Abstract
Background
Neutrophils and lymphocytes represent the larger percentage of all white blood cells, they vary with age, with a progressive increase of the ratio in the first years of life, and then tend to remain at similar levels in steady state condition during adult age. Neutrophils to lymphocytes-ratio (NL-ratio) was proposed as an effective and low-cost marker to monitor and predict the evolution of several clinical conditions. The main objective of the study is to analyze its temporal trend variation, over twenty years’ follow-up, according to age, sex, and main clinical diagnosis, in a large representative Italian population.
Methods
The InCHIANTI study enrolled representative samples from the registry list of two towns in Tuscany, Italy. Baseline data were collected in 1998, and last follow-up visits were made in 2015-18. 1343 out of the 1453 participants enrolled were included, and consented to donate a blood sample. All subjects were assessed and followed for life-style, clinical condition, physical performance, and underwent an instrumental diagnostic session.
Results
The NL-ratio showed a statistically significant interaction between birth-cohort and time of the study (p-value = 0.005). A gender dimorphism was recognized in the neutrophils absolute count and in the NL-ratio. Moreover, in female participants only, those who reported CHF had lower neutrophil-count and NL-ratio; whereas an increase in creatinine clearance was directly associated with NL-ratio. In male subjects, an increase of BMI was inversely associated with both NL-ratio and neutrophils-count during the follow-up; a similar association but in the opposite direction was observed in female participants.
Conclusion
NL-ratio is a more reliable predictor of healthy aging than absolute lymphocytes and/or neutrophils counts. It is associated with the changes induced by disease, lifestyle, and environmental challenges in the immune system. NL-ratio confirms the gender dimorphism in the occurrence of inflammation-driven diseases, thus providing additional evidence for the necessity of tailored sex-specific measures to prevent and treat such diseases.
Similar content being viewed by others
Introduction
The immune system is a homeostatic system that contributes to the appropriate function of the whole organism [1]. With aging, a less efficient immune system predisposes subjects to an increased risk of age-related morbidity and mortality [2]. Immunosenescence is explained by an imbalance between inflammatory and anti-inflammatory networks, resulting in the low-grade chronic pro-inflammatory status which has been termed inflammaging [3]. Underlying inflammatory processes have been recognized to be co-involved in the genesis and in the perpetuation of different ailments such as ischemic heart disease, stroke, cancer, diabetes mellitus, neurodegenerative conditions, osteoporosis and sarcopenia [4].
Immunosenescence is characterized by high inter-individual heterogeneity in adaptive and in innate immunity responses [5], though the efficacy of an immune response is also affected by changes in absolute numbers (or in the relative proportion) of immune cell subpopulations [1]. An increase in the frequency of an immune cell lineage does not necessarily reflect a good or bad response to a stressor, but according to the remodeling theory of aging, it should be considered the result of a successful or unsuccessful adaptation [6].
The recent COVID-19 pandemic has sparked a revived interest in the variation of cell counts such as neutrophils, lymphocytes, or their ratio, considered as possible markers of disease outcome, and not only in the elderly [7]. Consequently, a boosted circulating innate (neutrophilia) and depressed circulating adaptive immunity (lymphopenia) have been associated with worse disease outcomes and/or severe organ damage in different settings [8].
In more general terms, Wilson et al demonstrated a reduced neutrophil phagocytosis, with reduced trap formation, inaccurate migration and failure to prevent apoptosis, during aging [9]. The same group demonstrated that frailty, an augmented susceptibility to stress damage in the elderly, was associated with neutrophil chemotaxis defect in aged, compared to young not frail subjects [9].
T cells represent the overwhelming majority of circulating lymphocytes, with relative increase (despite absolute decrease of lymphocyte counts) with age [10]. With advancing age, naive T cells are gradually being replaced by highly differentiated memory and senescent cell types. Senescent T cells are dysfunctional immune cells, without division capacity, resistant to apoptosis, and secrete large amounts of pro-inflammatory mediators [11].
The neutrophil-to-lymphocyte ratio (NL-ratio) is a composite marker of the absolute peripheral count of both types of cells, and it has been used to indicate the immune-inflammatory activities of neutrophils and lymphocytes in several clinical conditions such as cancer progression [12], cardiovascular diseases [13], kidney diseases [14], and hypertension [15]. In the Rotterdam study, a long-standing population-based prospective cohort study on aging, NL-ratio levels were independently associated with an increased risk of cardiovascular and all-cause mortality, but not with cancer mortality [16].
No data reporting temporal trends in the variation of absolute counts of lymphocytes and neutrophils, and in their ratio, are available in an aging population, also accounting for gender differences, and in relation to the main clinical conditions. Therefore, the main objective of this study is to assess factors that affect age-trajectories of circulating neutrophils and lymphocytes in the InCHIANTI study, a large longitudinal study conducted in a representative population of the Italian population.
Results
The main characteristics of the population enrolled in the study, both at baseline and at subsequent follow-up times are reported in Table 1. The absolute number of deaths was reported as the number of events registered between consecutive times of the study, and as a percentage of the entire sample (n = 1453). Events reported in the last column (Follow-up 4), refer to deaths that occurred after the last follow-up (from 2015 to 2018). In a period of 20 years 851 deaths were registered (58.6% of the entire sample).
Chronological age represents the population’s mean age at the specific follow-up; whereas, age at baseline represents the mean age of those subjects who were alive, in the specific follow-up, at the enrollment in the study. The mean leukocytes count, and their ratio showed small variation across the study follow-up.
Age and time effect
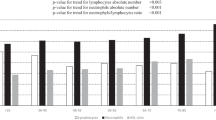
Lymphocyte counts decreased linearly according to year of birth-cohort (p < 0.001), and no significant differences were found across the follow-up times (Fig. 1.A). On the contrary, neutrophil counts had a direct association with birth-cohort, but different slopes for different times of the study were found (Fig. 1B; for the interaction p-value = 0.04). Also, the NL-ratio showed a linear direct association with birth cohort, and the slopes of this association varied at different follow-up times (Fig. 1C; for the interaction p-value = 0.005).
Sex effect
We observed no gender dimorphism for lymphocyte counts (Table 2 section-A); whereas for neutrophil counts and the NL-ratio different slopes were found for the association of sex with birth-cohort, but no differences for the interaction between the follow-up times of the study and sex (Table 2 sections B-C). Interestingly, the NL-ratio shows a multiplicative effect for age and time of the study (0.0008 ± 0.0003; p-value = 0.002).
Diseases effect
Lymphocyte counts were not associated with any of the diseases considered, namely: diabetes, stroke, cancer, congestive heart failure, and renal failure (data not reported). Due to gender dimorphism in the neutrophil counts and NL-ratio, subsequent analyses were conducted separately in males and females. In both sexes, not statistically significant first and second-order effect for neoplastic diseases, diabetes, and stroke could be found in the models with NL-ratio and neutrophil count variations as dependent variables (additional Tables 2–4).
In females only, congestive heart failure (CHF) was directly associated with the NL-ratio and neutrophil count (0.782 ± 0.403; p = 0.05, and 39.698 ± 12.068; p = 0.001, respectively), and for both markers the second order (age for CHF interaction) effect resulted in an attenuation of the associations (-0.011 ± 0.005; p = 0.03, and − 0.057 ± 0.015; p < 0.001, for NL-ratio and neutrophil counts, respectively) (Figs. 2.A and B and additional Table 5). A similar picture was observed for Creatinine Clearance (CC), which was directly associated with the NL-ratio and neutrophil count (0.004 ± 0.001; p = 0.005; 0.019 ± 0.005; p < 0.001, respectively), and for both, the second order (age for CC interaction) effect resulted in an attenuation of the associations (-0.0001 ± 0.00002; p-value < 0.001; -0.0003 ± 0.0001, p-value < 0.001, NL-ratio and neutrophil count respectively) (additional Table 6),
Other potential confounders
In male subjects, the interaction between the times of the study and BMI was inversely associated with both the NL-ratio and the neutrophil count (-0.006 ± 0.002; p < 0.001; -0.016 ± 0.005; p = 0.003, respectively). The opposite was observed for females, where a direct association was found (0.002 ± 0.001, p = 0.04; 0.007 ± 0.003, p = 0.03) (additional Table 7).
Finally, cigarette smoking and alcohol intake were not associated with any of the parameters evaluated (lymphocytes count, neutrophil count, and NL-ratio).
Discussion
The main findings of this study demonstrate that only the NL-ratio has a direct association with aging, which is demonstrated by a the statistically significant interaction between the birth-cohort effect and time effect. A gender dimorphism was found for the neutrophil absolute count and NL-ratio; for the same birth year, females had a lower number of neutrophils and a lower NL-ratio, compared to males.
Lymphocytes absolute number decreases inversely to birth-cohort, independently from sex. Sex modulates these two blood markers when categorized according to CHF and Creatinine Clearance. An inverse correlation between the birth-cohort and both neutrophils count, and NL-ratio was present only in females who developed CHF. In this same female group, higher values of all markers were measured in younger subjects, having similar CC values. Body mass index modulates the association between the blood markers and age differently according to sex, inversely in males and directly in females.
Aging
Circulating blood cells such as leukocytes, lymphocytes, and neutrophils, are widely utilized as markers of aging-related systemic inflammation. One of the most economical and widely available clinical markers of peripheral inflammation is the NL-ratio. Variation in the NL-ratio has previously been reported to be predictive of poorer prognosis [17], longer stay after hospitalization for major illnesses, frailty [18] and disability. Interestingly NL-ratio was also reported to be cross-sectionally associated with Alzheimer’s Dementia (AD) [19]; but in the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL cohort), longitudinal analysis of NL-ratio variation across time was limited for the problem of diagnosing AD, and unable to predict the transition from mild cognitive impairment to AD [20]. Such correlations were weak, and disappeared when age and sex were considered, indicating that those covariates, rather than the underlying disease process, drove the changes observed in the ratio [20]. In the Rotterdam study the NL-ratio levels were associated with an increased risk of all-cause mortality, independently from age; however the authors did not assess a potential association between age and NL-ratio [16]. In the InCHIANTI study, the NL-ratio showed a departure from a linear trend, but maintained a direct correlation with age, and, more interestingly, with the interaction between birth-cohort and times of the study (aging effect). This would possibly suggest that the NL-ratio might be a worthwhile and stable marker of healthy aging.
Both the innate and the adaptive immune systems are dynamically remodeled with aging, in a process which is known as immunosenescence, characterized by great heterogeneity [21]. Adaptive immunity changes can be tracked to a reduction of lymphocytes, mainly due to a decreased thymic output [22], and with an imbalanced CD4:CD8 ratio, a decrease in the number of CD4 cells and a contextual increase in the number of CD8 cells [23].
Peripheral T lymphocytes encompass a heterogeneous mix of cellular types, namely naive, effector, and memory cells. They have different lifespan and continuously recirculate through the lymphatic system. Investigations into the dynamics of T cell turnover have demonstrated that most peripheral T cells can remain in a quiescent state for prolonged periods, spanning months in rodents and even years in humans. Notably, typical naive T cells are known for their remarkable longevity as they persist as quiescent cells [24]. Conversely, effector and memory T cells exhibit a more rapid turnover, indicating their active involvement in immune responses [25]. Senescent-like features in highly differentiate T cells are characterized by a reduced proliferation rate, shorter telomere length and increased levels of p38 [26]. These senescent-like T cells express NK cell receptors and high levels of cytotoxic molecules, that might be responsible for tissue damage [27]. Also B cells show senescence signature mainly characterized by a pro-inflammatory senescence associated secretory phenotype [28]. An expanded subset of B cells that accumulate with age (age-associated B cells) [29] plays a significant role in various aspects of immunosenescence [30] and they are found at higher levels in autoimmune and autoinflammatory diseases [29]. The numbers of B lymphocytes are greatly reduced in aged individuals [10] but their function is important for the aging process.
Neutrophils are part of the innate immune system and represent the main effector cells against bacterial infections. Neutrophils also play a critical role in disease control, as in cancer [31]. The absolute number of neutrophils does not change significantly in immunosenescence [9]; they show a short life [32] (less than one day), coupled with a high production rate in the bone marrow (5–10×1010 neutrophils-day) [33]. During aging neutrophils show a decline in phagocytic functions that lead to an imbalance of tissue homeostasis [34]. Their senescence features include shortened telomere length, reduced proliferation, and a pro-inflammatory senescence associate secretory phenotype [34, 35].
In our study, the absolute number of circulating lymphocytes decreased with age independently from sex, whereas neutrophil count increased. This accounts for the declining NL ratio, however the reasons for the opposite trend are uncertain.
These results could be explained considering that immune system function undergoes a profound remodeling with age, even if it was extremely interindividual heterogeneous (immunobiography) [36]. Elderly subjects show an increased risk for infective and degenerative disease manifestations, and that is thought to be a phenomenon associated with a less efficient adaptive immune response [4]. On the contrary, some features of innate immunity seem to be preserved in immunosenescence; for example, inflammation is not dampened with age [37]. The aging-related exhaustion of the adaptive immune system recognized large and complex interactions of factors as genomic instability (shortening of telomeres) [38], epigenetic regulation (DNA methylation, histone modification, and noncoding RNAs) [39], damage to mitochondrial function (reducing energy availability), as well as hormones imbalance, multimorbidity and environmental causes[40, 41]. The prospective character of the study, with 20 years of follow-up (from 1998 to 2018), has enabled us to better capture this trend in the aging population.
Gender
Sex differences in the immune responses have been extensively demonstrated [40, 42]. Generally, females have a more efficient innate and adaptive immune response than males, and this apparent beneficial effect, is counterbalanced by female increased susceptibility to inflammatory and autoimmune diseases [43]. Reduced immune function in men might represent an example of pleiotropic antagonism, i.e. a side effect of positive selection for other traits, such as reproductive success or enhanced metabolism [44].
Lymphocyte absolute numbers did not differ between sexes, but a gender dimorphism was found in neutrophils count, with females showing higher numbers and NL-ratio, compared to males. With aging this sex-biased gap tended to be tapering, as demonstrated by the significant inverse interaction between age and sex [45]. These differences might plausibly be due to age-related changes in hormonal levels and immunological remodeling. For example, after a single i.m. injection of 17beta-oestradiol in male subjects, the number of neutrophils in blood doubled, although no significant changes in adhesion molecules were measured [46]. Androgens suppress the pro-inflammatory responses via inhibition of leukotriene formation in neutrophils [47]; progesterone enhance prostaglandin production of activated macrophages in female murine models inhibiting nitric-oxide production [48], whereas in a male murine model, estrogen treatment improves cellular immunity through NF-kB activation and reduced IL-6 production [49].
An alternative hypothesis explaining the gender differences considers the sexually dimorphic gene expression. The X chromosome is more complex than the Y chromosome, and contains many genes involved in immune function [50], which are thought to be partly responsible for the hyperresponsiveness of the female immune system; for example, males experience more frequent severe infections, on the contrary females are more prone to autoimmune diseases [51]. Those differences are attributable to X chromosome inactivation (XCI) during embryogenesis, female-specific mechanism to equalize gene expression between the sexes [45], and the genes escape silencing, a possible mechanism that would account for the overexpression of X-linked immune genes [52]. Approximately a quarter of X-linked genes are estimated to constitutively escape from XCI in humans and may contribute to the female autoimmune predisposition [52], with higher serum IgM, higher number of B cells, and higher percentage of CD4 + T-helper cells in females compared to males [52].
Diseases
Myocardial infarction is the leading cause of the development of CHF. Neutrophils are the first cells recruited to the site of myocardial injury, and they are of paramount importance in the clearance of necrotic tissue, for the local resolution of inflammation, and in tissue homeostasis [53]. To explain the reason for the increase in the neutrophil count and the NL-ratio only in female subjects with CHF, we must consider reasons beyond the immunological gender dimorphism. Sex differences are reported in heart tissue from the early embryogenesis, and they are epigenetically perpetuated. Those differences may have sex-specific repercussions during organogenesis, persisting through adulthood and producing a different response of tissues to injury [54].
Chronic kidney disease (CKD) progression has been associated with chronic inflammation and specifically with higher NL-ratio [55]. A recent meta-analysis confirmed the NL-ratio predictive value for death in CKD patients, for all causes and for specific cardiovascular mortality [55], suggesting also its use for monitoring inflammation, and renal insufficiency progression [56]. In the InCHIANTI data, creatinine clearance was directly associated with neutrophil count and NL-ratio, although in female subjects only. Younger subjects with low creatinine clearance showed higher levels of the two blood markers, and this figure is in agreement with previous findings in CKD [57].
Body composition
Lastly, in our study an increase in BMI across the follow-up times accompanies an increase of neutrophils count and NL-ratio in females, whereas in males for an increase in BMI a decrease in both parameters was found. Differences in body composition between sexes are well documented in aging; usually, women have a higher fat mass, and men have a higher muscle mass [58], and excessive fat deposition can cause a proinflammatory state due to adipose tissue secretion of cytokines, and disturbances in metabolic homeostasis [28]. Therefore, also sexual dimorphism in lipid metabolism must be considered and could explain the link among adipose tissue, aging, and chronic inflammation. Alternatively, starvation, malnutrition, as well as single nutrient deficiencies, may modify the balance between adaptive and innate immunity, probably as a consequence of bone marrow failure [59]. Moreover, intermitting fasting and/or caloric restriction response is gender-specific [60]. Even if, in females mice caloric restriction increases adipocytes infiltration, at least in the bone marrow [61] and in the liver [62], compared to males; those observations highlighted how different energy homeostasis is governed by sex hormones, and modulates chronic low grade inflammation.
Conclusion
In the InCHIANTI study, NL-ratio is a more reliable marker of healthy aging compared to absolute lymphocyte and neutrophil counts. Our results confirm also that immunosenescence shows a gender dimorphism, with an imbalance between adaptive and innate immunity. Disease manifestations such as age-related renal insufficiency and congestive heart failure influence immunosenescence in a gender-specific way. Lastly also lifestyle and more specifically body composition affect Inflammaging in a gender-specific way. Therefore, this study provides further evidence for the necessity of a tailored, sex-specific approach to prevention and therapy of age-related conditions.
Method
The InCHIANTI study protocol has been described in detail elsewhere [63]. Briefly, the study was designed by the Laboratory of Clinical Epidemiology of the Italian National Institute of Research and Care on Aging (INRCA, Florence, Italy), and was performed in two small towns in Tuscany. The baseline data were collected in 1998–2000; the three-year follow-up took place in 2001–2003, the six-year follow-up in 2004–2006, the nine-year follow-up in 2007–2009, the twelve-year follow-up 2013–2014, the last follow-up was conducted between 2015 and 2018.
Samples
1453 participants were enrolled at baseline in the InCHIANTI Study; a total of 4632 evaluations were considered (additional Table 1) for those subjects who had at least one assessment, and all variables of interest were available. Participants were all European subjects of Caucasian origin. The Ethical Committee of the Local Health Authority of Florence, Tuscany Region, approved the study protocol, and written informed consent was obtained from each participant.
Blood cell count analysis
Assessments of the number of red blood cells, white blood cells, platelets, hemoglobin concentration and hematometric values were performed through an automated system at the Laboratory of Clinical Chemistry and Microbiological Assays, SS. Annunziata Hospital, Azienda Sanitaria 10, Florence, Italy, using a Hematology SE 9000 Autoanalyzer (Sysmex, Kobe, Japan, provided by DASIT, Milano, Italy) for the Baseline and Follow-up 1 surveys, a Coulter LH 750 Hematology Autoanalyzer (Beckman Coulter Inc, Brea, CA, USA) for the Follow-up 2 and Follow-up 3 surveys, and a Sysmex XE 2100 (DASIT – Milano) for Follow-up 4..
Laboratory tests
Serum Creatinine Level (mg/dL) was measured by the Laboratory of Clinical Chemistry and Microbiological Assays, SS. Annunziata Hospital, Azienda Sanitaria 10, Florence, Italy, using a colorimetric assay (TP, Roche Diagnostics, GmbH, Mannheim, Germany) and a Roche analyzer (Roche Diagnostics, GmbH, Mannheim, Germany). At Baseline, the analyzer was a Hitachi 917. For the follow-ups it was a Modular P800 Hitachi. Glomerular filtration rate was calculated according to Cockcroft-Gault formula [64].
Statistical analysis
Descriptive data are shown as mean ± standard error, and as absolute number and percentages, for continuous and categorical variables respectively. The NL-ratio, due to the non-normal distribution, was log-transformed before the primary analysis. Linear mixed models with random intercept and random slope were applied using time since baseline as the time scale. We present four models that sequentially analyze the variation of blood cell count and their ratio: the first model considers year of birth-cohort, and possible second-order interaction between birth-cohort and time of the study; the second model considers sex effect and possible second-order interactions between sex and birth-cohort, and between sex and time of the study; the third model was sex-specific and considers as dependent variables in different models the effects of diseases (oncological diseases, diabetes, stroke, congestive heart failure, and renal insufficiency using of creatinine clearance); the last model, always stratified for sex considers the variation of BMI during the follow-up times. SAS version 9.4 for Windows (SAS Institute, Inc., Cary, NC) was used for all data processing and statistical analyses. We set the level of statistical significance at p < 0.05 (2-sided).
Availability of data and materials
The datasets used and/or analyzed during the current study are
available from the responsible authors for the InCHIANTI study (Luigi Ferrucci) on reasonable
request. Data of the InCHIANTI study is available to all researchers upon justified request using the
proposal form available on the InChianti website (https://www.nia.nih.gov/inchianti-study, accessed on 04/13/2023).
References
Martínez de Toda I, Maté I, Vida C, Cruces J, De la Fuente M. Immune function parameters as markers of biological age and predictors of longevity. Aging. 2016;8:3110–9. https://doi.org/10.18632/aging.101116.
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–32. https://doi.org/10.1038/s41591-019-0675-0.
Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. https://doi.org/10.1016/j.mad.2006.11.016.
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–90. https://doi.org/10.1038/S41574-018-0059-4.
Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front Immunol. 2019;10:2247. https://doi.org/10.3389/fimmu.2019.02247.
Franceschi C, Ostan R, Mariotti S, Monti D, Vitale G. The Aging Thyroid: A Reappraisal Within the Geroscience Integrated Perspective. Endocr Rev. 2019;40:1250–70. https://doi.org/10.1210/er.2018-00170.
Asperges E, Albi G, Zuccaro V, Sambo M, Pieri TC, Calia M, et al. Dynamic NLR and PLR in Predicting COVID-19 Severity: A Retrospective Cohort Study. Infect Dis Ther. 2023;12:1625–40. https://doi.org/10.1007/s40121-023-00813-1.
de Dios E, Rios-Navarro C, Perez-Sole N, Gavara J, Marcos-Garces V, Rodríguez E, et al. Similar Clinical Course and Significance of Circulating Innate and Adaptive Immune Cell Counts in STEMI and COVID-19. J Clin Med. 2020;9:3484. https://doi.org/10.3390/jcm9113484.
Wilson D, Drew W, Jasper A, Crisford H, Nightingale P, Newby P, et al. Frailty Is Associated With Neutrophil Dysfunction Which Is Correctable With Phosphoinositol-3-Kinase Inhibitors. Journals Gerontol Ser A. 2020;75:2320–5. https://doi.org/10.1093/gerona/glaa216.
Paganelli R, Scala E, Quinti I, Ansotegui IJ. Humoral immunity in aging. Aging (Milano). 1994;6:143–50. https://doi.org/10.1007/BF03324229.
Cao Dinh H, Njemini R, Onyema OO, Beyer I, Liberman K, De Dobbeleer L, et al. Strength Endurance Training but Not Intensive Strength Training Reduces Senescence-Prone T Cells in Peripheral Blood in Community-Dwelling Elderly Women. J Gerontol A Biol Sci Med Sci. 2019;74:1870–8. https://doi.org/10.1093/gerona/gly229.
Grilz E, Posch F, Königsbrügge O, Schwarzinger I, Lang IM, Marosi C, et al. Association of Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio with the Risk of Thromboembolism and Mortality in Patients with Cancer. Thromb Haemost. 2018;118:1875–84. https://doi.org/10.1055/s-0038-1673401.
Shah N, Parikh V, Patel N, Patel N, Badheka A, Deshmukh A, et al. Neutrophil lymphocyte ratio significantly improves the Framingham risk score in prediction of coronary heart disease mortality: insights from the National Health and Nutrition Examination Survey-III. Int J Cardiol. 2014;171:390–7. https://doi.org/10.1016/j.ijcard.2013.12.019.
Li H, Lu X, Xiong R, Wang S. High Neutrophil-to-Lymphocyte Ratio Predicts Cardiovascular Mortality in Chronic Hemodialysis Patients. Mediators Inflamm. 2017; 2017: 9327136. https://doi.org/10.1155/2017/9327136.
Jhuang Y-H, Kao T-W, Peng T-C, Chen W-L, Li Y-W, Chang P-K, et al. Neutrophil to lymphocyte ratio as predictor for incident hypertension: a 9-year cohort study in Taiwan. Hypertens Res. 2019;42:1209–14. https://doi.org/10.1038/s41440-019-0245-3.
Fest J, Ruiter TR, Groot Koerkamp B, Rizopoulos D, Ikram MA, van Eijck CHJ, et al. The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: The Rotterdam Study. Eur J Epidemiol. 2019;34:463–70. https://doi.org/10.1007/s10654-018-0472-y.
Tap L, Corsonello A, Di Rosa M, Fabbietti P, Formiga F, Moreno-González R, et al. Inflammaging and Blood Pressure Profiles in Late Life: The Screening for CKD among Older People across Europe (SCOPE) Study. J Clin Med. 2022;11. https://doi.org/10.3390/jcm11247311.
Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85 + Study. Mech Ageing Dev. 2012;133:456–66. https://doi.org/10.1016/J.MAD.2012.05.005.
Kara SP, Altunan B, Unal A. Investigation of the peripheral inflammation (neutrophil–lymphocyte ratio) in two neurodegenerative diseases of the central nervous system. Neurol Sci. 2022;43:1799–807. https://doi.org/10.1007/s10072-021-05507-5.
Rembach A, Watt AD, Wilson WJ, Rainey-Smith S, Ellis KA, Rowe CC, et al. An increased neutrophil–lymphocyte ratio in Alzheimer’s disease is a function of age and is weakly correlated with neocortical amyloid accumulation. J Neuroimmunol. 2014;273:65–71. https://doi.org/10.1016/J.JNEUROIM.2014.05.005.
Pawelec G. Age and immunity: What is immunosenescence? Exp Gerontol. 2018;105:4–9. https://doi.org/10.1016/j.exger.2017.10.024.
Kovtonyuk LV, Fritsch K, Feng X, Manz MG, Takizawa H. Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment. Front Immunol. 2016;7. https://doi.org/10.3389/fimmu.2016.00502.
Olsson J, Wikby A, Johansson B, Löfgren S, Nilsson B-O, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2001;121:187–201. https://doi.org/10.1016/S0047-6374(00)00210-4.
Candore G, Balistreri CR, Colonna-Romano G, Grimaldi MP, Lio D, Listi’ F, et al. Immunosenescence and Anti-Immunosenescence Therapies: The Case of Probiotics. Rejuvenation Res. 2008;11:425–32. https://doi.org/10.1089/rej.2008.0662.
Tough DF, Sprent J. Life span of naive and memory t cells. Stem Cells. 1995;13:242–9. https://doi.org/10.1002/STEM.5530130305.
Henson SM, Lanna A, Riddel NE, Franzese O, Macaulay R, Griffiths SJ, et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8+ T cells. J Clin Invest. 2014;124:4004–16. https://doi.org/10.1172/JCI75051.
Pereira BI, De Maeyer RPH, Covre LP, Nehar-Belaid D, Lanna A, Ward S, et al. Sestrins induce natural killer function in senescent-like CD8 + T cells. Nat Immunol. 2020;21:684–94. https://doi.org/10.1038/S41590-020-0643-3.
Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age-related diseases. Front Immunol. 2017;8. https://doi.org/10.3389/FIMMU.2017.01745.
Cancro MP, Age-Associated B, Cells. Annu Rev Immunol. 2020;38:315–40. https://doi.org/10.1146/ANNUREV-IMMUNOL-092419-031130.
Salvioli S, Monti D, Lanzarini C, Conte M, Pirazzini C, Giulia Bacalini M, et al. Immune System, Cell Senescence, Aging and Longevity - Inflamm-Aging Reappraised. Curr Pharm Des. 2013;19:1675–9. https://doi.org/10.2174/1381612811319090015.
Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, et al. Neutrophils: New insights and open questions. Sci Immunol. 2018;3. https://doi.org/10.1126/sciimmunol.aat4579.
Koenderman L, Tesselaar K, Vrisekoop N. Human neutrophil kinetics: a call to revisit old evidence. Trends Immunol. 2022;43:868–76. https://doi.org/10.1016/J.IT.2022.09.008.
Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–24. https://doi.org/10.1016/j.it.2010.05.006.
Lee KA, Flores RR, Jang IH, Saathoff A, Robbins PD. Immune Senescence, Immunosenescence and Aging. Front aging. 2022;3. https://doi.org/10.3389/FRAGI.2022.900028.
Pawelec G, Bronikowski A, Cunnane SC, Ferrucci L, Franceschi C, Fülöp T, et al. The conundrum of human immune system senescence. Mech Ageing Dev. 2020;192:111357. https://doi.org/10.1016/j.mad.2020.111357.
Franceschi C, Salvioli S, Garagnani P, de Eguileor M, Monti D, Capri M. Immunobiography and the Heterogeneity of Immune Responses in the Elderly: A Focus on Inflammaging and Trained Immunity. Front Immunol. 2017;8. https://doi.org/10.3389/FIMMU.2017.00982.
Marrella V, Facoetti A, Cassani B. Cellular Senescence in Immunity against Infections. Int J Mol Sci. 2022;23. https://doi.org/10.3390/IJMS231911845.
Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, Moore Z, et al. Measuring biological aging in humans: A quest. Aging Cell. 2020;19. https://doi.org/10.1111/acel.13080.
Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10:573–91. https://doi.org/10.18632/AGING.101414.
Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38. https://doi.org/10.1038/nri.2016.90.
Kriebs A. Measuring biological age. Nat Aging. 2022;2:457–9. https://doi.org/10.1038/s43587-022-00234-8.
Martínez de Toda I, González-Sánchez M, Díaz-Del Cerro E, Valera G, Carracedo J, Guerra-Pérez N. Sex differences in markers of oxidation and inflammation. Implications for ageing. Mech Ageing Dev. 2023;211:111797. https://doi.org/10.1016/j.mad.2023.111797.
McFall-Ngai M. Care for the community. Nature. 2007;445:153–3. https://doi.org/10.1038/445153a.
Zuk M. The Sicker Sex. PLoS Pathog. 2009;5:e1000267. https://doi.org/10.1371/journal.ppat.1000267.
Dodd KC, Menon M. Sex bias in lymphocytes: Implications for autoimmune diseases. Front Immunol. 2022;13. https://doi.org/10.3389/FIMMU.2022.945762.
Jilma B, Eichler HG, Breiteneder H, Wolzt M, Aringer M, Graninger W, et al. Effects of 17 beta-estradiol on circulating adhesion molecules. J Clin Endocrinol Metab. 1994;79:1619–24. https://doi.org/10.1210/jcem.79.6.7527406.
Pergola C, Dodt G, Rossi A, Neunhoeffer E, Lawrenz B, Northoff H, et al. ERK-mediated regulation of leukotriene biosynthesis by androgens: A molecular basis for gender differences in inflammation and asthma. Proc Natl Acad Sci. 2008;105:19881–6. https://doi.org/10.1073/pnas.0809120105.
Miller L, Alley EW, Murphy WJ, Russell SW, Hunt JS. Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukoc Biol. 1996;59:442–50. https://doi.org/10.1002/JLB.59.3.442.
Kovacs EJ, Plackett TP, Witte PL. Estrogen replacement, aging, and cell-mediated immunity after injury. J Leukoc Biol. 2004;76:36–41. https://doi.org/10.1189/jlb.1103538.
Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10:594–604. https://doi.org/10.1038/NRI2815.
Mage DT, Donner M. A genetic basis for the sudden infant death syndrome sex ratio. Med Hypotheses. 1997;48:137–42. https://doi.org/10.1016/S0306-9877(97)90280-2.
Syrett CM, Anguera MC. When the balance is broken: X-linked gene dosage from two X chromosomes and female-biased autoimmunity. J Leukoc Biol. 2019;106:919–32. https://doi.org/10.1002/JLB.6RI0319-094R.
Zimmer A, Bagchi AK, Vinayak K, Bello-Klein A, Singal PK. Innate immune response in the pathogenesis of heart failure in survivors of myocardial infarction. Am J Physiol Circ Physiol. 2019;316:H435–45. https://doi.org/10.1152/ajpheart.00597.2018.
Deegan DF, Karbalaei R, Madzo J, Kulathinal RJ, Engel N. The developmental origins of sex-biased expression in cardiac development. Biol Sex Differ. 2019;10:46. https://doi.org/10.1186/s13293-019-0259-1.
Zhao WM, Tao SM, Liu GL. Neutrophil-to-lymphocyte ratio in relation to the risk of all-cause mortality and cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail. 2020;42:1059–66. https://doi.org/10.1080/0886022X.2020.1832521.
Chen D, Xiao D, Guo J, Chahan B, Wang Z. Neutrophil-lymphocyte count ratio as a diagnostic marker for acute kidney injury: a systematic review and meta-analysis. Clin Exp Nephrol. 2020;24:126–35. https://doi.org/10.1007/S10157-019-01800-Y.
Altunoren O, Akkus G, Sezal DT, Ciftcioglu M, Guzel FB, Isiktas S, et al. Does neutrophyl to lymphocyte ratio really predict chronic kidney disease progression? Int Urol Nephrol. 2019;51:129–37. https://doi.org/10.1007/s11255-018-1994-7.
Kim S, Won CW. Sex-different changes of body composition in aging: a systemic review. Arch Gerontol Geriatr. 2022;102:104711. https://doi.org/10.1016/j.archger.2022.104711.
Caldiroli A, La Tegola D, Affaticati LM, Manzo F, Cella F, Scalia A et al. Clinical and Peripheral Biomarkers in Female Patients Affected by Anorexia: Does the Neutrophil/Lymphocyte Ratio (NLR) Affect Severity? Nutrients. 2023; 15. https://doi.org/10.3390/NU15051133.
Piotrowska K, Tarnowski M. Bone Marrow Adipocytes-Role in Physiology and Various Nutritional Conditions in Human and Animal Models. Nutrients. 2021;13. https://doi.org/10.3390/NU13051412.
Piotrowska K, Zgutka K, Kupnicka P, Chlubek D, Pawlik A, Baranowska-Bosiacka I. Analysis of Bone Mineral Profile After Prolonged Every-Other-Day Feeding in C57BL/6J Male and Female Mice. Biol Trace Elem Res. 2020;194:177–83. https://doi.org/10.1007/S12011-019-01758-8.
Piotrowska K, Tarnowski M, Zgutka K, Pawlik A. Gender Differences in Response to Prolonged Every-Other-Day Feeding on the Proliferation and Apoptosis of Hepatocytes in Mice. Nutr 2016, Vol 8, Page 176. 2016; 8: 176. https://doi.org/10.3390/NU8030176.
Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. https://doi.org/10.1111/j.1532-5415.2000.tb03873.x.
Garasto S, Fusco S, Corica F, Rosignuolo M, Marino A, Montesanto A, et al. Estimating Glomerular Filtration Rate in Older People. Biomed Res Int. 2014;2014:1–12. https://doi.org/10.1155/2014/916542.
Funding
The InCHIANTI study was supported as a “targeted project” (ICS 110.1/RS97.71) by the
Italian Ministry of Health and by the U.S. National Institute on Aging (contracts N01-AG-916413,
N01-AG-5-0002, and N01-AG-821336, and grant R01-AG-027012).
Author information
Authors and Affiliations
Contributions
Raffaello Pellegrino: conceptualization, interpretation of data, drafted the work. Roberto Paganelli: conceptualization, interpretation of data, drafted and revised the work. Angelo Di Iorio: conceptualization, acquisition, analysis, drafted the work. Stefania Bandinelli: design of the work, interpretation of data, revised the work. Antimo Moretti: analysis, interpretation of data, drafted the work. Giovanni Iolascon: analysis, interpretation of data, drafted the work. Eleonora Sparvieri: acquisition, analysis, interpretation of data, drafted the work. Domiziano Tarantino: acquisition, analysis, interpretation of data, drafted the work. Luigi Ferrucci: design, acquisition, analysis, interpretation of data, revised the text. All authors have read and agreed to the present version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The InCHIANTI study baseline was approved by the Ethical committee at INRCA, Ancona (protocol 14/CE, 28 February 2000) as the FU1 (protocol 45/01, 16 January 2001). InCHIANTI study FU2 and FU3 were approved by the Local Ethical Committee at Azienda Sanitaria Firenze (protocol n◦ 5/04, 12 May 2004) The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of INRCA di Ancona (Italy). Clinical Trial Registration: NCT01331512.
Consent to participate
Written informed consent was obtained from the patients to participate at the Study.
Competing interests
Roberto Paganelli and Luigi Ferrucci are members of the Editorial board of the Journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pellegrino, R., Paganelli, R., Di Iorio, A. et al. Temporal trends, sex differences, and age-related disease influence in Neutrophil, Lymphocyte count and Neutrophil to Lymphocyte-ratio: results from InCHIANTI follow-up study. Immun Ageing 20, 46 (2023). https://doi.org/10.1186/s12979-023-00370-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-023-00370-8