Abstract
Background
Bipolar disorder (BD) and schizophrenia (SZ) are the two main mental disorders with unknown etiology that significantly impact individuals’ quality of life. The potential pro-inflammatory role in their pathogenesis is postulated and Human Endogenous Retrovirus W (HERV-W) is an emerging candidate to modulate this pathogenic finding. HERVs, ancient retroviruses in the human genome, may play roles in inflammation and disease pathogenesis. Despite HERVs’ involvement in autoimmune diseases, their influence on mental disorders remains underexplored. Therefore, the aim of this study was to assess the level of HERV-W-env expression and the systemic inflammatory profile through the concentration of IL-2, IL-4, IL-6, IL-10, TNF-α and INF-γ cytokines in BD and SZ patients.
Results
All participants showed HERV-W-env expression, but its expression was higher in mental disorder patients (p < 0.01) than in control. When separated, SZ individuals exhibited higher HERV-W expression than the control group (p < 0.01). Higher serum levels of TNF-α and IL-10 were found in BD (p = 0.0001 and p = 0.001, respectively) and SZ (p = 0.01) and p = 0.01, respectively) than in the control group, while SZ showed decreased levels IFN-γ and IL-2 as compared to controls (p = 0.05) and BD patients (p = 0.05), respectively. Higher TNF-α/IL-4 and TNF-α/IL-10 ratios, and lower IFN-γ/IL-10 were observed in BD and SZ patients than controls. Significant negative correlation between HERV-W-env expression and IL-10 (r=-0.47 p < 0.05), as well as positive correlations between HERV-W-env expression and TNF-α/IL-10 or IFN-γ/IL-10 ratios (r = 0.48 p < 0.05 and r = 0.46 p < 0.05, respectively) were found in BD patients.
Conclusion
These findings suggest not only a potential link between HERV-W-env expression both in BD and SZ, but also a possible involvement of systemic inflammatory status in BD patients.
Similar content being viewed by others
Background
Bipolar disorder (BD) and Schizophrenia (SZ) are two main frequent and severe mental disorders that significantly compromise the quality of life and the social conditions of affected individuals [1]. Both disorders do not present any specific etiology, but genetic and environmental factors may be associated with the disease onset and clinical evolution of the disease [2, 3]. Despite the unknown etiology of the disease, it is utmost to emphasize the possible involvement of systemic pro-inflammatory status in the context of the disease’s pathogenesis [4, 5].
However, triggering this inflammatory response profile and sustaining this condition in both these diseases is still poorly understood. In this sense, Human Endogenous Retroviruses (HERV) may be highlighted not only as a potential candidate for promoting inflammatory response [6, 7], but might also be associated with these disease’s pathogenesis [8, 9].
HERVs are ancient retroviruses that have become integral components of the human genome, which originated from infections in our ancestor’s germline cells millions of years ago [10,11,12]. These retroviruses have been transmitted and perpetuated initially by horizontal transmission [13] and later inherited by Mendelian way [14], and today we know that HERVs compose 8% of the human genome [10,11,12, 15]. Over generations, retrotransposition events have contributed to the genomic diversity of these elements within the genome. However, HERVs have undergone mutations, resulting in their silencing by the presence of stop codon within the coding region, isolated genes/Long Terminal Repeats (LTRs) and incomplete sequences, which resulted in their inability to replicate [16]. Nevertheless, HERVs can still exhibit expression, and the virions may be formed through the combination of retroviral genes from various loci within the genome [13, 17,18,19].
Notably, these retroviruses play crucial roles in human physiology, e.g. the HERV-W-env protein, also known as Syncytin-1, assumes pivotal participation in human placentation by mediating the fusion of Syncytiotrophoblast during early nidation and placenta formation [20]. Additionally, the LTRs of HERVs may serve as promoters for human genes [21, 22]. Unfortunately, HERVs are also linked to the development of autoimmune diseases, such as Multiple Sclerosis, as it has been described in the past 30 years [23,24,25,26,27,28,29,30]. Interestingly, HERVs may promote an inflammatory response, and some pieces of evidence also point out that the inflammatory response may trigger HERV expression [6, 7], suggesting a looping between them.
HERVs have been postulated to play a role in BD and SZ diseases since there was a higher level of HERVs expression in brains Cerebrospinal fluid (CSF) and Peripheral Blood Mononuclear cells (PBMC) from patients with these diseases [31, 32]. Furthermore, higher concentration of some pro-inflammatory cytokines associated with HERV-W antigenemia [33] and direct role of HERV in the pathogenic reduction of neuronal density in the hippocampus region and expressive changes of dendritic morphologic were found in SZ patients [34]. Therefore, this evidence indicates possible and dynamic mechanisms for the role of HERVs, especially HERV-W, in BD and SZ’s pathogenesis.
Regarding systemic immune/inflammatory status in mental diseases, it was documented that the balance between some pro-inflammatory cytokines, such as TNF-α and IFN-γ, and anti-inflammatory cytokines, such as IL-4 and IL-10, which are involved in the T-helper type 1 (Th1) and T-helper type 2 (Th2) profiles, plays a corollary role in this mental disorders as BD [35] and SZ [36].
Although compelling evidence points out that HERVs might be associated with the pathogenesis of mental disorders, such as BD and SZ, the interplay between HERV-W-env expression in those patients and its systemic inflammatory status is still poorly understood. Based on this, here, we aimed to investigate both the HERV-W-env expression and the systemic inflammatory status in BD and SZ patients.
Methods
Study population
Patients with mental disorders (MD) (n = 48) were separated into two groups according to their diagnosis in Schizophrenia (SZ, n = 24) and Bipolar Disorder (BD, n = 24) groups. Another group with healthy individuals, paired by age without any previous report of autoimmune disease and mental disorders in the family, was included as a control group (n = 46). Sociodemographic data were collected from all volunteers through questionnaires. MD patients had previous diagnosis and were followed in the psychiatric outpatient clinic of Universidade Santo Amaro and in the Centro de Apoio de Atenção Psicossocial (CAPS), both located in the south region of São Paulo city, Brazil. Mini International Neuropsychiatric Interview (MINI) [37, 38] was used to confirm the diagnosis. Only patients out of the mania phase were included in the BD group. Symptoms of depression were assessed by Montgomery–Åsberg Depression Rating Scale [39] and symptoms of mania by Young Mania Rating Scale [40]. For the SZ group we recruited only patients who were not in a psychosis event. The study was approved by the ethical committee of Universidade Santo Amaro under protocol # 5.469.700. It is worth mentioning that the study was performed in agreement with the Declaration of Helsinki.
Blood sample collection and preparation
Blood samples were collected in tubes containing the anticoagulant EDTA for peripheral blood mononuclear cells (PBMCs) obtention, which was used to perform the molecular analysis, and in gel-barrier dry tubes for serum obtention, which was used to determine the systemic cytokine concentration.
PBMCs were obtained by Ficoll-HyPaque protocol and RNA was extracted by Trizol-chloroform Method. Briefly, Ficoll-Hypaque was added into whole blood in 1:1 proportion and centrifuged at 800 g for 20 min. Afterward, the upper solution (PBMC) was collected and washed repeatedly with sterile PBS and centrifugation until the removal of residual erythrocytes was removed entirely. Finally, cell pellet containing exclusively the PBMCs undergoing RNA extraction as follows: 1mL of Trizol was added into each of cell pellet samples and up-down was performed until complete homogenization, then 200µL of chloroform was added and samples were centrifuged at 10.000 g at -4oC. The upper phase was completely removed (around 600 µL) and then RNA was precipitated with Isopropanol 100% and washed twice with 70% Ethanol. RNA was resuspended in 40 µL H2O-Nuclease free. Rigorous decontamination of genomic DNA was performed with DNA-free turbo (Ambion). The absence of contaminant genomic DNA was confirmed by Real-Time PCR with primers complementary to the GAPDH gene with the absence of Reverse Transcriptase. RNA was quantified and then stored at -80oC. Around 150ng of RNA was used to synthesize the cDNA with a High-Capacity Reverse Transcription Kit.
HERV-W detection and relative quantification analysis
We used primers complementary to HERV-W-env [41] and GAPDH as housekeeping genes [42]. The RT-PCR mix included 0.1 µM of each primer and 1x of PCR Master Mix Sybr-Green one step (Merck). The cycling conditions for HERV-W were: 50oC for 2 min, 95oC for 10 min followed by 40 cycles of 95oC for 1 min, 50oC for 45 s and 60oC for 1 min. For GAPDH assay the cycling conditions were: 50oC for 2 min, 95oC for 10 min followed by 40 cycles of 95oC for 1 min and 60oC for 1 min. In both assays a previous step was added of 37oC for 30 min for cDNA synthesis and a final cycle to determine the melting temperature of the samples (55oC to 95oC). HERV activity expression was evaluated qualitatively (absence/presence) and quantitatively (level of expression). The level of expression was determined by the 2-ΔΔCt method where ΔCt = (HERV Ct- GAPDH Endogenous Control Ct)– (Average of ΔCt of all controls), and the results were represented as fold changes. In all cases, samples were considered positive for HERVs if the melting curve was the same or ± 0.3oC distinct from the control samples and therefore included in the relative quantification analysis.
Determination of systemic cytokine concentration
Serum concentrations of the cytokines IL-2, IL-4, IL-6, IL-10, TNF-α and INF-γ were determined by using the ELISA commercial kits (ThermoFisher), following the manufacturer’s instructions. The concentration of each cytokine was calculated through an appropriate standard curve that presented a correlation coefficient from 0.95 to 0.99, with coefficients of variance intra-assay varying from 2,5 to 4% and from 8 to 10% in inter-assay.
Statistical analysis
The sample size was collected by convenience. Descriptive data was obtained, and for the comparison of scores between different groups, both parametric and non-parametric tests were employed according to the normality distribution of the data. The tests used were as follows: The normality test was performed using the Shapiro-Wilk test, and the homogeneity of variance was evaluated using the Levene test. Mann-Whitney test was used to analyze HERV-W-env expression between MD and control groups, whereas the Kruskal-Wallis test was used to assess the differences between the BD, SZ, and control groups. In addition, Spearman’s rank coefficient correlation test was also applied. All tests were conducted under the assumption of a first-type error probability (alpha) of 5% (p < 0.05).
Results
Clinical and demographic findings of the volunteers
Table 1 summarizes the demographic characteristics of the volunteers included in the present study.
In addition to the demographic findings, it is noteworthy to mention that individuals in the BD group received a confirmed diagnosis at an average age of 26 ± 11.3 years, and the SZ group received a confirmed diagnosis at a similar average age (27 ± 10.9 years). In addition, both groups have been diagnosed for more than 15 years. It is essential to mention that most of the patients enrolled did not exhibit any other comorbidities, with only 8 (33%) in the BD group and 7 (25%) in the SZ group presenting additional inflammatory disorders, such as hypertension and dyslipidemia.
HERV-W-envexpression is upregulated in SZ patients
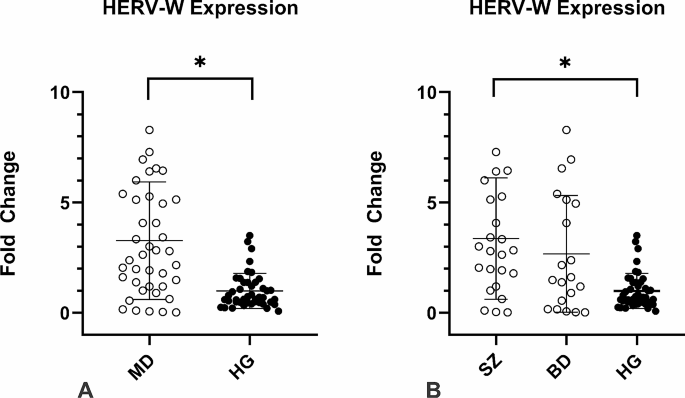
All participants of the study showed expression of HERV-W-env. However, the expression in MD patients was 3.3-fold higher on average than the control group (p < 0.01, Fig. 1A). When analyzing the HERV-W-env expression levels in the SZ and BD groups separately, the SZ group presented 3.3-fold higher on average than the control group (p < 0.01, Fig. 1B), whilst the BD group did not show significant differences in the HERV-W-env expression as compared to the control group (p = 0.54, Fig. 1B). In addition we have performed the analysis of HERV-W expression according to the therapeutic scheme, however no significant difference was found in any analysis.
HERV-W env relative expression in the individuals enrolled in the study. Figure 1 A: MD patients presented higher HERV-W expression *p < 0.01; Fig. 1B: HERV-W expression levels were assessed in MD individuals according to each group. SZ patients showed significantly higher levels of HERV-W expression than CG *p < 0.01, Mann-Whitney`s test
BD and SZ patients present higher concentration of some systemic cytokines
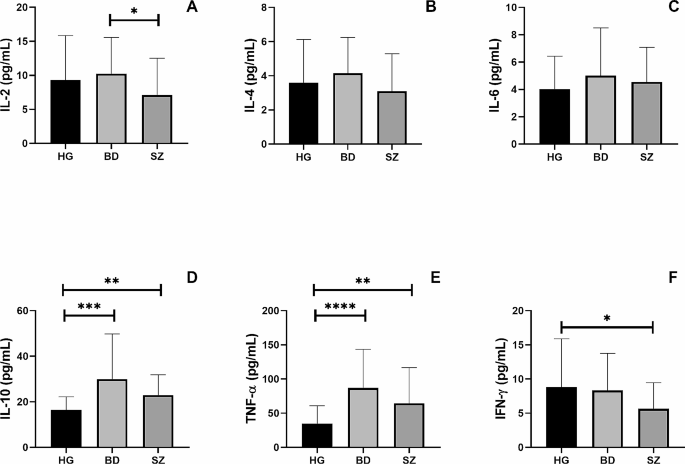
Figure 2 shows the systemic cytokine levels of IL-2 (Fig. 2A), IL-4 (Fig. 2B), IL-6 (Fig. 2C), IL-10 (Fig. 2D), TNF-α (Fig. 2E), and IFN-γ (Fig. 2F) in the volunteer groups. Both BD and SZ groups presented higher serum concentrations of IL-10 (p = 0.001 and p = 0.01, respectively) and TNF-α (p = 0.0001 and p = 0.01, respectively) than those observed in the control group. In addition, the SZ group showed lower circulating levels of IL-2 and IFN-γ than the BD group (p = 0.05) and the control group (p = 0.05), respectively.
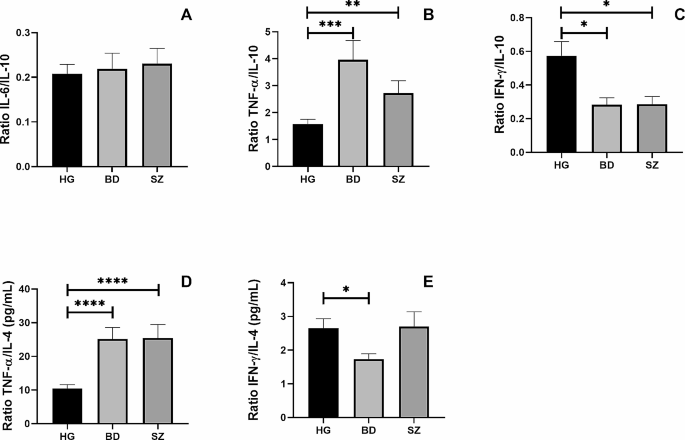
Additionally, Fig. 3 shows the ratio between the circulating levels of IL-6/IL-10 (Fig. 3A), TNF-α/IL-10 (Fig. 3B), IFN-γ/IL-10 (Fig. 3C), TNF-α/IL-4 (Fig. 3D), and IFN-γ/IL-4 (Fig. 4E) was also assessed. Higher TNF-α/IL-4 (p = 0.0001) and TNF-α/IL-10 (p = 0.001) ratios, as well as lower IFN-γ/IL-10 ratio (p = 0.05), were found in BD and SZ groups than in the control group. In addition, a lower IFN-γ/IL-4 ratio was observed in the BD group than in the control group (p = 0.05).
The ratio between pro-inflammatory and anti-inflammatory cytokines in HG, BD and SZ groups. The ratio between the concentrations of the pro-inflammatory cytokines IL-6 (A), TNF-α (B), and IFN-γ (C) and the anti-inflammatory cytokine IL-10 and the ratio between the concentrations of the pro-inflammatory cytokines TNF-α (D), IFN-γ (E) and the anti-inflammatory cytokine IL-4 in the plasma of patients enrolled in the study. Legend: BD = Bipolar Disorder Group, SZ = Schizophrenia Group, HG = Healthy individuals Group *= p < 0.05, **=p < 0.01, ***=p < 0.001, ****p < 0.0001
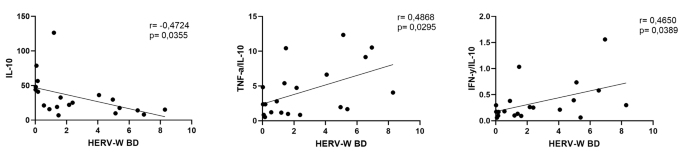
HERV-W-env expression correlates with the pro-inflammatory profile in BD patients
Figure 4 shows the significant results obtained in the Spearman coefficient correlation analysis. Exclusively in the BD group, it was observed a significant negative correlation (r=-0.4724) between the levels of HERV-W-env expression and serum IL-10 (Fig. 4A), as well as significant positive correlations between the HERV-W-env expression levels and TNF-α/IL-10 (r = 0.4868) (Fig. 4B) or IFN-γ/IL-10 (r = 0.4650) (Fig. 4C) ratios.
Discussion
Here, we have described a higher HERV-W-env expression in PBMCs obtained from MD patients compared to healthy individuals, and, specifically, the SZ group showed that this increase was 3.3-fold higher than HG (p < 0.01). This finding is not only in touch with previous studies that described higher levels of HERV-W expression in SZ patients [43], but can also provide additional evidence supporting the involvement of altered HERV-W-env activity in SZ patients. Moreover, and in an exciting way, SZ patients presented higher serum TNF-α concentrations than the control group, despite this finding not being positively correlated with HERV-W expression nor the pro- and anti-inflammatory cytokines ratios. This result not only corroborates the suggestion that the systemic inflammatory status has an essential role in SZ, but also might indicate that this condition was not closely associated with the HERV-W expression, even though its expression was also increased in those patients. Based on these facts, we can putatively suggest that systemic inflammatory status and HERV-W expression components may show distinct pathogenic roles in this disease. Previous reports in the literature have pointed out a direct involvement of neuronal pathological modifications with HERV-W-env expression and, particularly, some of these findings should be highlighted: neuronal apoptosis induction [44], structural and functional abnormalities in dopaminergic neurons that stimulates substantially the production of dopamine through Dopamine Receptor D2 (DRD2) and leads to alteration in sodium and calcium influx, which suggests a pivotal role of HERV-W not only in neuronal pathophysiology [45] but also in the reduction of hippocampal neurons density and the alteration of its dendritic and perikaryon morphology [46]. Although these previous data are very interesting in the context of HER-W expression and the nervous system, it is noteworthy to cite that our results were associated with the HERV-W-env expression in PBMC and not specifically concerning the central nervous system (CNS).
In a different way to SZ patients, the BD patients did not show significant differences in the HERV-W-env expression in PBMC as compared to the control group. This finding is in contrast with previous reports in the literature since it has been demonstrated higher levels of HERV-W expression both systemically [43] and in the brain [47] of BD patients. Even though we cannot affirm, some hypotheses can help us to understand this lack of a significant difference in HERV-W-env expression between BD and control groups found here: (i) since the HERV-W-env expression levels could putatively be impacted by different clinical manifestations in BD, it would be expected that BD patients in euthymic conditions could present a reduction on the HERV-W-env expression levels, however, it is paramount to mention that, until now, the dynamics of HERV expression in BD patients was not fully understood, thus a remarkable variation in their expression can also be presented in euthymic condition; and: (ii) despite no significant difference was found, a tendency for it could be observed (p = 0.054) and maybe this lack of statistically significant difference could be related to the number of BD patients enrolled in the study.
Previous findings report that both SZ and BD patients are associated with the deregulation of the systemic cytokine’s concentration. The main hypothesis is that the inflammatory condition may interfere specially in the blood and brain barrier permeability [48, 49]. It is supposed that in both BD and SZ, a pro-inflammatory profile is present and, chronically, could promote pathological modification in the CNS way before the disease’s onset. In this sense, it is known that not only genetic background added to the fundamental environmental factors is necessary to deregulate the balance of the immune-inflammatory responses [50] but also, as previously cited, that HERVs may elicit the inflammation in both physiological and pathological conditions [7, 51, 52]. Taken together, these pieces of information can corroborate our findings in which BD patients presented not only higher circulating TNF-α levels, a well-known pro-inflammatory cytokine associated with Th1 immune profile, than the control group but also a significant negative correlation between the levels of HERV-W-env expression and IL-10, a classical anti-inflammatory cytokine, besides significant positive correlations with TNF-α/IL-10 and IFN-γ/IL-10 ratios. Based on these data, it is clear that BD patients presented a prominent systemic pro-inflammatory status, which agrees with previous reports [53,54,55]. At this point, it is paramount to highlight that the ratio assessment has been considered as an accurate measure concerning the balance of pro- and anti-inflammatory cytokines in different contexts, including in MD patients [56]. Moreover, it is worth also mentioning that, although the pro-inflammatory status is present in BD patients, the triggers involved in this condition are yet to be found [57]. Thus, our findings can putatively suggest that HERV-W-env could be a potential player in this context. However, this hypothesis requires further investigation.
Beyond these findings, it is also of utmost importance to highlight that, regardless of the association with HERV-W-env expression, both BP and SZ groups showed a higher TNF-α/IL-4 ratio, which together with the higher TNF-α/IL-10 ratio, than the control group, which allows us to suggest that, in general, the T-help immune response was towards to Th1 profile, since there is a consensus that both TNF-α and IFN-γ are related to the Th1 immune profile, whereas the IL-4 is a classical cytokine of Th2 immune profile and IL-10 is closely associated with T regulatory (T-reg) immune profile [58]. In fact, according to the literature, increased TNF-α/IL-4 and IFN-γ/IL-4 ratios were verified in BP patients during manic episodes as compared with normal controls [59], and a relative predominance of the Th2 immune profile was evidenced in patients with acute exacerbation of schizophrenia [36]. Despite an imbalance in the Th1/Th2/Treg immune profile could be related to some worse outcomes both in BP and SZ patients, the results observed here allow us to putatively suggest that a “regulated” Th1 immune profile was present in the BP and SZ individuals enrolled in the present study since they showed not only higher circulating IL-10 levels but also lower IFN-γ/IL-10 ratio than the control group. In addition, the BP group also showed a significant reduction in the IFN-γ/IL-4 ratio compared to the control group, which corroborates our suggestion that although the Th1 immune response was predominant, this profile was not exacerbated at this point.
Among other limitations of the study formerly cited, our study presents another important limitation that should be mentioned since none of the patients enrolled in the study were under clinical episodes of BD and SZ in the sampling time, which might underestimate the level of HERV-W-env expression and cytokine concentration in thisstudy. Also, we were not able to gather enough patients to properly compare the levels of HERV-W-env expression and the systemic cytokine concentration according to the therapeutic scheme.
In summary, we have described high levels of HERV-W-env expression in SZ patients, which generally did not show a close association with the systemic cytokine levels. Therefore, in those patients, whereas the HERV-W-env may be related to SZ pathogenesis, their interplay with systemic inflammatory status was not evidenced, which allows us to suppose that HERV could act directly in a pathological manner, causing cellular changes capable of interfering with neuronal functioning, and not necessarily immune-inflammatory mediated. On the other hand, BD seems to present a distinct profile since a close association with the systemic inflammatory status was evidenced in those patients. Even though the HERV-W-env expression was not significantly higher in BD patients (p = 0.054), significant positive correlations between HERV-W-env expression and TNF-α/IL-10 and IFN-γ/IL-10 ratios, as well as significant negative correlation observed between the level of HERV-W-env expression and circulating IL-10, were found. Based on these findings, it is reasonable to consider that HERV-W-env may be modulating the inflammatory conditions of BD patients, which might propose a distinct pathogenic mechanism for this disease in contrast to SZ patients.
Finally, although significant findings have continually been described, subsequent studies should focus on understanding the natural role of HERVs in the systemic inflammatory profile in these diseases in order not only to identify possible interplay pathways between them in this context as well as to investigate whether HERV-W-env could represent a possible target candidate for treatment of both diseases. Additionally, and equally important, a comprehensive analysis of distinct HERV families upregulated in MD patients should also be a priority to improve the understanding of the dynamics of these retroelements’ expression in the MD pathogenesis.
Data availability
No datasets were generated or analysed during the current study.
References
Wang PS, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Borges G, Bromet EJ et al. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet (London, England) [Internet]. 2007 [cited 2023 Nov 27];370:841–50. https://pubmed.ncbi.nlm.nih.gov/17826169/.
Solmi M, Cortese S, Vita G, De Prisco M, Radua J, Dragioti E et al. An umbrella review of candidate predictors of response, remission, recovery, and relapse across mental disorders. Mol Psychiatry [Internet]. 2023 [cited 2023 Nov 27]; https://pubmed.ncbi.nlm.nih.gov/37957292/.
Fryar-Williams S, Tucker G, Strobel J, Huang Y, Clements P. Molecular Mechanism Biomarkers Predict Diagnosis in Schizophrenia and Schizoaffective Psychosis, with Implications for Treatment. Int J Mol Sci [Internet]. 2023 [cited 2023 Nov 27];24. https://pubmed.ncbi.nlm.nih.gov/37958826/.
Schmitt Junior AA, Primo de Carvalho Alves L, Padilha BL, da Rocha NS. Serum cytokine variations among inpatients with major depression, bipolar disorder, and schizophrenia versus healthy controls: a prospective true-to-life study. Ther Adv Psychopharmacol [Internet]. 2023 [cited 2023 Nov 27];13. https://pubmed.ncbi.nlm.nih.gov/36814596/.
Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry [Internet]. 2016 [cited 2023 Nov 27];21:1696–709. https://pubmed.ncbi.nlm.nih.gov/26903267/.
Helmy M, Selvarajoo K. Systems Biology to Understand and Regulate Human Retroviral Proinflammatory Response. Front Immunol [Internet]. 2021 [cited 2022 Aug 10];12. https://pubmed.ncbi.nlm.nih.gov/34867957/.
Rangel SC, da Silva MD, da Silva AL, dos Santos J, de MB, Neves LM, Pedrosa A et al. Human endogenous retroviruses and the inflammatory response: A vicious circle associated with health and illness [Internet]. Front. Immunol. 2022. https://www.frontiersin.org/articles/https://doi.org/10.3389/fimmu.2022.1057791.
Dolei A. Endogenous retroviruses and human disease. Expert Rev Clin Immunol [Internet]. 2006/01/01. 2006;2:149–67. http://www.ncbi.nlm.nih.gov/pubmed/20477095.
Wang X, Wu X, Huang J, Li H, Yan Q, Zhu F. Human endogenous retrovirus W family envelope protein (HERV-W env) facilitates the production of TNF-α and IL-10 by inhibiting MyD88s in glial cells. Arch Virol [Internet]. 2021 [cited 2022 Aug 10];166:1035–45. https://pubmed.ncbi.nlm.nih.gov/33438105/.
Weiss RA. The discovery of endogenous retroviruses. Retrovirology [Internet]. 2006 [cited 2015 Jun 23];3:67. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1617120&tool=pmcentrez&rendertype=abstract
Griffiths DJ. Endogenous retroviruses in the human genome sequence. Genome Biol [Internet]. 2001 [cited 2015 Jun 10];2:REVIEWS1017. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=138943&tool=pmcentrez&rendertype=abstract
Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG et al. The Sequence of the Human Genome. Science (80-) [Internet]. 2001 [cited 2017 Oct 24];291:1304–51. http://www.ncbi.nlm.nih.gov/pubmed/11181995.
Belshaw R, Pereira V, Katzourakis A, Talbot G, Paces J, Burt A et al. Long-term reinfection of the human genome by endogenous retroviruses. Proc Natl Acad Sci U S A [Internet]. 2004 [cited 2015 Aug 3];101:4894–9. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=387345&tool=pmcentrez&rendertype=abstract
Weiss RA. The discovery of endogenous retroviruses. Retrovirology [Internet]. 2006 [cited 2017 Sep 28];3:67. http://www.ncbi.nlm.nih.gov/pubmed/17018135.
Patience C, Wilkinson DA, Weiss RA. Our retroviral heritage. Trends Genet [Internet]. 1997 [cited 2015 Aug 3];13:116–20. http://www.ncbi.nlm.nih.gov/pubmed/9066271.
Mager DL, Goodchild NL. Homologous recombination between the LTRs of a human retrovirus-like element causes a 5-kb deletion in two siblings. Am J Hum Genet [Internet]. 1989 [cited 2017 Oct 24];45:848–54. http://www.ncbi.nlm.nih.gov/pubmed/2573998.
Perron H, Perin JP, Rieger F, Alliel PM. Particle-associated retroviral RNA and tandem RGH/HERV-W copies on human chromosome 7q: possible components of a chain-reaction triggered by infectious agents in multiple sclerosis? J Neurovirol [Internet]. 2000 [cited 2015 Mar 10];6 Suppl 2:S67-75. http://www.ncbi.nlm.nih.gov/pubmed/10871789.
Contreras-Galindo R, Kaplan MH, Markovitz DM, Lorenzo E, Yamamura Y. Detection of HERV-K(HML-2) viral RNA in plasma of HIV type 1-infected individuals. AIDS Res Hum Retroviruses [Internet]. 2006 [cited 2016 Apr 8];22:979–84. http://www.ncbi.nlm.nih.gov/pubmed/17067267.
Ruprecht K, Ferreira H, Flockerzi A, Wahl S, Sauter M, Mayer J et al. Human Endogenous Retrovirus Family HERV-K(HML-2) RNA Transcripts Are Selectively Packaged into Retroviral Particles Produced by the Human Germ Cell Tumor Line Tera-1 and Originate Mainly from a Provirus on Chromosome 22q11.21. J Virol [Internet]. 2008 [cited 2017 Oct 24];82:10008–16. http://www.ncbi.nlm.nih.gov/pubmed/18684837.
Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature [Internet]. 2000 [cited 2015 Jun 8];403:785–9. http://www.ncbi.nlm.nih.gov/pubmed/10693809.
Medstrand P, Landry JR, Mager DL. Long terminal repeats are used as alternative promoters for the endothelin B receptor and apolipoprotein C-I genes in humans. J Biol Chem [Internet]. 2001 [cited 2015 Jun 8];276:1896–903. http://www.ncbi.nlm.nih.gov/pubmed/11054415.
Ting CN, Rosenberg MP, Snow CM, Samuelson LC, Meisler MH. Endogenous retroviral sequences are required for tissue-specific expression of a human salivary amylase gene. Genes Dev [Internet]. 1992 [cited 2015 Jun 24];6:1457–65. http://www.ncbi.nlm.nih.gov/pubmed/1379564.
Voisset C, Blancher A, Perron H, Mandrand B, Mallet F, Paranhos-Baccala G. Phylogeny of a Novel Family of Human Endogenous Retrovirus Sequences, HERV-W, in Humans and Other Primates. AIDS Res Hum Retroviruses [Internet]. 1999 [cited 2017 Oct 25];15:1529–33. http://www.ncbi.nlm.nih.gov/pubmed/10580403.
Perron H, Geny C, Laurent A, Mouriquand C, Pellat J, Perret J et al. Leptomeningeal cell line from multiple sclerosis with reverse transcriptase activity and viral particles. Res Virol [Internet]. 1989/11/01. 1989;140:551–61. http://www.ncbi.nlm.nih.gov/pubmed/2482522.
do Olival GS, Faria TS, Nali LHS, de Oliveira ACP, Casseb J, Vidal JE et al. Genomic analysis of ERVWE2 locus in patients with multiple sclerosis: absence of genetic association but potential role of human endogenous retrovirus type W elements in molecular mimicry with myelin antigen. Front Microbiol [Internet]. 2013 [cited 2015 Jun 22];4:172. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3693062&tool=pmcentrez&rendertype=abstract
Nali LH, Olival GS, Montenegro H, da Silva IT, Dias-Neto E, Naya H et al. Human endogenous retrovirus and multiple sclerosis: A review and transcriptome findings. Mult Scler Relat Disord [Internet]. 2022 [cited 2022 Mar 15];57. https://pubmed.ncbi.nlm.nih.gov/34922254/.
Kremer D, Gruchot J, Weyers V, Oldemeier L, Göttle P, Healy L et al. PHERV-W envelope protein fuels microglial cell-dependent damage of myelinated axons in multiple sclerosis. Proc Natl Acad Sci U S A [Internet]. 2019 [cited 2021 Sep 4];116:15216–25. https://pubmed.ncbi.nlm.nih.gov/31213545/.
Mameli G, Poddighe L, Astone V, Delogu G, Arru G, Sotgiu S et al. Novel reliable real-time PCR for differential detection of MSRVenv and syncytin-1 in RNA and DNA from patients with multiple sclerosis. J Virol Methods [Internet]. 2009 [cited 2015 Jun 22];161:98–106. http://www.ncbi.nlm.nih.gov/pubmed/19505508.
Mameli G, Madeddu G, Mei A, Uleri E, Poddighe L, Delogu LG et al. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein-Barr virus latency: The missing link with multiple sclerosis? PLoS One [Internet]. 2013 [cited 2021 Sep 24];8. https://pubmed.ncbi.nlm.nih.gov/24236019/.
Dolei A, Uleri E, Ibba G, Caocci M, Piu C, Serra C. The aliens inside human DNA: HERV-W/MSRV/syncytin-1 endogenous retroviruses and neurodegeneration. J Infect Dev Ctries [Internet]. 2015 [cited 2016 Apr 2];9:577–87. http://www.ncbi.nlm.nih.gov/pubmed/26142666.
Huang W, Li S, Hu Y, Yu H, Luo F, Zhang Q et al. Implication of the env gene of the human endogenous retrovirus W family in the expression of BDNF and DRD3 and development of recent-onset schizophrenia. Schizophr Bull [Internet]. 2011 [cited 2023 Nov 30];37:988–1000. https://pubmed.ncbi.nlm.nih.gov/20100784/.
Karlsson H, Bachmann S, Schröder J, McArthur J, Torrey EF, Yolken RH. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proc Natl Acad Sci U S A [Internet]. 2001 [cited 2023 Nov 30];98:4634–9. https://pubmed.ncbi.nlm.nih.gov/11296294/.
Tamouza R, Meyer U, Foiselle M, Richard JR, Lu C, lieng, Boukouaci W et al. Identification of inflammatory subgroups of schizophrenia and bipolar disorder patients with HERV-W ENV antigenemia by unsupervised cluster analysis. Transl Psychiatry [Internet]. 2021 [cited 2023 Nov 28];11. https://pubmed.ncbi.nlm.nih.gov/34230451/.
Nagase H, Yao S, Ikeda S. Acute and chronic effects of exercise on mRNA expression in the skeletal muscle of two mouse models of peripheral artery disease. PLoS One [Internet]. 2017 [cited 2023 Aug 22];12. https://pubmed.ncbi.nlm.nih.gov/28771574/.
Kim YK, Myint AM, Lee BH, Han CS, Lee SW, Leonard BE et al. T-helper types 1, 2, and 3 cytokine interactions in symptomatic manic patients. Psychiatry Res [Internet]. 2004 [cited 2023 Dec 5];129:267–72. https://pubmed.ncbi.nlm.nih.gov/15661320/.
Avgustin Bojana, Wraber Branka TR. Increased Th1 and Th2 immune reactivity with relative Th2 dominance in patients with acute exacerbation of schizophrenia - PubMed. Croat Med J [Internet]. 2005 [cited 2023 Dec 5];46:268–74. https://pubmed.ncbi.nlm.nih.gov/15849849/.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J Clin Psychiatry [Internet]. 1998 [cited 2024 Mar 28];59:11980. https://www.psychiatrist.com/jcp/mini-international-neuropsychiatric-interview-mini.
Pettersson A, Modin S, Wahlström R, Af Winklerfelt Hammarberg S, Krakau I. The Mini-International Neuropsychiatric Interview is useful and well accepted as part of the clinical assessment for depression and anxiety in primary care: a mixed-methods study. BMC Fam Pract [Internet]. 2018 [cited 2024 Mar 28];19. https://pubmed.ncbi.nlm.nih.gov/29368585/.
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry [Internet]. 1979 [cited 2024 Mar 28];134:382–9. https://pubmed.ncbi.nlm.nih.gov/444788/.
Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry [Internet]. 1978 [cited 2024 Mar 28];133:429–35. https://pubmed.ncbi.nlm.nih.gov/728692/.
Nellåker C, Yao Y, Jones-Brando L, Mallet F, Yolken RH, Karlsson H. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology [Internet]. 2006 [cited 2015 Jul 2];3:44. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1539011&tool=pmcentrez&rendertype=abstract
de Jonge HJM, Fehrmann RSN, de Bont ESJM, Hofstra RMW, Gerbens F, Kamps WA et al. Evidence based selection of housekeeping genes. PLoS One [Internet]. 2007 [cited 2016 Jan 22];2:e898. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1976390&tool=pmcentrez&rendertype=abstract
Perron H, Hamdani N, Faucard R, Lajnef M, Jamain S, Daban-Huard C et al. Molecular characteristics of Human Endogenous Retrovirus type-W in schizophrenia and bipolar disorder. Transl Psychiatry [Internet]. 2012 [cited 2023 Nov 30];2. https://pubmed.ncbi.nlm.nih.gov/23212585/.
Li X, Wu X, Li W, Yan Q, Zhou P, Xia Y et al. HERV-W ENV Induces Innate Immune Activation and Neuronal Apoptosis via linc01930/cGAS Axis in Recent-Onset Schizophrenia. Int J Mol Sci [Internet]. 2023 [cited 2023 Nov 30];24. https://pubmed.ncbi.nlm.nih.gov/36769337/.
Yan Q, Wu X, Zhou P, Zhou Y, Li X, Liu Z et al. HERV-W Envelope Triggers Abnormal Dopaminergic Neuron Process through DRD2/PP2A/AKT1/GSK3 for Schizophrenia Risk. Viruses [Internet]. 2022 [cited 2023 Nov 30];14. https://pubmed.ncbi.nlm.nih.gov/35062349/.
Yao W, Zhou P, Yan Q, Wu X, Xia Y, Li W et al. ERVWE1 Reduces Hippocampal Neuron Density and Impairs Dendritic Spine Morphology through Inhibiting Wnt/JNK Non-Canonical Pathway via miR-141-3p in Schizophrenia. Viruses [Internet]. 2023 [cited 2023 Nov 28];15. https://pubmed.ncbi.nlm.nih.gov/36680208/.
Li F, Sabunciyan S, Yolken RH, Lee D, Kim S, Karlsson H. Transcription of human endogenous retroviruses in human brain by RNA-seq analysis. PLoS One [Internet]. 2019 [cited 2023 Nov 30];14. https://pubmed.ncbi.nlm.nih.gov/30605476/.
Altamura AC, Buoli M, Pozzoli S. Role of immunological factors in the pathophysiology and diagnosis of bipolar disorder: comparison with schizophrenia. Psychiatry Clin Neurosci [Internet]. 2014 [cited 2023 Dec 1];68:21–36. https://pubmed.ncbi.nlm.nih.gov/24102953/.
Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci [Internet]. 2010 [cited 2023 Dec 1];64:217–30. https://pubmed.ncbi.nlm.nih.gov/20602722/.
Giacomo F, Di, Strippoli M-PF, Castelao E, Amoussou JR, Gholam M, Ranjbar S et al. Risk factors for mood disorders among offspring of parents with bipolar disorder: Findings from a discordant-sibling study. Psychiatry Res [Internet]. 2023 [cited 2023 Dec 5];330:115615. https://pubmed.ncbi.nlm.nih.gov/38007982/.
Buzdin AA, Prassolov V, Garazha AV. Friends-Enemies: endogenous retroviruses are major transcriptional regulators of human DNA. Front Chem. 2017;5:35.
Perron H, Germi R, Bernard C, Garcia-Montojo M, Deluen C, Farinelli L et al. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler [Internet]. 2012 [cited 2015 Jun 22];18:1721–36. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3573672&tool=pmcentrez&rendertype=abstract
Rosenblat JD, McIntyre RS. Bipolar Disorder and Inflammation. Psychiatr Clin North Am [Internet]. 2016 [cited 2023 Dec 1];39:125–37. https://pubmed.ncbi.nlm.nih.gov/26876323/.
Saccaro LF, Crokaert J, Perroud N, Piguet C. Structural and functional MRI correlates of inflammation in bipolar disorder: A systematic review. J Affect Disord [Internet]. 2023 [cited 2023 Dec 1];325:83–92. https://pubmed.ncbi.nlm.nih.gov/36621677/.
Quidé Y, Bortolasci CC, Spolding B, Kidnapillai S, Watkeys OJ, Cohen-Woods S et al. Systemic inflammation and grey matter volume in schizophrenia and bipolar disorder: Moderation by childhood trauma severity. Prog Neuropsychopharmacol Biol Psychiatry [Internet]. 2021 [cited 2023 Dec 1];105. https://pubmed.ncbi.nlm.nih.gov/32540496/.
Maes M, Carvalho AF. The Compensatory Immune-Regulatory Reflex System (CIRS) in Depression and Bipolar Disorder. Mol Neurobiol [Internet]. 2018 [cited 2023 Dec 5];55:8885–903. https://pubmed.ncbi.nlm.nih.gov/29611101/.
Freund N, Juckel G. Bipolar Disorder: Its Etiology and How to Model in Rodents. Methods Mol Biol [Internet]. 2019 [cited 2023 Dec 1];2011:61–77. https://pubmed.ncbi.nlm.nih.gov/31273693/.
Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF et al. The Role of Aberrations in the Immune-Inflammatory Response System (IRS) and the Compensatory Immune-Regulatory Reflex System (CIRS) in Different Phenotypes of Schizophrenia: the IRS-CIRS Theory of Schizophrenia. Mol Neurobiol [Internet]. 2020 [cited 2023 Dec 5];57:778–97. https://pubmed.ncbi.nlm.nih.gov/31473906/.
Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord [Internet]. 2007 [cited 2023 Dec 5];104:91–5. https://pubmed.ncbi.nlm.nih.gov/17434599/.
Acknowledgements
Not applicable.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo Grants #2023/08773-0 and #2022/07567-5.
Author information
Authors and Affiliations
Contributions
SCR and MDS performed data collection, experimental analysis and data analysis and paper writing DGNF performed clinical analysis of the patients and applied the inclusion and exclusion in order to select the volunteersSNS performed data collection and experimental analysisJBA performed the inflammatory status analysis JRV performed the critical review of the manuscript KCNS performed the inflammatory status analysisIDT critical review of the manuscript and paper writingCNF performed the critical review of the manuscript, paper writing and study designMTS coordinated the molecular assays and data analysisLMN performed the clinical analysis of the patients and ALLB performed the study design, funding acquisition, paper writing and reviewingLHSN performed the study design, funding acquisition, paper writing, data analysis, paper reviewing.All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethical committee of Universidade Santo Amaro under protocol # 5.469.700. It is worth mentioning that the study was performed in agreement with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rangel, S.C., da Silva, M.D., Natrielli Filho, D.G. et al. HERV-W upregulation expression in bipolar disorder and schizophrenia: unraveling potential links to systemic immune/inflammation status. Retrovirology 21, 7 (2024). https://doi.org/10.1186/s12977-024-00640-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12977-024-00640-3