Abstract
Natural antibodies (nAbs) against aggregation-prone proteins have been found in healthy normal subjects. These proteins likely have a pathogenetic role in neurodegenerative diseases of ageing. They include the amyloid β (Aβ) protein which may play an important role in Alzheimer’s dementia (AD), and α-synuclein, a major determinant of Parkinson’s disease (PD). We measured nAbs to Aβ in a group of Italian patients with AD, vascular dementia, non-demented PD patients and healthy elderly controls. We found that Aβ antibody levels in AD were similar to age- and sex-matched controls, but contrary to our expectations, they were significantly reduced in PD. This may identify patients that could be more prone to amyloid aggregation.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the presence of protein aggregates of α-synuclein in the brain [1, 2]. The current view – similar to the amyloid theory for senile plaques formed by β amyloid (Aβ) in Alzheimer’s dementia (AD) – is that aggregation intermediates formed by these proteins are toxic to cells, whereas larger deposits of fibrils protect against neuronal damage [3,4,5,6,7]. It has been proposed that these amyloidogenic proteins can interact and promote each other’s aggregation [8,9,10,11]. Both Aβ and α-synuclein are targeted by natural antibodies (nAbs) present in normal healthy subjects [3, 12] as well as in patients [3, 13, 14]. In AD, levels of these nAbs were found to be lower than in healthy controls [15, 16] and most studies have confirmed this finding [17,18,19], despite a few discordant reports [20, 21]. The data obtained from immunization in transgenic mouse models of AD revealed that high levels of antibodies to Aβ prevented plaque formation and cognitive decline [22,23,24,25], thus leading to several but unsuccessful attempts to translate active and/or passive immunotherapy targeting Aβ to humans [12, 26, 27]. The development of monoclonal antibodies to Aβ oligomers [28] and the controversial FDA approval of aducanumab in selected cases of early mild AD [29] has sparked a debate on the role of Aβ in the pathogenesis of AD which has recently been very heated [30,31,32,33].
The significance of nAbs to α-synuclein in normal subjects and PD is much less clearly defined [13, 34, 35] and their levels have variously been found to be increased [36, 37], not differing [38] or decreased [39] in PD patients compared with controls. We set out to study the levels of nAbs anti-Aβ in the general population and patients with neurodegenerative diseases, including patients with dementia, both AD and vascular type (VaD), and a small cohort of non-demented PD cases.
Material and methods
Subjects
A total of 91 subjects (34 males and 57 females) was enrolled. Fifteen healthy donors aged > 65 years were selected from among volunteers at the Center of Excellence on Aging (CEA) of the UdA Foundation of the University “G. d’Annunzio” of Chieti. Other fifteen patients diagnosed with PD were selected at the Geriatric Clinic of the University Hospital. Thirty patients diagnosed with AD at the same clinic were consecutively recruited to the study. A group of 31 patients with dementia, diagnosed as vascular dementia (VaD) was also included. Diagnosis of probable AD was according to standard procedures and followed the NINCDS/ADRDA and DSM-III-R criteria [40]; Parkinson’s disease was diagnosed according to criteria valid at the time of enrollment [41] and the NINDS-AIREN criteria were applied for VaD [42], Table 1 reports the age and sex distribution of cases in each group, as well as some data of clinical importance. Age, sex, schooling and disease duration (from diagnosis) did nor significantly differ among the groups studied.
All subjects underwent a geriatric examination, aimed at evaluating neurological signs and symptoms of dementia; cognitive performance was assessed by the mini mental state evaluation (MMSE) score, which was corrected for age and schooling. A radiological assessment with CT or MRI scan was also performed to assess the brain vascular disease. A significantly lower MMSE score was found in patients with VaD and AD, and cases diagnosed with PD had a better CIRS (Table 1).
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of CEA (n. 2013/4); informed consent was obtained from all subjects involved in the study or their caregivers.
All subjects were residents in the same area of Central Italy living in the community. None was affected by major co-morbidities such as cancer, infectious or autoimmune diseases, or receiving immunosuppressive treatments at the time of recruitment. The main demographic and clinical data are reported in Table 1.
Venous blood was collected by venipuncture and allowed to clot for 30 min at room temperature, then centrifuged at 1,500 × g in a refrigerated centrifuge. Serum was then collected with a pipette, coded and stored in aliquots at -20 °C until tested.
Antibody measurements
Anti-Al-42 peptide antibody was determined by ELISA on coded serum specimens from all patients and elderly controls [43]. Briefly, microtiter wells were coated with 0.1 mg/ml human Al-42 peptide (Biosource International, Camarillo, CA) in 0.1 M sodium bicarbonate buffer (pH 9.6) at 4 °C overnight. The plates were then washed three times with PBS, 0.05% Tween 20, blocked with 10% newborn calf serum (Sigma, St Louis, MO) in water for 1 h at 37 °C, and washed again three times. An affinity-purified rabbit anti-Al-42 antibody (Biosource International,Camarillo, CA) served as a positive control. The coated plates were incubated for 1 h at 37 °C with three-fold serial dilutions of serum in PBS in a 12 row of wells starting with undiluted serum. The plates were then washed and incubated for 30 min at 37 °C with alkaline phosphatase-conjugated goat anti-human IgG antibodies (ICN Biomedical, Costa Mesa, CA), washed three times, and incubated for 30 min at room temperature with p-nitrophenylphosphate (Sigma, St Louis, MO). The absorbance at 405 nm was read on an automated plate reader (Molecular Devices, Sunnyvale, CA). The titer of sera was determined in comparison to an antibody standard included on each ELISA plate.
Analysis of the differences between the groups was performed by non-parametric statistics (Fisher’s exact test and Wilcoxon’s rank sum test).
Results
The groups did not differ for age, schooling and disease duration (from diagnosis), however, PD patients were mostly males, and with fewer comorbidities (Table 1).
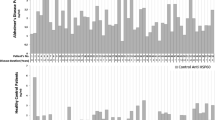
As shown in Table 2, levels of nAb to Aβ in PD patients were significantly lower (p < 0.05) than in healthy age- and sex-matched controls (p < 0.05) Their median titer was less than one third of controls (7.3 units vs. 23.2), and 73.3% of individual PD patient values were below the median of the controls. Although the median value in AD patients was 14.1 units with 63.3% of serum nAb levels below the median of the controls, this difference did not reach statistical significance. Finally, we analyzed a group of patients with VaD, which exhibited mean antibody titers similar to those found in normal controls, as expected (Fig. 1).
Serum levels of antibody to amyloid Aβ in 15 aged non-demented controls and 31 patients with dementia diagnosed as being of vascular origin (VaD); antibody levels did not differ between these groups. VaD cases could be subdivided in two subgroups, i.e. 14 with high (median 71.1) and 17 with low (median 6.8) antibody levels. VaD high patients had significantly more antibody to Aβ than old age controls (p = 0.047)
However, there was some evidence for two subgroups, neatly distinguishable as clusters above and below the median. When these were examined as distinct subgroups, the titers in VaD patients with high nAb to Aβ were significantly different from healthy aged controls (p < 0.05). Titers measured in these Italian subjects were much lower than those reported by the same method in other studies [43]. No correlation of Aβ natural autoantibodies with age, sex and MMSE score was found.
Discussion
In this pilot study of titers and distribution of nAbs to Aβ in the Italian population, we included both healthy aged subjects and patients with neurodegenerative diseases, both AD and PD, as well as patients diagnosed with VaD. Our hypothesis, based on published studies [5], was that anti-Aβ levels would be lower in AD compared to controls, but nAb titers were not found to be significantly different between normal subjects and AD. We had also included PD cases without clear cognitive impairment, to explore their nAbs levels to Aβ, since in this disease nAbs to α-synuclein are present [37]. Unexpectedly we found significantly reduced titers of nAbs to Aβ in these patients. There is only a small number of studies addressing this aspect, and the data are far from consistent, possibly due to technical problems in antibody detection methods [21, 43,44,45]. The major problem is the presence of antigen–antibody complexes in normal and patients’ sera, formed by anti-Aβ binding to soluble Aβ. This may lead to underestimation of antibody titers, and is only circumvented by acid dissociation of the bound antibody. The method has been applied to estimate the amount of anti-Aβ in commercial preparations of immunoglobulins for intravenous use (IGIV) [26, 44, 46, 47] to be used in passive immunization trials [12, 27, 48]. Aggregation of Aβ with other proteins such as tau [49] or α-synuclein [9, 11, 50] leads to possible formation of larger complexes, whereas soluble Ag-Ab complexes may trigger innate immunity and neuronal toxicity [18, 44, 51]. Recently, another protein, glycoprotein nonmetastatic melanoma protein B, has been implicated in α-synuclein induced neuronal damage [52], through colocalization detection. Another important issue is the specificity of the antibody detected in ELISA, because Aβ is known to exist in different forms with different conformational as well as linear epitopes. Protective antibodies are all those preventing the formation or promoting the dissociation of oligomeric Aβ, considered the neurotoxic form, binding to the mid-domain of the molecule [14, 21, 44, 48]. nAbs to oligomers have been studied [47, 48, 53], and they may be diagnostic tools [54,55,56,57] but we could not investigate the molecular species of Aβ targeted by our antibodies, so we can only presume that they are at least in part protective. However, the presence of high titers of antibodies to Aβ in about 45% of patients with VaD may indicate that excess antibody to some Aβ conformers has a potentially damaging role, as demonstrated for anti-Aβ in the development of the ARIA-like events characterizing cerebral amyloid angiopathy-related inflammation [24, 25, 58, 59]. These lesions have been detected also in patients treated with antibodies to Aβ [60, 61] and are probably due to rapid removal of Aβ fibrils which occurs with passive immunotherapy [28, 62]. Moreover, patients diagnosed with VaD might constitute a mixed group, some of them being both AD and VaD [63, 64], and the presence of two neatly distinct groups for anti-Aβ nAbs, as observed in Fig. 1, seems to be in agreement with such hypothesis.
Our findings show that in a phase of PD where memory and cognitive abilities are not impaired, anti-Aβ levels are greatly decreased compared to normal. This might bear on the possibility that amyloid precipitation of Aβ in fibrillar forms [65] may predate, and favour the aggregation of α-synuclein, as hypothesized in a pathogenetic scenario of PD [2, 10, 11, 49, 66,67,68]. Aβ aggregation is known to precipitate Tau deposition in fibrillar tangles in AD [49], and passive immunotherapy anti-Aβ might be useful in addition to anti-Tau in early phases of disease [69,70,71], similar to sequential anti-Aβ and anti-α-synuclein suggested in the case of PD [35, 55, 66, 72]. Passive Aβ immunotherapy has been shown to benefit with different success patients with early stage AD [30, 32, 33, 55, 73,74,75,76] since such natural antibodies may prevent oligomeric and fibrillary aggregation of neurotoxic peptides. Two recent trials of monoclonal antibodies to α-synuclein failed to meet their clinical endpoints in PD [77, 78] suggesting that targeting α-synuclein alone does not prevent progression of symptoms severity. There are possibly multiple pathways leading to neurodegeneration in PD [79], and partially interfering with one of them may not be a successful strategy [80].
We suggest that low levels of antibodies against Aβ may identify subgroups of cases in patients with PD, and possibly also AD and VaD, but their significance needs further evaluation.
Availability of data and materials
The data presented in this study are available on reasonable request from the corresponding author.
References
Spillantini MG. Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy are alpha-synucleinopathies. Parkinsonism Relat Disord. 1999;5(4):157–62.
Stroo E, Koopman M, Nollen EA, Mata-Cabana A. Cellular regulation of amyloid formation in aging and disease. Front Neurosci. 2017;11:64.
Weksler ME, Gouras G, Relkin NR, Szabo P. The immune system, amyloid-beta peptide, and Alzheimer’s disease. Immunol Rev. 2005;205:244–56.
Logovinsky V, Satlin A, Lai R, Swanson C, Kaplow J, Osswald G, et al. Safety and tolerability of BAN2401–a clinical study in Alzheimer’s disease with a protofibril selective Abeta antibody. Alzheimers Res Ther. 2016;8(1):14.
Li XW, Li XX, Liu QS, Cheng Y. Blood and Cerebrospinal Fluid Autoantibody to Abeta Levels in Patients with Alzheimer’s Disease: a Meta-Analysis Study. J Mol Neurosci. 2020;70(8):1208–15.
Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14(1):38–48.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62.
Volloch V, Olsen B, Rits S. Alzheimer’s Disease is Driven by Intraneuronally Retained Beta-Amyloid Produced in the AD-Specific, betaAPP-Independent Pathway: Current Perspective and Experimental Models for Tomorrow. Ann Integr Mol Med. 2020;2(1):90–114.
Subedi S, Sasidharan S, Nag N, Saudagar P, Tripathi T. Amyloid Cross-Seeding: Mechanism, Implication, and Inhibition. Molecules. 2022;27(6):1776.
Mandal PK, Pettegrew JW, Masliah E, Hamilton RL, Mandal R. Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer’s and Parkinson’s in dementia with Lewy body disease. Neurochem Res. 2006;31(9):1153–62.
Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30(21):7281–9.
Neff F, Wei X, Nolker C, Bacher M, Du Y, Dodel R. Immunotherapy and naturally occurring autoantibodies in neurodegenerative disorders. Autoimmun Rev. 2008;7(6):501–7.
Maetzler W, Apel A, Langkamp M, Deuschle C, Dilger SS, Stirnkorb JG, et al. Comparable autoantibody serum levels against amyloid- and inflammation-associated proteins in Parkinson’s disease patients and controls. PLoS One. 2014;9(2):e88604.
Liu YH, Wang J, Li QX, Fowler CJ, Zeng F, Deng J, et al. Association of naturally occurring antibodies to beta-amyloid with cognitive decline and cerebral amyloidosis in Alzheimer’s disease. Sci Adv. 2021;7(1):eabb0457.
Weksler ME, Relkin N, Turkenich R, LaRusse S, Zhou L, Szabo P. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp Gerontol. 2002;37(7):943–8.
Du Y, Dodel R, Hampel H, Buerger K, Lin S, Eastwood B, et al. Reduced levels of amyloid beta-peptide antibody in Alzheimer disease. Neurology. 2001;57(5):801–5.
Qu BX, Gong Y, Moore C, Fu M, German DC, Chang LY, et al. Beta-amyloid auto-antibodies are reduced in Alzheimer’s disease. J Neuroimmunol. 2014;274(1–2):168–73.
Jianping L, Zhibing Y, Wei Q, Zhikai C, Jie X, Jinbiao L. Low avidity and level of serum anti-Abeta antibodies in Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3):127–32.
Brettschneider S, Morgenthaler NG, Teipel SJ, Fischer-Schulz C, Burger K, Dodel R, et al. Decreased serum amyloid beta(1–42) autoantibody levels in Alzheimer’s disease, determined by a newly developed immuno-precipitation assay with radiolabeled amyloid beta(1–42) peptide. Biol Psychiatry. 2005;57(7):813–6.
Mruthinti S, Buccafusco JJ, Hill WD, Waller JL, Jackson TW, Zamrini EY, et al. Autoimmunity in Alzheimer’s disease: increased levels of circulating IgGs binding Abeta and RAGE peptides. Neurobiol Aging. 2004;25(8):1023–32.
Klaver AC, Coffey MP, Smith LM, Bennett DA, Finke JM, Dang L, et al. ELISA measurement of specific non-antigen-bound antibodies to Abeta1-42 monomer and soluble oligomers in sera from Alzheimer’s disease, mild cognitively impaired, and noncognitively impaired subjects. J Neuroinflammation. 2011;8:93.
Wang A, Das P, Switzer RC 3rd, Golde TE, Jankowsky JL. Robust amyloid clearance in a mouse model of Alzheimer’s disease provides novel insights into the mechanism of amyloid-beta immunotherapy. J Neurosci. 2011;31(11):4124–36.
Movsesyan N, Davtyan H, Mkrtichyan M, Petrushina I, Tiraturyan T, Ross T, et al. Low concentrations of anti-Abeta antibodies generated in Tg2576 mice by DNA epitope vaccine fused with 3C3d molecular adjuvant do not affect AD pathology. Hum Gene Ther. 2010;21(11):1569–76.
Morgan D. Mechanisms of A beta plaque clearance following passive A beta immunization. Neurodegener Dis. 2005;2(5):261–6.
Chantran Y, Capron J, Alamowitch S, Aucouturier P. Anti-Abeta Antibodies and Cerebral Amyloid Angiopathy Complications. Front Immunol. 2019;10:1534.
Dodel RC, Du Y, Depboylu C, Hampel H, Frolich L, Haag A, et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75(10):1472–4.
Dodel R, Rominger A, Bartenstein P, Barkhof F, Blennow K, Forster S, et al. Intravenous immunoglobulin for treatment of mild-to-moderate Alzheimer’s disease: a phase 2, randomised, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol. 2013;12(3):233–43.
Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–6.
Wang Y, Yan T, Lu H, Yin W, Lin B, Fan W, et al. Lessons from Anti-Amyloid-beta Immunotherapies in Alzheimer Disease: Aiming at a Moving Target. Neurodegener Dis. 2017;17(6):242–50.
Tian Hui Kwan A, Arfaie S, Therriault J, Rosa-Neto P, Gauthier S. Lessons Learnt from the Second Generation of Anti-Amyloid Monoclonal Antibodies Clinical Trials. Dement Geriatr Cogn Disord. 2020;49(4):334–48.
Piller C. Blots on a field? Science. 2022;377(6604):358–63.
Malik R, Kalra S, Bhatia S, Harrasi AA, Singh G, Mohan S, et al. Overview of therapeutic targets in management of dementia. Biomed Pharmacother. 2022;152:113168.
Luo JJ, Wallace W, Kusiak JW. A tough trek in the development of an anti-amyloid therapy for Alzheimer’s disease: do we see hope in the distance? J Neurol Sci. 2022;438:120294.
Akhtar RS, Licata JP, Luk KC, Shaw LM, Trojanowski JQ, Lee VM. Measurements of auto-antibodies to alpha-synuclein in the serum and cerebral spinal fluids of patients with Parkinson’s disease. J Neurochem. 2018;145(6):489–503.
Bach JP, Falkenburger BH. What autoantibodies tell us about the pathogenesis of Parkinson’s disease: an Editorial for “Measurements of auto-antibodies to alpha-synuclein in the serum and cerebral spinal fluids of patients with Parkinson’s disease” on page 489. J Neurochem. 2018;145(6):433–5.
Yanamandra K, Gruden MA, Casaite V, Meskys R, Forsgren L, Morozova-Roche LA. alpha-synuclein reactive antibodies as diagnostic biomarkers in blood sera of Parkinson’s disease patients. PLoS One. 2011;6(4):e18513.
Shalash A, Salama M, Makar M, Roushdy T, Elrassas HH, Mohamed W, et al. Elevated Serum alpha-Synuclein Autoantibodies in Patients with Parkinson’s Disease Relative to Alzheimer’s Disease and Controls. Front Neurol. 2017;8:720.
Papachroni KK, Ninkina N, Papapanagiotou A, Hadjigeorgiou GM, Xiromerisiou G, Papadimitriou A, et al. Autoantibodies to alpha-synuclein in inherited Parkinson’s disease. J Neurochem. 2007;101(3):749–56.
Besong-Agbo D, Wolf E, Jessen F, Oechsner M, Hametner E, Poewe W, et al. Naturally occurring alpha-synuclein autoantibody levels are lower in patients with Parkinson disease. Neurology. 2013;80(2):169–75.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44.
Vasconcellos LF, Pereira JS. Parkinson’s disease dementia: Diagnostic criteria and risk factor review. J Clin Exp Neuropsychol. 2015;37(9):988–93.
Pohjasvaara T, Mantyla R, Ylikoski R, Kaste M, Erkinjuntti T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences. Stroke. 2000;31(12):2952–7.
Szabo P, Relkin N, Weksler ME. Natural human antibodies to amyloid beta peptide. Autoimmun Rev. 2008;7(6):415–20.
Klaver AC, Patrias LM, Coffey MP, Finke JM, Loeffler DA. Measurement of anti-Abeta1-42 antibodies in intravenous immunoglobulin with indirect ELISA: the problem of nonspecific binding. J Neurosci Methods. 2010;187(2):263–9.
Szabo P, Mujalli DM, Rotondi ML, Sharma R, Weber A, Schwarz HP, et al. Measurement of anti-beta amyloid antibodies in human blood. J Neuroimmunol. 2010;227(1–2):167–74.
Balakrishnan K, Andrei-Selmer LC, Selmer T, Bacher M, Dodel R. Comparison of intravenous immunoglobulins for naturally occurring autoantibodies against amyloid-beta. J Alzheimers Dis. 2010;20(1):135–43.
Klaver AC, Patrias LM, Finke JM, Loeffler DA. Specificity and sensitivity of the Abeta oligomer ELISA. J Neurosci Methods. 2011;195(2):249–54.
Knight EM, Kim SH, Kottwitz JC, Hatami A, Albay R, Suzuki A, et al. Effective anti-Alzheimer Abeta therapy involves depletion of specific Abeta oligomer subtypes. Neurol Neuroimmunol Neuroinflamm. 2016;3(3):e237.
Do TD, Economou NJ, Chamas A, Buratto SK, Shea JE, Bowers MT. Interactions between amyloid-beta and Tau fragments promote aberrant aggregates: implications for amyloid toxicity. J Phys Chem B. 2014;118(38):11220–30.
Chau E, Kim JR. alpha-synuclein-assisted oligomerization of beta-amyloid (1–42). Arch Biochem Biophys. 2022;717:109120.
Trudler D, Nazor KL, Eisele YS, Grabauskas T, Dolatabadi N, Parker J, et al. Soluble alpha-synuclein-antibody complexes activate the NLRP3 inflammasome in hiPSC-derived microglia. Proc Natl Acad Sci U S A. 2021;118(15):e2025847118.
Diaz-Ortiz ME, Seo Y, Posavi M, Carceles Cordon M, Clark E, Jain N, et al. GPNMB confers risk for Parkinson’s disease through interaction with alpha-synuclein. Science. 2022;377(6608):eabk0637.
Klaver AC, Finke JM, Digambaranath J, Balasubramaniam M, Loeffler DA. Antibody concentrations to Abeta1-42 monomer and soluble oligomers in untreated and antibody-antigen-dissociated intravenous immunoglobulin preparations. Int Immunopharmacol. 2010;10(1):115–9.
Viola KL, Bicca MA, Bebenek AM, Kranz DL, Nandwana V, Waters EA, et al. The Therapeutic and Diagnostic Potential of Amyloid beta Oligomers Selective Antibodies to Treat Alzheimer’s Disease. Front Neurosci. 2021;15:768646.
Sim KY, Im KC, Park SG. The Functional Roles and Applications of Immunoglobulins in Neurodegenerative Disease. Int J Mol Sci. 2020;21(15):5295.
Paganelli A, Tarentini E, Benassi L, Kaleci S, Magnoni C. Mesenchymal stem cells for the treatment of psoriasis: a comprehensive review. Clin Exp Dermatol. 2020;45(7):824–30.
Paganelli A, Kaleci S, Benassi L, Pellacani G, Magnoni C. Mesenchymal stem cells and psoriasis: state of the art and future perspectives. Dermatol Ther. 2020;33(2):e13247.
Da Mesquita S, Papadopoulos Z, Dykstra T, Brase L, Farias FG, Wall M, et al. Meningeal lymphatics affect microglia responses and anti-Abeta immunotherapy. Nature. 2021;593(7858):255–60.
DiFrancesco JC, Longoni M, Piazza F. Anti-Abeta Autoantibodies in Amyloid Related Imaging Abnormalities (ARIA): Candidate Biomarker for Immunotherapy in Alzheimer’s Disease and Cerebral Amyloid Angiopathy. Front Neurol. 2015;6:207.
Filippi M, Cecchetti G, Spinelli EG, Vezzulli P, Falini A, Agosta F. Amyloid-Related Imaging Abnormalities and beta-Amyloid-Targeting Antibodies: A Systematic Review. JAMA Neurol. 2022;79(3):291–304.
Barakos J, Purcell D, Suhy J, Chalkias S, Burkett P, MarsicaGrassi C, et al. Detection and Management of Amyloid-Related Imaging Abnormalities in Patients with Alzheimer’s Disease Treated with Anti-Amyloid Beta Therapy. J Prev Alzheimers Dis. 2022;9(2):211–20.
Plowey ED, Bussiere T, Rajagovindan R, Sebalusky J, Hamann S, von Hehn C, et al. Alzheimer disease neuropathology in a patient previously treated with aducanumab. Acta Neuropathol. 2022;144(1):143–53.
Emrani S, Lamar M, Price CC, Wasserman V, Matusz E, Au R, et al. Alzheimer’s/Vascular Spectrum Dementia: Classification in Addition to Diagnosis. J Alzheimers Dis. 2020;73(1):63–71.
Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Dement. 2018;14(2):148–56.
Akhtar RS, Xie SX, Chen YJ, Rick J, Gross RG, Nasrallah IM, et al. Regional brain amyloid-beta accumulation associates with domain-specific cognitive performance in Parkinson disease without dementia. PLoS ONE. 2017;12(5):e0177924.
George S, Brundin P. Immunotherapy in Parkinson’s Disease: Micromanaging Alpha-Synuclein Aggregation. J Parkinsons Dis. 2015;5(3):413–24.
Kronimus Y, Albus A, Balzer-Geldsetzer M, Straub S, Semler E, Otto M, et al. Naturally Occurring Autoantibodies against Tau Protein Are Reduced in Parkinson’s Disease Dementia. PLoS One. 2016;11(11):e0164953.
Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, et al. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98(21):12245–50.
Davtyan H, Zagorski K, Rajapaksha H, Hovakimyan A, Davtyan A, Petrushina I, et al. Alzheimer’s disease Advax(CpG)- adjuvanted MultiTEP-based dual and single vaccines induce high-titer antibodies against various forms of tau and Abeta pathological molecules. Sci Rep. 2016;6:28912.
Kayed R, Lasagna-Reeves CA. Molecular mechanisms of amyloid oligomers toxicity. J Alzheimers Dis. 2013;33(Suppl 1):S67-78.
Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, et al. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain. 2010;133(Pt 5):1312–27.
Valera E, Masliah E. Immunotherapy for neurodegenerative diseases: focus on alpha-synucleinopathies. Pharmacol Ther. 2013;138(3):311–22.
Vaz M, Silva V, Monteiro C, Silvestre S. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin Interv Aging. 2022;17:797–810.
Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov. 2022;21(4):306–18.
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388(1):9–21.
Gandy S, Ehrlich ME. Moving the Needle on Alzheimer’s Disease with an Anti-Oligomer Antibody. N Engl J Med. 2023;388(1):80–1.
Pagano G, Taylor KI, Anzures-Cabrera J, Marchesi M, Simuni T, Marek K, et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N Engl J Med. 2022;387(5):421–32.
Lang AE, Siderowf AD, Macklin EA, Poewe W, Brooks DJ, Fernandez HH, et al. Trial of Cinpanemab in Early Parkinson’s Disease. N Engl J Med. 2022;387(5):408–20.
Calabrese V, Santoro A, Monti D, Crupi R, Di Paola R, Latteri S, et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. 2018;115:80–91.
Whone A. Monoclonal Antibody Therapy in Parkinson’s Disease - The End? N Engl J Med. 2022;387(5):466–7.
Acknowledgements
The authors wish to thank Prof. Marc E. Weksler and Dr. Paul Szabo, formerly at the Department of Medicine, Weill Medical College of Cornell University, New York, NY 10021, USA, for help with measurements of antibodies to Aβ.
Funding
This study has been funded in part by University “G. D’Annunzio” ex60% research grant to R.P. and A. Di I.
Author information
Authors and Affiliations
Contributions
Conceptualization, R.P. and A.Di I.; Methodology, A.Di I. and A.P.; Formal Analysis, A.Di I; Investigation, R.P.; Data Curation, G.P., A.P. and A.Di I.; Writing – Original Draft Preparation, R.P.; Writing – Review & Editing, G.P. and A.P. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the ASL2 Abruzzo (n.18–2013-176). Informed consent was obtained from each subject (or caregiver) included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Paganelli, R., Paganelli, A., Pawelec, G. et al. Natural IgG antibodies to β amyloid are decreased in patients with Parkinson’s disease. Immun Ageing 20, 13 (2023). https://doi.org/10.1186/s12979-023-00336-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-023-00336-w