Abstract
Background
Myasthenic crisis (MC) and disease exacerbation in myasthenia gravis (MG) are associated with significant lethality and continue to impose a high disease burden on affected patients. Therefore, we sought to determine potential predictors for MC and exacerbation as well as to identify factors affecting outcome.
Methods
We examined a retrospective, observational cohort study of patients diagnosed with MG between 2000 and 2021 with a mean follow-up of 62.6 months after diagnosis from eight tertiary hospitals in Germany. A multivariate Cox regression model with follow-up duration as the time variable was used to determine independent risk factors for MC and disease exacerbation.
Results
815 patients diagnosed with MG according to national guidelines were included. Disease severity at diagnosis (quantitative MG score or Myasthenia Gravis Foundation of America class), the presence of thymoma and anti-muscle specific tyrosine kinase-antibodies were independent predictors of MC or disease exacerbation. Patients with minimal manifestation status 12 months after diagnosis had a lower risk of MC and disease exacerbation than those without. The timespan between diagnosis and the start of immunosuppressive therapy did not affect risk. Patients with a worse outcome of MC were older, had higher MGFA class before MC and at admission, and had lower vital capacity before and at admission. The number of comorbidities, requirement for intubation, prolonged mechanical ventilation, and MC triggered by infection were associated with worse outcome. No differences between outcomes were observed comparing treatments with IVIG (intravenous immunoglobulin) vs. plasma exchange vs. IVIG together with plasma exchange.
Conclusions
MC and disease exacerbations inflict a substantial burden of disease on MG patients. Disease severity at diagnosis and antibody status predicted the occurrence of MC and disease exacerbation. Intensified monitoring with emphasis on the prevention of infectious complications could be of value to prevent uncontrolled disease in MG patients.
Graphical Abstract

Similar content being viewed by others
Background
Myasthenia gravis (MG) is an acquired autoimmune disorder of the neuromuscular junction characterized by dysfunction of the post-synaptic membrane [1]. Owing to improved treatment strategies and diagnostic tools, therapeutic outcomes have improved for the majority of MG patients [2]. However, a clinically distinct subgroup of patients, often referred to as refractory, remains symptomatic despite therapy [2, 3]. Exacerbation of disease and myasthenic crisis (MC) are frequent in these patients and substantially contribute to disease burden [4]. Despite diagnostic and therapeutic advances for the management of MG, patients experiencing MC continue to face a substantial mortality rate of approximately 5–12% [5, 6]. The requirement for hospitalisation, the associated burden of disease and the cost of available rescue therapies, underline the importance of the prevention and management of MC [7, 8].
Hindered by the rarity of MG, our understanding of the underlying pathophysiological mechanisms related to insufficient disease control remains fragmented. A range of potential triggers for the manifestation of MC or disease exacerbations have been observed including infections, surgery, adverse effects of medication, co-morbidity, pregnancy or tapering of immunosuppressive medication [9, 10]. Prognostic factors identifying patients at risk for MC or disease exacerbations remain incompletely understood and have only been characterized for MG patients presenting with a thymoma [11, 12]. However, factors predicting the occurrence of MC especially in patients without thymoma remain largely elusive. Finally, factors defining the outcome of MC are incompletely identified, but urgently needed to guide the clinical management of these patients. Our analysis aims at understanding factors predicting clinical deteriorations. We therefore analysed a cohort of 815 MG patients to identify potential risk factors for MC and disease exacerbations.
Methods
Study design and participants
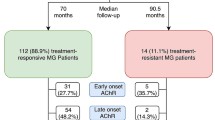
Our cohort study is a retrospective analysis of 815 patients from eight university hospitals in Germany (Charité—Universitätsmedizin Berlin and University Hospitals Cologne, Duesseldorf, Essen, Freiburg, Magdeburg, Muenster and Regensburg). Patients requiring intensive care were treated on specialized neurological intensive care units (NICU). Patients were identified by searching the on-site database for the corresponding ICD-10 code (ICD-10-GM-2019 G70.-). Overall, 1645 patients were screened, of whom 815 were included in the analysis (Fig. 1). Diagnosis of MG was established by characteristic clinical presentation in accordance with national guidelines [13], independent of disease duration or severity. All centres are certified as integrated myasthenia centre (iMC) by the German Myasthenia Gravis Society applying standardised clinical pathways for patient management. Diagnosis was supported by antibody findings and repetitive nerve stimulation. Antibody testing was performed by enzyme-linked- or radio-immunoassay (Euroline). Suspected cases without established diagnosis, with a change to their diagnosis (n = 609) or with insufficient case documentation were excluded (< 6 months of longitudinal documentation) (n = 127) (Fig. 1). The final cohort consisted of patients diagnosed between January 2000 and July 2021. Patients with an established diagnosis and sufficient longitudinal documentation of > 6 months were included during this time period. Socio-demographics (age, sex, disease duration), antibody (ab) status (acetylcholine-receptor (AChR), muscle specific receptor tyrosine kinase (MuSK), lipoprotein-related protein 4 (LRP4), seronegative), MG specific medication (cholinesterase-inhibitors, glucocorticoids, and long-term immunosuppressant’s), history of thymoma-status, and comorbidities were collected from patient’ charts. The follow-up strategy was standardized across centres. According to iMC standards, patients with a stable course were seen every 6 months and instable patients more frequently. MG-specific scoring was performed by the treating neurologist at the time of presentation.
Definitions
For this cohort analysis, we differentiated between MC and disease exacerbation as distinct clinical events.
A MC was defined as a rapid clinical decline requiring non-invasive ventilation, intubation or parenteral nutrition [14]. Dysphagia severe enough to require a nasogastric tube was also included as criterion for MC.
A disease exacerbation was defined as fulfilment all of the following criteria as adapted from national guidelines [15]:
-
Objective: QMG (quantitative myasthenia gravis) score [16] of ≥ 8 points and a minimum increase of ≥ 5 points from the previous visit. Ocular findings must not account for more than 5 points on the QMG score.
-
Subjective: progressive clinical deterioration due to weakness of bulbo-pharyngeal or limb muscles or reduced respiratory function impacting activities of daily living.
-
Period of time: progress of symptoms no longer than 30 days.
A clinical event matching both the definition of MC and disease exacerbation was classified as MC. The outcome of MC was defined according to the MGFA (Myasthenia Gravis Foundation of America) post-intervention status (MGFA–PIS) [17, 18]: Specifically, improved signifies that QMG score at hospital discharge was reduced by ≥ 3 points compared to pre-admission, worse signifies that QMG score hospital discharge was increased by ≥ 3 points compared to before the admission and unchanged signifies that neither the criteria for improved nor worse were met. Patients with worse outcome were discharged for further rehabilitation. The threshold was defined to be that a score of 3 points in a single item of the QMG score reflects severe impairment [18]. The cutoff between early-(EOMG) and late-onset (LOMG) MG was set at 50 years as previously defined [19]. Minimal manifestation status (MMS) was defined in accordance with the MGFA–PIS as no symptoms of functional limitation from MG but weakness on examination only detectable by examination [17, 20, 21]. For MMS, immunosuppressive therapy and symptomatic therapy, e.g., cholinesterase inhibitors, were permitted (analogous to MMS-3 as proposed by the MGFA–PIS) [17, 20, 21].
Standard protocol approvals, registrations, and patient consents
The study was approved by the local ethics committee and institutional review boards (no. AZ 2020-010-f-S, no. AZ 07/2017, 19-8973-BO, AZ 21-1265, AZ 21-1331). Data were anonymized and collected retrospectively according to the standardized requirements of the German register for myasthenia.
Statistical analysis
Statistical Analysis was performed using GraphPad Prism 9.3 (GraphPad Software, Inc., San Diego, CA) and R (R Core Team, 2020). Data were presented as median (IQR = interquartile range), mean (standard deviation = SD), or n (%). For univariate logistic regression, goodness of fit was assessed by Cox-Snell’s generalized R squared or Tjur’s Pseudo R squared as appropriate. Significance was assessed by the likelihood ratio test. The odds ratio (OR) was assessed using a multivariate Cox regression model with follow-up as the time variable. Experiencing at least one MC or disease exacerbation compared to no event was used as the status variable. For analysis of time between diagnosis and MC or disease exacerbation the Kaplan–Meier method was used. Statistical significance between survival curves was determined by a pairwise log rank test. Analysis of variance (ANOVA) testing was performed for the analysis of groups for continuous variables and Fisher’s exact test for categorial variables. To account for multiple comparisons, statistical significance was corrected by the false discovery rate (FDR). Anonymized data will be shared by request from any qualified investigator. For regression analysis of MGFA class II to IV, MGFA classes A and B were combined to allow for statistical analysis. Therefore, analysis is limited to MGFA classes without distinguishing the distribution of muscle weakness.
Results
Baseline characteristics and clinical features
Clinical and demographic data are presented in Table 1. Mean age at disease onset was 52.7 years (SD 20.0) and at diagnosis 53.5 years (SD 19.8). Early disease onset before the age of 50 years occurred in 300 patients (36.8%), while 510 cases (62.6%) were LOMG. The follow-up time was 62.6 months (SD 73.3) after diagnosis.
MGFA class at diagnosis was available for 782 patients (96.3%). 236 (28.9%) patients presented with ocular weakness (Class I); 309 (37.9%) with mild symptoms (Class II); 169 (20.8%) with moderate symptoms (Class III); 43 patients (5.3%) with severe muscle weakness (Class IV) and for 25 patients a history of intubation (3.0%) (Class V) was documented. Disease severity at diagnosis was classified by assessment of QMG score and was available for 687 patients (84.4%) [22]. Median QMG score at diagnosis was 4 points (IQR 2.0–8.0).
With respect to ab status, 714 (87.6%) patients were seropositive, whereas 86 (10.5%) were seronegative. The ab-status included anti-AChR-ab (n = 641), anti-MuSK-ab (n = 71), and anti-LRP4-ab (n = 2). 436 patients (53.5%) received corticosteroids following diagnosis with a mean dosage of 15 mg (SD 10). The average time between diagnosis and the start of the first immunosuppressive therapy (IST) was 1.3 years (SD 3.7). 451 patients (54.6%) received their first IST less than 1 year after diagnosis and were considered as early IST, while 111 patients (13.5%) received IST after 1 year or more and were considered late IST. The remaining patients did not receive IST during the observation period.
Predictive factors for MC and disease exacerbation
Overall, 217 patients (26.3%) experienced a MC during their disease course while 225 patients (27.6%) experienced a disease exacerbation. To assess potential risk factors for the occurrence of MC or disease exacerbation, we employed a model of univariate logistic regression (Additional file 1: Table S1). We assessed the risk for experiencing at least one MC or disease exacerbation compared to patients experiencing no event. Aiming to identify independent risk factors, we entered risk factors reaching statistical significance (p < 0.05) in univariate analysis in a model of multivariate Cox regression. In addition, we included clinical parameters (sex and age) as they were related to clinical outcomes in previous studies [5]. To avoid overfitting, factors facing high collinearity were excluded (age at manifestation, thymectomy, imaging suggestive of thymoma). Accordingly, multivariate analysis revealed that QMG score at diagnosis [OR 1.23 95% confidence interval (95%CI) 1.14–1.66, p < 0.0001], MGFA class at diagnosis (OR 1.83 95% CI 1.65–1.97, p < 0.001), anti-MuSK-ab (OR 2.18 95% CI 1.76–2.59, p < 0.05) and the presence of a thymoma (OR 3.71 95% CI 3.01–4.41, p < 0.0001) predicted the occurrence of MC as independent risk factors (Table 2). Multivariate analysis of risk factors for disease exacerbation identified generalized disease (OR 1.83 95% CI 1.23–2.39, p < 0.05), QMG score at diagnosis (OR 1.12 95% CI 1.09 to 1.44, p < 0.001), anti-MuSK-ab (OR 1.07 95% CI 1.01–1.28, p < 0.01) and the presence of a thymoma (OR 1.56 95% CI 1.29–2.07, p < 0.05) as independent risk factors. Next, we applied the Kaplan–Meier method to our data set. Here, we observed an inverse relation between MGFA class and the occurrence of MC (Fig. 2A, B). In addition, we observed that anti-MuSK-ab status correlates with the risk for experiencing MC (Fig. 2C) or disease exacerbation (Fig. 2D) (Table 2).
Survival analysis of MC and disease exacerbation. Survival curves displaying the time (in months) between diagnosis and the first MC (myasthenic crisis) or exacerbation. (A) Survival graph displaying the time to MC according to MGFA class. (B) Survival graph displaying the time to exacerbation according to MGFA class. (C) Survival graph displaying the time to MC according to anti-Musk-ab status. (D)Survival graph displaying the time to exacerbation according to anti-Musk-ab status. (E) Survival graph displaying the time to MC according to minimal manifestation status (MMS) at 12 months after diagnosis. (F) Survival graph displaying the time to exacerbation according to MMS at 12 months after diagnosis. Significance between survival curves was assessed by logrank testing. ****p < 0.0001 ***p < 0.001, **p < 0.01, *p < 0.05
Finally, we investigated whether therapeutic management of MG influences the occurrence of MC and exacerbation during the disease course. To assess the time between diagnosis and treatment as a potential risk factor, we separated the patient cohort by the time between diagnosis and the start of the first standard IST. Standard IST comprised of azathioprine, MMF, methotrexate and cyclosporine. Here, the risk for MC and disease exacerbation was not different for patients with early vs. late IST, respectively (MC: OR 0.38 95% CI 0.22–0.87, p = 0.79, exacerbation: OR 0.86 95% CI 0.65–0.99, p = 0.38). In addition to the time to treatment, we investigated the effect of treatment response on the occurrence of MC or exacerbation. We analysed the risk for MC and exacerbation for patients achieving MMS at 12 months after diagnosis and those who did not. To exclude bias due to patients presenting with MC or exacerbation as first manifestation, patients with a clinical event up to 6 months after diagnosis were excluded from the analysis of treatment response. Indeed, the risk was reduced for achieving MMS for MC (OR 0.32 95% CI 0.17–0.61, p = 0.002) and for exacerbation (OR 0.50 95% CI 0.34–0.70, p < 0.001). Next, using the Kaplan–Meier method we observed treatment non-responders as at risk to experience MC and exacerbations early in their disease as compared to treatment responders (Fig. 2E, F). To further dissect the importance of therapeutic management, we analyzed both cortisone treatment, as binary variable, and dosage, as continuous variable, as predictors for MC or exacerbation. Here, the risk for MC (OR 1.12 95% CI 1.05–1.33, p = 0.16) and exacerbation (OR 1.09 95% CI 1.01–1.45, p = 0.42) were similar for patients receiving cortisone following diagnosis compared with those who did not. In the group of cortisone-treated patients, assessment of cortisone dose did not reveal an association with the risk for MC (OR 1.27 95% CI 1.16–1.65, p = 0.23) or exacerbation (OR 1.52 95% CI 1.34–1.72, p = 0.18).
Factors determining the outcome of MC
Given the substantial mortality and lasting functional impairment associated with MC [5, 23], we further investigated potential factors affecting the outcome of MC. As detailed above, patients experiencing MC were grouped into three cohorts (improved, unchanged and worse). Clinical, demographic, diagnostic and therapeutic data were assessed for each cohort (Additional file 2: Table S2). Overall, 235 MC were recorded. In-hospital mortality was recorded for 6 patients (0.25%). To prevent bias (i.e., shorter ventilation time despite worse outcome), patients had died to MC were not included. Comparison of groups was performed on the remaining 229 MC. Recorded trigger factors are presented in Additional file 3: Table S3. Outcomes after MC were defined as improved for 143 MC (62.4%), unchanged for 33 MC (14.4%) and worse for 53 MC (23.2%) (Table 3).
Patients experiencing a worse outcome of following MC were older at the time of MC as compared to improved patients, while sex displayed no association with the outcome. MGFA class at admission as well as the last most recent measurement of MGFA class before prior to admission were lower in patients improving who improved. Interestingly, MC triggered by infections were was associated with a worse outcome. Consistent with previous reports, patients with a high number of comorbidities at admission had a worse outcome. Of note, vital capacity (VC) at admission, as well as the last recorded VC before MC, were was lower in patients worseningwho worsened. In addition, patients who were intubated, who had a longer time of mechanical ventilation or total hospital stay, and who developed pneumonia or sepsis had a poor outcome.
Finally, we analysed the impact of the available rescue therapies on the outcome of MC. Here, we compared the effect of IVIG (43 patients) vs. plasma exchange [PLEX (plasmapheresis) or IA (immunoadsorption)] (90 patients) vs. IVIG combined with plasma exchange (47 patients) vs. no rescue therapy (49 patients) (Table 3). Out of 49 patients with no rescue therapy, 31 were unable to receive therapy due to comorbidities (e.g., sepsis, renal failure), while 18 patient charts contained insufficient data on rescue treatments. Assessing the outcome of different rescue therapies revealed no differences between IVIG, plasma exchange, and the combination of both. However, patients receiving no rescue therapy had worse outcome compared to patients that received rescue therapy.
The six patients not surviving MC were on average 70 (SD 11.6) years. All 6 patients were intubated at admission, and the treating physician recorded an infection as the trigger for MC (pneumonia in all 6 cases). The average time of ventilation was 36.3 (SD32.5) days. Four patients died due to sepsis. One patient was treated with PLEX, one patient received both PLEX and IVIGs, while 4 patients did not receive rescue therapies.
Discussion
Despite therapeutic advances, 10–20% of MG patients experience MC during their disease course [3, 6, 24]. To ameliorate the burden of disease incurred by uncontrolled disease, identification of patients at risk for these events as well as factors and strategies promoting MC remission are of high importance for clinical practice. To guide identification and—by extension—management of patients at risk, we analysed a large cohort of MG patients, which reflected previously reported demographic and clinical characteristics [6]. In essence, our data implicate disease severity at diagnosis as a readily accessible and reliable predictor for MC. Treatment strategies should be tailored to the severity of initial symptoms, potentially reducing the likelihood for MC or exacerbation. In addition, our data underlines that the prevention and resolution of infections are pivotal factors defining MC outcome.
Previous observational studies regarding possible risk factors are mostly available for the subgroup of MG patients that received thymectomy [11, 12]. Investigating patients with and without thymoma, our study corroborates the presence of thymoma as a risk factor. Corroborating previous studies [25, 26], we also identified anti-MuSK-ab positivity as an independent risk factor for disease deterioration. Anti-MuSK-ab positive has also been associated with poor outcome of MC [27]. Interestingly, disease severity as assessed by clinical scoring was a robust predictor for patients at risk for MC or exacerbation, underlining the importance of standardized clinical evaluation of MG patients. Patients presenting with severe disease should receive intensified disease monitoring to recognize and, if possible, prevent the occurrence of MC.
Analysing the impact of disease management, we observed that treatment response influenced the risk for MC. Here, patients achieving MMS were at an reduced risk for MC and exacerbation than those who did not. MMS was proposed by the International Consensus Guidance for Management of MG as treatment target [17, 20]. We analyzed this parameter to understand if achieving the proposed treatment target is associated with a reduced risk for MC [17]. Treatment strategies were previously suggested to affect the course of disease in MG. As such, a recent meta-analysis suggested that cortisone treatment reduces the risk for secondary generalization for MG patients with ocular manifestation [28]. Thymectomy is also evidenced to improve clinical readouts over a 3-year time span as demonstrated in a recent, randomized, controlled trial [19, 20]. Taken together, successful treatment approaches appear to influence long-term outcomes.
Knowledge of factors affecting the outcome of MC are of high clinical importance to promote remission and functional independence [6], with most studies reporting factors associated with prolonged ventilation as a surrogate marker for clinical outcome of MC [6, 23, 29]. Following analysis of patients experiencing MC according to the MGFA post-intervention-status [17], we observed an association between prolonged ventilation time and a worse outcome, suggesting that ventilation time correlates with functional status at discharge. However, our cohort also revealed that VC might be a valuable biomarker for risk stratification of MC as VC predicted the outcome if assessed at admission. Interestingly, a previous retrospective cohort analysing 5 patients with MC found no link between VC and the need for mechanical ventilation [30]. Corroborating VC as a predictive biomarker in other neuromuscular diseases such as Guillain-Barré syndrome [31], our study contrasts the findings from the previous cohort with the difference potentially attributed to the substantial variance in cohort size implicating that monitoring and improvement of ventilation might allow clinicians to avert severe courses of MC. Intriguingly, an infectious trigger of MC was both frequent and associated with an unfavourable outcome compared to other triggers. Hence, prevention and early management of infection in MG patients, notably in MG patients with impaired ventilatory capacities, constitutes a cornerstone in the management of MC. We suggest that treatment of comorbidities making patients vulnerable to infection and resolute adhesion to vaccination protocols should be employed to reduce the risk of infection for MG patients.
The retrospective design of this study might be vulnerable to confounding factors as data were collected during routine clinical practice rather than a formal study setting making data sensitive to variation both in quantity and quality between individual patients and time points. Nonetheless, data quality was improved by collection according to standardised requirements of the German Myasthenia register. A focus on tertiary centers might introduce a bias towards severe cases. However, given the rarity of the disorder, most MG patients are treated in specialized centres [32]. Thus, our cohort is likely to be representative of the general MG population. Regarding the analysis of predictors, a potential limitation is that patients initially presenting with MC or exacerbation could not be included. These patients potentially constitute a distinct clinical subtype as they are expected to have fewer co-morbidities and are likely to be treated more aggressively [6]. Furthermore, definitions for MG exacerbation are heterogenous and diverging interpretations have been previously proposed, e.g., de Meel et al. included an increase in immunosuppressive therapy in their operational definition for exacerbation [33]. A caveat to the analysis of rescue therapies is that the subgroup of patients receiving no treatment for MC is biased to severe cases as these patients were often unable to be treated due to comorbidities (e.g., sepsis or renal failure).
Conclusions
Our study highlights that disease severity at diagnosis is a valuable clinical marker to identify patients at risk for MC or disease exacerbation. Intensified monitoring with emphasis on the prevention of infectious complications is pivotal for management of patients at risk.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- ab:
-
Antibody
- anti-AChR-ab:
-
Anti-acetylcholine-receptor-ab
- anti-LRP4-ab:
-
Anti-low-density lipoprotein receptor-related protein 4-ab
- anti-MuSK-ab:
-
Anti-muscle-specific tyrosine kinase-ab
- anti-VGCC-ab:
-
Anti-voltage-gated calcium channel-ab
- EOMG:
-
Early-onset MG
- FDR:
-
False discovery rate
- IA:
-
Immunoadsorption
- iMC:
-
Integrated Myasthenia Centre
- IQR:
-
Interquartile range
- IST:
-
Immunosuppressive therapy
- IVIG:
-
Intravenous immunoglobulin
- LOMG:
-
Late-onset MG
- MC:
-
Myasthenic crisis
- MG:
-
Myasthenia gravis
- MGFA:
-
Myasthenia Gravis Foundation of America
- MGFA–PIS:
-
Myasthenia Gravis Foundation of America post-intervention status
- MMF:
-
Mycophenolate-mofetil
- NICU:
-
Neurological Intensive Care Unit
- OR:
-
Odds ratio
- PLEX:
-
Plasmapheresis
- SD:
-
Standard deviation
References
Gilhus NE, et al. Myasthenia gravis—autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 2016;12:259–68.
Mantegazza R, Antozzi C. When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther Adv Neurol Disord. 2018; 1–11.
Schneider-Gold C, Hagenacker T, Melzer N, Ruck T. Understanding the burden of refractory myasthenia gravis. Ther Adv Neurol Disord. 2019;12:1–16.
Lacomis D. Myasthenic crisis. 2005. https://doi.org/10.1385/Neurocrit.
Alshekhlee A, Miles JD, Katirji B, Preston DC, Kaminski HJ. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology. 2009;72:1548–54.
Neumann B, et al. Myasthenic crisis demanding mechanical ventilation. Neurology. 2020;94:e299–313.
Barth D, Nabavi Nouri M, Ng E, Nwe P, Bril V. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology. 2011;76:2017–23.
Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Ann Neurol. 1997;41:789–96.
Gummi RR, Kukulka NA, Deroche CB, Govindarajan R. Factors associated with acute exacerbations of myasthenia gravis. Muscle Nerve. 2019;60:693–9.
Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1:16–22.
Geng Y, Zhang H, Wang Y. Risk factors of myasthenia crisis after thymectomy among myasthenia gravis patients: a meta-analysis. Medicine. 2020;99:e18622.
Xue L, et al. Risk factors of myasthenic crisis after thymectomy for thymoma patients with myasthenia gravis†. Eur J Cardiothorac Surg. 2017;52:692–7.
68 Diagnostik und Therapie der Myasthenia gravis und des Lambert-Eaton-Syndroms. in Leitlinien für Diagnostik und Therapie in der Neurologie. 2014. https://doi.org/10.1055/b-0034-37851.
Bedlack RS, Sanders DB. On the concept of myasthenic crisis. J Clin Neuromuscul Dis. 2002;4:40–2.
Schroeter M, Thayssen G, Kaiser J. Myasthenia gravis—exacerbation and crisis. Neurol Int Open. 2018;02:E10–5.
Bedlack RS, et al. Quantitative myasthenia gravis score: assessment of responsiveness and longitudinal validity. Neurology. 2005;64:1968–70.
Sanders DB, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87:419–25.
Katzberg HD, Barnett C, Merkies ISJ, Bril V. Minimal clinically important difference in myasthenia gravis: outcomes from a randomized trial. Muscle Nerve. 2014;49:661–5.
Wolfe GI, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375:511–22.
Narayanaswami P, et al. International consensus guidance for management of myasthenia gravis. Neurology. 2021;96:114–22.
America, T. F. of the M. S. A. B. of the M. G. F. of et al. Myasthenia gravis: recommendations for clinical research standards. Neurology. 2000;55: 16–23.
Besinger UA, et al. Myasthenia gravis: long-term correlation of binding and bungarotoxin blocking antibodies against acetylcholine receptors with changes in disease severity. Neurology. 1983;33:1316–21.
Thomas CE, et al. Myasthenic crisis: clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology. 1997;48:1253–60.
Maggi L, et al. Thymoma-associated myasthenia gravis: outcome, clinical and pathological correlations in 197 patients on a 20-year experience. J Neuroimmunol. 2008;201:237–44.
Evoli A, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. 2003;126:2304–11.
Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. 2011;44:36–40.
Konig N, et al. MuSK-antibodies are associated with worse outcome in myasthenic crisis requiring mechanical ventilation. J Neurol. 2021. https://doi.org/10.1007/s00415-021-10603-9.
Li M, et al. Do early prednisolone and other immunosuppressant therapies prevent generalization in ocular myasthenia gravis in Western populations: a systematic review and meta-analysis. Ther Adv Neurol Disord. 2019;12:1756286419876521.
Liu Z, et al. Predictors of extubation outcomes following myasthenic crisis. J Int Med Res. 2016;44:1524–33.
Rieder P, Louis M, Jolliet P, Chevrolet JC. The repeated measurement of vital capacity is a poor predictor of the need for mechanical ventilation in myasthenia gravis. Intensive Care Med. 1995;21:663–8.
Racca F, Del Sorbo L, Mongini T, Vianello A, Ranieri VM. Respiratory management of acute respiratory failure in neuromuscular diseases. Minerva Anestesiol. 2010;76:51–62.
Wiendl H. Diagnostik und Therapie der Myasthenia gravis und des Lambert-Eaton-Syndroms. 2014.
de Meel RHP, Lipka AF, van Zwet EW, Niks EH, Verschuuren JJGM. Prognostic factors for exacerbations and emergency treatments in myasthenia gravis. J Neuroimmunol. 2015;282:123–5.
Acknowledgements
We thank the patient and their families for their contribution.
Funding
Open Access funding enabled and organized by Projekt DEAL. PM is Einstein Junior Fellow funded by the Einstein Foundation Berlin, and is supported by grants from the Bundesministerium für Bildung und Forschung (Grants no. 16GW0191 and NUM-COVID 19—Organo-Strat 01KX2021).
Author information
Authors and Affiliations
Contributions
CN, FS and TR designed the study and methods. Formal analysis was done by CN and FS. Clinical data was provided by FS, CE, MP, CBS, NH, PM, EA, MÖ, DF, SS, SV, AG, HS, MS, BB, AT and TH. Resources were provided by SGM, AM, HW and TR. CN, FS and TR wrote the original draft. CE, MP, CBS, NH, PM, EA, EÖ, DF, SS, SV, AG, HS, MS, BB, AT, TH, SGM, AM and HW reviewed and edited the manuscript. Figures were created by CN, CBS and NH. Subversion was provided by SGM, AM, HW and TR. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee and institutional review boards (no. AZ 2020-010-f-S, no. AZ 07/2017, 19-8973-BO, AZ 21-1265, AZ 21-1331). Data were anonymized and collected retrospectively according to the standardized requirements of the German register for myasthenia.
Consent for publication
Not applicable.
Competing interests
Dr. Nelke reports no disclosures. Dr. Stascheit received speaking Honoria from Biogen and Alexion. Cand. med. Eckert reports no disclosures. Dr. Pawlitzki received speaker honoraria and travel/accommodation/meeting expenses from Novartis. Dr. Mergenthaler is on the Advisory Board of HealthNextGen Inc. and has equity interest in the company. His research is funded by the Bundesministerium für Bildung und Forschung (BMBF), the European Union, the Else Kröner-Fresenius Stiftung, the Volkswagen Stiftung, and the Einstein Foundation Berlin. Dr Schroeter reports no disclosures. Dr. Arat reports no disclosures. Dr. Özturk reports no disclosures. Prof. Föll reports no disclosures. Prof. Schreiber reports no disclosures. Prof. Vielhaber reports no disclosures. Dr. Gassa reports no disclosures. Dr. Stetefeld reports not disclosures. Prof. Schroeter reports no disclosures. Dr. Berger received travel grants and/or training expenses from Bayer Vital GmbH, IpsenPharma GmbH, Norvartis, Biogen GmbH and Genzyme, as well as lecture fees from Ipsen Pharma GmbH, Alexion Pharma GmbH, Merck, Sanofi Genzyme and Roche. Dr. Totzeck reports no disclosures. Dr. Hagenacker received speaker and advisory board honoraria from Alexion, Biogen and Roche. Prof. Meuth receives honoraria for lecturing, and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Bundesinstitut für Risikobewertung (BfR), Deutsche Forschungsgemeinschaft (DFG), Deutsche Multiple Sklerose Gesellschaft (DMSG), Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology and Alexion, Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. Prof. Wiendl receives honoraria for acting as a member of Scientific Advisory Boards, Biogen, Evgen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG, and Sanofi-Aventis as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Genzyme, TEVA, and WebMD Global. Prof. Wiendl is acting as a paid consultant for Abbvie, Actelion, Biogen, IGES, Johnson & Johnson, Novartis, Roche, Sanofi-Aventis, and the Swiss Multiple Sclerosis Society. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, the European Union, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster and RE Children’s Foundation, Biogen, GlaxoSmithKline GmbH, Roche Pharma AG, Sanofi-Genzyme. Prof. Meisel received speaker honoraria from Alexion, argnx, GRIFOLS and Hormosan. He received honoraria from Alexion, argnx, UCB, Janssen and Vitaccess for consulting services and financial research support from Octapharma and Alexion. Andreas Meisel is chairman of the medical advisory board of the German Myasthenia Gravis Society. Dr. Ruck reports grants from German Ministry of Education, Science, Research and Technology, grants and personal fees from Sanofi-Genzyme and Alexion; personal fees from Biogen, Roche and Teva; personal fees and nonfinancial support from Merck Serono, outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Suppl. Table 1. Risk factors for MC and exacerbation – Univariate analysis.
Additional file 2.
Suppl. Table 2. Clinical and demographic characteristics of included MC.
Additional file 3.
Suppl. Table 3. Trigger factors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nelke, C., Stascheit, F., Eckert, C. et al. Independent risk factors for myasthenic crisis and disease exacerbation in a retrospective cohort of myasthenia gravis patients. J Neuroinflammation 19, 89 (2022). https://doi.org/10.1186/s12974-022-02448-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-022-02448-4