Abstract
Background
Etanercept has been studied in doses up to 0.8 mg/kg/week (max 50 mg/week) in juvenile idiopathic arthritis (JIA) patients. In clinical practice higher doses are used off-label, but evidence regarding the relation with outcomes is lacking. We describe the clinical course of JIA-patients receiving high-dose etanercept (1.6 mg/kg/week; max 50 mg/week) in the BeSt for Kids trial.
Methods
92 patients with oligoarticular JIA, RF-negative polyarticular JIA or juvenile psoriatic arthritis were randomised across three treat-to-target arms: (1) sequential DMARD-monotherapy (sulfasalazine or methotrexate (MTX)), (2) combination-therapy MTX + 6 weeks prednisolone and (3) combination therapy MTX + etanercept. In any treatment-arm, patients could eventually escalate to high-dose etanercept alongside MTX 10mg/m2/week.
Results
32 patients received high-dose etanercept (69% female, median age 6 years (IQR 4–10), median 10 months (7–16) from baseline). Median follow-up was 24.6 months. Most clinical parameters improved within 3 months after dose-increase: median JADAS10 from 7.2 to 2.8 (p = 0.008), VAS-physician from 12 to 4 (p = 0.022), VAS-patient/parent from 38.5 to 13 (p = 0.003), number of active joints from 2 to 0.5 (p = 0.12) and VAS-pain from 35.5 to 15 (p = 0.030). Functional impairments (CHAQ-score) improved more gradually and ESR remained stable. A comparable pattern was observed in 11 patients (73% girls, median age 8 (IQR 6–9)) who did not receive high-dose etanercept despite eligibility (comparison group). In both groups, 56% reached inactive disease at 6 months. No severe adverse events (SAEs) occurred after etanercept dose-increase. In the comparison group, 2 SAEs consisting of hospital admission occurred. Rates of non-severe AEs per subsequent patient year follow-up were 2.27 in the high-dose and 1.43 in the comparison group.
Conclusions
Escalation to high-dose etanercept in JIA-patients who were treated to target was generally followed by meaningful clinical improvement. However, similar improvements were observed in a smaller comparison group who did not escalate to high-dose etanercept. No SAEs were seen after escalation to high-dose etanercept. The division into the high-dose and comparison groups was not randomised, which is a potential source of bias. We advocate larger, randomised studies of high versus regular dose etanercept to provide high level evidence on efficacy and safety.
Trial registration
Dutch Trial Register; NTR1574; 3 December 2008; https://onderzoekmetmensen.nl/en/trial/26585.
Similar content being viewed by others
Background
Pharmacological treatment of non-systemic juvenile idiopathic arthritis (JIA) has undergone substantial transformations during the past two decades [1]. Early initiation of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) such as methotrexate (MTX) and the growing availability of biologic DMARDs (bDMARDs) have led to improved clinical outcomes [2,3,4]. In addition, the treat-to-target approach has been adopted in clinical practice and incorporated into international recommendations [5].
Etanercept, a tumor necrosis factor (TNF-)inhibitor, is one of the most widely used bDMARDs for non-systemic JIA [6,7,8]. Commonly, etanercept is started at the labelled dose of 0.8 mg/kg/week (max. 50 mg/week) [9]. Higher doses (up to 1.6 mg/kg/week, max. 50 mg/week) are used off-label in clinical practice if the labelled dose is ineffective [10]. Even though data from Canada and the USA have shown that approximately 10% of JIA-patients treated with etanercept received doses > 40% above the labelled dose, the effects of such off-label high-dose etanercept treatment, including the efficacy and safety profile, are not sufficiently known [10, 11].
Therefore, our primary objective was to provide an in-depth description of the clinical course of non-systemic JIA-patients receiving high-dose etanercept as part of the BeSt for Kids trial [12]. As a comparison group, we present the same data of patients who did not escalate to high-dose etanercept despite eligibility according to trial-protocol (protocol deviations).
Methods
Trial design
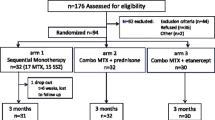
The BeSt for Kids (Dutch acronym for ‘treatment strategies for children’) study was described extensively elsewhere [12]. In short, this Dutch multi-centre trial evaluated three different treat-to-target regimens. Enrolment was from October 2009 to April 2014. It included 92 DMARD-naïve patients aged 2–16 years with new-onset oligoarticular JIA (n = 11), RF-negative polyarticular JIA (n = 73) or juvenile psoriatic arthritis (n = 8). Exclusion criteria comprised, among others, symptom duration ≥ 18 months, rheumatoid factor (RF) positivity and uveitis at enrolment. Please note that, due to the timing of patient recruitment right after diagnosis and efforts to start treatment early, oligoarticular JIA in this study comprises both persistent (≤ 4 joints affected in later progression) and extended oligoarthritis (> 4 joints affected in later progression).
Patients were randomised by variable block, stratified per centre and per oligoarticular or polyarticular disease, into three arms (1:1:1) with different initial treatments: (1) sequential DMARD-monotherapy (sulfasalazine or MTX), (2) combination-therapy of MTX and 6 weeks prednisolone, and (3) combination therapy of MTX and etanercept. Follow-up visits were planned every 3 months for two years. Median follow-up was 24.6 months.
The treatment target was defined by an adjusted ACRPedi50% at 3 months and, afterwards, by inactive disease in line with the Wallace 2004 criteria [13]. If not met, treatment was escalated according to the pre-specified treat-to-target protocol. In any treatment-arm patients could eventually escalate to high-dose etanercept (1.6 mg/kg/week, max 50 mg/week) alongside MTX (10 mg/m2/week; Fig. 1). High-dose etanercept was defined as escalation from the regular dose to a higher dose in line with this trial protocol. Due to the maximum absolute dose of 50 mg per week, the etanercept dose expressed in mg per kg bodyweight will be lower than 1.6 for patients weighing more than 31 kg.
Illustration of patient selection for current analyses based on a simplified flowchart of treatment steps in the BeSt for Kids trial
Legend
This simplified flowchart was designed to briefly illustrate patient numbers for current analyses. Exclusions and loss to follow-up are not shown. Please refer to the original BeSt for Kids report (including flowchart) for more details [12].
12 patients were not included in current analyses since they already received the maximum etanercept dose of 50 mg per week based on their body-weight (2 patients in arm 1, 8 in arm 2 and 2 in arm 3)
Abbreviations: MTX = methotrexate; mg = milligrams; SSZ = sulfasalazine; Pred = prednisolone;
ETN = etanercept
Patients studied
32 patients were escalated to high-dose etanercept after median 10 months (interquartile range (IQR) 7–16; ‘high-dose group’). Median etanercept dose at that point was 1.3 mg/kg/week (IQR 1.1–1.5).
11 other patients were eligible for high-dose etanercept at median 10 months (IQR 7–15), but did not proceed with this (‘comparison group’). These patients deviated from the trial-protocol through shared decision making with the treating paediatric rheumatologist. 10 of these patients continued etanercept in the regular dose, while one patient switched to infliximab. Reasons for not increasing the etanercept dose, as well as the alternative treatment decisions made, are described in more detail in Supplementary Table 1.
12 patients (two in arm 1, eight in arm 2 and two in arm 3) did not increase their etanercept dose as they already received the maximum of 50 mg per week based on their body-weight; these patients were not included in current analyses. For all patients that were included, bodyweight at the moment of eligibility for etanercept dose increase is presented in Supplementary Fig. 1.
Outcomes
Outcomes of interest were disease-activity measured by the JADAS-10 and its individual components [14], pain-intensity and functional impairments. A 0–100 mm visual analogue scale (VAS) was used to quantify pain intensity over the last 7 days, which was estimated by the parents for patients aged < 12 years, as described previously [15]. The number of active joints at each visit was assessed by a physician or physiotherapist blinded to treatment allocation (single-blinded study design). Functional impairments were assessed using the Childhood Health Assessment Questionnaire(CHAQ), ranging 0–3 with higher scores representing worse functioning [16].
In addition, the percentage of patients with inactive disease 6 months after eligibility for etanercept dose increase was calculated [15]. Inactive disease was defined by the Wallace 2004 criteria adjusted by physician’s global assessments < 10 mm indicating no active disease [12, 13]. The percentage of patients who subsequently lost this inactive disease criterium was also assessed.
Statistical analyses
Analyses in this study are descriptive. Direct statistical comparisons between the high-dose and comparison group were not performed since the trial was not powered accordingly [12].
Medians of clinical parameters were plotted from the moment of eligibility for etanercept dose increase onwards. The Wilcoxon rank-sum test was used to calculate whether clinical parameters had changed statistically significantly at 3 months after eligibility for etanercept dose increase. The rate of non-severe adverse events (AEs) was calculated per group by dividing the number of AEs registered after etanercept dose increase by the number of subsequent patient years follow-up. Moreover, the AE rate per patient year was calculated for the period until the moment of eligibility for etanercept dose increase.
In order to filter out potential treatment effects of bDMARD switching (rather than maintaining the etanercept dose), analyses were repeated after excluding patients in the comparison group who switched to another bDMARD.
IBM SPSS v29 was used. Two-sided p-values < 0.05 were considered statistically significant.
Results
Patients
Patient characteristics at inclusion and at the moment of eligibility for etanercept dose-increase are presented in Table 1. In the high-dose group, median age was 6 years at inclusion (IQR 4–10) and 69% were girls. In the comparison group, median age was 8 years at inclusion (IQR 6–9) and 73% were girls. The comparison group had a higher number of actively inflamed joints at inclusion (median (IQR) 11 (8–18) compared to 7 (5–11) in the high-dose group, (p = 0.022), otherwise clinical parameters were generally similar in both groups.
Clinical parameters over time
Follow-up was up to 2 years from baseline; median follow-up was 24.6 months. Clinical parameters over time from the moment of eligibility for etanercept dose increase are presented in Fig. 2. Overall, the clinical course seemed similar in both groups.
Clinical parameters over time from the moment of eligibility for etanercept dose increase for both the high-dose and the comparison group
Legend
Lines represent individual patients; squares and dots represent the group median
Timepoint zero represents the moment of eligibility for etanercept dose increase, which is not equivalent to the baseline visit of the BeSt for Kids trial and may differ from patient to patient
Abbreviations: JADAS = Juvenile Arthritis Disease Activity Score; VAS = visual analogue scale; CHAQ = childhood health assessment questionnaire; ESR = erythrocyte sedimentation rate
In the high-dose group, clinical measures of disease-activity improved largely within 3 months: median JADAS10 from 7.2 to 2.8 (p = 0.008), VAS-physician from 12 to 4 (p = 0.022), VAS-patient/parent from 38.5 to 13 (p = 0.003), VAS pain from 35.5 to 15 (p = 0.030), the number of active joints from 2 to 0.5 (p = 0.12) and functional status (CHAQ-score) from 0.63 to 0.50 (p = 0.047), while ESR remained stable (from 6 to 6; p = 0.32).
In the comparison group, a comparable pattern of clinical parameters over time was observed. After 3 months median JADAS10 improved from 8.8 to 1.9 (p = 0.017), VAS-physician from 16 to 0 (p = 0.24), VAS-patient/parent from 38 to 11.5 (p = 0.93), VAS pain from 22 to 7 (p = 0.67), the number of active joints from 2 to 0 (p = 0.29) and functional status (CHAQ-score) from 0.69 to 0.13 (p = 0.41), while ESR remained stable (from 7 to 8; p = 0.72).
Although in broad terms the clinical course appeared similar in both groups, at the 12 months timepoint specifically median JADAS10 and VAS pain were numerically lower (better) in the high-dose than in the comparison group (Fig. 2). However, at this point in time sample size was low (n = 11 in the high-dose group and n = 5 in the comparison group) and no direct statistical comparison was made.
Inactive disease
In both the high-dose and the comparison group the percentage of patients with inactive disease 6 months after eligibility for dose-increase was 56%.
Loss of inactive disease criteria after eligibility for high dose etanercept occurred in 8 patients in the high-dose group (25%) and in 3 patients (27%) in the comparison group.
Adverse events
No severe adverse events (SAEs) were recorded after etanercept dose-increase. In the comparison group there were 2 SAEs consisting of hospital admissions. One of these concerned supportive care for gastroenteritis. The other admission was for precautionary intravenous antiviral treatment due to mildly increased liver-enzymes together with a varicella infection (which can cause hepatitis in immunocompromised patients, but in this case the patient recovered without complications).
Non-severe adverse events (AEs) are summarised in Fig. 3 and presented in more detail in Supplementary Table 2. In the high-dose group, 18 out of 32 patients (56%) experienced 26 infectious AEs; 26 patients (81%) experienced 78 AEs of any sort. In the comparison group, 4 out of 11 patients (36%) experienced 4 infectious AEs; 6 patients (55%) experienced 17 AEs of any sort. Median time from eligibility for dose-increase until occurrence of the AE appeared similar in both groups (median 6 months (IQR 3–9) for AEs in the high-dose group; 7 months (IQR 4–12) for AEs in the comparison group). The rate of infectious AEs per patient year following eligibility for etanercept dose increase was 0.76 in the high-dose group and 0.34 in the comparison group. For AEs of any sort, rates were 2.27 and 1.43 per visit, respectively. Thus, AEs were numerically more frequent in the high-dose than in the comparison group.
Additional analyses
Findings were similar when one patient who switched to infliximab was excluded from the comparison group (Supplementary Figs. 2 and 3).
Next, we assessed AEs until eligibility for high-dose etanercept (Supplementary Fig. 4 and Supplementary Table 3). During this period, the overall rate of AEs per patient year was 3.26 in the high-dose group and 2.51 in the comparison group. Thus, the frequency of AEs was, numerically, already somewhat higher in the high-dose than in the comparison group before eligibility for etanercept dose-increase occurred.
Discussion
Although high-dose etanercept is used off-label in JIA-patients in clinical practice, supporting evidence is lacking. Therefore, we conducted a post-hoc analysis of the BeSt for Kids trial describing the clinical course of JIA-patients who received high-dose etanercept and of those who did not receive high-dose etanercept despite eligibility according to trial protocol. Parameters of disease-activity and the disease-burden developed largely similarly over time in both groups. No SAEs were seen after escalation to high-dose etanercept. Non-severe AEs were numerically more frequent in the high-dose than in the comparison group.
The question whether higher doses of etanercept can contribute to reaching treatment goals in JIA is highly relevant. Adequate treatment can contribute to improved quality of life for individual patients and parents [17]. In addition, there may be important social benefits such as reducing caregiver work-productivity loss [17, 18]. On the other hand, dose increase may have disadvantages such as increased costs and, hypothetically, dose-dependent side-effects [19] which are desired to be in proportion to the benefits of the treatment. If this is not the case, other strategies such as switching to another bDMARD might be more appropriate.
Nevertheless, literature on this topic is scarce. Takei et al. reported 8 JIA-patients receiving high-dose etanercept but lack a comparison group [11]. Another study found no significant differences regarding clinical outcomes and AEs between JIA-patients who were escalated from regular to high-dose bDMARDs on the one hand, and bDMARD-switchers on the other hand, but did not report specifically on the 14 patients who escalated to high-dose etanercept [10]. Our study adds to this scarce evidence by describing clinical outcomes and AEs in the largest-to-date group of JIA-patients who were escalated to high-dose etanercept, and in a relevant comparison group.
This study has several limitations, including its small group size and descriptive nature. In addition, escalating from regular to high-dose etanercept was not randomised and blinded. We observed similar clinical improvements in both groups, even though treatment was not changed in most patients in the comparison group. It is possible that the etanercept dose was not increased in the comparison group because further clinical improvement was expected by the paediatric rheumatologist or by the patient/parents. This would be indicative of (unmeasured) confounding by indication, which may lead to false-negative findings. On the other hand, due to the lack of randomisation and blinding, one may have expected a placebo response to increasing the etanercept-dose. Still, the clinical course was similar in both groups. Moreover, due to the open label design, patients and their parents knew when their etanercept dose was increased which may theoretically have led to more alertness to adverse events.
Furthermore, it is unknown whether the numerically higher rate of adverse events observed in this study is causally related to higher etanercept dosage. Exploring this further, we calculated rates of non-severe AEs per visit until eligibility for high-dose etanercept in both groups, and found that the frequency of AEs was, numerically, already somewhat higher in the high-dose than in the comparison group. This poses an additional challenge to interpretation of the data on AEs. Possibly, patients in the high-dose group may have been inherently more prone to the occurrence of AEs due to (unknown) confounders. For example, younger children may be more prone to AEs when using etanercept and median age was numerically lower in the high-dose than in the comparison group (median difference of 2 years, which was not statistically significant).
Altogether, these results should be interpreted with caution. Selection bias and confounding by indication should be considered as influencing factors. Future, preferably randomized studies would be needed to obtain higher level evidence.
Lastly, we would like to point out that etanercept in this study was given alongside 10 mg/m2 MTX per week. The recommended dose for MTX in JIA-patients is 10 to 15 mg/m2. It was deemed appropriate to dose MTX in the low-normal range given the context of treatment-to-target and tightly scheduled follow-up, allowing swift access to combination therapy. We acknowledge that in current times, considering current consensus and guidelines for JIA treatment [20, 21], the higher MTX dose of 15 mg/m2 could be preferred – also alongside etanercept.
Conclusions
In conclusion, escalation to high-dose etanercept in JIA-patients who were treated to target was generally followed by meaningful clinical improvement. However, similar improvements were observed in a smaller comparison group who did not escalate to high-dose etanercept. No SAEs were seen after escalation to high-dose etanercept. We advocate larger, randomised studies of high versus regular dose etanercept to provide high level evidence on efficacy and safety.
Data availability
Data are available from the corresponding author (e-mail; B.T.van_Dijk@lumc.nl) upon reasonable request.
Abbreviations
- AE:
-
Adverse event
- bDMARD:
-
Biologic DMARDs
- BeSt:
-
Behandelstrategieën (Dutch for ‘treatment strategies’)
- CHAQ:
-
Childhood Health Assessment Questionnaire
- csDMARD:
-
Conventional synthetic DMARD
- DMARD:
-
Disease-modifying anti-rheumatic drugs
- ESR:
-
Erythrocyte sedimentation rate
- ETN:
-
Etanercept
- IBM SPSS v29:
-
International Business Machines Corporation Statistical Package for the Social Sciences version 29
- IQR:
-
Interquartile range
- JADAS:
-
Juvenile Arthritis Disease Activity Score
- JIA:
-
Juvenile idiopathic arthritis
- LUMC:
-
Leiden University Medical Centre
- mg:
-
Milligrams
- mm:
-
Millimeters
- MTX:
-
Methotrexate
- Pred:
-
Prednisolone
- RF:
-
Rheumatoid factor
- SAE:
-
Severe adverse event
- SSZ:
-
Sulfasalazine
- TNF:
-
Tumor necrosis factor
- VAS:
-
Visual analogue scale
References
Stoll ML, Cron RQ. Treatment of juvenile idiopathic arthritis: a revolution in care. Pediatr Rheumatol Online J. 2014;12:13.
Albers HM, Wessels JA, van der Straaten RJ, Brinkman DM, Suijlekom-Smit LW, Kamphuis SS, et al. Time to treatment as an important factor for the response to methotrexate in juvenile idiopathic arthritis. Arthritis Rheum. 2009;61(1):46–51.
Prince FH, Geerdink LM, Borsboom GJ, Twilt M, van Rossum MA, Hoppenreijs EP, et al. Major improvements in health-related quality of life during the use of etanercept in patients with previously refractory juvenile idiopathic arthritis. Ann Rheum Dis. 2010;69(1):138–42.
Minden K, Niewerth M, Zink A, Seipelt E, Foeldvari I, Girschick H, et al. Long-term outcome of patients with JIA treated with etanercept, results of the biologic register JuMBO. Rheumatology (Oxford). 2012;51(8):1407–15.
Ravelli A, Consolaro A, Horneff G, Laxer RM, Lovell DJ, Wulffraat NM, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2018;77(6):819–28.
Grazziotin LR, Currie G, Twilt M, Ijzerman MJ, Kip MMA, Koffijberg H, et al. Real-world data reveals the complexity of disease modifying anti-rheumatic drug treatment patterns in juvenile idiopathic arthritis: an observational study. Pediatr Rheumatol. 2022;20(1):25.
Davies R, Carrasco R, Foster HE, Baildam EM, Chieng SEA, Davidson JE, et al. Treatment prescribing patterns in patients with juvenile idiopathic arthritis (JIA): analysis from the UK Childhood Arthritis prospective study (CAPS). Semin Arthritis Rheum. 2016;46(2):190–5.
Kip MMA, de Roock S, Currie G, Marshall DA, Grazziotin LR, Twilt M, et al. Pharmacological treatment patterns in patients with juvenile idiopathic arthritis in the Netherlands: a real-world data analysis. Rheumatology (Oxford). 2023;62(Si2):Si170–80.
European Medicines Agency. Enbrel Product Information. https://www.ema.europa.eu/en/documents/product-information/enbrel-epar-product-information_en.pdf (visited on 13 October 2023).
Correll CK, Shrader P, Dennos A, Phillips T, Shiff NJ, Verstegen RHJ, et al. Effectiveness and safety of high-dose biologics in Juvenile Idiopathic Arthritis in the Childhood Arthritis and Rheumatology Research Alliance. Arthritis Care Res (Hoboken). 2022;74(11):1770–9.
Takei S, Groh D, Bernstein B, Shaham B, Gallagher K, Reiff A. Safety and efficacy of high dose etanercept in treatment of juvenile rheumatoid arthritis. J Rheumatol. 2001;28(7):1677–80.
Hissink Muller P, Brinkman DMC, Schonenberg-Meinema D, van den Bosch WB, Koopman-Keemink Y, Brederije ICJ, et al. Treat to target (drug-free) inactive disease in DMARD-naive juvenile idiopathic arthritis: 24-month clinical outcomes of a three-armed randomised trial. Ann Rheum Dis. 2019;78(1):51–9.
Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31(11):2290–4.
Consolaro A, Giancane G, Schiappapietra B, Davì S, Calandra S, Lanni S, et al. Clinical outcome measures in juvenile idiopathic arthritis. Pediatr Rheumatol. 2016;14(1):23.
Spekking K, Anink J, de Boer P, Bergstra SA, van den Berg JM, Schonenberg-Meinema D, et al. Significant pain decrease in children with non-systemic juvenile idiopathic arthritis treated to target: results over 24 months of follow up. Pediatr Rheumatol. 2023;21(1):90.
Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37(12):1761–9.
Kuhlmann A, Schmidt T, Treskova M, López-Bastida J, Linertová R, Oliva-Moreno J, et al. Social/economic costs and health-related quality of life in patients with juvenile idiopathic arthritis in Europe. Eur J Health Econ. 2016;17(1):79–87.
García-Rodríguez F, Gamboa-Alonso A, Jiménez-Hernández S, Ochoa-Alderete L, Barrientos-Martínez VA, Alvarez-Villalobos NA, et al. Economic impact of Juvenile Idiopathic Arthritis: a systematic review. Pediatr Rheumatol. 2021;19(1):152.
Kip MMA, de Roock S, Currie G, Marshall DA, Grazziotin LR, Twilt M, et al. Costs of medication use among patients with juvenile idiopathic arthritis in the Dutch healthcare system. Expert Rev Pharmacoecon Outcomes Res. 2021;21(5):975–84.
Ferrara G, Mastrangelo G, Barone P, La Torre F, Martino S, Pappagallo G, et al. Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting. Pediatr Rheumatol. 2018;16(1):46.
Dutch Pediatric Society, Knowledge Institute of the Dutch Association of Medical Specialists. Juveniele idiopathische artritis [Dutch for. ‘Juvenile idiopathic arthritis] (JIA) 2018. Available from: richtlijnendatabase.nl/richtlijn/juveniele_idiopathische_artritis_jia (visited on 10 April 2024).
Acknowledgements
Not applicable.
Funding
The BeSt for Kids study is an investigator-initiated study which received financial support from Pfizer, who had no role in study design, data collection, data analysis, data interpretation, writing of an abstract, or decision to submit a manuscript for submission.
Author information
Authors and Affiliations
Contributions
RC, CA, DB and PH contributed to study conception and design. JB, DS, LS, MR, YK, RC, DB and PH contributed to data collection. BD performed the statistical analyses. BD, SB and PH drafted and edited the manuscript. All authors were involved in interpretation of the data and critically revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in compliance with the Helsinki Declaration. The BeSt for Kids trial was approved by the Institutional Review Board at the Leiden University Medical Centre (LUMC). Written informed consent was obtained from patients above 12 years of age and parents of all participating patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van Dijk, B.T., Bergstra, S.A., van den Berg, J.M. et al. Increasing the etanercept dose in a treat-to-target approach in juvenile idiopathic arthritis: does it help to reach the target? A post-hoc analysis of the BeSt for Kids randomised clinical trial. Pediatr Rheumatol 22, 53 (2024). https://doi.org/10.1186/s12969-024-00989-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-024-00989-x