Abstract
The aging process intricately involves immune system dynamics, with a crucial role in managing senescent cells (SNCs) and their senescence-associated secretory phenotypes (SASPs). Unfortunately, immunosenescence, a progressively dysregulated immunity with age, hampers effective SNC elimination, leading to accumulation, coupled with the release of SASPs, which, in turn, inhibits immunity and heightened susceptibility to aging-associated diseases (AADs). Natural killer (NK) cells, integral to the innate immune system, play a pivotal role in addressing SNCs swiftly. These cells also coordinate with other components of both innate and adaptive immunity to surveil and eliminate these cells. Accordingly, preserving NK cell function during aging is crucial for evading AADs and promoting healthy aging. Alternatively, NK-cell-based therapies present promising avenues for addressing the challenges associated with aging. Notable, recent studies in adoptive NK cell therapy have shown promise in rejuvenating immunosenescence, eliminating SNCs, and alleviating SASPs. This progress provides the proof-concept of adoptive NK cell therapy for senotherapy and holds promise as an emerging revolution in longevity therapeutics.
Similar content being viewed by others
Introduction
As the global population ages, the prevalence of associated diseases becomes increasingly apparent [1]. The pursuit of healthy aging, characterized by heightened resistance to lethal diseases, is the cornerstone of preventive medicine [2]. The aging process is a complex process involving cellular senescence and inflammation [3, 4], with the immune system playing a pivotal role in managing these aspects [3,4,5]. Timely clearance of senescent cells (SNCs) is central to maintaining tissue and organismal homeostasis [6, 7]. Unfortunately, immunosenescence, a progressively dysregulated immune state with age [8], fails to eliminate SNCs, leading to their accumulation. This often coincides with the release of senescence-associated secretory phenotypes (SASPs), inhibiting immunity and increasing vulnerability to aging-associated diseases (AADs) [3, 9, 10]. Consequently, targeting immunosenescence and SNCs emerges as a crucial therapeutic strategy to preserve and extend healthy aging [8, 11, 12]. While adaptive immunity has traditionally taken center stage in immunogerontological studies [11], growing evidence underscores the substantial impact of innate immunity in AADs [3,4,5]. Natural killer (NK) cells, integral to the innate immune system, uniquely identify and eliminate aberrant cells such as tumor and virus-infected cells [12,13,14,15,16]. Moreover, NK cells promptly address SNCs [4, 6, 7], and coordinate with other immune components through cytokine and chemokine production to surveil and eliminate cancer cells [17, 18]. Although whether the same occurs against SNCs remains to be determined. Evidence from healthy elderly individuals, especially those exhibiting physical fitness, independence in daily activities, or adequate cognitive function, the number and function of NK cells are highly preserved [19,20,21,22]. Conversely, diminished NK cell activity in elderly individuals is associated with disorders such as atherosclerosis [23] and an elevated risk of mortality [24, 25]. Accordingly, preserving NK cell function during aging is deemed crucial for healthy aging and longevity [4, 9]. Alternatively, NK-cell-based therapies, notably adoptive NK cell therapy, aligning with their established role in cancer and viral infection treatments [13, 16], show promise in rejuvenating immunosenescence, eliminating SNCs and alleviating SASPs, that lead to AADs [26,27,28]. This short review will delve into this issue to exploring the potential of adoptive NK cell therapy in fostering healthy aging and longevity.
Cellular senescence and aging-associated diseases
Cellular senescence, marked by irreversible cell growth arrest [29], serves crucial roles in development and tissue repair [6, 7], preventing abnormal cell proliferation and suppressing tumor growth [30] to maintain tissue homeostasis. However, with aging, SNCs progressively accumulate, often releasing SASPs [31]. This excessive presence of SNCs and SASPs disrupts tissue function, impacting neighboring cells and standing as a key contributor to AADs [10, 32, 33], ultimately curtailing the healthy lifespan [34]. Distinct populations of SNCs drive specific AADs, encompassing cancer [35], cardiovascular diseases [36], neurodegenerative diseases [37], and osteoarthritis [38]. Senolytic drugs, proven to eliminate SNCs in animal models and mitigate SASPs, exhibit promise in delaying or alleviating age-associated pathologies while extending lifespan [39]. Nevertheless, translating senolytic drugs to human use faces challenges due to the heterogeneity of SNCs and potential toxicity risks [40]. Therefore, exploring alternative methods that safely eliminate SNCs in humans is imperative.
NK cells: guardians against senescent cells
NK cells, emerging from the bone marrow, play a crucial role in identifying and eradicating aberrant cells, including SNCs [4, 13,14,15,16]. Beyond their direct elimination prowess, NK cells contribute to immune responses by secreting cytokines and chemokines, collaborating with other components of innate and adaptive immunity [17, 18]. Constituting 10–20% of the circulating lymphocyte pool, NK cells exhibit distinct subsets based on CD56 expression density. Roughly 90% of peripheral blood NK cells are CD56dim, known for heightened cytotoxicity but lower cytokine and chemokine production upon activation. In contrast, the 10% CD56bright NK cells excel in proliferation and produce a diverse array of cytokines and chemokines, albeit with minimal cytotoxicity [19]. The delicate equilibrium of NK cell function hinges on the interplay of activating and inhibiting signals through receptor interactions with ligands on target cells [41]. A plethora of NK cell receptors, ligands, and associated functions have been identified [42]. When activating signals surpass inhibitory ones beyond a critical threshold, NK cells spring into action, eliminating aberrant cells and secreting cytokines and chemokines that synchronize with other immune components. Notably, MHC class I chain-related (MIC) A/B and poliovirus receptor CD155, expressed on SNCs, act as activating receptor ligands (NKG2D and DNAM-1, respectively) [43]. Studies in vitro and in animal models have shown that NK cells actively eliminating SNCs [44, 45]. Thus, NK cells wield the ability to immune surveillance SNCs, instigating a response for their elimination [4, 5]. The orchestrated clearance of SNCs by NK cells is pivotal for upholding tissue homeostasis [4, 6, 7].
NK cell immunosenescence and its impact on aging-associated diseases
The immune system is pivotal in handling both foreign organisms and aberrant cells [8, 13,14,15,16]. Numerous studies underscore the involvement of immune cells—macrophages, NK cells, and cytotoxic T cells—in the ongoing surveillance of SNCs [4, 12]. Immunosenescence, a process characterized by declining immunity with age, is a hallmark of aging that affects both innate and adaptive immunity. It involves alterations in immune cells numbers, phenotypic and functional changes, and extends beyond defects in the clearance of SNCs, contributing to another hallmark of aging-inflammation [15, 46]. This decline results in the evasion of SNCs from immune detection, leading to their persistent accumulation and the release of SASPs [3]. This cumulative immunosenescence significantly contributes to the heightened occurrence of chronic conditions in older individuals, collectively referred to as AADs, encompassing cancer, cardiovascular diseases, neurodegenerative diseases, and osteoarthritis [35,36,37,38] (Fig. 1). The aging process brings about distinct changes in human NK cells. This includes a gradual decrease in the CD56bright/CD56dim subset ratio, phenotypic alterations (e.g., reduced expression of activating receptors like NKp30), an expansion of CD56dim CD57+ NK cells, and a decline in cytokine secretion and cytotoxicity against target cells [15, 20, 47,48,49,50]. Although the age-associated impairment of NK cells is well-documented in the context of tumor cells, it necessitates further elucidation regarding their efficacy in eliminating SNCs. Nonetheless, the immunosenescence of human NK cells emerges as a significant contributor to AADs [9, 12, 51], given their pivotal role in surveilling SNCs, the accumulation of which is a primary driver of SASPs with aging [10, 32, 33] (Fig. 1). NK cells, as innate immune responders, directly eliminate SNCs through granule exocytosis, with death-receptor pathways playing a limited role [52]. Furthermore, NK cells collaborate with other immune cells, such as macrophages and cytotoxic T cells, forming an interconnected network for comprehensive immunosurveillance against SNCs [17, 18] (Fig. 1). Impaired immunosurveillance accelerates the accumulation of SNCs and SASPs [52]. In light of this, the development of intervention strategies to rejuvenate or restore immunity, including NK cell function, holds promise for preventing and treating AADs. This approach envisions individuals aging without the burden of diseases [11]. Nonetheless, human studies emphasize that extrinsic factors, particularly lifestyle choices, significantly impact the number and function of NK cells [15]. Hence, promoting a healthy lifestyle becomes imperative, offering a favorable avenue to prevent NK cell immunosenescence and fostering successful aging [15].
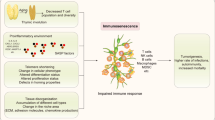
The schematic illustration outlines the potential of adoptive NK cell therapy in longevity therapeutics. Adoptive NK cell therapy directly targets and remove senescent cells (SNCs), resulting in the alleviation of senescence-associated secretory phenotypes (SASPs) and the rejuvenate and/or restore immunity. This intervention breaks the vicious cycle, ultimately reducing the burden of aging-associated diseases (AADs) (solid lines). Abbreviations: AADs, aging-associated diseases; NK cell, natural killer cell; SASPs, senescence-associated secretory phenotypes; SNCs, senescent cells
Adoptive NK cell therapy: a cutting-edge approach for aging and aging-associated diseases
Adoptive NK cell therapy entails introducing ex vivo activated NK cells directly into a patient. As meticulously reviewed by Myers et al., there’s ongoing exploration of various sources for therapeutic NK cells, potentially customizable to target SNCs [53]. Given their broad cytotoxicity and collaborative interactions with innate and adaptive immunity, NK cells emerge as promising candidates for senotherapy. Autologous NK cell therapy, involving the expansion of a patient’s own NK cells ex vivo, offers practical advantages in terms of ease of procurement and sidesteps challenges associated with HLA mismatch. This approach has undergone safety and tolerance assessments in cancer patients [54, 55]. Our research group has pioneered a feeder-cell-free method for substantial NK cell expansion, resulting in heightened expression of activating receptors, increased cytotoxicity, and elevated cytokine production compared to resting NK cells for clinical studies [54, 56]. Notably, these expanded NK cells demonstrated superior cytotoxicity against senescent fibroblasts in vitro [27]. Recent human studies underscore the effectiveness of adoptive autologous NK cell therapy in eliminating SNCs from peripheral blood mononuclear cells (PBMCs) and CD3+ T cells, as evidenced by markers p16 and β-galactosidase for up to 90 days post-infusion [27, 28]. This led to reduced specific T cell subsets, along with a decrease in well-defined inflammatory cytokines in both human [26] and mice [28]. This suggests a rejuvenated immunity, a reduced inflammatory burden [26, 28], and diminished senescence in various tissues of aged mice post-NK cell infusion, including the liver, kidney, lung, fat, and eye [28]. Moreover, documented cases reveal that two NK cell infusions alleviated human immunosenescence for more than a year [27]. Importantly, SNCs can activate NK cells, evidenced by increased expression levels of CD69 and perforin [28]. In essence, adoptive NK cell therapy holds the potential to rejuvenate and restore immunity by eliminating SNCs from PBMCs and tissues, thereby alleviating immunosenescence and SASPs (Fig. 1). This groundbreaking approach has transformative potential to mitigate the detrimental features of AADs, marking an innovative frontier in ultimate preventative medicine. Nevertheless, these results are not sufficiently powered to draw conclusion on longevity at this stage. Moreover, considering the therapeutic efficiency, establishing the preferred optional culture condition is crucial to ensure the expansion is non-senescent NK cells for improving NK cell functions. Furthermore, it would be necessary to verify whether aging impacts the expansion of NK cells.
Advantages of adoptive NK cell therapy compared with existing therapeutic modalities
Recent medical innovations have led to development of various methods and strategies to rejuvenate the immune system, such as nutrition, exercise, hormonal products, and other supplements. It must be stated that no well-established method beneficially impacts on the very complex nature of immunosenescence yet [11, 25]. There is also growing interest in the development of small-molecule drugs that target poorly defined molecular on SNCs and must be administered repeatedly overtime, producing substantial side effects [40]. Unlike small molecules, adoptive NK cell therapy has undergone safety and tolerance assessments and has the potential to persist and mediate the potent effects after single administration [26, 27]. Additionally, NK cell can migrate to SNCs in normal tissues by the local inflammatory environment produced by SNCs and eliminate them [28]. This in contrast to the immunosuppressive microenvironment created by tumor cells, which may impede the therapeutic effects. Consequently, adoptive NK cell therapy may have broad therapeutic potential mitigate the detrimental features of AADs.
Mechanistic insights and biomarkers of adoptive NK cell therapy for serotherapy
NK cells communicate with other immune components and promptly address SNCs [4, 6, 7, 44]. Chelyapov et al., described a studying in vitro where activated NK cells attack SNCs on highly cooperated level [27]. They assessed the senescent markers, p16 and β-galactosidase, in PBMCs before and after adoptive NK cell therapy from 5 healthy individuals, supporting the removal of immunosenescent cells in human [27]. Similarly, Tang et al., uncovered that senescence and exhaustion T cells were eliminated, and the secretions of SASP factors were decreased after adoptive NK cell therapy [26].
Urokinase plasminogen activator receptor (uPAR), upregulated on SNCs across different cell types, was paralleled with β-galactosidase-positive cells. Plasma levels of soluble uPAR positively correlate with the pace of aging in humans. uPAR chimeric antigen receptor (CAR) T cells can eliminate SNCs and improve aging-associated metabolic dysfunction [57]. Therefore, uPAR may serve as a suitable candidate biomarker of therapeutic efficacy.
Conclusions and future directions
Addressing aging and AADs through the immune elimination of SNCs and the management of age-related inflammation emerges as a promising strategy [4, 5, 12]. The evolution of NK cell research from its established roles in anti-tumor and viral immunity to the clearance of SNCs marks a significant advancement. Although in its early stages, initial studies on adoptive NK cell therapy showcase promising outcomes, including the mitigation of immunosenescence and SASPs, eliminating SNCs [26,27,28] (Fig. 1). While these preliminary findings are encouraging, further exploration is essential. Understanding the intricate mechanisms, clarifying the in vivo mode of action, determining the optimal timing for intervention in the aging process, and establishing the most effective therapeutic NK cell dosage are crucial aspects for deeper insights. Robust clinical trials involving larger cohorts are imperative to confirm the therapeutic role in addressing AADs and extending lifespan. Moreover, investigating alternative sources of therapeutic NK cells, such as allogenic NK cells, umbilical cord NK cells, stem cell-derived NK cells, and CAR-NK cells, holds substantial promise. Unraveling the complexities of preventing, delaying, or reversing NK cell immunosenescence will propel the revolution in longevity therapeutics, marking a paradigm shift in ultimate preventative medicine.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- AADs:
-
Aging-associated diseases
- CAR:
-
Chimeric antigen receptor
- NK cells:
-
Natural killer cells
- PBMCs:
-
Peripheral blood mononuclear cells
- SASPs:
-
Senescence-associated secretory phenotypes
- SNCs:
-
Senescent cells
- uPAR:
-
Urokinase plasminogen activator receptor
References
Kaeberlein M. Longevity and aging. F1000Prime Rep. 2013;5:5.
Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: the ultimate preventative medicine. Science. 2015;350:1191–3.
Fulop T, McElhaney J, Pawelec G, Cohen AA, Morais JA, Dupuis G, Baehl S, Camous X, Witkowski JM, Larbi A. Frailty, Inflammation and Immunosenescence. Interdiscip Top Gerontol Geriatr. 2015;41:26–40.
Antonangeli F, Zingoni A, Soriani A, Santoni A. Senescent cells: living or dying is a matter of NK cells. J Leukoc Biol. 2019;105:1275–83.
Kale A, Sharma A, Stolzing A, Desprez PY, Campisi J. Role of immune cells in the removal of deleterious senescent cells. Immun Ageing. 2020;17:16.
Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67.
Brighton PJ, Maruyama Y, Fishwick K, Vrljicak P, Tewary S, Fujihara R, Muter J, Lucas ES, Yamada T, Woods L, Lucciola R, Hou Lee Y, Takeda S, Ott S, Hemberger M, Quenby S, Brosens JJ. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Elife. 2017;6:e31274.
Weyand CM, Goronzy JJ. Aging of the immune system. Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13:S422–8.
Camous X, Pera A, Solana R, Larbi A. NK cells in healthy aging and age-associated diseases. J Biomed Biotechnol. 2012;2012:195956.
He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169:1000–11.
Fülöp T, Larbi A, Hirokawa K, Mocchegiani E, Lesourds B, Castle S, Wikby A, Franceschi C, Pawelec G. Immunosupportive therapies in aging. Clin Interv Aging. 2007;2:33–54.
Song P, An J, Zou MH. Immune clearance of senescent cells to combat ageing and chronic diseases. Cells. 2020;9:671.
Deng X, Terunuma H. Harnessing NK cells to control metastasis. Vaccines (Basel). 2022;10:2018.
Deng X, Terunuma H, Nieda M. Exploring the utility of NK cells in COVID-19. Biomedicines. 2022;10:1002.
Deng X, Terunuma H, Nieda M. Immunosurveillance of cancer and viral infections with regard to alterations of human NK cells originating from lifestyle and aging. Biomedicines. 2021;9:557.
Terunuma H, Deng X, Dewan Z, Fujimoto S, Yamamoto N. Potential role of NK cells in the induction of immune responses: implications for NK cell-based immunotherapy for cancers and viral infections. Int Rev Immunol. 2008;27:93–110.
Peterson EE, Barry KC. The natural killer-dendritic cell immune axis in anti-cancer immunity and immunotherapy. Front Immunol. 2021;11:621254.
Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE 3rd. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006;66:517–26.
Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40.
Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18:1613–20.
Ligthart GJ, Schuit HR, Hijmans W. Natural killer cell function is not diminished in the healthy aged and is proportional to the number of NK cells in the peripheral blood. Immunology. 1989;68:396–402.
Sansoni P, Cossarizza A, Brianti V, Fagnoni F, Snelli G, Monti D, Marcato A, Passeri G, Ortolani C, Forti E, Fagiolo U, Passeri M, Franceschi C. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–73.
Bruunsgaard H, Pedersen AN, Schroll M, Skinhøj P, Pedersen BK. Decreased natural killer cell activity is associated with atherosclerosis in elderly humans. Exp Gerontol. 2001;37:127–36.
Ogata K, Yokose N, Tamura H, An E, Nakamura K, Dan K, Nomura T. Natural killer cells in the late decades of human life. Clin Immunol Immunopathol. 1997;84:269–75.
Mocchegiani E, Muzzioli M, Giacconi R, Cipriano C, Gasparini N, Franceschi C, Gaetti R, Cavalieri E, Suzuki H. Metallothioneins/PARP-1/IL-6 interplay on natural killer cell activity in elderly: parallelism with nonagenarians and old infected humans. Effect of zinc supply. Mech Ageing Dev. 2003;124:459–68.
Tang X, Deng B, Zang A, He X, Zhou Y, Wang D, Li D, Dai X, Chen J, Zhang X, Liu Y, Xu Y, Chen J, Zheng W, Zhang L, Gao C, Yang H, Li B, Wang X. Characterization of age-related immune features after autologous NK cell infusion: protocol for an open-label and randomized controlled trial. Front Immunol. 2022;13:940577.
Chelyapov N, Nguyen TT, Gonzalez R. Autologous NK cells propagated and activated ex vivo decrease senescence markers in human PBMCs. Biochem Biophys Rep. 2022;32:101380.
Bai Z, Yang P, Yu F, Li Z, Yao Z, Martinez J, Li M, Xu H. Combining adoptive NK cell infusion with a dopamine-releasing peptide reduces senescent cells in aged mice. Cell Death Dis. 2022;13:305.
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. Cellular senescence: defining a path forward. Cell. 2019;179:813–27.
Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27-31.
Byun HO, Lee YK, Kim JM, Yoon G. From cell senescence to age-related diseases: differential mechanisms of action of senescence-associated secretory phenotypes. BMB Rep. 2015;48:549–58.
Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69:S4-9.
Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–35.
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–9.
Milanovic M, Fan DNY, Belenki D, Däbritz JHM, Zhao Z, Yu Y, Dörr JR, Dimitrova L, Lenze D, Monteiro Barbosa IA, Mendoza-Parra MA, Kanashova T, Metzner M, Pardon K, Reimann M, Trumpp A, Dörken B, Zuber J, Gronemeyer H, Hummel M, Dittmar G, Lee S, Schmitt CA. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553:96–100.
Shakeri H, Lemmens K, Gevaert AB, De Meyer GRY, Segers VFM. Cellular senescence links aging and diabetes in cardiovascular disease. Am J Physiol Heart Circ Physiol. 2018;315:H448–62.
Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128:1208–16.
Jeon OH, David N, Campisi J, Elisseeff JH. Senescent cells and osteoarthritis: a painful connection. J Clin Invest. 2018;128:1229–37.
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–56.
Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, Kellogg TA, Khosla S, Koerber DM, Lagnado AB, Lawson DK, LeBrasseur NK, Lerman LO, McDonald KM, McKenzie TJ, Passos JF, Pignolo RJ, Pirtskhalava T, Saadiq IM, Schaefer KK, Textor SC, Victorelli SG, Volkman TL, Xue A, Wentworth MA, Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–56.
Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–58.
Bellora F, Castriconi R, Dondero A, Carrega P, Mantovani A, Ferlazzo G, Moretta A, Bottino C. Human NK cells and NK receptors. Immunol Lett. 2014;161:168–73.
Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foà R, Santoni A. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–11.
Sagiv A, Burton DG, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S, Golani O, Polic B, Krizhanovsky V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging. 2016;8:328–44.
Zang J, Ye J, Zhang C, Sha M, Gao J. Senescent hepatocytes enhance natural killer cell activity via the CXCL-10/CXCR3 axis. Exp Ther Med. 2019;18:3845–52.
Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–46.
Le Garff-Tavernier M, Béziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, Debré P, Merle-Beral H, Vieillard V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9:527–35.
Gayoso I, Sanchez-Correa B, Campos C, Alonso C, Pera A, Casado JG, Morgado S, Tarazona R, Solana R. Immunosenescence of human natural killer cells. J Innate Immun. 2011;3:337–43.
Brauning A, Rae M, Zhu G, Fulton E, Admasu TD, Stolzing A, Sharma A. Aging of the immune system: focus on natural killer cells phenotype and functions. Cells. 2022;11:1017.
Hazeldine J, Hampson P, Lord JM. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell. 2012;11:751–9.
Solana C, Tarazona R, Solana R. Immunosenescence of natural killer cells, inflammation, and Alzheimer’s disease. Int J Alzheimers Dis. 2018;2018:3128758.
Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, Windheim J, Tsoory M, Schirmbeck R, Amit I, Geiger H, Krizhanovsky V. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9:5435.
Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85–100.
Terunuma H, Deng X, Nishino N, Watanabe K. NK cell-based autologous immune enhancement therapy (AIET) for cancer. J Stem Cells Regen Med. 2013;9:9–13.
Nahi H, Chrobok M, Meinke S, Gran C, Marquardt N, Afram G, Sutlu T, Gilljam M, Stellan B, Wagner AK, Blomberg P, Holmqvist PH, Walther-Jallow L, Mellström K, Liwing J, Gustafsson C, Månsson R, Klimkowska M, Gahrton G, Lund J, Ljungman P, Ljunggren HG, Alici E. Autologous NK cells as consolidation therapy following stem cell transplantation in multiple myeloma. Cell Rep Med. 2022;3:100508.
Deng X, Terunuma H, Nieda M, Xiao W, Nicol A. Synergistic cytotoxicity of ex vivo expanded natural killer cells in combination with monoclonal antibody drugs against cancer cells. Int Immunopharmacol. 2012;14:593–605.
Amor C, Fernández-Maestre I, Chowdhury S, Ho YJ, Nadella S, Graham C, Carrasco SE, Nnuji-John E, Feucht J, Hinterleitner C, Barthet VJA, Boyer JA, Mezzadra R, Wereski MG, Tuveson DA, Levine RL, Jones LW, Sadelain M, Lowe SW. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat Aging. 2024;4:336–49.
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, X.D. and H.T.; literature review, X.D.; writing—original draft preparation, X.D.; writing—review and editing, X.D. and H.T. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
X.D. and H.T. hold patents for feeder-cell-free NK cell expansion culture technology, are stockholders and employed by the company of Biotherapy Institution of Japan Inc., a biotech startup company developing NK-cell-based immunotherapy and Mesenchymal Stromal Cell (MSC)-based products, such as adipose MSCs, and MSC-derived conditioned medium (CM) concentrates and extracellular vesicle (EV) isolates (StemSup®) for clinical research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deng, X., Terunuma, H. Adoptive NK cell therapy: a potential revolutionary approach in longevity therapeutics. Immun Ageing 21, 43 (2024). https://doi.org/10.1186/s12979-024-00451-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-024-00451-2