapy (ART). Initiation of ART during early or acute HIV infection not only reduces viremia, thereby halting onward transmission of HIV, but also may facilitate HIV remission by reducing the size of the latent HIV reservoir and preserving immune function. In Thailand, many efforts have been made to reduce the time from HIV infection to diagnosis and from diagnosis to treatment, especially among men who have sex with men and transgender women. Successfully identifying and initiating ART in individuals with acute HIV infection has been leveraged to conduct groundbreaking studies of novel strategies to achieve HIV remission, including studies of broadly-neutralizing HIV-specific monoclonal antibodies and candidate therapeutic vaccines. These efforts have mostly been deployed in Bangkok and future efforts should include other urban and more rural areas. Continued progress in HIV prevention, screening, and treatment will position Thailand to substantially limit new infections and may pave the way for an HIV cure.
Similar content being viewed by others
Introduction
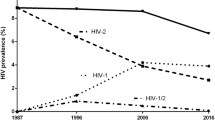
In Thailand, there are an estimated 440,000 people living with HIV (PLWH) and 15,000 die of AIDS-related illnesses annually [1]. Despite having the highest prevalence of HIV in Asia, Thailand has gained fame for tremendously effective deployment of HIV prevention programs that reduced the number of annual new HIV infections from 115,000 in 1992 to 6400 in 2016 [2,3,4]. These public health interventions were most successful in decreasing HIV transmission among reproductive age adults populations and people who inject drugs (PWID) [2, 5].
Building upon this success, Thailand has set the ambitious goal of stopping AIDS by 2030 [6]. As part of the strategy to achieve this goal, the country intends to increase HIV testing coverage for key populations, including men who have sex with men (MSM), transgender people, PWID and sex workers. PLWH identified via this expanded screening will be referred immediately for antiretroviral therapy (ART) to decrease HIV transmission, improve clinical outcomes and achieve rapid viral suppression. Since 2014, the Thailand HIV guidelines have recommended ART initiation as soon as possible, regardless of CD4 cell count [7], forerunning similar recommendations from the World Health Organization (WHO) [8]. The same national guidelines also recommended pre-exposure prophylaxis (PrEP) as part of combination HIV prevention packages for people who are HIV-uninfected and at high-risk of HIV acquisition.
Thailand is moving towards a concrete realization of early diagnosis and treatment, which plays a role not only in the prevention of onward transmission of HIV, but also in minimizing the size of the HIV reservoir and preserving immune function [9, 10]. This progress in management of HIV infection affirms Thailand as a key country in developing and implementing potential strategies to achieve an HIV cure [11].
Size and effectiveness of HIV screening programs
In order to diagnose HIV soon after the infection is acquired, screening programs must be available to populations at the highest risk of infection. In Asia, nearly 65% of new infections occur in MSM, clients of sex workers and other sexual partners of key populations [12]. However, HIV screening programs among MSM are still scarce in Asia and there is a low rate of regular HIV testing [13,14,15]. According to recent data from Thailand, HIV testing coverage, defined as receipt of a test in the last 12 months, was only 29% among MSM, compared with 58% among female sex workers and 61% among PWID [16]. Compared to data from 2008 to 2009, testing coverage remained stable in PWID (59.7%) and had increased in both MSM and sex workers (21.3% and 35.2%, respectively) [17]. Prevention programs are more extensive and effective in the capital city, Bangkok, than in the rest of Thailand [18, 19]. Persistent obstacles to HIV screening include people’s inability to self-identify or admit HIV risk, HIV-related stigma, and concern about side effects of ART [11, 20].
Thailand has successfully piloted a number of strategies to increase access to HIV testing for key populations, including MSM. HIV prevalence in MSM is almost ten times higher than in reproductive age adults nationwide [3] and is a staggering 28.6% in Bangkok [21]. Incentives to motivate key populations to access HIV testing include providing free tests and rapid results. Delayed test results have been linked to increased loss to follow-up after testing [22]. HIV rapid diagnostic testing can be conducted by well-trained lay providers with test accuracy similar to those performed by healthcare professionals, which is an implementation strategy that could make screening programs more widespread and improve HIV testing coverage [23].
Lay providers, who themselves may be members of or work closely with key populations, can design and deliver health services that are well tailored and responsive to the specific needs of key populations with non-discrimination and quality standards. In 2016, these key population lay providers under the key population-led health services (KPLHS) model contributed to 42% of all HIV testing and 35% of all HIV diagnoses made among MSM and transgender women (TGW) in Thailand [22].
Mobile clinics and peer-led HIV testing services have been shown to increase both access to HIV screening and HIV knowledge, especially among younger participants [24]. In addition, advances in technology such as online HIV testing services help with both the quantity and quality of HIV screening for at-risk MSM and TGW [25, 26]. Other strategies to encourage uptake of HIV prevention interventions could include provision of ancillary services such as screening for other sexually transmitted infections [22], offering self-testing as an adjunct or alternative to clinic-based HIV screening [26] and guaranteeing access to gender affirmative hormone treatment among TGW [27].
HIV PrEP uptake among key populations
Thailand has included PrEP in its national HIV guidelines as a prevention method for people at high risk of infection since 2014 [7]. A number of programs have since then made PrEP available to key populations, including MSM, TGW, and individuals in serodiscordant couples. PrEP-30, launched in Thai Red Cross Anonymous Clinic in December 2014, was the first available PrEP service in Thailand, providing nonsubsidized PrEP for a fee of 30 THB ($1 USD) per day [28]. In November 2016, the Thai Ministry of Public Health launched PrEP2START, a public health capacity-building program that provides free PrEP to anyone at risk in eight provinces [29]. The Princess PrEP Program, supported by the Princess Soamsawali HIV Prevention fund at The Thai Red Cross AIDS Research Center, was the first key population-led PrEP program for MSM, transgender populations, sex workers, and people who use drugs, providing PrEP through eight community-based clinics in four provinces [30]. By the end of 2018, there were over 6000 people in Thailand accessing PrEP [31], and the Thai National Health Security Office announced that PrEP would be made available through the national health insurance system as early as the end of 2019 [32, 33].
Decreasing time from diagnosis to ART initiation
Once HIV infection occurs, proviral HIV DNA rapidly integrates into resting and memory CD4 cells, where it remains transcriptionally silent [34]. This latent reservoir represents the major barrier to HIV cure. Starting ART during acute HIV infection (AHI) substantially reduces the HIV reservoir as compared to ART initiation during chronic HIV infection [9, 10]. A study conducted in Pattaya and Bangkok demonstrated that ART initiation within 5 days of HIV infection increased the likelihood of having no detectable HIV DNA in central memory CD4 cells [35]. Therefore, shortening the time between diagnosis and ART initiation may facilitate HIV cure when combined with other novel interventions.
The test-and-treat approach combines periodic HIV testing and immediate ART initiation. At-risk populations screened within this intervention strategy are motivated to initiate ART as soon as possible, thereby achieving a life expectancy similar to HIV-uninfected people [22, 36, 37]. In 2012, 810 Thai MSM and TGW were enrolled in a test-and-treat study in Bangkok, Ubon Ratchathani, Lampang and Mahasarakam that newly-diagnosed 134 (16.5%) PLWH [38]. Immediate ART initiation was recommended to all participants diagnosed with HIV and the acceptance rate was 83% [38].
Between 2015 and 2016 another test-and-treat study was conducted at five hospitals that served MSM and TGW in four Thai provinces [39]. Many participants received HIV testing for the first time, revealing that a previously-unreached key population was being screened [40]. Among those with incident HIV infection, 86.1% initiated ART and 58.6% of ART initiators did so within 2 weeks of diagnosis [39]. Although ART uptake in this program was higher than had been reported from other areas in Thailand [41], it still fell short of the UNAIDS 90-90-90 target.
At the Thai Red Cross AIDS Research Centre (TRCARC) in Bangkok, physicians offer same-day ART to participants who fulfill eligibility criteria, such as the exclusion of active tuberculosis, cryptococcal meningitis and other opportunistic infections. Same-day ART was accepted and initiated by 89.5% of 3443 individuals with newly-diagnosed HIV between July 2017 and April 2019 [42]. Successes in deploying test-and-treat programs in Thailand’s large cities need to be emulated in other parts of the country to fully realize the potential of this HIV prevention strategy.
Diagnosis and treatment during acute HIV infection
AHI is defined as the first few weeks after HIV transmission, before the HIV antibody response has fully developed. AHI can be diagnosed by the presence of HIV RNA in the blood in the absence of HIV antibodies or by the detection of HIV antibodies by sensitive third or fourth generation antibody tests while less-sensitive second generation antibody tests and Western blot remain nonreactive or indeterminate [43]. The use of newer generation HIV test kits that detect both HIV antigens and antibodies has increased sensitivity to detect the earliest phases of HIV infection [44]. In one study in Thailand, addition of nucleic acid testing to to an HIV screening algorithm based on the 4th generation enzyme immunoassay raised the number of AHI diagnosis from 12 to 17 per 10,000 samples tested [45].
Since 2009, the Anonymous Clinic at the TRCARC has screened for AHI in over 300,000 people presenting for voluntary HIV testing and over 600 have enrolled for immediate ART and longitudinal follow-up in the RV254/SEARCH010 cohort (NCT00796146). The median duration since estimated HIV exposure was 19 (range: 3–61) days and 99% initiated ART within 1 week of AHI diagnosis [46].
ART initiation during AHI, has been shown to limit the size of the HIV reservoir and to preserve immune function [47]. Studies conducted in the RV254/SEARCH010 cohort have highlighted other benefits of early treatment, such as the potential to prevent or limit gut inflammation [48] and neurological impairment [49].
Viral suppression rates on ART
PLWH who are virally-suppressed on ART experience improved clinical outcomes as compared to viremic individuals and cannot transmit HIV. For these reasons, achieving viral suppression is a cornerstone of HIV management and the third “90” in the UNAIDS 90-90-90 targets to end AIDS. Hoenigl et al. showed that viral suppression was rapid after early ART initiation in either AHI or chronic HIV infection, with median time to undetectable viremia being 12 weeks (interquartile range, IQR: 4–24 weeks) in each group [50]. At TRCARC, PLWH who started ART on the day of HIV diagnosis were 2.2 times more likely to be virally suppressed when compared to PLWH who started ART later after diagnosis [51]. In the RV254/SEARCH010 cohort, participants who started ART during Fiebig stage I reached viral suppression in a median of 8 weeks (IQR: 4–12), while all other Fiebig stages achieved viral suppression in a median of 12 weeks (IQR: 8–16), showing a statistically significant difference in time to viral suppression that favored earlier ART initiation even within the setting of acute infection [52]. As HIV is a chronic disease, viral suppression needs to be maintained lifelong, which for most PLWH means taking daily medication with strict adherence for decades. When ART is started during chronic HIV infection, virologic failure rates have been reported to be 10–20% at 24 weeks [53, 54]. Virologic failure is less common in individuals who initiate ART during AHI, observed in only 1.1% of 264 Thai PLWH at 24 weeks [52].
Between July 2017 and April 2019, at the TRCARC in Bangkok 89.8% of HIV infected persons on ART who received viral load testing were virally suppressed [42].
These results highlight successes in achieving viral suppression in Thailand once HIV has been diagnosed, but there is room to improve. In Thailand, recent data showed that more than 95% of PLWH knew their status, 72% were on ART and 62% were virally suppressed [12]. Successful programs to promote HIV testing and earlier ART initiation need to be scaled up nationwide in order to achieve the UNAIDS 90-90-90 targets.
HIV cure in Thailand
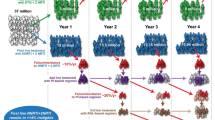
The ongoing RV254/SEARCH010 cohort in Bangkok has proven to be fertile ground for the development and implementation of HIV remission trials, leveraging evidence that the lower reservoir size and preserved immune function of individuals who start ART during acute infection might facilitate viral control in the absence of ART. The study has shown that execution of observational and interventional research during the period surrounding acute HIV infection is safe, feasible, and acceptable to participant populations [55, 56]. This includes research that involves invasive procedures such as lumbar puncture and lymph node biopsy [57, 58]. To date, four HIV remission clinical trials have been completed using the cohort as a source population (Tables 1 and 2).
RV411 was a study of analytic treatment interruption (ATI) in 8 participants who started ART during the earliest stage of AHI (Fiebig I) and were treated for a median of 2.8 years. Following ATI, all participants experienced viral rebound above 20 copies/mL at a median of 26 (range 13–48) days. This single-arm study demonstrated that very early ART alone was not sufficient to control or eradicate HIV [59].
RV397 was a randomized, placebo-controlled clinical trial evaluating the safety and efficacy of a broadly neutralizing human monoclonal antibody (VRC01) targeted against the HIV CD4 binding site in 18 adults who initiated ART during AHI [60]. Participants were closely monitored and restarted ART when plasma HIV RNA was above 1000 copies/mL on two separate measurements. VRC01 modestly delayed the time to viral rebound, which occurred at a median of 14 days after ATI in the placebo group and 26 days after ATI in the VRC01 group (p = 0.051). One VRC01 recipient maintained undetectable peripheral HIV RNA through week 42. This randomized study demonstrated that VRC01 monotherapy was insufficient to maintain viral suppression in most individuals, even in this carefully selected population [60].
In a test of the “kick and kill” strategy, 15 acutely-treated participants were randomized to receive either ART alone or in combination with vorinostat (a latency reversal agent), maraviroc (an entry inhibitor), and hydroxychloroquine (an immune modulator) [61]. At week 10 all medications were stopped, and ATI was begun. Time to viral rebound > 1000 copies/mL, which occurred at a median of 22 days, did not differ significantly between the intervention and placebo arms. No changes were observed both in total HIV DNA in peripheral blood mononuclear cells (PBMCs), in T cell and soluble immune activation markers. Furthermore, ART duration, total HIV DNA in PBMCs, single copy HIV RNA and CD4/CD8 ratio did not predict time to viral load.
RV405 was a randomized, placebo-controlled study of a therapeutic vaccine using an Adenovirus type 26 vector prime and modified vaccinia Ankara boost combination with mosaic inserts in HIV-infected adults who initiated ART during AHI. A total of 26 participants were enrolled in the active vaccine (n = 17) and placebo (n = 9) arms. As in all ATI studies, participants were monitored frequently, and ART was reinitiated when viral rebound was detected [62]. The study showed that the vaccine regimen was safe, well-tolerated, and induced a robust immunologic response; but that it resulted in only a slight delay in time to viral rebound after ATI. Future trials may investigate therapeutic vaccine regimens with the addition of immunomodulators and different immunogens.
Conclusions
Thailand has emerged as a pioneer in efforts to prevent, treat, and ultimately cure HIV with an ambitious national strategy to stop the AIDS epidemic in the country by 2030. Successful pilot studies of expanded HIV screening and test-and-treat programs now need to be scaled up to reach diverse populations throughout the country, including key underserved populations such as MSM, TGW, and PWID. Thailand has been uniquely successful in providing prevention modalities and initiating ART in individuals with acute HIV infection, yielding a valuable source population for the conduct of groundbreaking studies of novel strategies to achieve HIV remission. Thailand is poised to continue to have a leading role in HIV prevention and cure research thanks to the combined efforts from communities of key and affected populations, researchers, government and policy makers.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the conduct of this review.
Abbreviations
- AHI:
-
acute HIV infection
- AIDS:
-
acquired immune deficiency syndrome
- ART:
-
antiretroviral therapy
- ATI:
-
analytic treatment
- IQR:
-
interquartile range
- KPLHS:
-
key population-led health services
- MSM:
-
men who have sex with men
- O2O:
-
Online-to-Offline
- PBMC:
-
peripheral blood mononuclear cell
- PLWH:
-
people living with HIV
- PrEP:
-
pre-exposure prophylaxis
- PWID:
-
people who inject drugs
- TGW:
-
transgender women
- TRCARC:
-
Thai Red Cross AIDS Research Centre
- UNAIDS:
-
the Joint United Nations Programme on HIV/AIDS interruption
- WHO:
-
World Health Organization
References
Country factsheets Thailand 2017. UNAIDS; https://www.unaids.org/en/regionscountries/countries/thailand. Accessed 14 Apr 2019.
Siraprapasiri T, Ongwangdee S, Benjarattanaporn P, Peerapatanapokin W, Sharma M. The impact of Thailand’s public health response to the HIV epidemic 1984–2015: understanding the ingredients of success. J Virus Erad. 2016;2(Suppl 4):7–14.
Data book 2017. UNAIDS; https://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf. Accessed 14 Apr 2019.
Punyacharoensin N, Viwatwongkasem C. Trends in three decades of HIV/AIDS epidemic in Thailand by nonparametric backcalculation method. AIDS. 2009;23:1143–52.
Chaivooth S, Bhakeecheep S, Ruxrungtham K, Teeraananchai S, Kerr SJ, Teeraratkul A, et al. The challenges of ending AIDS in Asia: outcomes of the Thai National AIDS Universal Coverage Programme, 2000–2014. J Virus Erad. 2017;3:192–9.
UNAIDS. Thailand launches new national strategy to end the AIDS epidemic by 2030. http://www.unaids.org/en/resources/presscentre/featurestories/2017/September/20170915_Thailand_NSP. Accessed 20 Apr 2019.
Manosuthi W, Ongwandee S, Bhakeecheep S, Leechawengwongs M, Ruxrungtham K, Phanuphak P, et al. Guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2014, Thailand. AIDS Res Ther. 2015;12:12.
World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf. Accessed 22 June 2019.
Lewin SR, Deeks SG, Barré-Sinoussi F. Towards a cure for HIV—are we making progress? Lancet. 2014;384:209–11.
Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12:607–14.
Phanuphak P, Lo YR. Implementing early diagnosis and treatment: programmatic considerations. Curr Opin HIV AIDS. 2015;10:69–75.
Data book 2018. UNAIDS; https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf. Accessed 25 Apr 2019.
Chow EP, Wilson DP, Zhang L. The rate of HIV testing is increasing among men who have sex with men in China. HIV Med. 2012;13:255–63.
Morineau G, Nugrahini N, Riono P, Nurhayati, Girault P, Mustikawati DE, et al. Sexual risk taking, STI and HIV prevalence among men who have sex with men in six Indonesian cities. AIDS Behav. 2011;15:1033–44.
Aung T, McFarland W, Paw E, Hetherington J. Reaching men who have sex with men in Myanmar: population characteristics, risk and preventive behavior, exposure to health programs. AIDS Behav. 2013;17:1386–94.
Thailand Country Snapshot 2018. UNAIDS; https://www.aidsdatahub.org/sites/default/files/country_review/Thailand_Country_Card_Sept%202018.pdf. Accessed 30 Apr 2019.
UNGASS Country Progress Report: Thailand 2010, National AIDS Prevention and Alleviation Committee; http://data.unaids.org/pub/report/2010/thailand_2010_country_progress_report_en.pdf. Accessed 30 Apr 2019.
Evaluation of the National HIV prevention program for key affected population, migrant workers and prisoners, Institute for Population and Social Research, Mahidol University, 2013; https://www.aidsdatahub.org/sites/default/files/publication/Evaluation_of_the_National_HIV_prevention_program_for_KAP_Migrants_Prisoners_2013.pdf. Accessed 30 Apr 2019.
Johnston LG, Steinhaus MC, Sass J, Sirinirund P, Lee C, Benjarattanaporn P. Recent HIV testing among young men who have sex with men in Bangkok and Chiang Mai: HIV testing and prevention strategies must be enhanced in Thailand. AIDS Behav. 2016;20:2023–32.
Sapsirisavat V, Phanuphak N, Sophonphan J, Egan JE, Langevattana K, Avihingsanon A, et al. Differences between men who have sex with men (MSM) with low CD4 cell counts at their first HIV test and MSM with higher CD4 counts in Bangkok, Thailand. AIDS Behav. 2016;20(Suppl 3):398–407.
Prevention Gap Report 2016, UNAIDS; https://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Accessed 1 May 2019.
Maek-a-nantawat W, Phanuphak N, Teeratakulpisarn N, Pakam C, Kanteeranon T, Chaiya O, et al. Attitudes toward, and interest in, the test-and-treat strategy for HIV prevention among Thai men who have sex with men. AIDS Care. 2014;26:1298–302.
Wongkanya R, Pankam T, Wolf S, Pattanachaiwit S, Jantarapakde J, Pengnongyang S, et al. HIV rapid diagnostic testing by lay providers in a key population-led health service programme in Thailand. J Virus Erad. 2018;4:12–5.
Wasantioopapokakorn M, Manopaiboon C, Phoorisri T, Sukkul A, Lertpiriyasuwat C, Ongwandee S, et al. Implementation and assessment of a model to increase HIV testing among men who have sex with men and transgender women in Thailand, 2011–2016. AIDS Care. 2018;30:1239–45.
Anand T, Nitpolprasert C, Trachunthong D, Kerr SJ, Janyam S, Linjongrat D, et al. A novel Online-to-Offline (O2O) model for pre-exposure prophylaxis and HIV testing scale up. J Int AIDS Soc. 2017;20:21326.
Phanuphak N, Anand T, Jantarapakde J, Nitpolprasert C, Himmad K, Sungsing T, et al. What would you choose: Online or Offline or Mixed services? Feasibility of online HIV counselling and testing among Thai men who have sex with men and transgender women and factors associated with service uptake. J Int AIDS Soc. 2018;21:e25118.
Janamnuaysook R, Samitpol K, Ketwongsa P, Chancham A, Kongkapan J, Mingkwanrungruang P, et al. Integrated gender affirmative hormone treatment services improve access to and retention in HIV testing, syphilis testing and pre-exposure prophylaxis (PrEP) service uptake among transgender women in Thailand. IN: AIDS 2018: 22nd international AIDS conference, Amsterdam, Netherlands, July 23–27, 2018. Abstract THAC0204.
Colby D. PrEP or Peril: rolling out PrEP in the private sector without subsidy or government support. In: AIDS 2018: 22nd international AIDS conference, Amsterdam, Netherlands, July 23–27, 2018.
Suraratdecha C, Stuart RM, Manopaiboon C, Green D, Lertpiriyasuwat C, Wilson DP, et al. Cost and cost-effectiveness analysis of pre-exposure prophylaxis among men who have sex with men in two hospitals in Thailand. J Int AIDS Soc. 2018;21:e25129.
Phanuphak N, Sungsing T, Jantarapakde J, Pengnonyang S, Trachunthong D, Mingkwanrungruang P, et al. Princess PrEP program: the first key population-led model to deliver pre-exposure prophylaxis to key populations by key populations in Thailand. Sex Health. 2018;15:542–55.
Vannakit R, Thammatach-aree J, Rungtanatada K, Linjongrat D, Janyam S, Chanlearn P, et al. From “nice-to-have” to “necessary”: increases in domestic financing and perceived value of key population-lead HIV services by the Thai government as international donor funding transitions. In: 10th international conference on HIV science, July 21–24, 2019, Mexico City. Abstract TUAD0302.
Funding boost for healthcare. http://www.nationmultimedia.com/detail/Economy/30364003. Accessed 16 Aug 2019.
NHSO board adds PrEP for high risk populations to fiscal year 2020 budget; https://www.nhso.go.th/frontend/NewsInformationDetail.aspx?newsid=MjUzMg. Accessed 16 Aug 2019.
Siliciano JD, Siliciano RF. Recent developments in the search for a cure for HIV-1 infection: targeting the latent reservoir for HIV-1. J Allergy Clin Immunol. 2014;134:12–9.
Ananworanich J, Chomont N, Eller LA, Kroon E, Tovanabutra S, Bose M, et al. HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine. 2016;11:68–72.
Vannakit R, Jantarapakde J, Pengnongyang S, Pankam T, Trachunthong D, Pussadeeet K, et al. High linkage to ART and HIV RNA suppression among HIV-positive MSM and TG, along with high PrEP uptake among HIV-negative MSM and TG, through community-led health service model in Thailand. In: IAS 2017: 9th IAS conference on HIV science, Paris, France, July 23–26, 2017. Abstract TUPED1313.
Phanuphak N, Pattanachaiwit S, Pankam T, Teeratakulpisarn N, Chamnan P, Pathipvanich P, et al. Sexually transmitted infections and HIV RNA levels in blood and anogenital compartments among Thai men who have sex with men before and after antiretroviral therapy: implication for treatment as prevention programme. J Int AIDS Soc. 2018;21:e25186.
Phanuphak N, Chamnan P, Pathipvanich P, Thongpaen S, Nonenoy S, Jantarapakde J, et al. Factors associated with uptake of immediate ART regardless of CD4 count among Thai MSM and TG in the Test and Treat program. In: AIDS 2014: 20th international AIDS conference, Melbourne, Australia, July 20–25, 2014. Abstract WEPE431.
Ongwandee S, Lertpiriyasuwat C, Khawcharoenporn T, Chetchotisak P, Thiansukhon E, Leerattanapetch N, et al. Implementation of a test, treat, and prevent HIV program among men who have sex with men and transgender women in Thailand, 2015–2016. PLoS ONE. 2018;13:e0201171.
Seekaew P, Pengnonyang S, Jantarapakde J, Sungsing T, Rodbumrung P, Trachunthong D, et al. Characteristics and HIV epidemiologic profiles of men who have sex with men and transgender women in key population-led test and treat cohorts in Thailand. PLoS ONE. 2018;13:e0203294.
Thai MoH/NAC/UNAIDS “AIDS Zero Portal: Data use tool”. http://aidszeroportal.org. Accessed 16 Aug 2019.
Personal communication from Pich Seekaew, PREVENTION, Thai Red Cross AIDS Research Centre, Bangkok, Thailand.
Branson BM, Stekler JD. Detection of acute HIV infection: we can’t close the window. J Infect Dis. 2012;205:521–4.
Ananworanich J, Fletcher JLK, Pinyakorn S, van Griensven F, Vandergeeten C, Schuetz A, et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology. 2013;10:56.
De Souza MS, Phanuphak N, Pinyakorn S, Trichavaroj R, Pattanachaiwit S, Chomchey N, et al. Impact of nucleic acid testing relative to antigen/antibody combination immunoassay on the detection of acute HIV infection. AIDS. 2015;29:793–800.
Kroon E, Pham PT, Sirivichayakul S, Trichavaroj R, Colby DJ, Pinyakorn S, et al. Transmission dynamics among participants initiating antiretroviral therapy upon diagnosis of early acute HIV-1 infection in Thailand. AIDS. 2018;32:2373–81.
Takata H, Buranapraditkun S, Kessing C, Fletcher JL, Muir R, Tardif V, et al. Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection. Sci Transl Med. 2017. https://doi.org/10.1126/scitranslmed.aag1809.
Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10:e1004543.
Valcour VG, Spudich SS, Sailasuta N, Phanuphak N, Lerdlum S, Fletcher JLK, et al. Neurological response to cART vs. cART plus integrase inhibitor and CCR1 antagonist initiated during acute HIV. PLoS ONE. 2015;10:e0142600.
Hoenigl M, Chaillon A, Moore DJ, Morris SR, Mehta SR, Gianella S, et al. Rapid HIV viral load suppression in those initiating antiretroviral therapy at first visit after HIV diagnosis. Sci Rep. 2016;6:32947.
Seekaew P, Teeratakulpisarn N, Surapuchong P, Teeratakulpisarn S, Amatavete S, Jomja P, et al. Same-day ART initiation in HIV/STI testing center in Bangkok, Thailand: initial results from implementation research. In: AIDS 2018: 22nd international AIDS conference, Amsterdam, Netherlands, July 23–27, 2018. Abstract THAC0403.
Crowell TA, Phanuphak N, Pinyakorn S, Kroon E, Fletcher JL, Colby D, et al. Virologic failure is uncommon after treatment initiation during acute HIV infection. AIDS. 2016;30:1943–50.
Gallien S, Flandre P, Nguyen N, De Castro N, Molina JM, Delaugerre C. Safety and efficacy of coformulated efavirenz/emtricitabine/tenofovir single-tablet regimen in treatment-naive patients infected with HIV-1. J Med Virol. 2015;87:187–91.
Campbell TB, Smeaton LM, Kumarasamy N, Flanigan T, Klingman KL, Firnhaber C, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9:e1001290.
Sacdalan C, Crowell T, Colby DJ, Kroon E, Chan P, Pinyakorn S, et al. Brief report: safety of frequent blood sampling in research participants in an acute HIV infection cohort in Thailand. J Acquir Immune Defic Syndr. 2017;76:98–101.
Henderson GE, Waltz M, Meagher K, Cadigan RJ, Jupimai T, Isaacson S, et al. Going off antiretroviral treatment in a closely monitored HIV “cure” trial: longitudinal assessments of acutely diagnosed trial participants and decliners. J Int AIDS Soc. 2019;22:e25260.
Chintanaphol M, Sacdalan C, Chottanapund S, Pinyakorn S, Crowell TA, Kroon E, et al. Brief report: safety and tolerability of inguinal lymph node biopsy in individuals with acute HIV infection in Thailand. J Acquir Immune Defic Syndr. 2018;79:244–8.
Chan P, Hellmuth J, Colby D, Kroon E, Sacdalan C, Fletcher J, et al. Safety of lumbar puncture procedure in an international research setting during acute HIV infection. J Virus Erad. 2018;4:16–20.
Colby DJ, Trautmann L, Pinyakorn S, Leyre L, Pagliuzza A, Kroon E, et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. 2018;24:923–6.
Crowell TA, Colby DJ, Pinyakorn S, Intasan J, Benjapornpong K, Tanjnareel K, et al. HIV-specific broadly-neutralizing monoclonal antibody, VRC01, minimally impacts time to viral rebound following treatment interruption in virologically-suppressed, HIV-infected participants who initiated antiretroviral therapy during acute HIV infection. In: IAS 2017: 9th IAS conference on HIV science, Paris, France, July 23–26, 2017. Abstract TUAB0106LB.
Kroon E, Ananworanich J, Eubanks K, Intasan J, Pinyakorn S, Chomont N, et al. Effect of Vorinostat, Hydroxychloroquine and Maraviroc combination therapy on viremia following treatment interruption in individuals initiating ART during acute HIV infection. In: AIDS 2016: 21st international AIDS conference Durban, South Africa, July 18–22, 2016. Abstract 10535.
Ananworanich J. AD26 & MVA vaccines in acutely treated HIV: safety, immunogenicity and viral rebound. In: Keystone symposia on functional cures and the eradication of HIV, Whistler, British Columbia, March 24–28, 2019.
Acknowledgements
The authors gratefully acknowledge the contribution to this work by the Thai Red Cross AIDS Research Centre and USAMC-AFRIMS in Bangkok.
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense, nor the Henry M. Jackson Foundation for the Advancement of Military Medicine. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25.
Funding
This work was supported by a cooperative agreement between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense [W81XWH-18-2-0040]. The funders had no role in the design of the collection, analysis, and interpretation of data.
Author information
Authors and Affiliations
Contributions
TC, DC, EK, NP, CM conceptualized this review. CS, RR performed the literature search. CM authored the first draft of the manuscript. PS performed data extraction. JA, PP reviewed and commented on initial and final drafts of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
JA has received honoraria for participating in advisory meetings for ViiV Healthcare, Gilead, Merck, Roche and AbbVie. RR has received speaker fees and travel support from Gilead. CM, TC, EK, CS, PS, PP, DC, NP declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Muccini, C., Crowell, T.A., Kroon, E. et al. Leveraging early HIV diagnosis and treatment in Thailand to conduct HIV cure research. AIDS Res Ther 16, 25 (2019). https://doi.org/10.1186/s12981-019-0240-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12981-019-0240-4