Abstract
Background
The use of e-cigarettes has increased during the past few years. Exposure to e-cigarette liquids, whether intentional or accidental, may lead to adverse events our aim was to assess factors associated with e-cigarette exposures across European Union Member States (EU MS).
Methods
A retrospective analysis of exposures associated with e-cigarettes reported to national poison centers was performed covering incidents from 2012 to March 2015 from 10 EU MS. De-identified and anonymous raw data was acquired.
Results
In total, 277 incidents were reported. Unintentional exposure was the most frequently cited type of exposure (71.3%), while e-cigarette refill vials were responsible for the majority of the reported incidents (87.3%). Two-thirds of all exposures (67.5%) occurred as ingestion of e-liquids, which was more frequent among children (≤ 5 years, 6–18 years) compared to adults (87.0% vs. 59.3% vs. 57.6%, p < 0.001 respectively), exposure via the respiratory (5.4% vs. 22.2% vs. 22.2%, p < 0.001) were more frequent among paediatric patients while ocular routes (2.2% vs. 3.7% vs. 11.4%, p = 0.021) were more frequent among adults. Logistic regression analyses indicated that paediatric incidents (≤ 5 years) were more likely to be through ingestion (adjusted Odds Ratio [aOR] = 4.36, 95% Confidence Interval [C.I.]: 1.87–10.18), but less likely to have a reported clinical effect (aOR = 0.41, 95% C.I.: 0.21–0.82).
Conclusions
Our study highlighted parameters related to e-cigarette exposure incidents in 10 EU MS, the results of which indicate that consideration should be given to the design features which may mitigate risks, thereby protecting users, non-users and especially children.
Similar content being viewed by others
Background
The European Tobacco Products Directive (TPD) 2014/40/EU, regulates e-cigarettes across 28 European Member States (EU MS) and includes provisions to harmonise the safety and technical design specifications for e-cigarettes and their refill containers [1]. The TPD and its accompanying implementing acts regulate many aspects of e-cigarette design including but not limited to the volume of the refill container, the nicotine and ingredient content and tamper resistant and child-resistant refill containers [2]. In the USA, the food and Drug administration (FDA) in 2016 also finalized a deeming rule covering the manufacture, import, packaging, labelling, advertising, promotion, sale, and distribution of Electronic Nicotine Delivery Systems (ENDS) in the United States [3].
E-cigarette refill liquids contain nicotine and a wide variety of chemical components such as humectants, flavours, and potential impurities [4, 5]. Exposure to such liquids, whether intentional or accidental, may lead to adverse events [6]. Indeed, several recent reports from poison centers, primarily from the United States (US) and the United Kingdom (UK), have revealed a significant increase in the number of reported incidents of poisoning with e-cigarette liquids and associations with specific demographic characteristics [4, 5]. However, data from across Europe, are only sporadically available, mostly in the form of case reports in the literature [7,8,9].
Due to the fact that the e-cigarette market and respective experimentation or use has been documented to increase over the past few years in Europe [10, 11], it is of significant interest to evaluate the current landscape with regards to adverse exposures to e-cigarette liquids, especially in light of the recent adoption of technical design parameters that e-cigarettes will need to adhere to within the context of alignment with the provisions of the European TPD.
In light of the above our aim was to assess the factors associated with e-cigarette exposure incidents reported to poison centers across multiple EU MS.
Methods
Data collection
A retrospective analysis of exposures associated with e-cigarettes as reported to national poison centers was conducted, within the context of a study prepared for the Directorate General for Health and Food Safety (EU DG Health and Food Safety), (Chafea/2014/Health/17).
A request for raw data was sent to poison centers within the 22 EU MS. Ten poison centers from equal EU MS (Sweden, The Netherlands, Ireland, Portugal, Austria, Slovakia, Lithuania, Hungary, Croatia and Estonia) agreed to provide raw data covering incidents from 2012 to March 2015. However, three EU MS (Ireland-53 out of 90 incidents, Croatia-3 incidents and Estonia- 10 incidents) were only able to provide summary statistics for the specific incidents reported to their centers, due to lack of detailed data or confidentiality regulations. These 66 incidents were excluded from the statistical analysis and hence all statistical analyses were performed on the 277 incidents from the remaining eight EU MS (Sweden, Ireland-37 out of 90 incidents, The Netherlands, Portugal, Austria, Slovakia, Lithuania and Hungary).
All information requested and received was de-identified and anonymous. Data on age (Paediatric incidents: ≤5 years, 6–18 years, adults: ≥19 years); gender (male, female); route of exposure (ingestion, respiratory, dermal and ocular); initial type of exposure (e-cigarette refill liquid, e-cigarette non refillable, unknown); management of incident (residence/on site, hospital, ambulance, other/unknown); reported clinical outcome (minor effects, moderate effects, major effects, death); and reason of exposure (intentional, unintentional, abuse, misuse, suspected suicide or unknown reason) were collected from the archive of each poison center. For the purpose of our analyses intentional, abuse and misuse were grouped together as intentional exposures.
Statistical analysis
All variables are displayed as frequencies and percentages (n, %), unless stated otherwise. Chi-square tests were used for associations between categorical variables. Multivariable binary logistic regression models were performed to investigate the association of having an effect from poisoning and route of exposure with age, gender and exposure characteristics. Level of significance was set at p < 0.05 and regression results are presented as adjusted Odds Ratios (aOR) with 95% Confidence Intervals (CI). Data were analyzed using the SPSS statistical package version 21.0 (SPSS, Chicago, IL).
Results
Incidents, geographical distribution and time trends
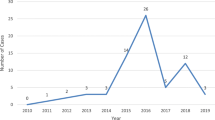
Data for a total of 343 incidents were initially reported while 277 were analysed. Sweden reported the highest number of incidents (n = 121) followed by Ireland (n = 37) and the Netherlands (n = 78), while Portugal (n = 25), Austria (n = 8), Slovakia (n = 5), Lithuania (n = 2) and Hungary (n = 1) followed with a smaller number of incidents. Among all analysed e-cigarette poisoning incidents, 42.7% (n = 118) were among children and the remaining 57.2% (n = 158) were among adults. There was approximately equal exposure incidents among males and females (50.6% vs. 49.4%). An increase in the number of reported exposures during the reporting period was noted, 10 in 2012, 85 in 2013 and 140 in 2014.
Exposure characteristics
Unintentional exposure was the most frequently cited exposure (71.3%, n = 196), followed by intentional (17.8%, n = 49), abuse/misuse (n = 21) and suspected suicide (n = 3). The distribution of incidents via exposure routes and clinical outcomes is depicted in Table 1. A wide range of symptoms were reported, of which vomiting (20.3%), dizziness (14.5%), nausea (13.8%) and throat conditions (9.1%) were the most frequently reported in both children and adults. Abdominal conditions, eye conditions, headache, diarrhoea, breathing conditions and tremor were also reported. The majority of incidents were managed on site (70.1%), 56 incidents (23.7%) were managed in a hospital and 4 incidents (1.7%) in an ambulance setting.
The majority of recorded exposure incidents had a favourable outcome. In 39.4% of them, no effect was reported and a further 53.8% of the incidents resulted in only minor effects, 6.3% reported moderate effects and 1 case reported a major clinical outcome. No deaths were recorded as a result of an e-cigarette exposure within the data collection period and within our data collected.
As depicted in Table 2, age was associated with the recorded medical outcome, as among incidents which reported clinical effects 65.6% were adults and 34.9% children (p = 0.002). Age was also associated with intentional poisoning as the majority of those who reported intentional poisoning using e-liquids were 19 years old or older (p < 0.001).
Poisoning though ingestion was the most frequent exposure route both for adults and paediatric patients (both those ≤5 years and between 6 and 18 years). Exposure via ingestion was more frequent among paediatric patients (≤5 years) (87.0% vs. 59.3 vs. 57.6 p < 0.001) compared with children of 6–18 years and adults, while respiratory exposure was more frequent among adults, and children of 6–18 years of age compared to those ≤5 (22.2% vs. 22.2% vs. 5.4%, p = 0.002).
Ingestion was the most frequently recorded route of exposure in both intentional and unintentional incidents (54.8% and 71.9% respectively, p = 0.008), while exposure via the respiratory track was the most frequent route of intentional exposure (41.1% vs. 7.1%, p < 0.001). Finally, as shown in Table 2, vomiting, dizziness and nausea were the most common clinical symptoms in both intentional and unintentional exposures.
Overall, among incidents that recorded a medical outcome (minor-moderate or major), 54.8% of incidents were associated with ingestion, 28.6% with inhalation, 9.5% of ocular and 7.9% with dermal exposure.
Regression analysis
Logistic regression analysis assessing factors associated with reporting a clinical outcome indicated that paediatric incidents ≤5 years of age were less likely to lead to clinical effects (aOR 0.41, 95% C.I.: 0.21–0.82) in comparison to older incidents. Gender, intention and type of refill device did not have a significant impact on reporting a clinical effect as outlined in Table 3.
Table 4 depicts the logistic regression analysis that evaluated the factors associated with each route of exposure. Paediatric incidents ≤5 years were 4.36 times more likely to be exposed through ingestion in comparison to adults (95% C.I.: 1.87–10.18) while they were less likely to be exposed through an ocular route (aOR 0.10, 95%C.I: 0.02–0.48). Respiratory exposure was less likely to occur unintentionally (aOR 0.15, 95% C.I.: 0.07–0.32), while dermal exposure was 13.2 times more likely to occur unintentionally (aOR 13.22, 95% C.I.: 1.70–102.56). Refillable e-cigarette liquids were associated more often with exposures via ingestion (aOR 13.05, 95% C.I.:3.11-) and less often via the ocular route (aOR 0.14, 95% C.I.: 0.03–0.71) in comparison to incidents attributed to non-refillable/unknown e-cigarette devices.
Discussion
This is the first study to report exposure incidents attributable to e-cigarettes, as recorded by poison centers in multiple EU MS. Our results indicated that unintentional exposure to e-cigarette refill liquids were responsible for the majority of the reported incidents. The most frequent route of exposure occurred as ingestion or respiration.
The human toxicity of nicotine has become increasingly relevant in the past few years through marketing of new nicotine-containing products, such as smokeless tobacco and liquids for electronic nicotine delivery systems (electronic cigarettes) that are readily available in most countries [12]. This phenomenon has resulted in unprecedented access to potentially toxic doses of nicotine and other harmful compounds in the home [13].
Our results are quite similar in comparison to the US Poison Center report [14]. Vomiting, nausea and dizziness were the most frequent symptoms reported in incidents of e-cigarette exposure both in the US and the EU. Dizziness and throat conditions were more frequent in the EU compared to the US, but the overall profile of clinical effects was quite similar [14]. While one death was recorded in the US dataset, there were no deaths among the European incidents [14].
It is likely that the above symptoms are attributable primarily to nicotine, as historical or experimental evidence has indicated that low doses of nicotine may have stimulant effects (e.g., tachycardia), while vomiting is common following ingestion. Signs of central nervous system toxicity may include ataxia and seizures, while as doses increase, loss of nicotinic receptor specificity may occur and result in signs of muscarinic cholinergic toxicity, including extreme secretions and gastrointestinal disturbance. The highest levels of poisoning can result in neuromuscular blockade, respiratory failure, and death [13]. While the aforementioned symptoms are in line with nicotine poisoning, we cannot rule out that they may also be partially attributable to other ingredients within e-cigarette liquids, which could potentially be harmful if used inappropriately, therefore further research is needed to evaluate the impact of these substances on human health. Our finding that the majority of incidents reported in poison centers refer to unintentional exposure is also consistent with published data [14]. However, several incidents of intentional poisoning –suicide attempts- through ingestion have also been reported in the literature [15,16,17,18,19,20,21,22].
Our results also indicated that paediatric patients are more likely to be exposed through ingestion of e-cigarette liquids, which is a subject of concern. This finding is in line with data from the UK National Poisons Information Service, which indicated that 36.4% of the exposure incidents were for children younger than 4 years old [17] while a CDC report noted that young children accounted for more than 50% of the incidents [4].
Our results also indicated an increase in the number of exposure incidents during the study period, a factor which we hypothesize is associated with the increase in popularity, and hence availability, of e-cigarettes and their refill vials in Europe [11]. Nevertheless, the number of incidents reported to national poison centers were not proportional to either the country’s population or the prevalence of e-cigarette use. Therefore, they might also reflect reporting procedures or differences in the products used in each country [11]. Similarly, events related to e-cigarette product exposure reported to the American Association of Poison Control Centers increased from 2011 to 2014 [23,24,25]. A recent retrospective analysis of exposures associated with nicotine and tobacco products among children younger than 6 years old conducted using US National Poison Data System data also indicated a continuing increase in the number of exposures associated with e-cigarettes, which increased almost 15-fold between 2012 and 2015. The increase in the number of exposure incidents and the identified age specific epidemiology of exposures highlights the need for the implementation of design features that could mitigate the incidence of e-cigarette poisonings [26].
Within the EU, the TPD implementing acts stipulate the need to incorporate child and tamper resistant caps, a parameter that may reduce paediatric incidents attributable to ingestion, while Article 20(3)(g) of the TPD requires EU Member States to ensure that electronic cigarettes and their refill containers have a mechanism that ensures refilling without leakage, a fact that would mitigate the likelihood of dermal/ocular exposures to users. Our study was completed before the implementation of such provisions, therefore further prospective studies are needed so as to assess if the proposed technical parameters would impact the epidemiological trends and characteristics of exposure incidents in EU MS.
This is the first study to report exposure incidents recorded by poison centers in multiple EU MS, results of which are imperatively needed so as to map the existing status quo of e-cigarette exposure incidents before the implementation of the regulations of the TPD in 2016. Despite the above, our analysis has limitations as results cannot be generalized beyond the ten EU MS that contributed data in this analysis, however the alignment with findings from US poison centers indicate the potential common epidemiology of exposure incidents attributable to e-cigarettes. Furthermore as data was retrospectively collected and de-identified and hence limited to those obtained from archive records, further in depth analysis of the data were restricted due to the small sample size of a number of exposure characteristics which led in some cases to wide confidence intervals. Further research using larger datasets is warranted.
Conclusions
Our study highlighted specific exposure characteristics and routes associated with involuntary exposure to e-cigarette refill liquids, with pediatric incidents more likely to be attributable to ingestion. These results indicate that consideration should be given in the technical design features of e-cigarette refill vials, which may limit the risk of accidental ingestion and risks associated with the refilling processes and thereby protect both users and non-users, and especially children. Further research is needed after the implementation of the legislative design features outlined in the TPD so as to assess their impact on the incidence and characteristics of future exposure incidents.
Abbreviations
- ENDS:
-
Electronic Nicotine Delivery Systems
- EU DG Health and Food Safety:
-
European Directorate General for Health and Food Safety
- EU MS:
-
European Member States
- FDA:
-
Food and Drug Administration
- TPD:
-
Tobacco Products Directive
- UK:
-
United Kingdom
- US:
-
United States
References
DIRECTIVE 2014/40/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 3 April 2014 on the approximation of the laws, regulations and administrative provisions of the Member States concerning the manufacture, presentation and sale of tobacco and related products and repealing Directive 2001/37/EC, OJ 2014 L 127/1. 2001.
Commission Implementing Decision (EU) 2016/586 of 14 April 2016 on technical standards for the refill mechanism of electronic cigarettes. 2016. https://ec.europa.eu/health/tobacco/key_documents_en#anchor0. Accessed 15 Feb 2017.
US Food and Drug Administration (FDA), Vaporizers, E-Cigarettes, and other Electronic Nicotine Delivery Systems (ENDS). 2017. http://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm). Accessed 15 Feb 2017.
Chatham-Stephens K, Taylor E, Melstrom P, Bunnell R, Wang B, Apelberg B, et al. Notes from the field: calls to poison centers for exposures to electronic cigarettes--United States, September 2010-February 2014. MMWR. 2014;63:292–3.
Public Health England, National Poisons Information Service Report 2014/15. http://www.npis.org/NPISAnnualReport2014-15.pdf. Accessed 15 Feb 2017.
Dinakar C, O’Connor GT. The Health Effects of Electronic Cigarettes. N Engl J Med. 2016;375:1372–81.
Pajarre-Sorsa S, Saukkonen M, Hoppu K. Calls concerning electronic cigarettes to the Finnish Poison Information Center. Clin Toxicol. 2014;52:337.
De La Oliva US, Conejo Menor JL. Exposures to electronic cigarettes: Calls to the poison center in Spain. Revista Espanola de Medicina Legal. 2014;40(4):146–9.
Davanzo F, Settimi L, Milanesi G, Giordano F, Sesana FM, Celentano A, et al. Surveillance of hazardous exposures to electronic cigarettes in Italy. Clin Toxicol. 2014;52:336–7.
European Commission. Study on the identification of potential risks to public health associated with the use of refillable electronic cigarettes and development of technical specifications for refill mechanisms, 2016. http://ec.europa.eu/health//sites/health/files/tobacco/docs/potentialrisks_specs_refillableecigarettes.pdf. Accessed 15 Feb 2017.
Filippidis FT, Laverty AA, Gerovasili V, Vardavas CI. Two-year trends and predictors of e-cigarette use in 27 European Union member states. Tob Control. 2017;26(1):98–104.
Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014;88:5–7.
Bassett RA, Osterhoudt K, Brabazon T. Nicotine poisoning in an infant. N Engl J Med. 2014;370(23):2249–50.
Vakkalanka JP, Hardison LS Jr, Holstege CP. Epidemiological trends in electronic cigarette exposures reported to US Poison Centers. Clin Toxicol. 2014;52(5):542–8.
Valento M. Nicotine poisoning following ingestion of e-Liquid. Clin Toxicol. 2013;51(7):683–4.
Lindberg SW, Ebbehoej N, Bang J, Christensen LB. Nicotine poisoning related to the use of e-cigarettes. Clin Toxicol. 2015;53(4):242–3.
Thomas E, Spears RA, Alldridge G, Krishna CV, Thompson JP, Eddleston M, et al. E-cigarette liquid refills-a safe beverage? Analysis of enquiries to the UK National Poisons Information Service from 2007 to 2013. Clin Toxicol. 2014;52:338–9.
Ordonez J, Forrester MB, Kleinschmidt K. Electronic cigarette exposures reported to poison centers. Clin Toxicol. 2013;51(7):685.
Schipper EM, Graaff LC, Koch BC, Brkic Z, Wilms EB, Alsma J, et al. A new challenge: suicide attempt using nicotine fillings for electronic cigarettes. Br J Clin Pharmacol. 2014;78(6):1469–71.
Eberlein CK, Frieling H, Köhnlein T, Hillemacher T, Bleich S. Suicide Attempt by Poisoning Using Nicotine Liquid For Use in Electronic Cigarettes. Am J Psychiatr. 2014;171(8):891.
Christensen LB, van't Veen T, Bang J. Three cases of attempted suicide by ingestion of nicotine liquid used in e-cigarettes. Clin Toxicol. 2013;51(4):290.
Bartschat S, Mercer-Chalmers-Bender K, Beike J, Rothschild MA, Jübner M. Not only smoking is deadly: fatal ingestion of e-juice—a case report. Int J Legal Med. 2015;129(3):481–6.
Bronstein AC, Spyker DA, Cantilena LR Jr, Rumack BH, Dart RC. 2011 annual report of the American Association of Poison Control Centers’ National Poison data system (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10):911–1164.
Mowry JB, Spyker DA, Cantilena LR Jr, Bailey JE, Ford M. 2012 Annual report of the American association of poison control centers’ national poison data system (NPDS): 30th annual report. Clin Toxicol. 2013;51(10):949–1229.
Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 annual report of the American association of poison control centers’ National poison data system (NPDS): 31st annual report. Clin Toxicol. 2014;52(10):1032–283.
Kamboj A, Spiller HA, Casavant MJ, Chounthirath T, Smith GA. Pediatric exposure to e-cigarettes, nicotine, and tobacco products in the United States. Pediatrics. 2016;9:e20160041.
Acknowledgements
This work was produced under the EU Health Programme (2008-2013) in the frame of a service contract with the Consumers, Health, Agriculture and Food Executive Agency (CHAFEA) acting on behalf of the European Commission. The content of this report represents the views of the EUREST Consortium and is its sole responsibility; it can in no way be taken to reflect the views of the European Commission and/or Chafea or any other body of the European Union.
The full report of the EUREST Consortium is available from here: http://ec.europa.eu/health//sites/health/files/tobacco/docs/potentialrisks_specs_refillableecigarettes.pdf
Funding
This research was supported the EU Health Programme (2008–2013) in the frame of a service contract with the Consumers, Health, Agriculture and Food Executive Agency (CHAFEA) acting on behalf of the European Commission.
Availability of data and materials
Data sharing is not applicable to this article.
Author information
Authors and Affiliations
Contributions
CIV, CG, AIV, MNT, AMT, PKB, were involved in the study conception and design. FTF was responsible for the data analysis. MO, RK, IV, LS, AA, SP, RT, LG, FR, DG, HS, AB, ED provided the data. All authors contributed to the discussion, interpreted the findings, helped write, reviewed/edited the manuscript for intellectual content, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Vardavas, C.I., Girvalaki, C., Filippidis, F.T. et al. Characteristics and outcomes of e-cigarette exposure incidents reported to 10 European Poison Centers: a retrospective data analysis. Tob. Induced Dis. 15, 36 (2017). https://doi.org/10.1186/s12971-017-0141-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12971-017-0141-z