Abstract
Background
Analyzed the clinical features and treatment process of the patient suffering from immunodeficiency with systemic lupus erythematosus(SLE)-like syndrome in a novel mutation of PRKDC.
Case presentation
The patient had multiple positive auto-antibodies, chest CT and bronchoscopy showed Diffuse alveolar hemorrhage(DAH), and psychiatric symptoms showed brain atrophy by magnetic resonance imaging (MRI). Whole exon sequencing showed that novel complex heterozygous mutations of PRKDC gene (C. 1777 − 710_1777-709INSA (IVS16/IC16), C.1337T > A(p.Phe446Tyr). The mature B cell (CD19 + CD27 + CD38 dimIgD IgM-) were absent. The treatment of high-dose methylprednisolone (MP) and cyclophosphamide(CTX) can quickly relieve the symptoms of the patient.
Conclusion
We described the case of an infant immunodeficiency with SLE like-syndrome, which may cause by PRKDC mutation, treated successfully with high-dose MP and CTX.
Similar content being viewed by others
Introduction
Childhood systemic lupus erythematosus (cSLE) has been a common autoimmune disease in children, mainly characterized by multi-system chronic lesions, including skin, joint, kidney, nerve, cardiovascular, and lung damage, accompanied by extensive auto-antibody formation [1, 2]. Diffuse alveolar hemorrhage (DAH) syndrome is a very serious and fatal interstitial lung disease with an average mortality of 50%, caused by autoimmune diseases, especially SLE [3,4,5].
SLE has been mainly related to the immune dysfunction of B cells. Abnormal activation of B cells can generate multiple auto-antibodies, leading to the formation of immune complexes [6]. PRKDC encodes DNA-dependent protein kinase (DNA-PK),which is abundantly expressed in almost mammalian cells [7, 8]. Studies show that PRKDC mutation influenced production T/B cells and V(D)J rearrangement, which act as major triggers of auto-antibodies and autoimmune disease production [9, 10]. PRKDC mutation is seldom reported on autoimmune diseases and no related studies on SLE, especially for the treatment and prognosis of SLE with this gene mutation.
Here, we found a novel compound heterozygous mutation of PRKDC in a male cSLE for the performances of severe acute diffuse alveolar hemorrhage, multiple positive auto-antibodies, psychiatric abnormalities and multiple pathogen infections. Both parents were found to be heterozygous for the mutation. Aggressive plasma exchange, high-dose methylprednisolone pulse combined with cyclophosphamide (CTX) immunosuppression therapy significantly improved the clinical symptoms of the children. These results suggested that PRKDC mutations may induce T/B cell dysfunction and immune leakage, promoted the formation of auto-antibodies, which caused severe SLE with DAH symptoms.
Case presentation
An 8-month-old male child was admitted to the hospital because of cough and hemoptyses for more than one month with butterfly erythema and hepatomegaly. An episode of acute onset of alveolar hemorrhage (hemoglobin 8 g/L, Fib 0.5 g/L), pulmonary infection (Enterobacter cholera, Pseudomonas Aeruginosa, Enterobacter cloacae, Mycoplasma pneumoniae, Parainfluenza virus. Acinetobacter and oligoxomonas maltophilia were detected in alveolar lavage fluid with Next Generation Sequencing(NGS), hypoxemia and psychosis were presented. Personal history: The patient was a full-term natural birth baby, and had retarded psychomotor development manifested as dysphasia and poor active grip with regular rehabilitation treatment at the age of 4 months old. Family history: His father worked in the administrative department of electronic components waste recycling. The parents and the sister were in good health. His father’s antinuclear antibody spectrum showed suspicious positive anti-SM antibody and weak positive anti-RNP/Smith antibody. The mother and the sister were negative in routine of auto-antibodies, immunity and hematuria detecting. Past history: He had been hospitalized twice for bronchopneumonia and moderate anemia. Laboratory text of autobiographies showed, positive antinuclear antibody (ANA) (nuclear granular + cytosolic) 1:640, anti-double, stranded DNA (dsDNA) and others were detect in the patient.
The Whole-exome showed novel compound heterozygous mutation of PRKDC was detect in the patient (Fig. 1), which had been reported to be pathogenic for this variant to involve in the pathogenesis of immunodeficiency type 26 [9]. The CD19 + CD27 + CD38dim IgD/IgM of classical immunoglobulin class conversion B lymphocytes decreased significantly which be converted from IgM to IgG, IgA, IgE, etc. [11] (Table 1). The polymorphism of B-cell receptor (BCR) and T-cell receptor(TCR) were limited in patients with PRKDC mutations (Fig. 2). Above the result present the patient had the deficiency in the VDJ rearrangement with secondary immune disorder.
Distinctive annular erythematosus skin lesions and sanger sequencing of compound heterozygous mutation in the patient. A. Distinctive annular erythematosus skin lesions of the patient. B. Sanger sequencing showed the compound heterozygous mutation of the patient. C. PRKDC gene characteristics and domain distribution
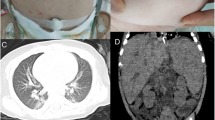
The treatment was given MP combined with CTX immunosuppression, and the dose was adjusted according to the ratio of T/B/NK cell subsets (Fig. 3), supplemented with plasma exchange three times to remove auto-antibodies and inflammatory mediators, and anti-infection treatment were performed with cefoperazone sodium and sulbactam sodium, teicoplanin, azithromycin and voriconazole according to the etiological examination. Finally, the patient’s blood oxygen and hemoglobin were stable, and pulmonary exudation was improved obviously in assessment of high-resolution chest CT (Fig. 4). Chest High-resolution CT showed the imaging changes of DAH (Fig. 5). Bronchoscope record: hemorrhagic diffuse alveolar in left and right side of the bronchial (Fig. 6).
Discussion and conclusions
SLE was proved to be a typical autoimmune disease and the damage to B cell tolerance checkpoints may be the main reason for the production of auto-antibodies. Antibodies, as secretory Immunoglobulin (Ig), are produced by B lymphocytes and are Y-type protein complexes composed of IgH and IgL pairs connected by disulfide bonds. IgH and IgL have variable and constant regions, respectively, in which the variable region specifically recognizes and binds antigens. Variable region coding genes are produced by V (D) J recombination. In this case, we found the DAH as the first episode symptom of cSLE with genetic compound heterozygous mutation in PRKDC gene. PKCDC regulated apoptosis and proliferation of B cells and it had been reported that mutation of PRKDC can cause type 26 immune deficiency (Table 2), mainly manifested by impaired differentiation of T and B cells, due to DNA-PKCs deficiency and V(D)J rearrangement obstruction [12].
At the early stage of the disease, we found that the patient presented with nervous system lesions such as dysphoria, irritability, hypertonia and other neurological manifestations which was indicated as brain atrophy by MRI. After treatment with MP and cyclophosphamide, the above symptoms disappeared and brain atrophy resumed. In this patient, Brain atrophy may be associated with the development of symptoms of lupus of the nervous system. A Kalinowska-Lyszczarz reported that the same classifiers were identified in a subgroup analysis that included patients with a short disease duration. In SLE brain atrophy was the main determinant of brain volume. Different correlation patterns between volumetric and clinical data may suggest that is mostly associated with age in SLE.
DNA PKcs-the key partners of autoimmune regulator play an important role in regulating human autoimmune responses, especially in systemic lupus erythematosus. Anne-laure Mathieu found that PRKDC mutation reduced the level of DNA-PKCs, causing autoimmune diseases [9]. In addition, the accumulation of anti-Ku and DNA-PKCs antibodies related to the non-homologous DNA end-joining pathway were detected in SLE patients with PRKDC polymorphism [13,14,15]. Above all, we suggested the PRKDC mutation can causes the occurrence of SLE.
Conclusions
Here we described the case of an infant immunodeficiency with SLE like- syndrome, which cause by PRKDC mutation, treated successfully with high-dose MP and CTX. The novel compound heterozygous mutation of PRKDC may be relate to dysregulates B-cell proliferation, promotes auto-antibody formation.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
19 August 2023
Child’s eyes in figure 1 have been blurred out.
References
Wu CH, Chen CA, Lin SH, et al. Increased risk of early-onset childhood systemic lupus erythematosus for children born to affected parents: a nationwide child-parent cohort study[J]. Front Immunol. 2022;13:966809.
Rotstein GI, Pinto NF, Lobo A, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in patients with childhood systemic lupus erythematosus: a real-world interventional multi-centre study[J]. Lupus. 2020;29(8):934–42.
Reddy HG, Maynes EJ, Saxena A, et al. Utilization of extracorporeal life support for diffuse alveolar damage and diffuse alveolar hemorrhage: a systematic review[J]. Artif Organs. 2021;45(6):559–68.
Jiang M, Chen R, Zhao L, et al. Risk factors for mortality of diffuse alveolar hemorrhage in systemic lupus erythematosus: a systematic review and meta-analysis[J]. Arthritis Res Ther. 2021;23(1):57.
Xu T, Zhang G, Lin H, et al. Clinical characteristics and risk factors of diffuse alveolar hemorrhage in systemic Lupus Erythematosus: a systematic review and Meta-analysis based on Observational Studies[J]. Clin Rev Allergy Immunol. 2020;59(3):295–303.
Al-Adhoubi NK, Bystrom J. Systemic lupus erythematosus and diffuse alveolar hemorrhage, etiology and novel treatment strategies[J]. Lupus. 2020;29(4):355–63.
Casey JP, Nobbs M, McGettigan P, et al. Recessive mutations in MCM4/PRKDC cause a novel syndrome involving a primary immunodeficiency and a disorder of DNA repair[J]. J Med Genet. 2012;49(4):242–5.
Kurosawa A. Autophosphorylation and Self-Activation of DNA-Dependent protein Kinase[J]. Genes (Basel), 2021,12(7).
Mathieu AL, Verronese E, Rice GI, et al. PRKDC mutations associated with immunodeficiency, granuloma, and autoimmune regulator-dependent autoimmunity[J]. J Allergy Clin Immunol. 2015;135(6):1578–88.
Cowan MJ, Gennery AR. Radiation-sensitive severe combined immunodeficiency: the arguments for and against conditioning before hematopoietic cell transplantation–what to do?[J]. J Allergy Clin Immunol. 2015;136(5):1178–85.
Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors[J]. Nature. 2007;449(7161):473–7.
Abdul-Razak HH, Rocca CJ, Howe SJ, et al. Molecular evidence of genome editing in a mouse model of Immunodeficiency[J]. Sci Rep. 2018;8(1):8214.
Spielmann L, Nespola B, Severac F, et al. Anti-Ku syndrome with elevated CK and anti-Ku syndrome with anti-dsDNA are two distinct entities with different outcomes[J]. Ann Rheum Dis. 2019;78(8):1101–6.
Adarsh MB, Kavadichanda CG, Shah S, et al. Anti Ku antibody is associated with haematological manifestations but not with overlap features in systemic lupus erythematosus[J]. Clin Exp Rheumatol. 2021;39(Suppl 128):3–4.
van der Spek J, Groenwold RH, van der Burg M, et al. TREC based newborn screening for severe combined immunodeficiency disease: a systematic Review[J]. J Clin Immunol. 2015;35(4):416–30.
Acknowledgements
Not applicable.
Funding
This work was supported by the Basic and applied basic research foundation of guangdong province enterprise mutual funds on the project, (grant number 2021A1515220043) and Zhongnanshan Medical Foundation of Guangdong Province (grant number 202102010343) and the Science and Technology Program of Guangzhou (grant number 202102010276).
Author information
Authors and Affiliations
Contributions
LHW and ZYW analyzed clinical and FC data analysis of patient immune function; LHW offered the T and B cell receptor sequencing to detect the V(D)J rearrangement of the patient; ZBY and summarized clinical data and interpretation: DHC was responsible for writing articles; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Patient consent for publication
The research proposal for human menstrual blood collection was approved by the Ethics Committee of The first hospital affiliated to Guangzhou Medical University (approval no.20220514). All volunteers participating in the experiment signed the informed consent.
Ethics approval and consent to participate
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, H., Zhang, Y., Zhang, B. et al. A novel PRKDC mutation caused B lymphocytes V(D)J rearrangement disorder in the SLE-DAH like symptoms patient. Pediatr Rheumatol 21, 84 (2023). https://doi.org/10.1186/s12969-023-00840-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-023-00840-9