Abstract
The anti-HIV activity of natural killer (NK) cells could be induced fast enough to potentially prevent the establishment of HIV infection. Epidemiological studies identified two genotypes encoding NK receptors that contribute to NK cell function, that were more frequent in people who remained uninfected despite multiple HIV exposures than in HIV-susceptible subjects. NK cells from carriers of the *h/*y+B*57 genotype have higher NK cell functional potential and inhibit HIV replication in autologous HIV-infected CD4+ T cells (iCD4) more potently than those from carriers of non-protective genotypes. HIV suppression depends on the secretion of CC-chemokines that block HIV entry into CD4+ cells. NK cell education and the effect of HIV infection on iCD4 cell surface expression of MHC-I antigens both influenced NK cell responses to autologous iCD4. The second KIR3DS1 homozygous protective genotype encodes an activating receptor that upon interacting with its HLA-F ligand on iCD4 induces anti-viral activity.
Similar content being viewed by others
Background
Some individuals remain uninfected despite high levels of exposure to HIV. Persons with this profile are called HIV exposed seronegative subjects (HESN) [1]. Understanding why HESN are resistant to HIV infection has the potential to identify correlates of protection from infection. As early as 2003, Scott-Algara et al. found that stimulating NK cells from HESN enrolled in a Vietnamese injection drug user cohort led to activation of higher levels of cytolysis and cytokine/chemokine secretion than did stimulating NK cells from HIV-infected persons or from seroconverters even before they seroconverted [2]. This was the first report suggesting that HESN may have NK cells with superior functionality.
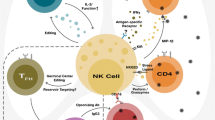
Natural killer cells are important in innate host defences. They function in early responses to transformed and virus-infected cells, including to HIV-infected CD4+ T cells (iCD4) [3]. They express a plethora of cell surface receptors whose engagement initiate signals that culminate in inhibition or activation of effector functions. NK cell activity is regulated by the integration of signals from these receptors. An important family of NK receptors (NKR) are the killer immunoglobulin-like receptors (KIR). Their inhibitory counterparts (iKIR) use a subset of major histocompatibility complex class I (MHC-I) antigens as ligands. iKIR/HLA engagement educate developing NK cells for functionality and suppress the function of mature NK cells so that they are tolerant to normal self cells expressing MHC-I antigens [4]. HIV infected cells downmodulate HLA-A, -B and -C using Nef- and Vpu-mediated mechanisms and upregulate ligands for activating NKRs (aNKR) [5, 6]. This leads to NK cell activation of anti-HIV functions. NK cells express pre-stored IFN-γ transcripts, granzyme and perforin and are ready to lyse targets within minutes of activation [7]. They also secrete CCL3, CCL4 and CCL5, which bind and block CCR5, the co-receptor for HIV entry into CD4+ T cells [8].
Epidemiological studies reported that certain KIR/HLA genotypes were associated with slower time to AIDS in HIV-infected individuals implicating NK cells in HIV control [9, 10]. NK cells from individuals carrying the KIR3DS1/Bw4*80I combination, associated with slow progression to AIDS, were superior to those from carriers of either the receptor or ligand alone, in suppressing HIV replication in vitro [11]. The frequency of KIR/HLA genotypes in HESN and in HIV susceptible subjects enrolled in an HIV primary infection cohort was compared to address whether NK cells also play a role at the level of protection from HIV infection. HESN had higher frequencies of a high expression KIR3DL1 homozygous (hmz) genotype termed KIR3DL1*h/*y co-carried with HLA-B*57 (*h/*y+B*57) and the KIR3DS1hmz genotype [12, 13]. A survival analysis performed on longitudinally followed HESN and HIV seroconverters found that time to seroconversion was significantly longer in individuals with, than without, these protective genotypes [14].
Functionality of NK cells from carriers of *h/*y+B*57 genotypes
HLA-null cell stimulation of NK cells reveals their functional potential, which is directly related to how potently they were educated during development [15]. HLA-null stimulation of NK cells from *h/*y+B*57 carriers generated higher frequencies of NK cells expressing CD107 (a marker for degranulation) and secreting IFN-γ and TNF-α than NK cells from carriers of other KIR3DL1hmz/HLA-B genotypes [16, 17]. These triple functional cells exhibited a higher intensity of each of these functions than their mono-functional counterparts [17]. Thus, the *h/*y+B*57 genotype encoded iKIR receptor/HLA-B*57 ligand combinations that were particularly potent educating pairs that supported the development of NK cells with high functional activity. NK cells from *h/*y+B*57 carriers inhibited the replication of autologous iCD4 cells more potently than those from carriers of non-protective KIR/HLA genotypes [18]. Induction of NK cell inhibition of HIV replication was dependent on contact with autologous iCD4s. However, once NK cells were activated, their secreted products were sufficient to inhibit HIV replication. Among these, were the CC-chemokines CCL3, CCL4 and CCL5 detected in NK/iCD4 co-culture supernatants as well as by intra-cellular NK cell staining. Levels of CC-chemokines were highest in NK cells from *h/*y+B*57 carriers and higher in stimulated KIR3DL1+ than KIR3DL1− NK cells [18]. Neutralization of all three CC-chemokines in NK/iCD4+ co-cultures reversed inhibition of HIV replication [18]. Thus, the ability of NK cells to inhibit HIV replication is, at least in part, due to their ability to secrete CC-chemokines that block HIV infection of CD4+ T cells [8].
Determinants of NK cell responsiveness to autologous iCD4
Natural killer cells respond to stimulation with autologous iCD4. By using fluorochrome conjugated antibody panels it is possible to gate on NK cell populations expressing defined inhibitory NKRs (iNKR) and to detect anti-viral functions such as secretion of IFN-γ/CCL4 and expression of CD107a by flow cytometry. This strategy has been used to explore the factors governing how NK cells from HIV-uninfected persons respond to their first encounter with autologous iCD4.
NKG2A/CD94 is an iNKR expressed on almost all CD56bright and on ~40% of CD56dim NK cells. The ligand for NKG2A in HLA-E, a non-classical MHC-Ib antigen whose cell surface expression depends on binding highly conserved peptides from the leader sequence of many HLA-A, B, C and G MHC-I molecules [19]. HLA-E is expressed on many cell types and maintained on iCD4 [20]. As NKG2A is an iNKR, the interaction of NKG2A with HLA-E on iCD4 should inhibit NK cell activation. However, the highest frequency of functional NK cells induced by autologous iCD4 were NKG2A+ NK cells within both the CD56bright and CD56dim compartments [21]. This NKG2A+ NK cell activation occurs because HLA-E on iCD4 presents an invariant HIV Gag derived peptide that abrogates NKG2A recognition and prevents negative signalling through this receptor [22].
KIR3DL1+ NK cells are educated in HLA-Bw4 donors and remain uneducated in HLA-Bw6 individuals expressing no HLA-Bw4 antigens. Autologous iCD4 triggered higher frequencies of responsive KIR3DL1+ NK cells from Bw4 than Bw6 donors [21, 23]. The frequency of responsive KIR3DL1+ NK cells was higher when from subjects carrying 1 copy than 2 copies of HLA-Bw4 [23]. This finding is consistent with the ability of HIV Nef to downmodulate co-dominantly expressed HLA antigens on iCD4 to a threshold low enough to interrupt negative signaling through KIR3DL1. It may be easier to achieve this threshold in Bw4/Bw6 heterozygotes encoding half the number of HLA-Bw4 antigens than do Bw4hmz [5, 24]. It is notable that secretion of CCL4, which has the potential to limit HIV infection, was the main contributor to total NK cell responsiveness to iCD4 [8, 21, 23].
HLA-C antigens can be dichotomized into C1 and C2 sub-groups. The HIV isolate used to infect CD4 cells in these studies retained cell surface HLA-C, making it available for interaction with KIR2DL3 on NK cells educated through this receptor, the consequence of which is to inhibit KIR2DL3+ NK cell activation. KIR2DL3+ NK cells are educated in HLA-C1 donors but remain largely uneducated in HLA-C2hmz [25]. The frequency of KIR2DL3+ NK cells from C1hmz responding to autologous iCD4 was lower than that of C2hmz. As for KIR3DL1+ NK cells, a ligand dosage effect was observed where iCD4 from C1hmz suppressed KIR2DL3+ NK cell function more potently than did those from C1/C2 heterozygotes. The 2 copies of C1 ligand expressed on iCD4 from C1hmz likely engaged KIR2DL3 more potently that the CI expressed on iCD4 from C1/C2 heterozygotes, resulting in more potent inhibition of KIR2DL3+ NK cell function [23]. HLA-C is expressed on the cell surface at lower levels than HLA-A and -B antigens [26]. This may also contribute to the less potent KIR2DL3+ NK cell inhibitory capacity of iCD4 from C1/C2 carriers than C1hmz subjects. Secretion of CCL4 was the main contributor to the total KIR2DL3+ NK cell responsiveness to iCD4.
Functionality of KIR3DS1+ NK cells from KIR3DS1hmz
The KIR3DS1hmz genotype is also associated with protection from HIV infection [13, 14]. The notion that the ligand for KIR3DS1 was a subset of Bw4 antigens with an isoleucine at position 80 of the HLA heavy chain (Bw4*80I) has recently been debunked in favor the non-classical MHC-Ib antigen, HLA-F [27]. HLA-F is expressed on the HLA-null 721.221 and K562 cells and on HIV-infected cells [27].
The KIR genetic region is not only polymorphic but also polygenic. Next generation sequencing of this region showed that genes in the centromeric and telomeric KIR regions are in linkage disequilibrium with each other and separated by a recombination hotspot [28]. KIR3DS1 lies in a telomeric KIR B haplotype region know as TB01. When KIR3DS1 is present so is the linked adjacent gene, KIR2DL5 [28]. The linkage disequilibrium (LD) between the 2 genes raises the possibility that genes in LD with KIR3DS1 are responsible for the observed association of the KIR3DS1hmz genotype with HIV resistance observed in HESN. Although the KIR genes in the TB01 region generally segregate together, their gene products are stochastically expressed in NK cell populations. When NK cells from KIR3DS1hmz expressing all possible combinations of KIR3DS1 and KIR2DL5 were stimulated with HLA-F+ 721.221 HLA-null cells, only the NK cell populations expressing KIR3DS1 responded above background levels while expression of KIR2DL5 made no contribution (Jackson et al., submitted). Thus, KIR3DS1+ NK cells are functional and activatable by HLA-F+ cells. Further investigation is needed to confirm that these NK cells also respond to autologous iCD4 and that responsiveness can be ascribed to the interaction of KIR3DS1 with HLA-F on iCD4.
Conclusions
Genotypes encoding NKRs that are overrepresented in HESN confer NK cells with superior functional potential to HLA-null cells and with superior responses to autologous iCD4. NK cell education is important in the responsiveness of NK cell populations to iCD4. The higher functionality, in terms of CCL4 secretion, of educated KIR3DL1+ NK cells may be a mechanism underlying the superior viral control and slower time to AIDS observed in epidemiological studies for some carriers of KIR3DL1hmz/Bw4 compared to carriers of other KIR/HLA genotypes [10]. The functionality of NK cell populations to autologous iCD4 is not only dependent on their education but also on HIV-mediated changes in MHC-I expression levels. An important component of the anti-viral activity of stimulated NK cells is secretion of CC-chemokine, which can block HIV entry into new CD4+ target cells.
That NK cells can play a role at the level of protection from HIV infection and in slowing HIV disease course prompts a consideration of how to harness their anti-HIV activity for preventative and therapeutic vaccine development. It may be possible to activate NK cells for HIV directed effector functions by using vaccines that induce anti-HIV specific antibodies whose Fc portions interact with the aNKR CD16. Mechanisms such as this one are likely what accounted for the success of the RV144 Thai HIV vaccine trial in preventing HIV infection [29, 30]. Another avenue to explore that is in its infancy at present, is to find ways to induce memory-like NK cells that may be able to respond faster and more potently to HIV exposure than conventional NK cells [31].
References
Meyers AF, Fowke KR. International symposium on natural immunity to HIV: a gathering of the HIV-exposed seronegative clan. J Infect Dis. 2010;202(Suppl 3):S327–8.
Scott-Algara D, Truong LX, Versmisse P, David A, Luong TT, Nguyen NV, Theodorou I, Barre-Sinoussi F, Pancino G. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171(11):5663–7.
Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376.
Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–13.
Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10(6):661–71.
Apps R, Del Prete GQ, Chatterjee P, Lara A, Brumme ZL, Brockman MA, Neil S, Pickering S, Schneider DK, Piechocka-Trocha A, et al. HIV-1 Vpu mediates HLA-C downregulation. Cell Host Microbe. 2016;19(5):686–95.
Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198(7):1069–76.
Oliva A, Kinter AL, Vaccarezza M, Rubbert A, Catanzaro A, Moir S, Monaco J, Ehler L, Mizell S, Jackson R, et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest. 1998;102(1):223–31.
Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31(4):429–34.
Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, Colombo S, Brown EE, Shupert WL, Phair J, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39(6):733–40.
Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, Streeck H, Waring M, Meier A, Brander C, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204(12):3027–36.
Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS. 2008;22(12):1487–91.
Boulet S, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS. 2008;22(5):595–9.
Tallon BJ, Bruneau J, Tsoukas CM, Routy JP, Kiani Z, Tan X, Bernard NF. Time to seroconversion in HIV-exposed subjects carrying protective versus non protective KIR3DS1/L1 and HLA-B genotypes. PLoS ONE. 2014;9(10):e110480.
Johansson S, Johansson M, Rosmaraki E, Vahlne G, Mehr R, Salmon-Divon M, Lemonnier F, Karre K, Hoglund P. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med. 2005;201(7):1145–55.
Boulet S, Song R, Kamya P, Bruneau J, Shoukry NH, Tsoukas CM, Bernard NF. HIV protective KIR3DL1 and HLA-B genotypes influence NK cell function following stimulation with HLA-devoid cells. J Immunol. 2010;184(4):2057–64.
Kamya P, Boulet S, Tsoukas CM, Routy JP, Thomas R, Cote P, Boulassel MR, Baril JG, Kovacs C, Migueles SA, et al. Receptor–ligand requirements for increased NK cell poly-functional potential in *h/*y+B57 HIV-1 infected slow progressors. J Virol. 2011;85:5949–60.
Song R, Lisovsky I, Lebouche B, Routy JP, Bruneau J, Bernard NF. HIV protective KIR3DL1/S1-HLA-B genotypes influence NK cell-mediated inhibition of HIV replication in autologous CD4 targets. PLoS Pathog. 2014;10(1):e1003867.
Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187(5):813–8.
Ward JP, Bonaparte MI, Barker E. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS. 2004;18(13):1769–79.
Lisovsky I, Isitman G, Song R, DaFonseca S, Tremblay-McLean A, Lebouche B, Routy JP, Bruneau J, Bernard NF. A higher frequency of NKG2A+ than of NKG2A− NK cells respond to autologous HIV-infected CD4 cells irrespective of whether they co-express KIR3DL1. J Virol. 2015;89(19):9909–19.
Davis ZB, Cogswell A, Scott H, Mertsching A, Boucau J, Wambua D, Le GS, Planelles V, Campbell KS, Barker E. A conserved HIV-1-derived peptide presented by HLA-E renders infected T-cells highly susceptible to attack by NKG2A/CD94-bearing natural killer cells. PLoS Pathog. 2016;12(2):e1005421.
Lisovsky I, Isitman G, Tremblay-McLean A, Song R, DaFonseca S, Lebouche B, Routy JP, Bruneau J, Bernard NF. The differential impact of natural killer (NK) cell education via KIR2DL3 and KIR3DL1 on CCL4 secretion in the context of in vitro HIV infection. Clin Exp Immunol. 2016;186:336–46.
Brodin P, Lakshmikanth T, Mehr R, Johansson MH, Duru AD, Achour A, Salmon-Divon M, Karre K, Hoglund P, Johansson S. Natural killer cell tolerance persists despite significant reduction of self MHC class I on normal target cells in mice. PLoS ONE. 2010;5(10):e13174.
Moesta AK, Parham P. Diverse functionality among human NK cell receptors for the C1 epitope of HLA-C: KIR2DS2, KIR2DL2, and KIR2DL3. Front Immunol. 2012;3:336.
Apps R, Meng Z, Del Prete GQ, Lifson JD, Zhou M, Carrington M. Relative expression levels of the HLA class-I proteins in normal and HIV-infected cells. J Immunol. 2015;194(8):3594–600.
Garcia-Beltran WF, Holzemer A, Martrus G, Chung AW, Pacheco Y, Simoneau CR, Rucevic M, Lamothe-Molina PA, Pertel T, Kim TE, et al. Open conformers of HLA-F are high-affinity ligands of the activating NK-cell receptor KIR3DS1. Nat Immunol. 2016;17(9):1067–74.
Pyo CW, Guethlein LA, Vu Q, Wang R, Abi-Rached L, Norman PJ, Marsh SG, Miller JS, Parham P, Geraghty DE. Different patterns of evolution in the centromeric and telomeric regions of group A and B haplotypes of the human killer cell Ig-like receptor locus. PLoS ONE. 2010;5(12):e15115.
Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–20.
Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–86.
Reeves RK, Li H, Jost S, Blass E, Li H, Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, et al. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol. 2015;16(9):927–32.
Acknowledgements
The author wishes to acknowledge the contribution of the study participants.
Competing interests
The author declares that she has no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Boards of the Centre Hospitalier de l’Université de Montréal and the McGill University Health Centre. All subjects provided written informed consent for the collection of samples and subsequent analyses.
Funding
This work was supported by the Canadian Institutes for Health Research (CIHR) MOP 123800 and MOP-142494 and the Fonds de la Recherche du Québec-Santé (FRQ-S) AIDS and Infectious Diseases Network. The funding bodies played no role in the design, analysis, interpretation or writing of this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bernard, N.F. Natural killer (NK) cell receptor-HLA ligand genotype combinations associated with protection from HIV infection: investigation of how protective genotypes influence anti HIV NK cell functions. AIDS Res Ther 14, 38 (2017). https://doi.org/10.1186/s12981-017-0172-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12981-017-0172-9