Abstract
Objectives
To determine the influence of HLA-B27 positivity on risk of developing chronic nonbacterial osteomyelitis (CNO).
Methods
HLA-B*27 genotype was assessed in 3 European CNO populations and compared with local control populations (572 cases, 33,256 controls). Regional or whole-body MRI was performed at diagnosis and follow-up in all cases which reduces the risk of disease misclassification. Genotyping was performed using either next generation DNA sequencing or PCR based molecular typing. Statistical analysis used Fisher’s exact test with Bonferroni correction and a fixed effects model for meta-analysis of odds ratios.
Results
HLA-B*27 frequency was higher in all 3 populations compared with local controls (combined odds ratio (OR) = 2.2, p-value = 3 × 10–11). This association was much stronger in male compared with female cases (OR = 1.99, corrected p-value = 0.015). However, the HLA-B*27 status was not statistically significantly associated with co-occurrence of psoriasis, arthritis or inflammatory bowel disease.
Conclusion
Carriage of HLA-B*27 is associated with greater risk of developing CNO, particularly in male cases.
Similar content being viewed by others
Introduction
Chronic nonbacterial osteomyelitis (CNO) is a rare autoinflammatory bone disease (OMIM 259,680), predominantly affecting children and adolescents with an estimated prevalence of 1 per 105–106 [1]. It is characterised by relapsing episodes of localised bone inflammation, most commonly affecting long bone metaphyses, clavicles, vertebrae and pelvis [1]. Long-term sequelae can include psychosocial complications, scoliosis and leg-length discrepancy [1]. It is associated with other inflammatory conditions, in particular inflammatory arthritis, psoriasis and inflammatory bowel disease, although the reported co-occurrence of these conditions varies widely [1,2,3].
HLA-B*27 testing is recommended in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) consensus treatment and monitoring recommendations due to the possible association between CNO and HLA-B*27 associated inflammatory disease [4].
There is controversy regarding the role of HLA-B*27 in CNO. While an association was proposed almost 20 years ago, many subsequent studies fail to replicate this. However, of the 17 studies both supporting and refuting this association, 14 involved HLA-B*27 testing in 43 or fewer participants (range 6 – 43) [3, 5,6,7,8,9,10]. Three studies involved HLA-B*27 typing in 72, 86 and 163 participants; none showed an association with CNO [1, 11, 12]. In addition, the control population data and ethnicity of participants is not reported in all studies; ethnicity may be of particular relevance when looking at small studies. Therefore, the relationship between HLA-B*27 and CNO remains unclear.
In this study we examined HLA-B*27 status in three European CNO populations compared with local control populations. We also assessed whether HLA-B*27 was associated with major demographic or clinical variables. Our data reveal this variant to be a risk allele for CNO, particularly in male cases.
Patients and methods
Study population
Three European cohorts of children with CNO were included in the study. The Irish cohort included children attending the National Centre for Paediatric Rheumatology in Children’s Health Ireland between September 2018 and December 2021. The Bristol cohort included children who attended Bristol Children’s Hospital between 2010 and 2021. All patients in the Irish and Bristol cohorts met the Bristol criteria for CNO. All MRI scans were assessed and reported on by a radiologist as part of the diagnostic process, independent of this study. The German National Paediatric Rheumatologic Database (NPRD) includes patients from over 60 centres in Germany and Austria. Since 2009, it has gathered clinical and sociodemographic information on CNO from clinicians and patients using standardised questionnaires. A confirmed diagnosis of CNO by an expert was required for inclusion in the NPRD. Confirmation of diagnosis for the NPRD was based on clinically symptomatic inflammatory lesions with the exclusion of other relevant diagnoses as described in the previously published one-year outcome data, with diagnosis confirmed by the same authors (CR, KM, HG) for all cases [13]. The same methodology was applied to any additional cases recruited from 2018–2020 which were included in this study but not described in that paper [13]. CNO data recorded in the NPRD between 2016 and 2020 were included in this study with the most recent entry extracted for each patient.
Ethics approval
The study received ethics approval from Children's Health Ireland at Crumlin (GEN/572/17) and Charite Medical University of Berlin (NPRD). Data collection for this study received an ethics exemption from Bristol University Hospital (UK). Informed consent/assent was obtained locally at CHI and NPRD sites.
Data collection
Detailed demographic, clinical, laboratory and radiological data were recorded on all patients. MRI diagnosis of lesions was based on the presence of bone marrow hyperintensity, bony expansion, soft tissue hyperintensity and complications such as vertebral compression or pathological fracture and the exclusion of alternative diagnoses such as enthesitis-related arthritis (ERA). Whole-body MRI data was included for all participants in the Irish and Bristol cohorts but was not available from the NPRD cohort. Ethnicity was recorded in the Irish and Bristol cohorts but not in the NPRD cohort. Ethnicity was self-reported in response to an open question.
HLA typing in the Bristol and NPRD cohorts
Molecular HLA-B*27 typing using polymerase chain reaction (PCR) was performed on patients attending tertiary paediatric rheumatology services at centres participating in the NPRD at the treating clinician’s discretion. All patients attending Bristol Children’s Hospital (UK) are being tested using PCR typing and HLA-B*27 typing is being requested on all patients as part of routine phlebotomy regardless of the site of CNO lesion or co-morbidities. Patients who had HLA-B*27 testing completed prior to 31.3.2022 were included in this study.
HLA prediction in the Irish cohort
Whole exome sequencing was performed on genomic DNA from whole blood as part of an ongoing study. Samples were sent to either University of Leeds Next Generation Sequencing laboratory or Novogene UK for whole exome sequencing using Illumina HiSeq 3000 and NovaSeq 6000, respectively, with 150 bp paired-end reads. Reads were aligned to the hg19 reference genome using BWA software, duplicates removed using Picard tools and GATK software used to realign indels and call variants. HLA alleles were predicted from whole exome sequencing results using Optitype software [14] through the Nextflow nf-core/hlatyping pipeline (version 1.1.5). Molecular PCR-based HLA typing in the same cohort was performed at the treating clinician’s discretion.
Population control data
HLA imputed data was obtained from the UK Biobank (project number 80917) [15]. HLA imputation from single nucleotide polymorphism (SNP) data was performed in the UK Biobank as previously described using the HLA*IMP:02 algorithm [15, 16]. Self-reported ethnicity was used to match the Irish and Bristol cohorts with an appropriate UK Biobank control population; all cases and controls for the Irish cohort were ethnically Irish while the proportion of different ethnicities in the Bristol cohort were matched to the UK Biobank data. Data from the German Bone Marrow Registry publicly available on the Allele Frequency Net Database (AFND), accessed at allelefrequencies.net [17], was used as control data for the NPRD cohort. HLA typing on this cohort used reverse sequence specific oligonucleotide probe (SSOP) and included participants are those who self-report as German living in Germany.
Statistical analysis
Statistical analysis was performed in Rstudio (version 1.1.456). Fisher’s exact test was performed to determine odds ratios (OR) and 95% confidence interval (95% CI) using R package stats (version 4.1.0). Bonferroni correction was used to correct for multiple testing. Heterogeneity between study populations was tested using Cochrane’s Q test and I2 statistic. Significant heterogeneity was defined as a Q test p-value < 0.01 and/or an I2 > 50%; a fixed effect model is appropriate for meta-analysis if there is a lack of significant heterogeneity. A fixed effects model was used for the meta-analysis of odds ratios using R package meta (version 5.2.0).
Patient and public involvement
Patients were not involved in the study design. A patient research partner was involved in developing plain English information regarding the Irish arm of the study. After publication, results will be disseminated through a plain English newsletter, co-edited by a patient research partner.
Results
Detailed phenotypic data from the NPRD [13] and Irish cohorts [18] were previously published; all cohorts are similar to previously published CNO cohorts in terms of median age of onset, female predominance, multifocal disease and co-morbidities. Phenotypic data for the cohorts is summarised in Table 1.
All patients in the Irish and Bristol cohorts underwent whole-body MRI. CNO lesions were most frequently identified in the long bones of the lower limbs in all cohorts; the distribution of lesions in the Irish and Bristol cohorts is shown in Table 2. In the Irish and Bristol cohorts, 0/9 and 6/8 of those with inflammatory arthritis had axial involvement, respectively. 2/8 in the Bristol cohort met the criteria for enthesitis-related arthritis (ERA) during follow-up; these patients met criteria for CNO clinically and radiologically but subsequently developed sacroiliitis clinically and on imaging. None of the Irish cohort met the criteria for ERA. In those with axial arthritis/ERA in these cohorts, all had MRI signs consistent with CNO at sites distant from entheseal points, in addition to lesions at sites not classically associated with ERA such as clavicular or mandibular lesions. Those with peripheral arthritis had clinical or radiologic signs of synovitis distant from any documented bony lesion.
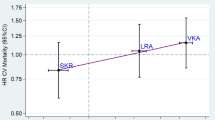
In the Irish cohort, HLA class I prediction was successful from all 50 WES samples. Molecular HLA-B*27 typing was performed in 45/50 of this cohort and agreed with OptiType prediction in all cases. Meta-analysis of the pooled odds ratios from the three European cohorts is shown in Fig. 1.
HLA-B*27 was not associated with an increased risk of psoriasis or inflammatory bowel disease associated with CNO after a median of 2.5 years of follow-up. There was a non-significant trend toward association with an increased risk of inflammatory arthritis. However, there was a statistically significant association between HLA-B*27 and CNO in male compared with female patients (Table 3).
The previously reported association between HLA-B*27 and CNO affecting bones of the foot [13] was not replicated in the Irish and Bristol cohorts, although there was a non-significant trend toward association in the Irish cohort.
Discussion
This is the first multicentre study to describe a possible genetic risk factor in sporadic CNO. HLA-B*27 is known to be associated with an increased risk of inflammatory diseases including uveitis and spondyloarthritis with the HLA-B*27 positive subtype of these diseases more frequently associated with male sex. HLA-B*27 is associated with a more chronic and extensive disease course in male children and adolescents with enthesitis-related arthritis (ERA) and with a better response to anti-TNF treatment in ankylosing spondylitis [19, 20]. In acute anterior uveitis, differing cellular infiltrates were recently identified in the aqueous humor of HLA-B*27-positive compared to HLA-B*27-negative patients [21].
The relationship between CNO and HLA-B*27-associated inflammatory disease, particularly enthesitis-related arthritis (ERA) or ankylosing spondylitis, is unclear. An association between CNO and inflammatory arthritis affecting a joint distant from the bony lesion has been reported in several studies [3, 12, 18]. However, most studies do not state whether the arthritis is axial or peripheral. Furthermore, in CNO-associated arthritis, particularly those meeting diagnostic criteria for ERA, there is a risk that MRI signs of enthesitis could mimic CNO lesions leading to misclassification. Individual-level MRI results and detailed phenotypic data were reviewed for all patients in the Irish and Bristol cohorts to mitigate the risk of bias being introduced in this way. Although detailed MRI data was not available from the NPRD cohort for this study, data from participants which included the subset described here were reviewed by three authors of a previous study in order to confirm the diagnosis of CNO [13]. Many rheumatologists believe that CNO and the related condition Synovitis Acne Pustulosis Hyperostosis Osteitis (SAPHO) syndrome belong to the same spectrum of disease as ERA/ankylosing spondylitis [22] with many current CNO treatments extrapolated from ERA treatment pathways [23]. The trend toward association between HLA-B*27 positive CNO and inflammatory arthritis seen in this study requires longer term follow-up to determine disease evolution over time. This follow-up is ongoing and may add clarity to the relationship between CNO and ERA/ankylosing spondylitis.
Recent data from the 1-year follow up of the German NPRD showed a statistically significant association between HLA-B*27 and both involvement of the bones of the foot and progression to second-line treatment with a disease modifying antirheumatic drug (DMARD) during the first 12 months of follow-up [13]. These findings were not replicated in the other two cohorts in this study. It is important to note, however, that the absence of consensus treatment plans prior to 2018 [4], with resultant differences in local prescribing practices, makes it difficult to interpret the rate of progression to second-line treatment between different centres.
One limitation of this study is the relatively short duration of follow-up. This is of particular importance in terms of establishing whether there are differences in disease course dependent on HLA-B*27 status. In addition, it is well established that the rate of HLA-B*27 varies significantly between ethnic groups. Therefore, the use of a control population based on ethnicity rather than geographic location for the NPRD cohort would be preferable. Patients from the NPRD and Bristol cohorts were included on the basis of completed HLA-B*27 typing. While no clear phenotypic differences were seen between this subset and the overall CNO population in these centres, this is another limitation of the study. Testing in the Bristol cohort was performed irrespective of the sites involved in CNO or presence of co-morbidities. However, testing in the NPRD cohort was determined by the treating clinician and, therefore, may have resulted in selection bias. Within the Irish cohort, the 50 who responded to the invitation to participate did not differ in terms of demographics or phenotype from those who did not respond.
Conclusion
Further research is required to establish whether the HLA-B*27 positive subtype of CNO is phenotypically different from HLA-B*27 negative disease. Based on other HLA-B*27-associated diseases, there may be differences in treatment response dependent on HLA-B*27 status. It would be of interest to establish whether the inflammatory infiltrate differs from HLA-B*27 negative CNO. A comparative study of bone biopsy samples may help to establish whether HLA-B*27 positive CNO is a pathologically distinct disease, as is the case in acute anterior uveitis [21]. Overall, this study supports consistent inclusion of HLA-B*27 typing in patients with CNO in order to better understand its role in disease pathogenesis.
Availability of data and materials
Data are available upon reasonable request. Deidentified datasets are available from Dr O’Leary (ORCID 0000–0001-9201–8221). Control population data from the UK Biobank is available by applying to directly to the UK Biobank.
Abbreviations
- CNO:
-
Chronic nonbacterial osteomyelitis
- NPRD:
-
National Paediatric Rheumatologic Database
- AFND:
-
Allele Frequency Net Database
- ERA:
-
Enthesitis related arthritis
- DMARD:
-
Disease modifying antirheumatic drug
- CARRA:
-
Childhood Arthritis and Rheumatology Research Alliance
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SAPHO:
-
Synovitis Acne Pustulosis Hyperostosis Osteitis
References
Wipff J, Costantino F, Lemelle I, Pajot C, Duquesne A, Lorrot M, et al. A large national cohort of French patients with chronic recurrent multifocal osteitis. Arthritis Rheumatol. 2015;67(4):1128–37.
Schnabel A, Range U, Hahn G, Berner R, Hedrich CM. Treatment response and longterm outcomes in children with chronic nonbacterial osteomyelitis. J Rheumatol. 2017;44(7):1058–65.
Skrabl-Baumgartner A, Singer P, Greimel T, Gorkiewicz G, Hermann J. Chronic non-bacterial osteomyelitis: a comparative study between children and adults. Pediatr Rheumatol Online J. 2019;17(1):49.
Zhao Y, Wu EY, Oliver MS, Cooper AM, Basiaga ML, Vora SS, et al. Consensus Treatment Plans for Chronic Nonbacterial Osteomyelitis Refractory to Nonsteroidal Antiinflammatory Drugs and/or With Active Spinal Lesions. Arthritis Care Res. 2018;70(8):1228–37.
Miettunen PM, Wei X, Kaura D, Reslan WA, Aguirre AN, Kellner JD. Dramatic pain relief and resolution of bone inflammation following pamidronate in 9 pediatric patients with persistent chronic recurrent multifocal osteomyelitis (CRMO). Pediatr Rheumatol Online J. 2009;7:2.
Kaiser D, Bolt I, Hofer M, Relly C, Berthet G, Bolz D, et al. Chronic nonbacterial osteomyelitis in children: a retrospective multicenter study. Pediatr Rheumatol. 2015;13(1):25.
Girschick HJ, Raab P, Surbaum S, Trusen A, Kirschner S, Schneider P, et al. Chronic non-bacterial osteomyelitis in children. Ann Rheum Dis. 2005;64(2):279–85.
Concha S, Hernández-Ojeda A, Contreras O, Mendez C, Talesnik E, Borzutzky A. Chronic nonbacterial osteomyelitis in children: a multicenter case series. Rheumatol Int. 2020;40(1):115–20.
Handrick W, Hörmann D, Voppmann A, Schule R, Reichardt P, Tröbs RB, et al. Chronic recurrent multifocal osteomyelitis - Report of eight patients. Pediatr Surg Int. 1998;14(3):195–8.
Job-Deslandre C, Krebs S, Kahan A. Chronic recurrent multifocal osteomyelitis: Five-year outcomes in 14 pediatric cases. Jt Bone Spine. 2001;68(3):245–51.
Jansson A, Renner ED, Ramser J, Mayer A, Haban M, Meindl A, et al. Classification of non-bacterial osteitis: Retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology. 2007;46(1):154–60.
Girschick H, Finetti M, Orlando F, Schalm S, Insalaco A, Ganser G, et al. The multifaceted presentation of chronic recurrent multifocal osteomyelitis: a series of 486 cases from the Eurofever international registry. Rheumatology (Oxford). 2018;57(7):1203–11.
Reiser C, Klotsche J, Hospach A, Berendes R, Schnabel A, Jansson AF, et al. First-year follow-up of children with chronic nonbacterial osteomyelitis—an analysis of the German National Pediatric Rheumatologic Database from 2009 to 2018. Arthritis Res Ther. 2021;23(1):281.
Szolek A, Schubert B, Mohr C, Sturm M, Feldhahn M, Kohlbacher O. OptiType: Precision HLA typing from next-generation sequencing data. Bioinformatics. 2014;30(23):3310–6.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nat. 2018;562(7726):203–9.
Dilthey AT, Gourraud P-A, Mentzer AJ, Cereb N, Iqbal Z, McVean G. High-Accuracy HLA Type Inference from Whole-Genome Sequencing Data Using Population Reference Graphs. Franke A, editor. PLOS Comput Biol. 2016;12(10):e1005151.
Gonzalez-Galarza FF, McCabe A, Dos SEJM, Jones J, Takeshita L, Ortega-Rivera ND, et al. Allele frequency net database (AFND) 2020 update: gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Res. 2020;48(D1):D783–8.
O’Leary D, Wilson AG, MacDermott EJ, Lowry C, Killeen OG. Variability in phenotype and response to treatment in chronic nonbacterial osteomyelitis; the Irish experience of a national cohort. Pediatr Rheumatol. 2021;19(1):1–8.
Berntson L, Nordal E, Aalto K, Peltoniemi S, Herlin T, Zak M, et al. HLA-B27 predicts a more chronic disease course in an 8-year followup cohort of patients with juvenile idiopathic arthritis. J Rheumatol. 2013;40(5):725–31.
Baraliakos X, Koenig AS, Jones H, Szumski A, Collier D, Bananis E. Predictors of Clinical Remission under Anti-tumor Necrosis Factor Treatment in Patients with Ankylosing Spondylitis: Pooled Analysis from Large Randomized Clinical Trials. J Rheumatol. 2015;42(8):1418–26.
Kasper M, Heming M, Schafflick D, Li X, Lautwein T, ZuHorste MM, et al. Intraocular dendritic cells characterize HLA-B27-associated acute anterior uveitis. Elife. 2021;1(10):67396.
Furer V, Kishimoto M, Tsuji S, Taniguchi Y, Ishihara Y, Tomita T, et al. The Diagnosis and Treatment of Adult Patients with SAPHO Syndrome: Controversies Revealed in a Multidisciplinary International Survey of Physicians. Rheumatol Ther. 2020;7(4):883.
Sinnappurajar P, Roderick M, Ramanan AV. The neglected and untreated pains of CRMO and SAPHO syndrome. Rheumatology (Oxford). 2022;61(9):3509–10.
Acknowledgements
We would like to thank all the families who participated through the NPRD, Children’s Health Ireland and Bristol Royal Children’s Hospital.
We are grateful to the NPRD/ GKJR study group for their participation. Special thanks go to Martina Niewerth, Nadine Grösch and Jana Hörstemann for their careful supervision and maintenance of the registry. We thank all physicians, who enrolled patients more than 10 patients: Hermann Girschick: Vivantes Klinkum Friedrichshain, Klinik für Kinder- und Jugendmedizin; Christiane Reiser: Landeskrankenhaus Bregenz, Abteilung für Kinder- u. Jugendheilkunde; Ralf Trauzeddel: Helios Klinikum Berlin-Buch, Klinik für Kinder- und Jugendmedizin, Fachambulanz Kinderrheumatologie; Tilmann Kallinich: Universitätsmedizin Berlin—Charité, Campus Virchow-Klinikum, Otto-Heubner-Centrum für Kinder- und Jugendmedizin, Sozialpädiatrisches Zentrum—Rheumaambulanz; Carl Thiem Klinikum Cottbus, Klinik für Kinder- und Jugendmedizin; Antje Nimtz-Talaska: Kinderarztpraxis; Frank Dressler: Medizinische Hochschule Hannover, Kinderklinik, Rheumatologische Ambulanz; Daniel Windschall: St. Josef-Stift Sendenhorst, Abt. Kinder- und Jugendrheumatologie; Maria Haller: Kinderarztpraxis; Markus Hufnagel: Universitätsklinikum Freiburg, Zentrum für Kinder- und Jugendmedizin, Sektion Päd. Infektiologie und Rheumatologie; Georg Leipold: Gemeinschaftspraxis Kinder- und Jugendärzte; Almut Meyer-Bahlburg: Universitätsmedizin Greifswald, KöR, Abt. Allgemeine Pädiatrie, Pädiatrische Rheumatologie und Immunologie; Michael Borte: Städtisches Klinikum St. Georg, Klinik für Kinder- und Jugendmedizin, Ambulanz für Immunologie, Infektiologie, Rheumatologie; Ute Derichs: Johann-Gutenberg-Universität Mainz, Zentrum für Kinder- und Jugendmedizin, Immunologie / Rheumatologie; Kirsten Mönkemöller: Kinderkrankenhaus der Stadt Köln, Kinder- und Jugendmedizin, Ambulanz Nephrologie/ Rheumatologie; Gerd Horneff: Asklepios Kinderklinik St. Augustin, Zentrum für Allgemeine Pädiatrie und Neonatologie; Johannes-Peter Haas: Deutsches Zentrum für Kinder- und Jugendrheumatologie (Garmisch-Partenkirchen); Rainer Berendes: Kinderkrankenhaus St. Marien, Kinderrheumatologie; Reinhard Berner: Universitätsklinikum Carl Gustav Carus, Klinik und Poliklinik für Kinder- und Jugendmedizin; Peggy Rühmer: Helios Vogtland-Klinikum Plauen, Fachambulanz der Klinik für Kinder- und Jugendmedizin; Georg Heubner: Städtisches Klinikum Dresden-Neustadt, Klinik für Kinder- und Jugendmedizin; Toni Hospach: Olgahospital Stuttgart, Klinik für Kinderheilkunde und Jugendmedizin, Kinderrheumatologie; Regina Hühn: Martin-Luther-Universität Halle-Wittenberg; Jürgen Grulich-Henn: Universitätsklinikum Heidelberg, Zentrum für Kinder- und Jugendmedizin—Kinderheilkunde I, Ambulanz für Kinder- und Jugendrheumatologie; Frank Weller-Heinemann: Klinikum Bremen Mitte—Professor-Hess-Kinderklinik; Axel Sauerbrey: Helios Klinikum Erfurt, Klinik für Kinder- und Jugendmedizin; Claudia Koch: Universitätsklinikum Ulm, Klinik für Kinder- und Jugendmedizin, Spezialambulanz Rheumatologie und Autoimmunerkrankungen; Betina Rogalski: Kinderrheumatologische Privatpraxis.
Funding
The National Pediatric Rheumatological Database was funded by the German Children Arthritis Foundation (Deutsche Kinder-Rheumastiftung), AbbVie, Chugai, Pfizer, GSK and.
Novartis. Dr O’Leary’s work was funded by the National Children’s Research Centre, Dublin, Ireland (grant code G/17/1) and the AbbVie SOBI Nordic Pharma Newman Fellowship in Rheumatology through the UCD Newman Foundation.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. DO’L had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: DO’L, AR, MR, OGK, AGW. Acquisition of data: DO’L, ZM, DAJ, JK, KM, MR, AR, OGK, AGW. Analysis and interpretation of data: DO’L, DAJ, JK, KM, MR, AR, OGK, AGW.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study received ethics approval from Children's Health Ireland at Crumlin—GEN/572/17 (Ireland) and Charite Medical University of Berlin (NPRD, Germany). Data collection for this study received an ethics exemption from Bristol University Hospital (UK). Informed consent/assent was obtained locally at CHI and NPRD sites.
Consent for publication
Not applicable.
Competing interests
No potential conflicts of interest relevant to this article were reported by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
O’Leary, D., Al Julandani, D.A., Zia, M. et al. HLA-B*27 is associated with CNO in a European cohort. Pediatr Rheumatol 21, 52 (2023). https://doi.org/10.1186/s12969-023-00826-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-023-00826-7