Abstract
Background
Primary immunodeficiencies are immunological disorders caused by gene mutations involved in immune system development and activation. Recently, activated phosphoinositide 3-kinase delta syndrome (APDS) due to mutations in the phosphoinositide 3-kinase (PI3K), phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit delta gene (PIK3CD), and phosphoinositide 3-kinase regulatory subunit 1 (PIK3R1) genes have been reported to induce a combined immunodeficiency syndrome leading to senescent T cells, lymphadenopathy, and immunodeficiency. The exact diagnosis of these deficiencies is essential for treatment and prognosis. In recent years, targeted treatment with selective PI3Kd inhibitors has had a significant effect on controlling the symptoms of these patients.
Case presentation
In this case report, we represent a 27-month-old girl with recurrent fever, an increased level of inflammatory markers, and erythema nodosum, who was referred to the rheumatology clinic. In the course of evaluations, because of the lack of clinical improvement with usual treatments, and a history of frequent respiratory infections, combined immunodeficiency was diagnosed in the immunological investigations. Moreover, whole-exome sequencing was performed for her.
Conclusion
The genetic analysis found a novel variant of PIK3CD (c.1429 G > A) in the patient. Following daily antibiotic prophylaxis and monthly IV therapy, the patient’s frequent infections and fevers were controlled.
Similar content being viewed by others
Introduction
Primary immune deficiency (PID) includes a wide variety of diseases in which the immune system is impaired or incapable of performing its functions normally, putting the individual at risk for infections, cancer, and autoimmune conditions. It has been recognized genetically that there are more than 480 distinct disorders, and new diseases are continually being discovered [1]. In most cases, primary immunodeficiency disorders are inherited from parents, but some are acquired [2, 3].There are different reports on the prevalence of PID worldwide. It is estimated that about 1 per 10,000 people are involved with PID [4, 5].
The p110δ catalytic subunit widely produced in immune cells is encoded by the phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit delta gene (PIK3CD). The mutation of this gene can result in lymphadenopathy and combined immunodeficiency. Mutations that cause this gene gain or loss of function have been reported to induce immunodeficiency in different ways [6].
PIDs can occur at any age, and diagnosing them accurately and promptly necessitates high suspicion and specific testing. A wide variety of clinical manifestations occur with PIDs, but most increase the risk of infection. Many PIDs result in frequent infections and may go unnoticed in general care. Diagnosing and treating diseases as early as possible is imperative to avoid considerable disease-related morbidity and improve patient outcomes [7, 8]. In this case report, we presented a girl with a novel mutation in the PIK3CD gene that has not been reported until now, to the best of our knowledge.
Case presentation
A 27-month-old girl was referred due to recurrent fever, arthritis, and erythema nodosum to the rheumatology clinic of Mofid Children’s Hospital (Tehran, Iran). She was the second alive child of consanguineous marriage (G2P2L2) with a 3800-gram birth weight and a history of neurodevelopmental delays in walking (at the age of 21 months) and speaking (at the age of 24 months). There is no positive point in the family history, except for the mother, who had hypothyroidism and a history of frequent respiratory infections in childhood.
She had a history of several hospital admissions after her birth. First, she was admitted at two months old with a fever and high levels of ESR and CRP. She received antibiotics for five days and was discharged with no signs or symptoms but still had high ESR and CRP.
At 8 months old, she was evaluated due to failure to thrive (FTT). During patient examinations, the low heart rate, muffled heart sound, and massive pericardial effusion were found in her echocardiography. The pericardial effusion caused her to be hospitalized for 16 days in the intensive care unit (ICU). Pericardial effusion was tapped without any infectious resources. Pericardial effusion was treated with prednisolone, and she was discharged in good condition. Two months later, she was hospitalized again with recurrent pericardial effusion for 19 days. In evaluations, she had hypothyroidism and a little pericardial effusion. She was treated with dexamethasone, ibuprofen, and levothyroxine.
Fourth and fifth hospitalizations occurred due to high-grade fever and elevated ESR and CRP levels at the 13th and 18th months of old, respectively.
She got COVID-19 at 24 months old and was admitted to the hospital for the 6th time because of her past medical history. She had only a fever as a COVID-19 manifestation. After 4 months, she got COVID-19 again and had gastrointestinal manifestations without any adverse effects.
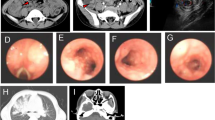
At the age of 27 months, the patient was referred to the rheumatology clinic due to several episodes of high grade fever, inflammatory pericardial effusion, and active left knee arthritis with an increase in inflammatory marker levels without any localized origin, as well as an erythematous plaque on the leg and a history of multiple hospitalizations. A skin biopsy revealed septal panniculitis compatible with erythema nodosum. The patient was thoroughly evaluated in terms of rheumatology, her height, and weight were 82 cm and 10 kg in physical examination, respectively. We found blonde hair, frontal bossing, and macular rashes on limbs, and eventually, according to the lack of response to treatment and the history of respiratory infections, an immunological examination including CBC, CD markers, and immunoglobulins levels was performed. The clinical manifestations of the patient are shown in Fig. 1.
Low serum IgG levels, decreased CD4/CD8 ratio and CD27 level, and a poor response to candida in the lymphocyte transformation test (LTT) were detected (Table 1). Intravenous immunoglobulin (IVIG) and prophylactic antibiotics were started which led to the control of the patient’s fever and other symptoms.
After about 5 months of the follow-up, the patient was hospitalized due to polyuria, polydipsia, tachypnea, and lethargy with the diagnosis of diabetic ketoacidosis and was discharged with injectable insulin. Whole-exome sequencing was performed on the patient’s whole blood sample. Variant interpretation of interested variants was accomplished through the American College of Medical Genetics and Genomics (ACMG). A novel heterozygous variant (c.1429 G > A; p.Glu477Lys) was found in the PIK3CD gene (Table 2). The variant was validated in the patient, and segregation analysis showed the mother is the carrier for the variant. According to the ACMG guideline, this variant can be classified as a Variant of Unknown Significance (VUS).
Discussion
In this report, we described a girl with a novel mutation in the PIK3CD gene. She had a recurrent fever, and erythema nodosum with the manifestations of combined immunodeficiency in immunological investigations. She experienced several episodes of viral and bacterial infections, autoimmune disorders (hypothyroidism and type 1 diabetes mellitus), and auto-inflammatory manifestations (left knee arthritis, pericardial effusion).
Frequent infections were considered the main manifestation of primary immune deficiencies, but in recent years, non-infectious manifestations related to this group of diseases have received more attention. These can be the first complaint for a patient to visit the doctor [9].
In the largest cohort study on 53 patients with this type of immunodeficiency conducted by Tanya I. Coulter et al., upper and lower respiratory tract infections were the most common symptoms, which were seen in 98% of patients. Our patient has suffered from recurrent bacterial sinusitis and pneumonia since early infancy. The autoimmune or inflammatory diseases were reported in 34% of the patients. Among them, only 3 patients had autoimmune thyroid disease in adulthood, two patients suffered from arthritis, and one patient had pericardial effusion, but our patient experienced all three of these very rare manifestations in infancy. Global developmental or isolated speech delays were detected in 19% of patients [10]. According to the results of this study, our patient also had motor and verbal development delays.
Frequent respiratory infections can lead to lung damage and bronchiectasis, so it is very important to pay attention to this point in the follow-up of patients. Quick and timely diagnosis can prevent such serious injuries in patients [11]. Nonmalignant lymphoproliferative manifestations are among the common manifestations in patients, but they were not seen in our patient [12].
Considering the susceptibility of these patients to chronic viral infections such as Epstein Barr Virus (EBV), the risk of cancer in them is higher than in the normal population [13]. Fortunately, our patient has not been infected with chronic viral infections such as Epstein’s, etc., but we should always keep this point in mind during follow-up.
Immunodeficiency may be accompanied by cardiac tamponade; however, it is not common. Farouji et al. reported a 35-year-old woman with immune deficiency presented with cardiac tamponade [14]. Our patient had a massive pericardial effusion that could have led to tamponade, but her early diagnosis saved her life.
Early diagnosis and management of a patient with PI3KCD deficiency are important.
Somatic and germline mutations in genes involved in the PI3Kd pathway can result in different clinical and laboratory manifestations. Somatic mutations are associated with cancer. Both overactivation and underactivation of PI3Kd as a result of germline mutations could lead to immunodeficiency and immune dysregulation. Recently, more than 200 such patients have been detected [13]. The immunologic workups of our patient were compatible with combined immunodeficiency. Besides, she had manifestations of immune dysregulation.
To our knowledge, the variant of the PIK3CD gene (c.1429 G > A) found in this study has not previously been reported. Lu et al. reported two cases with frequent pulmonary infections, sinusitis, and a positive anti-neutrophil cytoplasmic antibody (ANCA). The whole exome sequencing revealed a Glu1021Lys (c.3061 G > A) mutation in the PIK3CD gene [15]. Moreover, a fourteen-year-old girl reported by Nichols-Vinueza et al. had a PIK3CD mutation (c.1546 G > A) and a history of severe atopy, saddle nose deformity, nasal septal perforation, repeated infections, and increased IgE and eosinophilia [16]. Further, she had a mid-face deformity and recurrent infections compared with our patient. It seems that mutations in PIK3CD are accompanied by midface malformations. Moreover, our report showed that a novel variant in PIK3CD gene in exon 11 related to recurrent infection as well as some facial and dermal involvement. Antibiotic prophylaxis and immunoglobulin replacement therapy (intravenous immunoglobulin or subcutaneous immunoglobulin) are among the main treatments in most patients. Bacterial infections were decreased in 89% of patients in the Elkaim et al. APDS2 cohort with long-term IRT, and 61% of patients received antibiotic prophylaxis [17].
Sirolimus, the mTOR inhibitor, appears to be effective in controlling lymphoproliferative symptoms in these patients [18]. The findings suggests that HSCT is curative in younger patients with life threatening complications of APDS [19].
PI3Kδ inhibitors are new drugs recently used in the treatment of this group of patients. They have had satisfactory results in resolving lymphoproliferation, but it seems that they do not replace a hematopoietic stem cell transplant [20, 21].
In our practice, we performed supportive treatments for the patient, like IVIG infusions and antibiotic therapy. Considering the poor outcome of this disease, bone marrow transplantation is one of the decisions for the patient; however, she has not had a proper, fully HLA matched donor yet.
Conclusion
The variant in PIK3CD (c.1429 G > A) is a novel mutation that can cause immunodeficiency in patients. This gene can be presented at an early age. Whole-exome sequencing is a valuable method for diagnosing patients with an immunodeficiency that physicians do not have any direct clue about. The exact genetic diagnosis would be helpful for controlling the disease and providing appropriate treatment.
Data Availability
Not applicable.
Abbreviations
- PI3K:
-
Phosphoinositide 3-kinase
- APDS:
-
Activated phosphoinositide 3-kinase delta syndrome
- PIK3CD :
-
Phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit delta
- PID:
-
Primary immune deficiency
- FTT:
-
Failure to thrive
- ICU:
-
Intensive care unit
- LTT:
-
Lymphocyte transformation test
- IVIG:
-
Intravenous immunoglobulin
- ACMG:
-
American College of Medical Genetics and Genomics
- VUS:
-
Variant of Unknown Significance
- ANCA:
-
Anti-neutrophil cytoplasmic antibody
- WBC:
-
White Blood cell
- Hb:
-
Hemoglobin
- PLT:
-
Platelet
- ANA:
-
AntiNuclear Antibody
- RF:
-
Rheumatoid Factor
- Anti Ds DNA:
-
Anti Double stranded DNA
- Anti CCP:
-
Anti Cyclic Citrullinated Peptide
- Anti SSA Ab:
-
anti–Sjögren’s-syndrome-related antigen A autoantibodies
- Anti SSB:
-
anti–Sjögren’s-syndrome-related antigen B autoantibodies
- ESR:
-
Erythrocyte Sedimentation Rate
- CRP:
-
C-reactive Protein
- Ig:
-
Immunoglobulin
- PHA:
-
Phytohemagglutinin
- BCG:
-
Bacillus Calmette-Guerin
References
Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40(1):24–64.
Chi CY, Lin CH, Ho MW, Ding JY, Huang WC, Shih HP, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-γ autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine. 2016;95(25):e3927.
Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2 Suppl 2):182–94.
Rhim JW, Kim KH, Kim DS, Kim BS, Kim JS, Kim CH, et al. Prevalence of primary immunodeficiency in Korea. J Korean Med Sci. 2012;27(7):788–93.
van Zelm MC, Condino-Neto A, Barbouche MR. Editorial: primary Immunodeficiencies Worldwide. Front Immunol. 2019;10:3148.
Zhang Q, Ma H, Ma J, Wang D, Zhao Y, Wang T, et al. Clinical and genetic analysis of immunodeficiency-related diseases associated with PIK3CD mutations. Pediatr Invest. 2018;2(4):257–62.
Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186–205. e1-78.
Shehata N, Palda V, Bowen T, Haddad E, Issekutz TB, Mazer B, et al. The use of immunoglobulin therapy for patients with primary immune deficiency: an evidence-based practice guideline. Transfus Med Rev. 2010;24(Suppl 1):28–50.
Goudouris ES. Immunodeficiencies: non-infectious manifestations. Jornal de pediatria. 2021;97(Suppl 1):24–s33.
Coulter TI, Chandra A, Bacon CM, Babar J, Curtis J, Screaton N, et al. Clinical spectrum and features of activated phosphoinositide 3-kinase δ syndrome: a large patient cohort study. J Allergy Clin Immunol. 2017;139(2):597–606e4.
Angulo I, Vadas O, Garçon F, Banham-Hall E, Plagnol V, Leahy TR, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Sci (New York NY). 2013;342(6160):866–71.
Aghamohamadi NZMA. Activated PI3K-Delta syndrome: pathogenesis, clinical manifestations, diagnosis, classification, and management. Immunol Genet J. 2020;3(3):8–15.
Nunes-Santos CJ, Uzel G, Rosenzweig SD. PI3K pathway defects leading to immunodeficiency and immune dysregulation. J Allergy Clin Immunol. 2019;143(5):1676–87.
Farouji I, Damati A, Chan KH, Ramahi A, Chenitz K, Slim J, et al. Cardiac Tamponade Associated with Human Immunodeficiency Virus-Associated Immune Complex kidney disease. J global Infect Dis. 2021;13(3):151–3.
Lu M, Gu W, Sheng Y, Wang J, Xu X. Case Report: activating PIK3CD mutation in patients presenting with granulomatosis with polyangiitis. Front Immunol. 2021;12:670312.
Nichols-Vinueza DX, Su HC, Rao VK, Bayer DK, Ferguson PJ, Parker R, et al. Granulomatosis with polyangiitis and severe systemic Eosinophilia due to a Novel PIK3CD mutation. J Allergy Clin Immunol. 2019;143(2):AB425.
Elkaim E, Neven B, Bruneau J, Mitsui-Sekinaka K, Stanislas A, Heurtier L, et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase δ syndrome 2: a cohort study. J Allergy Clin Immunol. 2016;138(1):210–8e9.
Coulter TI, Cant AJ. The treatment of activated PI3Kδ syndrome. Front Immunol. 2018;9:2043.
Nademi Z, Slatter MA, Dvorak CC, Neven B, Fischer A, Suarez F, et al. Hematopoietic stem cell transplant in patients with activated PI3K delta syndrome. J Allergy Clin Immunol. 2017;139(3):1046–9.
Notarangelo LD. Hematopoietic stem cell transplantation for activated phosphoinositide 3-kinase δ syndrome: who, when, and how? J Allergy Clin Immunol. 2019;143(1):91–3.
Maccari ME, Abolhassani H, Aghamohammadi A, Aiuti A, Aleinikova O, Bangs C, et al. Disease Evolution and Response to Rapamycin in activated phosphoinositide 3-Kinase δ syndrome: the european Society for Immunodeficiencies-Activated phosphoinositide 3-Kinase δ Syndrome Registry. Front Immunol. 2018;9:543.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NSh and SSh wrote the manuscript. NSh, ZCh, LGh, ShH, SSh, SSh and RSh visited the patient, analyzed and interpreted the clinical and laboratory data of the patient. All authors revised the manuscript. All authors approved the final manuscript and agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The consent for publication was obtained from the patient.
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shashaani, N., Chavoshzadeh, Z., Ghasemi, L. et al. Immunodeficiency due to a novel variant in PIK3CD: a case report. Pediatr Rheumatol 21, 71 (2023). https://doi.org/10.1186/s12969-023-00859-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-023-00859-y