Abstract
Background
Phase contrast (PC) cardiovascular magnetic resonance (CMR) in the ascending aorta (AAo) is widely used to calculate left ventricular (LV) stroke volume (SV). The accuracy of PC CMR may be altered by turbulent flow. Measurement of SV at another site is suggested in the presence of aortic stenosis, but very few data validates the accuracy or inaccuracy of PC in that setting. Our objective is to compare flow measurements obtained in the AAo and LV outflow tract (LVOT) in patients with aortic stenosis.
Methods
Retrospective analysis of patients with aortic stenosis who had CMR and echocardiography. Patients with mitral regurgitation were excluded. PC in the AAo and LVOT were acquired to derive SV. LV SV from end-systolic and end-diastolic tracings was used as the reference measure. A difference ≥ 10% between the volumetric method and PC derived SVs was considered discordant. Metrics of turbulence and jet eccentricity were assessed to explore the predictors of discordant measurements.
Results
We included 88 patients, 41% with bicuspid aortic valve. LVOT SV was concordant with the volumetric method in 79 (90%) patients vs 52 (59%) patients for AAo SV (p = 0.015). In multivariate analysis, aortic stenosis flow jet angle was a strong predictor of discordant measurement in the AAo (p = 0.003). Mathematical correction for the jet angle improved the concordance from 59 to 91%. Concordance was comparable in patients with bicuspid and trileaflet valves (57% and 62% concordance respectively; p = 0.11). Accuracy of SV measured in the LVOT was not influenced by jet eccentricity. For aortic regurgitation quantification, PC in the AAo had better correlation to volumetric assessments than LVOT PC.
Conclusion
LVOT PC SV in patients with aortic stenosis and eccentric jet might be more accurate compared to the AAo SV. Mathematical correction for the jet angle in the AAo might be another alternative to improve accuracy.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Phase-contrast (PC) is the main approach to quantify blood flow parameters with cardiovascular magnetic resonance (CMR). It is widely accepted and used to assess valvular and congenital heart diseases [1, 2]. Flow volume can be measured by the acquisition of a cross-sectional image of the vessel or area of interest, in which fluid velocity is calculated for every pixel [1, 3]. However, the accuracy of PC may be altered by the presence of turbulent flow. Such turbulence typically occurs in patients with heart valve diseases and stenotic or regurgitant jets, which are associated with flow acceleration/deceleration and intravoxel dephasing [4,5,6]. Regurgitant or stenotic valves are also associated with flow eccentricity, increasing the difficulty to optimize the correct imaging plane.
PC in the ascending aorta (AAo) is widely used to calculate forward stroke volume (SV) and regurgitant aortic flow; those variables are essential to assess mitral and/or aortic regurgitation [5, 7]. Aortic regurgitation can be measured directly from the PC sequence, while mitral regurgitation is computed as the difference between aortic SV and left ventricular (LV) SV obtained volumetrically from a short axis stack. Blood flow quantification in the AAo is however potentially altered in the presence of aortic valve disease, and SV measurement at another site [pulmonary valve or left ventricular outflow tract (LVOT)] is sometime suggested [5, 8, 9]. However, it is not clear if and at which severity the presence of aortic valve disease can invalidate AAo PC measurements [10, 11], and there are few data comparing SV obtained from different sites in the presence of valve disease. These issues are relevant as patients with multiple valve diseases are frequently encountered and increasingly assessed by CMR [5, 7].
Our objectives are to compare flow measurements obtained by PC in the ascending aorta (SVAAo) and the LVOT (SVLVOT) in patients with various degrees of aortic stenosis. We have selected a population without significant mitral regurgitation so that SV obtained by volumetric method (SVVM) from LV tracings can be used as a reference.
Methods
Patient population
A total of 88 patients prospectively recruited in the ongoing PROGRESSA study (NCT 01679431) between 2011 and 2015 were retrospectively analyzed. Included patients had either aortic stenosis (Vmax > 2 m/s), bicuspid aortic valve (with or without stenosis), and controls without valve disease. Patients were excluded if they had symptomatic aortic stenosis, any mitral valve disease (mitral stenosis or > trace mitral regurgitation), LV ejection fraction (LVEF) < 50%, rheumatic valve disease or endocarditis, previous aortic/mitral valve repair or replacement, previous ascending aorta repair or replacement, if they were pregnant/lactating or if they had contraindications to gadolinium. More details about inclusion/exclusion criteria were previously described [12]. Patients underwent transthoracic echocardiogram (TTE) and CMR within 3 months. The study was approved by the Ethics Committee of the Quebec Heart and Lung Institute and patients signed a written informed consent at the time of inclusion.
Doppler echocardiographic measurements
All Doppler echocardiographic examinations were acquired using commercially available ultrasound machines (iE33 and EPIQ, Philips Healthcare, Best, Netherlands) and according to the current recommendations of the American Society of Echocardiography [13, 14]. Images were analyzed offline in a core laboratory. Aortic regurgitation and mitral regurgitation were graded using a multiparametric approach as suggested by guidelines [14, 15]. All patients with more than mild mitral regurgitation severity were excluded for the purpose of this study.
Cardiovascular magnetic resonance measurements
CMR was performed using 1.5 and 3T CMR scanners (Achieva or Ingenia, Philips Healthcare). Cardiac morphology and function were assessed by balanced steady-state free precession sequences at 30 phases per cardiac cycle in held end-expiration. Standard planes included 8–14 contiguous parallel short-axis (8 mm thickness, 0 mm gap) covering the entire cardiac volume, 2-chamber, 4-chamber and two orthogonal LVOT planes. Typical parameters at 1.5T were TR/TE 3.2/1.6 ms, flip angle 60º, and NEX of 1, in-plane spatial resolution of 1.6 × 2 mm. Equivalent acquisition parameters at 3T were TR/TE 2.8/1.3 ms, flip angle 45°, and NEX of 1, in-plane spatial resolution of 1.7 mm × 2 mm, 7 mm slice thickness, 0 mm gap. LV volumes and LVEF were measured by contour analysis of end-diastolic and end-systolic phases of the short-axis stack. LV SVVM was calculated as the difference between LV end-diastolic and end-systolic volumes. To reflect different practices of CMR post-processing, LV volumes and SV were computed with and without including the papillary muscles and major trabeculations in the blood pool.
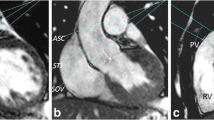
Using double-oblique long-axis views of the LVOT and aortic valve, through-plane PC imaging was performed during breath-hold at two sites: (1) LVOT, 5–10 mm below the aortic annulus in mid-systole and (2) AAo, 10-mm above the aortic annulus (Fig. 1). The imaging PC sequence was planned parallel to the aortic valve annulus plane as previously described [16]. Flow imaging parameters consisted of TR/TE = 4.29–4.92/2.52–3.05 ms, flip angle = 15°, 24 phases, pixel spacing = 1.32–2.07 mm, slice thickness = 10 mm, acquisition matrix = 256 × 208. For each patient, peak aortic jet velocity measured by TTE was used as a starting point to define CMR encoding velocity in the AAo [CMR encoding velocity = (1.25–1.5) × peak jet velocity] with further adjustment in case of aliasing. Forward systolic flows (SVAAo and SVLVOT) were computed using semi-automated tracings. Regurgitant volume was measured by PC at both sites and also estimated using the difference between right ventricular (RV) and LV SVs as these patients did not have significant mitral or tricuspid regurgitation [15]. We have evaluated the eccentricity of the aortic stenosis jet by assessing the angle between the aortic valve plane and the aortic jet in double-oblique long axis images. An angle of 90° reflects a jet flow parallel to the vessel orientation, and lower angles reflects jet eccentricity (Fig. 2). The angle was measured in 2 cross sectional planes, and the lowest measured angle was registered. Mathematical correction of the measured SVAAo for the eccentricity angle was performed [corrected flow = measured flow/sin(angle)] as illustrated in Additional file 1: Figure S1). All analyses were done with cvi42 software (version 5.6.4, Circle Cardiovascular Imaging, Calgary, Alberta, Canada).
Locations of Phase-contrast flow measurements. A, B show double oblique orthogonal planes of the left ventricular (LV) outflow tract (LVOT) with the corresponding slice planes for the LVOT (blue lines) and ascending aorta (green lines). Phase-contrast images for measurement of flow at the LVOT and ascending aorta are shown in C, D
Statistical analyses
Normal distribution of continuous variables was assessed using the Shapiro–Wilk test. Continuous data were expressed as mean ± standard deviation or median [interquartile range], and categorical variables as percentages. Correlation and agreement (95% confidence intervals) between SVAAo and SVLVOT as compared with SVVM were assessed by Spearman correlations and Bland–Altman comparisons [17]. Paired Student t tests were used to test for significance of any overestimation or underestimation. A margin of error of ± 10% between PC derived SV and SVVM was considered concordant measurements, and any difference exceeding this limit was considered a significant underestimation or overestimation. Receiver operating curves (ROC) were performed to derive the best thresholds for each parameter associated with discordance. Variability of measurements for SVLVOT and SVAA vs SVVM were stratified according to aortic stenosis severity, aortic valve morphology and eccentricity of the jet. Statistical analyses were performed with STATA (version 15.3, Stata Corporation, College Station, Texas, USA). A two-sided p value < 0.05 was considered significant.
Results
Study population
Aortic stenosis severity ranged from none to severe [peak velocity 2.3 (1.7–3.0) m/s, mean gradient 13 (4, 7–19) mmHg]. Demographic, echocardiographic and CMR characteristics are depicted in Table 1. A bicuspid aortic valve was present in 36 (41%) patients. No patient had more than trace mitral regurgitation. Aortic diameter was higher in bicuspid vs tricuspid patients (3.6 ± 0.5 vs 3.4 ± 0.4 cm respectively, p = 0.037). Thirty-three (38%) patients were scanned at 1.5T and 55 (62%) at 3T.
Forward stroke volume estimation according to different methods
LV SVs by different methods are shown in Table 1. Overall, correlation between SVAAo and SVLVOT was excellent (r = 0.89, p < 0.001, Additional file 1: Figure S2). However, SVAAo lead to lower SV values than SVLVOT, while SVVM was statistically higher than both SVLVOT and SVAAo (both p < 0.001, Additional file 1: Table S1). Exclusion of the papillary muscles from the blood pool led to significantly lower end-diastolic and end-systolic volumes and significantly higher LVEF (Table 1). SV, albeit with a statistically significant difference (86 ± 20 ml vs 87 ± 20 ml excluding and including papillary muscles within LV mass respectively), was clinically comparable (average difference 1 ± 5 ml). SVLVOT was concordant with SVVM in 90% of the cases, vs 59% for SVAAo (p < 0.001, Fig. 3). Similar results were obtained when papillary muscles were excluded from blood pool (93% vs 59% concordance for SVLVOT vs SVAAo respectively, p < 0.001). CMR field strength had no impact on the discordance between SVAAo and SVLVOT compared to SVVM (p = 0.12). The use of background static tissue correction in discordant cases did change the SV by an average of 1 ± 1 ml in the LVOT and 1 ± 1 ml in the aorta, without changing the concordant/discordant status in any case.
Agreement between PCAA, PCLVOT and volumetric method. Upper Panels: Bland–Altman plots comparing stroke volume (SV) estimated by phase contrast (PC) at the LVOT, AAo and corrected AAo flow respectively as compared to the reference (volumetric method). Data presented included the papillary muscles in the blood pool (similar results obtained by excluding them). Solid red lines: mean bias ± 2 standard deviations. Dashed green line: level of zero bias. Pie charts show the proportion of concordance, over- and under-estimation of SV for 3 methods
Factors associated with discordance between SVAAo and SVVM
There was no relationship between the degree of underestimation of SV estimated by SVAAo as compared to SVVM and peak aortic velocity (r = − 0.14, p = 0.19). Difference between SVAAo and SVVM was related to the jet angle (more discordance in more eccentric jets, Additional file 1: Figure S3). ROC analysis suggested an angle of 85 degrees as the best threshold to predict SVAAo vs SVVM discordance (Additional file 1: Figure S4). A jet angle < 85º was present in 45 (51%) patients and was more frequent as aortic stenosis severity increases [aortic sclerosis: 3 (10%); mild aortic stenosis: 22 (58%); moderate aortic stenosis 10 (77%); severe aortic stenosis: 5 (83%), p < 0.001]. In central jets (angle 85–90°), bias between SVAAo and SVVM was lower than for eccentric jets (absolute difference 6 ± 6 ml vs 12 ± 9 ml respectively, p < 0.001). Concordance of SVAAo was significantly higher in central vs eccentric jets (Fig. 4). Mathematical correction for the eccentricity angle however restored the concordance with SVVM (91% concordance after correction) with lower overall bias (Table 1).
There was a non-significant trend for better concordance in patients with trileaflet vs bicuspid valves (Fig. 4). Patients with a bicuspid valve had however more frequently eccentric jets than those with trileaflet morphology (65% vs 35%, p < 0.001) and had higher peak aortic velocities [2.7 (2.4–3.0) m/s vs 2.2 (1.9–2.4 m/s), p < 0.01]. After multivariate adjustment for valve morphology, eccentricity and peak aortic velocity, the only variable that remained associated with discordance between SVAAo and SVVM was eccentricity of the jet, either as a continuous variable (jet angle) or dichotomized as central jet/eccentric jet (Table 2). There was no association between the aortic diameter and the degree of discordance between SVAAo and SVVM (p = 0.26).
Factors associated with discordance between SVLVOT and SVVM
There was no significant predictor of SVLVOT/SVVM discordance for the studied variables (peak aortic velocity: p = 0.22, jet angle: p = 0.21, aortic diameter: p = 0.58, valve morphology: p = 0.54).
Assessment of aortic regurgitation
The grade of aortic regurgitation as determined by echocardiography was none/trace in 66 (75%) patients, mild in 16 (18%) and moderate in 6 (7%) patients. No patients had severe aortic regurgitation as per exclusion criteria. Regurgitant volume by PC in the LVOT was 30% smaller vs the values obtained in the aorta in the whole cohort (3 ± 3 ml vs 5 ± 4 ml, p < 0.01) and a similar numerical trend was observed in the 6 patients with moderate AR (10 ± 8 vs 14 ± 7 ml, p = 0.25). Regurgitant volume estimated in the AAo correlated better with the difference between RV and LV SVs (Additional file 1: Figure S5).
Discussion
The main findings of this study are: (1) in patients with aortic stenosis, SVLVOT has better overall agreement to volumetric measurements than SVAAo; (2) jet eccentricity is the main factor associated with discordant SVAAo; (3) mathematical correction using measured SV and eccentricity angle corrected the discordance in our population and (4) consistent with previous studies and current recommendation, aortic regurgitant volume is likely underestimated when assessed in the LVOT. To the best of our knowledge this is the first study to formally explore the validity of PC CMR measurement site in a population of this size with various degrees of aortic stenosis severity.
Aortic stenosis, turbulent jets and phase-contrast CMR
PC-CMR is a powerful, accurate and reproducible non-invasive tool to assess blood flow [1, 18]. However, some caveats should be considered: (1) Acquisition plane should be reasonably perpendicular to the direction of flow; this direction is not always in line with the anatomic orientation of the cavity/vessel in which the flow is measured. In some cases, 2 or more jets differentially oriented may co-exist, and can also change their direction throughout the cardiac cycle. Also, high velocity jets may provoke signal loss due to flow acceleration and intravoxel dephasing [10]. Therefore, aortic stenosis is challenging as it presents both problems: high velocity jets which are frequently eccentric. Use of a plane upstream of the stenotic lesion (LVOT) might circumvent these problems.
Interestingly, there was no relation between SVAAo discordance and peak aortic velocity in our cohort. This suggest that aortic stenosis hemodynamic severity might not be by itself a reason to use another site to measure SV. Importantly, the most severe spectrum of aortic stenosis is underrepresented in the current cohort, and this absence of relation could be related to low statistical power. Also, PC-CMR imaging has made advances since its first implementation, with shorter echo-times minimizing the impact of accelerating flow. SVAAo was acquired at a plane in the ascending aorta approximately 10 mm from the valve, which is distal to the vena contracta [16]. Previous works showed that shorter echo-times and distance from the stenosis can reduce the error in PC assessment of flow [6, 10, 16]. While we did not acquire flow data more distally in the AAo, it is likely that the discordance would decrease as the measurement site moves away from the stenosis.
The only factor independently associated with the degree of discordance between SVAAo and SVVM in our cohort was jet eccentricity. It is known that stroke volume measurement requires an imaging plane positioned orthogonal to the main direction of flow [1, 3, 18]. However, in cases of eccentric jets (which are misaligned to the main longitudinal axis of the aorta), optimal PC planning can be extremely challenging and time-consuming. Measurement of SV at the LVOT showed improved accuracy compared to the AAo. Interestingly, mathematical correction for the eccentricity angle improved the concordance which became similar to what is observed with the LVOT measures. Regarding valve morphology, previous studies have shown that in bicuspid patients, flow measurement at the AAo lead to underestimation of forward flow [19]. However, bicuspid valves are frequently associated with complex flow patterns. Our results suggest that bicuspid valve morphology is more often associated with eccentric jets, but otherwise not directly associated with the degree of discordance.
Clinical relevance for CMR and aortic regurgitation quantification.
CMR is increasingly suggested to assess the severity of mitral regurgitation and aortic regurgitation. Several studies have evaluated the reliability of mitral regurgitation quantification by CMR, including systematic review of more than 30 studies [20,21,22]. The PC plane used to derive aortic forward flow was mostly the AAo at sinotubular junction. Most studies do not mention the use of SVLVOT or another site as an alternative to SVAAo, and did not include patients with concomitant aortic stenosis; however coexisting aortic and mitral diseases are frequent in real life practice. It is estimated that up to 20% of patients have at least two moderate valvular pathologies and this will likely expand in the future as the prevalence is constantly increasing [23, 24]. Regarding aortic regurgitation quantification, potential limitations of PC have been discussed in the presence of non-laminar flow [25]. Our study was not designed to assess the best PC plane for aortic regurgitation and is limited by a small number of patients with significant aortic regurgitation. Nevertheless, our results show 30% difference between LVOT and AAo, while the AAo correlated better with RV-LV SV differences. This is consistent with previous studies [11] and suggest that in the case of mixed aortic disease with both stenosis and regurgitation, using 2 sites (LVOT for forward flow; AAo for regurgitant flow) might provide the best assessment.
Despite its good performance, the use of LVOT can be limited in case of subvalvular flow acceleration (hypertrophic cardiomyopathy/sub-aortic membrane). In this case, the selection of an alternative site to confirm forward SV is advisable. The use of either an aortic plane as distal as possible from the flow turbulence, right sided PC planes or combination of both can be considered. Suggested approaches for PC planning in different clinical scenarios are presented in Table 3.
Limitations
Our data are from a single center study, and the population limited to the inclusion criteria of the PROGRESSA study with a low prevalence of severe aortic stenosis. We cannot exclude that very high maximal velocity aortic stenosis can influence SV measurement in the aorta, even in central jets. AAo dilatation is a potential cause of flow turbulence; while the association with discordance was not significant, we had few patients with dilated AAo. Also, few patients had significant aortic regurgitation because of the specific nature of the cohort study which includes mainly patients with aortic stenosis. There was no phantom correction for the flow acquisitions. The aortic phase contrast sequences were planned relatively close to the valve (10 mm): a plane closer to the aortic arch was not assessed but could have decreased discordance. The mathematical correction used in this study does not take into account the whole complexity of flow turbulence and will require validation in other cohorts. PC planning in the LVOT can be potentially challenging, however imaging quality was excellent in all cases with good concordance with SVVM. The assessment of other sites (right sided PC, combination of descending aorta/superior vena cava) has not been explored. Coronary flow can explain in part the difference between SV measured in AA vs LVOT—however as coronary perfusion is in diastole and we have measured systolic forward flow, this component has likely a minor impact. Finally, time-resolved 3D PC (4D-flow) was not performed. 4D flow has been shown to overcome some of the 2D PC-CMR limitations and is an extremely promising tool in this field [26].
Conclusion
Aortic stenosis can negatively influence the PC SVAAo. SVLVOT has overall better agreement with SVVM than SVAAo, especially in patients with eccentric jets. Therefore, flow jet direction rather than aortic stenosis severity alone should be assessed to select the best plane for SV measurement. However, LVOT plane underperforms for aortic regurgitation quantification. Thus, the use of an additional PC-CMR plane at the LVOT in addition to -but not instead of- the conventional plane at the AAo might be preferable in patients with mixed aortic disease. Mathematical correction of SVAAo for eccentric jets should be explored in future studies.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the ongoing status of the main clinical study, but are available from the corresponding author on reasonable request.
Abbreviations
- AAo:
-
Ascending aorta
- CMR:
-
Cardiovascular magnetic resonance
- LV:
-
Left ventricle/left ventricular
- LVEDV:
-
Left ventricular end-diastolic volume
- LVEF:
-
Left ventricular ejection fraction
- LVESV:
-
Left ventricular end-systolic volume
- LVOT:
-
Left ventricular outflow tract
- PC:
-
Phase-contrast
- RV:
-
Right ventricle/right ventricular
- RVEDV:
-
Right ventricular end-diastolic volume
- RVEF:
-
Right ventricular ejection fraction
- RVESV:
-
Right ventricular end-systolic volume
- SV:
-
Stroke volume
- SVAAo :
-
Stroke volume measured at the ascending aorta (phase-contrast)
- SVLVOT :
-
Stroke volume measured at the LVOT (phase-contrast)
- SVVM :
-
Stroke volume measured using volumetric method
- TTE:
-
Transthoracic echocardiography
References
Nayak KS, Nielsen J-F, Bernstein MA, Markl M, D. Gatehouse P, M. Botnar R, et al. Cardiovascular magnetic resonance phase contrast imaging. J Cardiovasc Magn Reson. 2015;17(1).
Myerson SG. Heart valve disease: investigation by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:7.
Lotz J, Meier C, Leppert A, Galanski M. cardiovascular flow measurement with phase-contrast MR imaging: basic facts and implementation. Radiographics. 2002;22(3):651–71.
O’Brien KR, Cowan BR, Jain M, Stewart RAH, Kerr AJ, Young AA. MRI phase contrast velocity and flow errors in turbulent stenotic jets. J Magn Reson Imaging. 2008;28(1):210–8.
Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30(4):303–71.
O’Brien KR, Gabriel RS, Greiser A, Cowan BR, Young AA, Kerr AJ. Aortic valve stenotic area calculation from phase contrast cardiovascular magnetic resonance: the importance of short echo time. J Cardiovasc Magn Reson. 2009;11(1):49.
Chambers JB, Garbi M, Nieman K, Myerson S, Pierard LA, Habib G, et al. Appropriateness criteria for the use of cardiovascular imaging in heart valve disease in adults: a European Association of Cardiovascular Imaging report of literature review and current practice. Eur Heart J Cardiovasc Imaging. 2017;18(5):489–98.
Uretsky S, Argulian E, Narula J, Wolff SD. Use of cardiac magnetic resonance imaging in assessing mitral regurgitation: current evidence. J Am Coll Cardiol. 2018;71(5):547–63.
Garg P, Swift AJ, Zhong L, Carlhäll C-J, Ebbers T, Westenberg J, et al. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat Rev Cardiol. 2020;17(5):298–312.
Muzzarelli S, Monney P, O’Brien K, Faletra F, Moccetti T, Vogt P, et al. Quantification of aortic flow by phase-contrast magnetic resonance in patients with bicuspid aortic valve. Eur Heart J Cardiovasc Imaging. 2014;15(1):77–84.
Lee E, Richards B, Lu JC, Mahani MG, Dorfman AL, Balasubramanian S, et al. Phase-contrast magnetic resonance quantification of aortic regurgitation in patients with turbulent aortic flow. J Comput Assist Tomogr. 2019;43(2):317–22.
Capoulade R, Mahmut A, Tastet L, Arsenault M, Bédard E, Dumesnil JG, et al. Impact of plasma Lp-PLA2 activity on the progression of aortic stenosis: the PROGRESSA study. J Am Coll Cardiol Img. 2015;8(1):26–33.
Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30(4):372–92.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.
Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for non invasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303–71.
Garcia J, Kadem L, Larose É, Clavel MA, Pibarot P. Comparison between cardiovascular magnetic resonance imaging and transthoracic doppler echocardiography for the estimation of valve effective orifice area in patients with aortic stenosis. J Cardiovasc Magn Reson. 2011;13:25.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10.
Karamitsos TD, Myerson SG. The role of cardiovascular magnetic resonance in the evaluation of valve disease. Prog Cardiovasc Dis. 2011;54(3):276–86.
Krieger EV, Lee J, Branch KR, Hamilton-Craig C. Quantitation of mitral regurgitation with cardiac magnetic resonance imaging: a systematic review. Heart. 2016;88(23):1864–70.
Mehta NK, Kim J, Siden JY, Rodriguez-Diego S, Alakbarli J, Di Franco A, et al. Utility of cardiac magnetic resonance for evaluation of mitral regurgitation prior to mitral valve surgery. J Thorac Dis. 2017;9(Suppl 4):S246–56.
Myerson SG, D’Arcy J, Christiansen JP, Dobson LE, Mohiaddin R, Francis JM, et al. Determination of clinical outcome in mitral regurgitation with cardiovascular magnetic resonance quantification. Circulation. 2016;133(23):2287–96.
Iung B. A prospective survey of patients with valvular heart disease in Europe: the euro heart survey on valvular heart disease. Eur Heart J. 2003;24(13):1231–43.
Unger P, Pibarot P, Tribouilloy C, Lancellotti P, Maisano F, Iung B, et al. Multiple and mixed valvular heart diseases. Circulation: Cardiovasc Imaging. 2018;11(8).
Lee JC, Branch KR, Hamilton-Craig C, Krieger EV. Evaluation of aortic regurgitation with cardiac magnetic resonance imaging: a systematic review. Heart. 2018;104(2):103–10.
Garcia J, Barker AJ, Markl M. The role of imaging of flow patterns by 4D flow MRI in aortic stenosis. JACC Cardiovasc Imaging. 2019;12(2):252–66.
Archer GT, Elhawaz A, Barker N, Fidock B, Rothman A, van der Geest RJ, et al. Validation of four-dimensional flow cardiovascular magnetic resonance for aortic stenosis assessment. Sci Rep. 2020;10(1):10569.
Acknowledgements
Not applicable.
Funding
Dr. Guzzetti was supported by a research grant from the Quebec Heart & Lung Institute Foundation. Mr. Tastet was supported by a doctoral scholarship from Fonds de Recherche en Santé-Québec (FRSQ). Dr. Pibarot holds the Canada Research Chair in Valvular Heart Diseases and a Foundation Scheme Grant (FDN-143225 from the Canadian Institutes of Health Research); has received a grant from the Foundation of the Québec Heart and Lung Institute; has echocardiography core laboratory contracts with Edwards Lifesciences, for which he receives no direct compensation; and has a research contract with Medtronic. Dr. Clavel has core laboratory contracts with Edwards Lifesciences, for which she receives no direct compensation; and has received a research Grant from Medtronic. Dr. Clavel holds a New National Investigator award from the Heart and Stroke Foundation of Canada and an Early Career Investigator award from Canadian Institutes of Health Research; Dr Beaudoin has received funding from the Fonds de Recherche Québec-Santé, Canadian Institute for Health Research (Grant #399323) and from the Foundation of the Quebec Heart and Lung Institutes.
Author information
Authors and Affiliations
Contributions
HPR, EG and JB have acquired the data, performed the analyses and drafted the manuscript; MS, LT, EL, PB and MAC have contributed to data acquisition and performed important revisions to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Quebec Heart and Lung Institute and patients signed a written informed consent at the time of inclusion.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guzzetti, E., Racine, HP., Tastet, L. et al. Accuracy of stroke volume measurement with phase-contrast cardiovascular magnetic resonance in patients with aortic stenosis. J Cardiovasc Magn Reson 23, 124 (2021). https://doi.org/10.1186/s12968-021-00814-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12968-021-00814-4