Abstract
The global pandemic of coronavirus disease 2019 (COVID-19) caused by infection with severe acute respiratory suyndrome coronavirus 2 (SARS-CoV-2) is now entering its 4th year with little evidence of abatement. As of December 2022, the World Health Organization Coronavirus (COVID-19) Dashboard reported 643 million cumulative confirmed cases of COVID-19 worldwide and 98 million in the United States alone as the country with the highest number of cases. While pneumonia with lung injury has been the manifestation of COVID-19 principally responsible for morbidity and mortality, myocardial inflammation and systolic dysfunction though uncommon are well-recognized features that also associate with adverse prognosis. Given the broad swath of the population infected with COVID-19, the large number of affected professional, collegiate, and amateur athletes raises concern regarding the safe resumption of athletic activity (return to play, RTP) following resolution of infection. A variety of different testing combinations that leverage the electrocardiogram, echocardiography, circulating cardiac biomarkers, and cardiovascular magnetic resonance (CMR) imaging have been proposed and implemented to mitigate risk. CMR in particular affords high sensitivity for myocarditis but has been employed and interpreted non-uniformly in the context of COVID-19 thereby raising uncertainty as to the generalizability and clinical relevance of findings with respect to RTP. This consensus document synthesizes available evidence to contextualize the appropriate utilization of CMR in the RTP assessment of athletes with prior COVID-19 infection to facilitate informed, evidence-based decisions, while identifying knowledge gaps that merit further investigation.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Overview of COVID-19 and myocardial inflammation
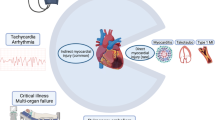
Coronavirus disease 19 (COVID-19) infection can result in a varying severity of manifestations affecting multiple organ systems. While respiratory illness is the most common clinical manifestation of COVID-19, cardiovascular involvement can also occur. Cardiovascular manifestations associated with COVID-19 include myocardial infarction [1], myocarditis [2], arrhythmia [3], and stress cardiomyopathy [4]. Cardiac biomarker (troponin) elevation is a commonly reported abnormality in COVID-19, occurring in 20–36% of patient hospitalized with COVID-19, and is associated with greater disease severity including need for mechanical ventilation and increased risk of death [5, 6]. The underlying pathophysiologic mechanism of troponin elevation is incompletely understood and is likely multifactorial in etiology resulting from systemic illness and upregulation of systemic inflammatory and prothrombotic pathways [7, 8]. While myocarditis may be suspected in patients with elevated cardiac biomarkers and there is an association between COVID-19 infection and myocarditis, it is important to note that direct viral infection of the myocardium caused by COVID-19 has been uncommonly confirmed by histologic analyses [9]. For example, in an autopsy study of 39 COVID-19 infected patients in Germany, 62% had evidence of the viral genome within the heart, though findings did not meet histopathologic criteria (i.e., inflammatory infiltrate) for myocarditis [10]. That said, a more recent report did convincingly show evidence of cardiomyocyte COVID-19 infection with resultant cardiac injury and increased macrophage abundance [11]. In this context, while prior studies have shown troponin elevation to correlate with severity of illness and extent of COVID-19 viremia [7, 12, 13], it is unknown whether troponin release simply mirrors disease severity or has mechanistic implications for worsened prognosis.
Regarding severity of illness, COVID-19 infection can result in a wide spectrum of disease manifestations ranging from no symptoms to critical illness and can be grouped into the following categories [14]: 1. Asymptomatic or pre-symptomatic (no signs or symptoms of infection despite positive severe acute respiratory syndrome coronavirus 2 (SAR-CoV-2) virologic test), 2. Mild illness (upper respiratory infection and other mild symptoms without shortness of breath or abnormal chest imaging), 3. Moderate illness (lower respiratory disease on clinical or imaging assessment and oxygen saturation (SpO2) ≥ 94%), 4. Severe illness (SpO2 < 94%, partial pressure oxygen (PaO2)/FiO2 < 300 mmHg, respiratory rate > 30 breaths/min, or lung infiltrates > 50%) and 5. Critical illness (respiratory failure, septic shock and/or multiple organ dysfunction). Whereas some COVID-19 survivors recover quickly, others have a more prolonged course of illness due to persistent symptoms (Long COVID syndromes which are now collectively referred to as Post-Acute Sequelae of SARS-CoV-2 infection [PASC]) [15]. For example, among 143 patients with resolved COVID-19 (2 negative polymerace chain reaction (PCR) tests) who required hospitalization, 87% had at least one ongoing cardiopulmonary symptom – including fatigue (53%), dyspnea (43%), and chest pain (22%), and nearly half (44%) had worsened quality of life (QOL) at 60 days after acute infection [16]. While wide variability in time to symptom resolution has been reported, recovery time appears to be associated with pre-existing risk factors as well as severity of acute COVID-19 illness [16,17,18].
Imaging of cardiac involvement
Cardiovascular imaging plays an important role in the evaluation of COVID-19 patients with suspected cardiac involvement. Transthoracic echocardiogram (TTE) evidenced left ventricular (LV) and right ventricular (RV) dysfunction has been commonly reported in acute COVID-19 infection requiring hospitalization, occurring in up to 41% and 15% of affected patients, respectively [19]. Such TTE evidence of ventricular contractile dysfunction during acute infection has been shown to provide incremental prognostic utility to clinical and biomarker indices. In convalescent patients with COVID-19, patients with LV and RV dysfunction on initial TTE have shown improvement following recovery from acute illness [20, 21]. For example, on prospective TTE longitudinal follow up of 79 hospitalized patients with COVID-19 pneumonia, prevalence of RV and LV abnormalities decreased from 51 to 19% and 13% to 9% following recovery, respectively [21]. Similarly, in the World Alliance Societies of Echocardiography-COVID study, patients with impaired LV and RV longitudinal strain at baseline had significant improvement on follow up (LV: − 14.5% ± 2.9% vs. − 16.7% ± 5.2%, p < 0.001 and RV: − 15.2% ± 3.4% vs. − 17.4% ± 4.9%, p = 0.004, respectively) [20], supporting the general notion that the acute functional decline associated with infection is reversible in some patients in whom dynamic changes may be attributable to hemodynamic or other transient consequences of acute systemic illness. Further, there is likely a heterogeneity of pathologies responsible for contractile dysfunction, some of which are irreversible (i.e. myocyte necrosis in context of hypoperfusion) and others which are transient (i.e. stunning/contractile depression in context of acute systemic illness).
Cardiovascular magnetic resonance (CMR) is uniquely capable of characterizing myocardial tissue properties in-vivo, enabling assessment of pattern and functional sequelae of cardiac injury, including evaluation for myocardial edema present in myocarditis (Fig. 1). While CMR exams during acute COVID-19 illness have been less frequently performed owing to associated critical illness and concerns regarding patient monitoring and transmission during prolonged imaging, an initial case report described CMR abnormalities including LV dysfunction, abnormal T2 times consistent with interstitial edema, pericardial effusion and a non-ischemic pattern of diffuse late gadolinium enhancement (LGE) [2]. More recently, CMR abnormalities including impaired LV function via strain and high prevalence of myocardial edema on T2 weighted imaging (56%) have been described in 25 patients with acute COVID-19 infection who underwent imaging within 10 days of initial COVID-19 symptoms [22]. Supporting findings in prior longitudinal TTE studies that demonstrate functional recovery [20, 21], in this cohort, there was low prevalence of irreversible myocardial necrosis with only one patient demonstrating LGE (4%).
Representative cardiovascular magnetic resonance (CMR) examples of altered myocardial substrate in patients following acute coronavirus disease 2019 (COVID-19) infection. Note focal fibrosis (yellow arrows) accompanied by increased T2 (black arrows) on parametric mapping consistent with myocardial edema
Role of CMR in the evaluation of suspected acute myocarditis
Clinical presentations of acute inflammatory cardiomyopathy or myocarditis include acute coronary syndrome-like presentation, new-onset or worsening chronic heart failure, life-threatening arrhythmia, and cardiogenic shock [23]. A diagnosis of myocarditis is made using one or more diagnostic tests including electrocardiography (ECG), blood markers of myocardial injury (troponin-T or -I), endomyocardial biopsy, and cardiac imaging. Common cardiac imaging tests used to diagnose myocarditis include TTE (which can identify functional and anatomic sequelae) and CMR (which can concomitantly identify alterations in myocardial function, anatomy, and tissue properties). CMR is a key test in the contemporary assessment of patients with suspected myocarditis and is often used to establish the diagnosis owing to its unparalleled capacity to characterize myocardial tissue [23,24,25].
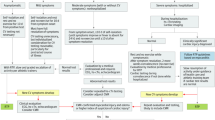
In 2009, an “International Consensus Group on CMR Diagnosis of Myocarditis” comprised of 22 experts published recommendations (dubbed the “Lake Louise Criteria” or LLC for where they met in Alberta, Canada) on the indications for CMR, the protocol, and analyses for the diagnosis of myocarditis [26]. The criteria were revised in 2018 to incorporate evidence that parametric mapping techniques (including native T1 and T2 mapping) could be used to identify myocarditis among patients with sufficient pre-test probability. The update was prompted by the development of contemporary CMR mapping techniques, allowing efficient measurement of myocardial native T1 and T2 relaxation times and several studies describing high sensitivity, specificity, and diagnostic accuracy of mapping techniques in the CMR assessment of suspected myocarditis [27]. The use of three integrated approaches involves LGE for highlighting focal myocardial injury, T1 mapping to identify diffuse myocardial fibrosis, and T2 mapping to reveal (diffuse) myocardial edema. The “JACC Scientific Expert Panel” comprised of 11 experts published updated criteria [27] (Fig. 2) recommending that CMR provides strong evidence for acute myocarditis if criteria in each of 2 categories were met:
-
1)
Abnormal T1-based marker for myocardial injury on T1 mapping – abnormal native (non-contrast) T1 or extracellular volume fraction (ECV) – or LGE imaging (in a non-ischemic pattern),
-
2)
Abnormal T2-based marker for myocardial edema on T2 mapping or T2-weighted imaging.
Overview of the updated Lake Louise Criteria [27]. ECV extracellular volume fraction, LGE late gadolinium enhancement, LV left ventricular, T2W T2 weighted.
Overview of T1 and T2 mapping in acute myocarditis
T1 and T2 relaxation times are determined by the tissue composition, interstitial and intra-cellular milieus, and external factors such as the magnetic field strength and the methods of measurement, including the hardware and the software platforms. Since T1 and T2 mapping techniques vary by the magnetic field, hardware, and pulse sequences used, the normal ranges of myocardial T1 and T2 values are derived from healthy individuals imaged locally on the same CMR scanner or equivalent systems. Abnormal T1 and T2 relaxation times (i.e. outside the normal range) help with the detection and diagnosis of myocardial pathology. Using T1 and T2 mapping techniques, global or regional myocardial T1 or T2 relaxation times can be obtained with pixel-level resolution. Myocardial ECV can be measured using pre-contrast and post-contrast T1 mapping and incorporating the hematocrit value. Recommendations and challenges in the clinical application of T1 and T2 mapping are covered in detail in a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) [28].
Abnormal native T1 and ECV suggest an expansion of the extracellular or interstitial space, which could occur globally or regionally due to various pathologies including acute myocardial inflammation and edema, vasodilation, hyperemia, and capillary leak, myocardial necrosis, and myocardial fibrosis. Abnormal native T1 may also reflect intracellular edema [28]. LGE also depicts extracellular pathology, and unlike native T1 or ECV, it generally reflects irreversible myocardial damage. This could be necrosis with accompanying inflammatory changes such as edema in the acute setting, or fibrosis in the chronic setting. Abnormal T2 is principally a marker for increased water content, either intracellularly or extracellularly, related to inflammation and edema.
CMR to diagnose COVID-19 myocardial involvement in athletes
CMR has been widely used to detect COVID-19 myocardial involvement in athletes with either COVID-19 or asymptomatic SARS-CoV-2 infection with great variation in application. The use of CMR has been routine in all athletes at some institutions, whereas others have employed a strategy of limited use of CMR to investigate abnormalities on other tests such as troponins, TTE, and ECG (termed “triad testing”). Using the updated LLC, a proportion of athletes have been diagnosed with COVID-19 myocardial involvement based on abnormalities on T1 and T2 mapping, often without LGE. It is important to emphasize that established CMR measures of cine volume and function or myocardial damage as identified by LGE are better validated than the more novel mapping techniques. Thus, the application of CMR and the updated LLC to diagnose COVID-19 myocardial involvement in athletes with either COVID-19 but no symptoms of myocarditis or asymptomatic SARS-CoV-2 infection, and its use in RTP decision-making requires nuance because of certain caveats enumerated below.
First, the limited specificity of the T1- and T2-mapping-based criteria in the updated LLC combined with the low prevalence of COVID-19 myocardial involvement in general—and particularly in young, previously healthy athletes—leads to a low positive predictive value for the criteria in this patient group. Second, most patients in the validation studies of myocarditis diagnosed on CMR have LGE, which has been validated more extensively than mapping abnormalities in histologically proven viral myocarditis [29, 30]. Third, while several prognostic studies have established an adverse prognostic significance for LGE in patients with myocarditis [31,32,33], including a study with > 10 years of follow-up [33], there are no prognostic data for abnormalities on T1 or T2 mapping in the absence of LGE. For ECV, there is at least one study describing its prognostic value in patients with suspected myocarditis, but ECV only maintained an independent association with outcomes independent of LGE at a relatively high ECV threshold (ECV > 0.35) [34]. Fourth, the literature validating the use of CMR for myocarditis using pathology and outcome data involves symptomatic patients with inflammation largely limited to the heart. Indeed, there are several papers on abnormalities describing T1 and/or T2 mapping independent of, or in the absence of, LGE in asymptomatic patients comprising a wide gamut of systemic inflammatory conditions including autoimmune diseases such as systemic lupus erythematosus [35,36,37] or systemic sclerosis [37,38,39], sarcoidosis [40,41,42], or infectious diseases such as human immunodeficiency virus (HIV) [43, 44]. Simultaneously, there are a dearth of data regarding T1 and/or T2 mapping abnormalities in the absence of LGE from the perspectives of pathology validation, natural evolution over time into cardiac damage in the form of necrosis and/or fibrosis, or prognostic implications. Finally, although rare, COVID-19 vaccine-related myocarditis shares similar reported CMR features with COVID-19 myocardial involvement in athletes, and occurs almost exclusively in adolescents and young adults [45,46,47], the same demographic as many athletes. Given the reported findings of T1 and/or T2 mapping abnormalities, without irreversible cardiac damage (e.g., LGE) in systemic inflammatory conditions, it is plausible that COVID-19, an infection featuring significant systemic inflammation, could be accompanied by myocardial inflammation without direct cardiac involvement by SARS-CoV-2 or irreversible damage, calling to question the prognostic significance of these findings.
Summary of published studies describing CMR in the recovered athlete
Early during the COVID-19 pandemic, several studies reported a high prevalence of cardiac involvement detected on CMR after recovery from COVID-19, even among patients who were initially asymptomatic or minimally symptomatic during the acute infection [48,49,50]. However, there was high variability among studies for presence of cardiac involvement (ranging 26–78% prevalence) and of methods used to quantify and report myocardial tissue characterization. The plethora of early data showing a high prevalence of myocardial abnormalities post infection led to heightened concern regarding the safety of athletes preparing to return to play (RTP) after COVID-19 infection. These early observations led to the initiation of clinical CMR studies specifically focused on athletes post infection to evaluate for myocardial injury.
As the pandemic continued, additional small, single-center observational studies of collegiate and professional athletes undergoing CMR assessment for RTP eligibility post COVID-19 infection reported variable prevalence of cardiac involvement by CMR (ranging from 0 to 100%, Table 1) [48, 51,52,53,54,55,56,57,58,59,60,61,62]. A more detailed review of the literature can be found in the American College of Cardiology Solution Set Oversight Committee’s Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play [63]. Again, in many of these studies, non-standardized methods were used for CMR-determined cardiac involvement and cardiac abnormalities did not meet LLC for myocarditis. Moreover, in the majority of these published studies, control groups of uninfected athletes were not included as a comparator. Thus, these studies provide little evidence on whether similar findings might be seen in myocardial remodeling in highly conditioned athletes [64] who had not previously had COVID-19 infection. Moreover, without meeting LLC, many studies reported findings which were not specific for myocarditis. A large study in professional athletes used a tiered testing approach consisting of the commonly employed “triad” testing of cardiac troponin, 12-lead ECG and TTE, with CMR performed only when clinically indicated or when suggested by abnormal initial testing. Using this strategy, cardiac involvement was found to be only 0.6% [65]. Finally, in a study of 147 COVID-19 positive athletes which did have athletic (N = 59) and healthy athletic controls (n = 56), CMR showed no differences in volumetric, functional or tissue characteristics between athletes with prior COVID-19 infection and matched healthy athletes. While 4.7% (n = 7) of COVID-19 positive athletes had findings consistent with myocarditis, none were asymptomatic [61].
More recently, several large cohort studies in athletes used the updated 2018 LLC [27] to determine cardiac involvement by CMR. Although the LLC was originally developed for the diagnosis of myocarditis in symptomatic patients, these criteria were adapted to ascertain whether athletes had definite, probable, or possible myocarditis post COVID-19, some of whom were asymptomatic. Important modifications to the LLC criteria included considering supplemental information such as reduced LV ejection fraction (LVEF) and pericardial involvement more strongly [60, 65]. Also, ensuring that T1 and T2 abnormalities colocalized in the same myocardial region was important in the reduction of variability and improvement of specificity. In a large cohort study of 1,597 athletes (Big Ten COVID-19 Cardiac Involvement registry), Daniels et al.reported a prevalence of 2.3% of clinical and subclinical myocarditis using the modified LLC definitions (Table 1) [66]. The most common CMR abnormalities detected were elevated T2 indicative of edema and non-ischemic patterns of LGE. However, the authors acknowledged limitations including the lack of standardized timing from COVID-19 infection to cardiac testing (discussed in detail below) and institutional differences in CMR interpretation.
Another large registry study, the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) [60] investigated cardiac involvement post-COVID-19 among competitive athletes using the same modified LLC approach. In this study, 198 athletes underwent primary CMR screening whereas another 119 underwent CMR only if clinically indicated per “triad” non-invasive screening or clinical judgement. The authors reported a low prevalence of cardiac involvement (ranging from 0.5% to 3.0%) and no adverse cardiac events in the short term in over 3,000 infected athletes after resumption of normal athletic activities with definite, probable, or possible cardiac involvement. Furthermore, they noted an over fourfold increase in diagnostic yield when CMR was performed when indicated by “triad” testing as opposed to widespread screening. Based on these results, the authors concluded that CMR was most useful in athletes with a high pretest probability of cardiac involvement defined by abnormalities on “triad” testing or the presence of cardiopulmonary symptoms. Although the registry studies overall indicate a low prevalence of COVID-19 related cardiac involvement in athletes, the limitations of the studies include variability of CMR interpretation with no centralized imaging core lab, use of LLC in asymptomatic individuals with unclear clinical implications and lack of a control group. [60, 65, 66]
Application of triad testing in the RTP assessment
Clinical data characterizing hospitalized patients with severe COVID-19 that suggested a high prevalence of cardiac injury as defined by cardiac troponin elevation stimulated concern about athlete safety following COVID-19 infection thereby prompting the development of post-infectious RTP screening protocols [67, 68]. Initial expert consensus recommendations suggested cardiac “triad” testing (i.e. ECG, cardiac troponin, and TTE) for competitive athletes with symptoms following COVID-19 infection [69]. This conservative approach was presented during the initial global sports hiatus when clinical experience with infected but otherwise healthy athletes was minimal. As summarized above, widespread implementation of “triad” testing during the subsequent return of organized athletics provided an abundance of data leading to several important scientific advances. Multi-center registry data at both the professional and collegiate levels, including one collegiate registry examining the role of mandatory CMR imaging, demonstrated a lower than anticipated prevalence of clinically relevant post-infectious cardiac involvement (~ 0.5%) [60]. In addition, these databases established links between the severity of acute infection, the presence of symptoms during return to exercise, and the likelihood of acute cardiac inflammation. Ongoing research continues to test the prognostic utility of CMR in COVID-19 survivors, including optimal screening algorithms in athletic and non-athletic cohorts, patient profiles that predict increased diagnostic yield for CMR based testing, and specific findings on CMR (including functional and tissue substrate alterations) most associated with residual clinical symptoms, impaired quality of life, and long-term clinical risk.
These observations have led to refinement of RTP screening protocols with an emphasis on limiting testing to athletes at highest risk for cardiac complications following infection. While ongoing research is focused on refining risk profiles and screening algorithms, current expert consensus recommendations suggest cardiac testing only among athletes infected with COVID-19 who require hospitalization or those who develop cardiac symptoms (chest pain, syncope, palpitations, dyspnea) during or after the acute phase of infection [63]. These recommendations are consistent with the clinical approach in any other viral syndrome during which cardiopulmonary symptoms develop raising concern for myocarditis. Symptoms suggestive of acute cardiac inflammation include chest pain at rest or with exertion, subjective or objective tachyarrhythmia, or a heart failure syndrome. Athletes with one or more of these symptoms are at moderate or high pretest probability of having acute cardiac inflammation and should undergo comprehensive evaluation prior to return to training and competition. This evaluation should include cardiac “triad” testing with an emphasis on detecting findings related to acute cardiac inflammation (Table 2). The specificity of 12-lead ECG to identify injury patterns [70] or TTE [71] to identify altered cardiac structure and function in the context of acute cardiac inflammation will be maximized when compared to pre-infection baseline values when available.
Lack of standardization in CMR techniques resulting in variability in findings
One key cause of variability in prevalence of cardiac involvement in both athletes and non-athletes post COVID-19 stems from lack of standardized interpretation of CMR abnormalities. Several different strategies of image analysis have been implemented across COVID-19 CMR studies including reporting: (1) any T1, T2 and LGE abnormality detected even in isolation (qualitative visual assessment and mapping) (2) reporting abnormalities according to LLC and (3) modifying LLC to require co-localization of T1 and T2 abnormalities and including supportive findings such as pericarditis or reduced left ventricular ejection fraction (LVEF). Given the heterogeneity of methods of image acquisition, type and field strength of scanners, and variability in interpreting and reporting tissue characterization abnormalities, it is not surprising that high variability of cardiac involvement exists between studies. Furthermore, this highlights the need to better standardize CMR metrics to quantify cardiac involvement in myocarditis, particularly as they pertain to T1 and T2 measures which can be variable. Finally, even in large cohort studies, the number of true myocarditis cases are few. In this context, it is likely that a uniform strategy of CMR screening of all athletes who have recovered from COVID-19 with no clinical findings or symptomatology would result in the identification of a substantial number of cases with abnormal CMR findings, for whom clinical, therapeutic, and long-term prognostic relevance is uncertain. Given the fact that substantial equipoise exists regarding this issue, evidenced based data are lacking at present to support such a uniform screening strategy. Moreover, to date, there are no outcomes studies in COVID-19 positive athletes with abnormal CMR findings. Thus, based on current available evidence, these positive CMR findings in asymptomatic athletes (without ancillary testing indicative of contractile dysfunction, electrical, or biomarker alterations) are most likely unhelpful in guiding physicians and coaches alike in determining RTP.
Technical considerations regarding heterogeneity in LGE and mapping techniques
Among imaging modalities applied to studying pathological changes in the heart after COVID-19 infection, CMR has played a prominent role because the relaxographic (i.e. T1 and T2) properties of myocardial tissue are relatively sensitive to changes at the cellular and molecular level. Whereas the overarching approach used with CMR for tissue characterization follows a relatively standardized schema to assess patterns of altered myocardial tissue substrate, it is worth noting that each of its components may vary in terms of pulse sequence, parameter settings, and post-processing thereby potentially introducing substantial heterogeneity with respect to prevalence and extent and magnitude of such abnormalities.
Regarding LGE, it is well-established that spatial resolution varies in relation to acquisition scheme, and that improved spatial resolution provides improved scar/fibrosis detection. Among 20 COVID-19 survivors, Bustin et al. reported that focal fibrosis was evident on high-resolution LGE-CMR (isotropic voxel size 0.6 cm3) in 67% of patients (n = 12), among whom conventional LGE-CMR (voxel size 1.5 × 1.5 × 4.0 mm) was interpreted as negative or inconclusive in 33% (4/12) [72]. All segments with fibrosis on conventional LGE images were also identified on high resolution LGE, and an additional 8 segments had fibrosis evident only on high resolution LGE—which yielded significant increased prevalence of LV segments with fibrosis (16% vs. 13%, p < 0.01). It is also important to recognize that LGE signal intensity varies in relation to magnetic field strength, and that prevalence of LGE has been shown to vary in relation to signal intensity thresholds [73, 74]—concepts of substantial importance given that differential diagnostic thresholds have been used to define prevalence of LGE in COVID-focused research (Additional file 1: Table S1) [48,49,50,51,52, 61, 72, 75,76,77,78,79,80,81,82,83,84,85,86,87]. Last, it should be noted that some groups have reported that LGE can occur in high endurance athletes in the absence of COVID-19 infection [64, 88, 89], raising the possibility that observed patterns might be a consequence of increased LV wall stress, altered myocardial perfusion gradients, or hemodynamic sequelae of athletic competition itself. While the underlying mechanism for this association is uncertain, it is known that the finding of LGE itself does not provide temporal information—highlighting the importance of adjunctive CMR approaches to elucidate time course of myocardial injury.
Parametric mapping, like LGE, is subject to heterogeneity in pulse sequence parameters that can provide an important source of variability with respect to diagnostic yield, in addition to the differences of native T1 with magnetic field strength. For T1 mapping, prior COVID-19 studies have used an array of pulse sequences that vary with respect to saturation/inversion pulse design, sampling interval, and fitting algorithm [48,49,50,51,52, 61, 72, 75,76,77,78,79,80, 82,83,84, 86, 87]. Similarly, among the T2 mapping studies reported, different pulse sequences, fitting algorithms, and signal equations have been used to estimate decay curves [48, 49, 51, 52, 61, 72, 76,77,78,79,80, 82,83,84,85,86,87]—each of which is capable of impacting the derived T2. Additionally, as is the case for LGE, variable spatial resolution provides a potential source of data heterogeneity of particular importance to the post-COVID athlete, given that endurance and strength trained athletes can manifest differential LV remodeling [90, 91] and that some studies have reported athletes to manifest increased LV trabeculations [92] (providing a source of partial voxel admixture of LV blood pool and myocardium). In this context, it is worthwhile noting that prior COVID-19 studies of athletic and non-athletic cohorts have used a variety of pulse sequences, different thresholds for delineation of myocardial tissue substrate abnormalities, and performed CMR at variable time points after COVID-19 (see Additional file 1: Table S1). Each of these factors, as well as inherent differences in population characteristics and study design may contribute to heterogeneity in prevalence of reported myocardial tissue substrate abnormalities.
It remains a challenge to reconcile the findings of the various reported studies of COVID-19 infected athletes from different centers as the schemes for data acquisition vary by center and published studies have not included controls with the same scanner and protocol to provide center-specific reference ranges. These studies are therefore not consistent with best practice recommendations from CMR expert panels on T1/T2 mapping [28]. One approach to overcome the limited comparability of T1/T2 parameters could be to consider relative changes of T1/T2 with respect to the center-specific results in healthy controls. This still leaves open the question to what degree controls should match clinical characteristics of COVID-19 patients with respect to key indices (e.g. age, gender, cardiovascular disease risk factors)—some studies have expanded additional efforts to address this issue [50]. Fig. 3 illustrates an example of an approach to standardize the results for native T1 differences based on published studies that include T1 reference values for from their own center [22, 48,49,50, 75, 76, 78, 80, 83,84,85, 87, 93,94,95]. This type of meta-analysis can identify factors such as the time of recovery since infection as a source of disease-related variability of native myocardial T1 in COVID-19 survivors.
Meta-regression for percent differences of CMR native T1 in COVID-19 studies. The percentage difference of the means for native T1 in COVID-19 and control groups regresses with the time since the original Covid-19 diagnosis. The studies selected are for adult, non-athletic cohorts which also include native T1 results for a control group scanned under equivalent conditions (i.e. same field strength, scanner, T1 mapping technique). The continuous line and dashed lines correspond to the predicted mean and confidence intervals obtained from a meta-regression model for the ratio of means of native T1 for COVID-19 and control groups. The size of the data points is proportional to the weighs given in the meta-regression analysis. The last name of each study’s first author appears next to the data points. All ratios of means of native T1 were converted to percentage differences for illustration in this figure. The native T1 at the upper bound of its normal range in controls corresponds to an approximately 5% relative to its mean [22, 48,49,50, 75, 76, 78, 80, 83,84,85, 87, 93,94,95]
When to consider CMR testing in the RTP assessment
Evidence suggests that athletes with cardiac symptoms, severe acute COVID-19 infection requiring hospitalization, and/or abnormalities on “triad” testing should undergo CMR imaging [63]. While CMR is an invaluable diagnostic tool in the setting of clinically suspected myocarditis, available evidence does not support its widespread use as a primary screening tool following COVID-19 infection among competitive athletes [96]. This recommendation is based on an appraisal of the fundamental characteristics of a “good” screening test. Effective screening tests should be easy to administer, inexpensive, reliable, valid, and should address a disease process that represent a significant public burden. Compared to other forms of screening testing, CMR is generally somewhat more expensive and available largely at tertiary care or academic medical centers [97]. These limitations render CMR an impractical screening test for the large number (potentially thousands if not more) of competitive athletes who contract COVID-19. Further, as noted above, there is a need for standardized CMR analyses, contextual interpretation of available literature, and need for further study of the prognostic significance of CMR findings including parametric mapping abnormalities in isolation. Available clinical surveillance data from prospective registries as summarized above suggest exceptionally low rates of adverse events among athletes evaluated by “triad” testing in isolation thereby suggesting that abnormalities detected only by CMR (without symptoms or triad testing abnormalities) are likely of little clinical relevance.
What should constitute a positive CMR for myocarditis
As noted in this statement, CMR abnormalities that could be consistent with evidence of persistent myocardial inflammation and/or myocardial scarring from prior inflammation are derived from (1) parametric mapping results above the upper limit of normal for that specific acquisition sequence and field strength and (2) LGE. LGE can be conceived as (1) likely unrelated and of questionable pathological significance (such as in septal insertional LGE), (2) likely unrelated but pathological “chronic” patterns (prior subendocardial LGE in an ischemic pattern or prior high signal intensity LGE observed in the context of a wall thickening pattern suggestive of hypertrophic cardiomyopathy (HCM), or (3) likely related “acute” LGE patterns attributable to recent COVID-19 (sub-epicardial LGE or mid-myocardial LGE particularly involving the non-septal walls) co-localized to parametric abnormalities. In this context, CMR findings should be interpreted with this qualification scheme in mind and should fall into one of the following adjudications: (1) no myocarditis (normal native T1, T2, ECV and no LGE), (2) possible myocarditis (abnormal native T1 and/or T2, normal ECV, and present but non-specific LGE), and (3) probable myocarditis (abnormal native T1 and/or T2 or abnormal ECV, or present LGE in a pattern consistent with acute myocarditis and co-localized to parametric abnormalities). It is important to reiterate that available outcomes data for mapping techniques are limited relative to more rigorously validated imaging biomarkers (such as cine structural and functional parameters or LGE), and that clinical decision making is best predicated upon the latter, more highly substantiated parameters.
Use of CMR in follow-up imaging
Consideration of the utilization and timing of repeat CMR among athletes with prior abnormal CMR testing is well described in the aforementioned Expert Consensus Decision Pathway document [63]. In the context of inconsistent or conflicting testing results, a shared decision-making model is reasonable to balance the potential risk of athletic endeavors with the implications of cessation of activity. Moreover, in the competitive athlete, cessation of athletic activity may have significant psychological, financial, and educational implications. The ultimate decision to compete or restrict must be individualized and will depend in part on the quality and significance of the abnormal testing, risk tolerance, and the benefits of competition.
Research priorities and unanswered questions
This effort to consolidate expert opinions in this statement was motived by the observed variability in utilization and interpretation of CMR in the clinical practice of the RTP assessment. With the exception of the larger registry studies, recommendations put forth here are drawn largely upon presently available case series and cohorts that do not in themselves confer sufficient evidence on which to base definitive recommendations. Furthermore, there is considerable overlap in the CMR imaging applications of the RTP recommendation with assessment of non-athletes with symptoms of PASC [63]. For this reason, investigations funded by the significant $1.1 billon investment committed by the National Institutes of Health (NIH) to address PASC will likely inform the interpretation of testing results, including CMR, in the RTP assessment. Similarly, CMR features of myocarditis following mRNA COVID-19 vaccine is another area of overlap. A more thorough discussion of CMR in the assessment of PASC or post-mRNA vaccine myocarditis is beyond the scope of this document. Table 3 reflects important knowledge gaps relevant to CMR in the RTP (and PASC) assessment which will be addressed through future investigations leveraging various study designs, registries, and multi-center observational studies.
Conclusions
The vast numbers of recreational, collegiate, and professional athletes infected by COVID-19 has placed many clinicians in the unenviable position of rendering “clinical clearance” for resumption of athletic activities and mitigation of adverse cardiac risk. CMR is an indispensable tool to identify myocardial inflammation owing to COVID-19 infection and current literature suggests that CMR should be applied judiciously in selected cases of symptomatic COVID-19 and abnormal “triad” testing. Future studies will further inform the prognostic significance of the diversity of reported CMR findings to shape clinical action taken.
Availability of data and materials
Not applicable.
Abbreviations
- CMR:
-
Cardiovascular magnetic resonance
- Covid-19:
-
Coronavirus disease 2019
- ECG:
-
Electrocardiogram
- ECV:
-
Extracellular volume fraction
- LGE:
-
Late gadolinium enhancement
- LLC:
-
Lake Louise Criteria
- LV:
-
Left ventricle/left ventricular
- LVEF:
-
Left ventricular ejection fraction
- PASC:
-
Post-acute sequelae Covid-19
- PCR:
-
Polymerase chain reaction
- QOL:
-
Quality of life
- RTP:
-
Return to play
- RV:
-
Right ventricle/right ventricular
- SAR-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TTE:
-
Transthoracic echocardiogram
References
Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, et al. ST-Segment elevation in patients with covid-19 - a case series. N Engl J Med. 2020;382:2478–80.
Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:819–24.
Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811–8.
van Osch D, Asselbergs FW, Teske AJ. Takotsubo cardiomyopathy in COVID-19: a case report. Haemodynamic and therapeutic considerations. Eur Heart J Case Rep. 2020;4:1–6.
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–10.
Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 Infection. J Am Coll Cardiol. 2020;76:533–46.
Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950–73.
Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7.
Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50: 107300.
Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss H-P, et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiology. 2020;5:1281–5.
Verma AK, Olagoke O, Moreno JD, Rezaee N, Ma P, Liu J, Javaheri A, Lavine K, Masood MF, Lin CY. SARS-CoV-2-associated myocarditis: a case of direct myocardial injury. Circ Heart Fail. 2022;15: e008273.
Siddiqi HK, Weber B, Zhou G, Regan J, Fajnzylber J, Coxen K, Corry H, Yu XG, DiCarli M, Li JZ, et al. Increased Prevalence of Myocardial Injury in Patients with SARS-CoV-2 Viremia. Am J Med. 2021;134:542–6.
Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, Huang H, Luo YC, Zhou X, Liu ZY, et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106:1154–9.
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Accessed 31 Jan 2022. https://www.covid19treatmentguidelines.nih.gov/.
Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4: e2111417.
Carfi A, Bernabei R, Landi F. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324:603–5.
Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, Doucet L, Berkani S, Oliosi E, Mallart E, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–6.
Tenforde MW, Billig Rose E, Lindsell CJ, Shapiro NI, Files DC, Gibbs KW, Prekker ME, Steingrub JS, Smithline HA, Gong MN, et al. Characteristics of Adult Outpatients and Inpatients with COVID-19 - 11 Academic Medical Centers, United States, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:841–6.
Kim J, Volodarskiy A, Sultana R, Pollie MP, Yum B, Nambiar L, Tafreshi R, Mitlak HW, RoyChoudhury A, Horn EM, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76:1965–77.
Karagodin I, Singulane CC, Descamps T, Woodward GM, Xie M, Tucay ES, Sarwar R, Vasquez-Ortiz ZY, Alizadehasl A, Monaghan MJ, et al. Ventricular changes in patients with acute COVID-19 infection: follow-up of the world alliance societies of echocardiography (WASE-COVID) Study. J Am Soc Echocardiogr. 2022;35:295–304.
Moody WE, Liu B, Mahmoud-Elsayed HM, Senior J, Lalla SS, Khan-Kheil AM, Brown S, Saif A, Moss A, Bradlow WM, et al. Persisting adverse ventricular remodeling in COVID-19 survivors: a longitudinal echocardiographic study. J Am Soc Echocardiogr. 2021;34:562–6.
Chen BH, Shi NN, Wu CW, An DA, Shi YX, Wesemann LD, Hu J, Xu JR, Shan F, Wu LM. Early cardiac involvement in patients with acute COVID-19 infection identified by multiparametric cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2021;22:844–51.
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(2636–48):2648a–2648d.
Tschope C, Cooper LT, Torre-Amione G, Van Linthout S. Management of myocarditis-related cardiomyopathy in adults. Circ Res. 2019;124:1568–83.
Law YM, Lal AK, Chen S, Cihakova D, Cooper LT Jr, Deshpande S, Godown J, Grosse-Wortmann L, Robinson JD, Towbin JA, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021;144:e123–35.
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–87.
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–76.
Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19:75.
Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–8.
Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–90.
Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved Systolic Function: ITAMY Study. J Am Coll Cardiol. 2017;70:1977–87.
Grani C, Eichhorn C, Biere L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70:1964–76.
Greulich S, Seitz A, Muller KAL, Grun S, Ong P, Ebadi N, Kreisselmeier KP, Seizer P, Bekeredjian R, Zwadlo C, et al. Predictors of mortality in patients with biopsy-proven viral myocarditis: 10-year outcome data. J Am Heart Assoc. 2020;9: e015351.
Grani C, Biere L, Eichhorn C, Kaneko K, Agarwal V, Aghayev A, Steigner M, Blankstein R, Jerosch-Herold M, Kwong RY. Incremental value of extracellular volume assessment by cardiovascular magnetic resonance imaging in risk stratifying patients with suspected myocarditis. Int J Cardiovasc Imaging. 2019;35:1067–78.
Puntmann VO, D’Cruz D, Smith Z, Pastor A, Choong P, Voigt T, Carr-White G, Sangle S, Schaeffter T, Nagel E. Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. Circ Cardiovasc Imaging. 2013;6:295–301.
Zhang Y, Corona-Villalobos CP, Kiani AN, Eng J, Kamel IR, Zimmerman SL, Petri M. Myocardial T2 mapping by cardiovascular magnetic resonance reveals subclinical myocardial inflammation in patients with systemic lupus erythematosus. Int J Cardiovasc Imaging. 2015;31:389–97.
Mayr A, Kitterer D, Latus J, Steubing H, Henes J, Vecchio F, Kaesemann P, Patrascu A, Greiser A, Groeninger S, et al. Evaluation of myocardial involvement in patients with connective tissue disorders: a multi-parametric cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2016;18:67.
Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Rai AB, Matthews PM, Robson MD, Moon J, Wordsworth PB, Neubauer S, et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis–a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson. 2014;16:21.
Galea N, Rosato E, Gigante A, Borrazzo C, Fiorelli A, Barchetti G, Trombetta AC, Digiulio MA, Francone M, Catalano C, et al. Early myocardial damage and microvascular dysfunction in asymptomatic patients with systemic sclerosis: A cardiovascular magnetic resonance study with cold pressor test. PLoS ONE. 2020;15: e0244282.
Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med. 2014;189:109–12.
Greulich S, Kitterer D, Latus J, Aguor E, Steubing H, Kaesemann P, Patrascu A, Greiser A, Groeninger S, Mayr A, et al. Comprehensive cardiovascular magnetic resonance assessment in patients with sarcoidosis and preserved left ventricular ejection fraction. Circ Cardiovasc Imaging. 2016;9: e005022.
Puntmann VO, Isted A, Hinojar R, Foote L, Carr-White G, Nagel E. T1 and T2 mapping in recognition of early cardiac involvement in systemic sarcoidosis. Radiology. 2017;285:63–72.
Luetkens JA, Doerner J, Schwarze-Zander C, Wasmuth JC, Boesecke C, Sprinkart AM, Schmeel FC, Homsi R, Gieseke J, Schild HH, et al. Cardiac magnetic resonance reveals signs of subclinical myocardial inflammation in asymptomatic HIV-Infected Patients. Circ Cardiovasc Imaging. 2016;9: e004091.
Ntusi N, O’Dwyer E, Dorrell L, Wainwright E, Piechnik S, Clutton G, Hancock G, Ferreira V, Cox P, Badri M, et al. HIV-1-related cardiovascular disease is associated with chronic inflammation, frequent pericardial effusions, and probable myocardial edema. Circ Cardiovasc Imaging. 2016;9: e004430.
Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021;6:1202–6.
Truong DT, Dionne A, Muniz JC, McHugh KE, Portman MA, Lambert LM, Thacker D, Elias MD, Li JS, Toro-Salazar OH, et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults. Circulation. 2022;145:345–56.
Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, Parker MA, Kim RJ. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–201.
Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330–9.
Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, Patel R, Chacko L, Brown JT, Coyle C, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–78.
Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265–73.
Brito D, Meester S, Yanamala N, Patel HB, Balcik BJ, Casaclang-Verzosa G, Seetharam K, Riveros D, Beto RJ 2nd, Balla S, et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2021;14:541–55.
Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–8.
Starekova J, Bluemke DA, Bradham WS, Eckhardt LL, Grist TM, Kusmirek JE, Purtell CS, Schiebler ML, Reeder SB. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6:945–50.
Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, Slaughter JC, Fitch W, Hughes SG, Soslow JH. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR). Circulation. 2021;143:609–12.
Fikenzer S, Kogel A, Pietsch C, Lavall D, Stobe S, Rudolph U, Laufs U, Hepp P, Hagendorff A. SARS-CoV2 infection: functional and morphological cardiopulmonary changes in elite handball players. Sci Rep. 2021;11:17798.
Hwang CE, Kussman A, Christle JW, Froelicher V, Wheeler MT, Moneghetti KJ. Findings from cardiovascular evaluation of national collegiate athletic association division I collegiate student-athletes after asymptomatic or mildly symptomatic SARS-CoV-2 infection. Clin J Sport Med. 2022;32:103–7.
Malek LA, Marczak M, Milosz-Wieczorek B, Konopka M, Braksator W, Drygas W, Krzywanski J. Cardiac involvement in consecutive elite athletes recovered from Covid-19: A magnetic resonance study. J Magn Reson Imaging. 2021;53:1723–9.
Vago H, Szabo L, Dohy Z, Merkely B. Cardiac magnetic resonance findings in patients recovered from COVID-19: initial experiences in elite athletes. JACC Cardiovasc Imaging. 2021;14:1279–81.
Hendrickson BS, Stephens RE, Chang JV, Amburn JM, Pierotti LL, Johnson JL, Hyden JC, Johnson JN, Philip RR. Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: results of an algorithm-guided screening. Circulation. 2021;143:1926–8.
Moulson N, Petek BJ, Drezner JA, Harmon KG, Kliethermes SA, Patel MR. Baggish AL and Outcomes Registry for Cardiac Conditions in Athletes I. SARS-CoV-2 Cardiac Involvement in Young Competitive Athletes. Circulation. 2021;144:256–66.
Szabo L, Juhasz V, Dohy Z, Fogarasi C, Kovacs A, Lakatos BK, Kiss O, Sydo N, Csulak E, Suhai FI, et al. Is cardiac involvement prevalent in highly trained athletes after SARS-CoV-2 infection? A cardiac magnetic resonance study using sex-matched and age-matched controls. Br J Sports Med. 2022;56:553–60.
Petek BJ, Moulson N, Baggish AL, Kliethermes SA, Patel MR, Churchill TW, Harmon KG, Drezner JA. Prevalence and clinical implications of persistent or exertional cardiopulmonary symptoms following SARS-CoV-2 infection in 3597 collegiate athletes: a study from the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA). Br J Sports Med. 2021. https://doi.org/10.1136/bjsports-2021-104644.
Gluckman TJ, Bhave NM, Allen LA, Chung EH, Spatz ES, Ammirati E, Baggish AL, Bozkurt B, Cornwell WK 3rd, Harmon KG, et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;79:1717–56.
Domenech-Ximenos B, Sanz-de la-Garza M, Prat-Gonzalez S, Sepulveda-Martinez A, Crispi F, Duran-Fernandez K, Perea RJ, Bijnens B, Sitges M. Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes. J Cardiovasc Magn Reson. 2020;22:62.
Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, Phelan D, Kim JH, Meeuwisse W, Sills AK, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2022;6:745–52.
Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, Jeudy J, Mattson SE, Law IH, Borchers J, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021;6:1078–87.
Kim JH, Levine BD, Phelan D, Emery MS, Martinez MW, Chung EH, Thompson PD, Baggish AL. Coronavirus Disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6:219–27.
Perillo Filho M, Francisco RC, Garcia TG, Teixeira MF, Bassaneze B, Albuquerque LCA, Alo ROB, Colombo C, Ghorayeb N. Sports in Covid-19 times: heart alert. Arq Bras Cardiol. 2020;115:303–7.
Phelan D, Kim JH, Chung EH. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) Infection. JAMA Cardiol. 2020;5:1085–6.
Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM, La Gerche A, Ackerman MJ, Borjesson M, Salerno JC, et al. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol. 2017;69:1057–75.
Baggish AL, Battle RW, Beaver TA, Border WL, Douglas PS, Kramer CM, Martinez MW, Mercandetti JH, Phelan D, Singh TK, et al. Recommendations on the use of multimodality cardiovascular imaging in young adult competitive athletes: a report from the American Society of Echocardiography in collaboration with the society of cardiovascular computed tomography and the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2020;33:523–49.
Bustin A, Sridi S, Gravinay P, Legghe B, Gosse P, Ouattara A, Roze H, Coste P, Gerbaud E, Desclaux A, et al. High-resolution free-breathing late gadolinium enhancement cardiovascular magnetic resonance to diagnose myocardial injuries following COVID-19 infection. Eur J Radiol. 2021;144: 109960.
McAlindon E, Pufulete M, Lawton C, Angelini GD, Bucciarelli-Ducci C. Quantification of infarct size and myocardium at risk: evaluation of different techniques and its implications. Eur Heart J Cardiovasc Imaging. 2015;16:738–46.
Klem I, Heiberg E, Van Assche L, Parker MA, Kim HW, Grizzard JD, Arheden H, Kim RJ. Sources of variability in quantification of cardiovascular magnetic resonance infarct size - reproducibility among three core laboratories. J Cardiovasc Magn Reson. 2017;19:62.
Joy G, Artico J, Kurdi H, Seraphim A, Lau C, Thornton GD, Oliveira MF, Adam RD, Aziminia N, Menacho K, et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. JACC Cardiovasc Imaging. 2021;14:2155–66.
Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F, Okell T, Sheerin F, Xie C, Mahmod M, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31: 100683.
Wang H, Li R, Zhou Z, Jiang H, Yan Z, Tao X, Li H, Xu L. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23:14.
Li X, Wang H, Zhao R, Wang T, Zhu Y, Qian Y, Liu B, Yu Y, Han Y. Elevated extracellular volume fraction and reduced global longitudinal strains in participants recovered from COVID-19 without clinical cardiac findings. Radiology. 2021;299:E230–40.
Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, Goldring J, Jacobs M, Lamb LE, Negus R, et al. COVID-19: Myocardial injury in survivors. Circulation. 2020;142:1120–2.
Ng MY, Ferreira VM, Leung ST, Yin Lee JC, Ho-Tung Fong A, To Liu RW, Man Chan JW, Wu AKL, Lung KC, Crean AM, et al. Patients recovered from COVID-19 show ongoing subclinical myocarditis as revealed by cardiac magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2476–8.
Wojtowicz D, Dorniak K, Lawrynowicz M, Rejszel-Baranowska J, Fijalkowska J, Kulawiak-Galaska D, Szurowska E, Kozinski M. Spectrum of lesions visualized in cardiac magnetic resonance imaging in COVID-19-related myocarditis: findings from a pilot study of the TRICITY-CMR trial. Cardiol J. 2021;28:976–8.
Webster G, Patel AB, Carr MR, Rigsby CK, Rychlik K, Rowley AH, Robinson JD. Cardiovascular magnetic resonance imaging in children after recovery from symptomatic COVID-19 or MIS-C: a prospective study. J Cardiovasc Magn Reson. 2021;23:86.
Urmeneta Ulloa J, Martinezde Vega V, Salvador Montanes O, Alvarez Vazquez A, Sanchez-Enrique C, Hernandez Jimenez S, Sancho Garcia FD, Lopez Ruiz L, Recio Rodriguez M, Pizarro G, et al. Cardiac magnetic resonance in recovering COVID-19 patients. Feature tracking and mapping analysis to detect persistent myocardial involvement. Int J Cardiol Heart Vasc. 2021;36:100854.
Galea N, Marchitelli L, Pambianchi G, Catapano F, Cundari G, Birtolo LI, Maestrini V, Mancone M, Fedele F, Catalano C, et al. T2-mapping increase is the prevalent imaging biomarker of myocardial involvement in active COVID-19: a Cardiovascular Magnetic Resonance study. J Cardiovasc Magn Reson. 2021;23:68.
Luetkens JA, Isaak A, Ozturk C, Mesropyan N, Monin M, Schlabe S, Reinert M, Faron A, Heine A, Velten M, et al. Cardiac MRI in Suspected Acute COVID-19 Myocarditis. Radiol Cardiothorac Imaging. 2021;3: e200628.
Breitbart P, Koch A, Schmidt M, Magedanz A, Lindhoff-Last E, Voigtlander T, Schmermund A, Mehta RH, Eggebrecht H. Clinical and cardiac magnetic resonance findings in post-COVID patients referred for suspected myocarditis. Clin Res Cardiol. 2021;110:1832–40.
Clark DE, Dendy JM, Li DL, Crum K, Dixon D, George-Durrett K, Parikh AP, Wassenaar JW, Hughes SG, Soslow JH. Cardiovascular magnetic resonance evaluation of soldiers after recovery from symptomatic SARS-CoV-2 infection: a case-control study of cardiovascular post-acute sequelae of SARS-CoV-2 infection (CV PASC). J Cardiovasc Magn Reson. 2021;23:106.
Tahir E, Starekova J, Muellerleile K, von Stritzky A, Munch J, Avanesov M, Weinrich JM, Stehning C, Bohnen S, Radunski UK, et al. Myocardial fibrosis in competitive triathletes detected by contrast-enhanced CMR correlates with exercise-induced hypertension and competition history. JACC Cardiovasc Imaging. 2018;11:1260–70.
Breuckmann F, Mohlenkamp S, Nassenstein K, Lehmann N, Ladd S, Schmermund A, Sievers B, Schlosser T, Jockel KH, Heusch G, et al. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009;251:50–7.
Pluim BM, Zwinderman AH, Laarse A, Wall EE. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–44.
La Gerche A, Taylor AJ, Prior DL. Athlete’s heart: the potential for multimodality imaging to address the critical remaining questions. JACC Cardiovasc Imaging. 2009;2:350–63.
Caselli S, Ferreira D, Kanawati E, Di Paolo F, Pisicchio C, Attenhofer Jost C, Spataro A, Jenni R, Pelliccia A. Prominent left ventricular trabeculations in competitive athletes: A proposal for risk stratification and management. Int J Cardiol. 2016;223:590–5.
Cassar MP, Tunnicliffe EM, Petousi N, Lewandowski AJ, Xie C, Mahmod M, Samat AHA, Evans RA, Brightling CE, Ho LP, et al. Symptom Persistence Despite Improvement in Cardiopulmonary Health - Insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine. 2021;41: 101159.
Pan C, Zhang Z, Luo L, Wu W, Jia T, Lu L, Liu WV, Qin Y, Hu F, Ding X, et al. Cardiac T1 and T2 Mapping Showed Myocardial Involvement in Recovered COVID-19 Patients Initially Considered Devoid of Cardiac Damage. J Magn Reson Imaging. 2021;54:421–8.
Kravchenko D, Isaak A, Zimmer S, Mesropyan N, Reinert M, Faron A, Pieper CC, Heine A, Velten M, Nattermann J, et al. Cardiac MRI in patients with prolonged cardiorespiratory symptoms after mild to moderate COVID-19. Radiology. 2021;301:E419–25.
Phelan D, Kim JH, Drezner JA, Elliott MD, Martinez MW, Chung EH, Krishan S, Levine BD, Baggish AL. When to consider cardiac MRI in the evaluation of the competitive athlete after SARS-CoV-2 infection. Br J Sports Med. 2022;56:425–6.
Petersen SE, Friebel R, Ferrari V, Han Y, Aung N, Kenawy A, Albert TSE, Naci H. Recent trends and potential drivers of non-invasive cardiovascular imaging use in the United States of America and England. Front Cardiovasc Med. 2020;7: 617771.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
FLR: conceived and designed the research; analyzed and interpreted the data; handled funding and supervision; drafted the Contribution; made critical revision of the Contribution for important intellectual content. ALB: drafted the Contribution; made critical revision of the Contribution for important intellectual content. AGH: drafted the Contribution; made critical revision of the Contribution for important intellectual content. JJH: analyzed and interpreted the data; made critical revision of the Contribution for important intellectual content. JK: drafted the Contribution; made critical revision of the Contribution for important intellectual content. KGO: conceived and designed the research; drafted the Contribution; made critical revision of the Contribution for important intellectual content. GR: made critical revision of the Contribution for important intellectual content. CS: drafted the Contribution; made critical revision of the Contribution for important intellectual content. JWW: analyzed and interpreted the data; drafted the Contribution; made critical revision of the Contribution for important intellectual content. PKW: drafted the Contribution; made critical revision of the Contribution for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
FLR: Research grant support from NIH/NHLBI (R01HL139671), Alnylam Pharmaceuticals, Akcea Therapeutics, and Pfizer, and consulting support from Attralus.
ALB: Dr. Baggish has received funding from the National Institute of Health/ National Heart, Lung, and Blood Institute, the National Football Players Association, and the American Heart Association to study issues relevant to this manuscript and receives compensation for his role as team cardiologist from the US Olympic Committee / US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University.
AGH: Research grant support from NIH/NHLBI (1R01HL147660).
MJH: Research grant support from NIH/NIA (1R01AG052282).
JK: Research grant support from NIH/NHLBI (R01HL159055).
KGO: Research contract from NIH/NIBIB (75N9202D00018).
Research grant from American College of Radiology Fund for Collaborative Research in Imaging (FCRI) titled: Transcatheter Aortic Valve Replacement (TAVR) Registry.
GR: None.
CS: Research grant support from NIH/NHLBI (K23HL132011) and University of Minnesota Clinical and Translational Science Institute K-R01 Transition to Independence Grant (supported by the National Institutes of Health grant UL1TR002494),
JWW: Research support from NIH/NHLBI (R01HL151686, R01HL159055), General Electric Healthcare.
PKW: Research support from NIH/NHLBI (R01HL150891, R01HL142297), NIH/NCATS (UL1TR002345), NIH/NIBIB (T32EB021955), Siemens, Bayer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is co-published in the journals Journal of Cardiovascular Magnetic Resonance and Circulation: Cardiovascular Imaging. https://doi.org/10.1186/s12968-022-00907-8 and https://doi.org/10.1161/CIRCIMAGING.122.014106
Supplementary Information
Additional file 1.
Supplemental Table 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ruberg, F.L., Baggish, A.L., Hays, A.G. et al. Utilization of cardiovascular magnetic resonance (CMR) imaging for resumption of athletic activities following COVID-19 infection: an expert consensus document on behalf of the American Heart Association Council on Cardiovascular Radiology and Intervention (CVRI) Leadership and endorsed by the Society for Cardiovascular Magnetic Resonance (SCMR). J Cardiovasc Magn Reson 24, 73 (2022). https://doi.org/10.1186/s12968-022-00907-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12968-022-00907-8