Abstract
Background
The prognostic value of left atrial (LA) size and function in hypertrophic cardiomyopathy (HCM) is well recognized, but LA function is difficult to routinely analyze. Fast LA long-axis strain (LA-LAS) analysis is a novel technique to assess LA function on cine cardiovascular magnetic resonance (CMR). We aimed to assess the association between fast LA-LAS and adverse clinical outcomes in patients with HCM.
Methods
359 HCM patients and 100 healthy controls underwent routine CMR imaging. Fast LA-LAS was analyzed by automatically tracking the length between the midpoint of posterior LA wall and the left atrioventricular junction based on standard 2- and 4-chamber balanced steady-state free precession cine-CMR. Three strain parameters including reservoir strain (εs), conduit strain (εe), and active strain (εa) were assessed. The endpoint was set as composite adverse events including cardiovascular death, resuscitated cardiac arrest, sudden cardiac death aborted by appropriate implantable cardioverter-defibrillator discharge, and hospital admission related to heart failure.
Results
During an average follow-up of 40.9 months, 59 patients (19.7%) reached endpoints. LA strains were correlated with LA diameter, LA volume index (LAVI) and LA empty fraction (LAEF) (all p < 0.05). In the stepwise multivariate Cox regression analysis, εs and εe (hazard ratio, 0.94 and 0.89; p = 0.019 and 0.006, respectively) emerged as independent predictors of the composite adverse events. Fast LA εs and LA εe are stronger prognostic factors than LA size, LAVI and the presence of left ventricular late gadolinium enhancement.
Conclusions
Fast LA reservoir and conduit strains are independently associated with adverse outcomes in HCM.
Similar content being viewed by others
Background
Hypertrophic cardiomyopathy (HCM), characterized by unexplained increase in left ventricular (LV) wall thickness, is a common inherited cardiac disorder, with a prevalence of approximately 0.2% in the general population [1,2,3]. A recent retrospective cohort study involving 4893 adult HCM patients from seven European referral centers reported that during a median follow-up of 6.2 years, 14.7% patients reached the composite end point of all-cause mortality, heart transplant, and aborted sudden cardiac death (SCD), representing an excess mortality and morbidity compared to the general population [4]. However, identification of high risk HCM patients remains challenging.
The complex pathophysiology of HCM include LV outflow tract (LVOT) obstruction, LV diastolic dysfunction, mitral regurgitation, and myocardial ischemia [2, 5]. Left atrial (LA) enlargement and elevated LA pressure are consequences of these pathophysiologic processes and reduced LA compliance may be due to intrinsic atrial myopathy [6,7,8]. Several studies have reported that LA size is significantly associated with adverse clinical outcomes in patients with HCM; particularly relevant to the identification of patients at a risk of heart failure (HF)-related death [1, 9]. LA function could more better reflect LA pathophysiology and providing additional prognostic value in HCM [8].
Yang et al.found LA reservoir and conduit dysfunction before LA enlargement in patients with non-obstructive HCM by cardiovascular magnetic resonance (CMR) feature tracking (FT) [10]. More importantly, Hinojar et al.conducted a CMR-FT study and demonstrated that LA strain was associated adverse outcome [11]. But the CMR-FT algorithm automatically tracks 48 points of the LA based on various anatomic elements which is challenging to use with the complex LA anatomy, including the pulmonary veins and LA appendages [12,13,14].
To overcome this challenge, Leng et al.proposed a fast LA long-axis strain (LA-LAS) method for quantifying long LA deformation using routine two- and four-chamber cine-CMR image. Fast LA-LAS is less affected by the complex LA anatomy as it only involves the automatic tracking of 3 points. Compared with standard CMR-FT, they showed excellent reliability, comparability and 55% reduction in assessment time [13]. More importantly, they also confirmed that LA reservoir strain (εs) and conduit strain (εe) can provide incremental prognostic value in ST-segment elevation myocardial infarction [15].
Nevertheless, the correlation between LA-LAS and HCM prognosis remains unclear. In this study, we aimed to evaluate the prognostic value of LA-LAS, measured using a fast semi-automated CMR method, in patients with HCM.
Methods
Study population and design
The study population included HCM patients who underwent clinical CMR imaging between August 2011 and October 2017, and 100 healthy controls. Healthy subjects were chosen from our database to match the age of the HCM population [16]. The HCM diagnostic criteria recommended by the latest European Society of Cardiology guidelines were applied: normal LV size with LV wall thickness (WT) ≥ 15 mm in ≥ 1 LV myocardial segments that was not explained by the secondary cause of hypertrophy or WT of ≥ 13 mm with a family history of HCM [1]. The obstructive LVOT was defined as an instantaneous peak Doppler LVOT pressure gradient of ≥ 30 mm Hg with provocation or at rest [1]. The exclusion criteria included known causes of myocardial hypertrophy, contraindication for CMR, and atrial fibrillation at the time of imaging. The study protocol was approved by the Institutional Ethics Committee of West China Hospital, Sichuan University. Written informed consent was obtained from all study participants.
CMR imaging
All participants were examined using a 3 T CMR scanner (Magnetom Tim Trio; Siemens Healthineers, Erlangen, Germany) with a 32-channel phased array cardiac coil. The consecutive short-axis covering the entire LV and three long-axis (2-, 3-, and 4-chamber) cine series were acquired using balanced steady-state free precession (bSSFP) during breath-hold (field of view = 320–340 mm2; slice thickness = 8 mm with no gap; repetition time = 3.4 ms; echo time = 1.3 ms; flip angle = 50°; acquisition matrix = 256 × 144; acquired temporal resolution = 42 ms; spatial resolution = 1.4 × 1.3 mm2). Late gadolinium enhancement (LGE)-CMR was performed using an inversion recovery turbo fast low-angle shot sequence with phase-sensitive reconstruction and acquired 10–15 min after the intravenous administration of a contrast agent (Magnevist; Bayer Schering Pharma, Berlin, Germany, 0.15 mmol/kg body weight; flip angle = 20°; matrix = 256 × 144).
Imaging analysis
All images were analyzed using post-processing software (Medis suit 2.1, QMass 8.1, Qstrain 2.0, Medis, Leiden, the Netherlands). Biventricular volume and function, LV mass, maximal WT were assessed as previously described [16, 17]. The presence of LGE was visually assessed by two independent observers (YC and JS with more than 10 years of CMR experience) who were blinded to the clinical data. Semi-automated quantification of LGE was performed using the myocardium signal intensity of 6 standard deviations (SD) from the normal myocardium and calculated as the proportion of the LV myocardium, as described previously [16, 18].
LA measurements
LA size was measured at the end of the LV systolic phase on 3-chamber cine images. maximal LA volume (LAV) at LV end-systole (LAV max), LA volume at LV diastole before LA contraction (LAV p-ac) and minimal LA volume at LV end-diastole (LAV min) were measured using the biplane area-length method by tracing the LA endocardial contour manually in 2- and 4-chamber cine views with the exclusion of pulmonary veins and LA appendage. Total LA empty fraction (LAEF), passive LAEF, and active LAEF were calculated using LA volumetric parameters and were consistent with those used in our previous study [19].
Fast LA-LAS
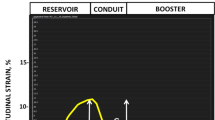
LA-LAS was acquired by semi-automated tracking of the length between the midpoint of posterior LA wall and the atrioventricular junction on cine-CMR 2- and 4-chamber views (Fig. 1). Analysis was performed using a custom-written program in Matlab software (R2017a, The Mathworks, Natick, MA, USA). The midpoint of posterior LA wall was selected at the intersection point of the LA long axis and posterior LA wall. The AV junctions were defined as the mitral valve insertion points at the inferior and anterior annular insertion points on the 2-chamber view and the lateral and septal borders of the annulus on the 4-chamber view. The AV junction points and the mid-point of the posterior LA wall were manually placed at end-diastole and were then automatically tracked on other cardiac phases using the method of template matching. The automatic tracking results were manually adjusted when these points failed to track on the cine images with slight artifacts. The analysis method of fast LA-LAS was adopted from the method published and validated against CMR-FT method [13].
Fast left atrial (LA) long-axis strain (LA-LAS) was measured in 2- and 4-chamber views. Three small squares were manually selected at three anatomical reference points that were automatically tracked in the cardiac cycle. D: the length between the midpoint of posterior LA wall and the left atrioventricular junction on standard cine-CMR 2-chamber (Danterior and Dinferior) and 4-chamber (Dseptal and Dlateral) views. ms millisecond
LA-LAS (ε) was calculated using the following formula: ε (t) = [D (t) − D0] × 100/D0 (t: time points in the cardiac cycle, 0: LV end-diastolic phase). Fast LA-LAS was assessed at three phases: εs, εe, and actrive strain (εa). Finally, mean values of LA strain in 2- and 4-chamber views were used for further analyses.
Reproducibility
To examine variability, a sample of 30 patients and 30 healthy controls were randomly selected for reproducibility assessment. For intra-observer variability, the same investigator analyzed LA-LAS of the selected sample at least two weeks later blinded to the first analysis results. For inter-observer variability, a different investigator who were blinded to the clinical data and results by the first observer reanalyzed the data.
Clinical follow-up
The follow-up period was the duration between the initial clinical evaluation, which was performed at the first CMR, and October 2019. Follow-up information was obtained from direct communication with patients or their family members. The combined outcome was defined as a composite of cardiovascular death, resuscitated cardiac arrest, SCD aborted by appropriate implantable cardioverter-defibrillator discharge (ICD), and hospital admission related to HF.
Statistical analysis
Categorical variables are expressed as N (%); continuous variables are expressed as mean ± SD or median with interquartile range. Student’s t-test was used to compare two normally distributed variables. For comparing the proportions of categorical variables, chi-square test and Fisher’s exact test were applied, as appropriate. The correlation between fast LA strains and other parameters was assessed using Pearson’s and Spearman’s correlation coefficients, as appropriate. Receiver operating curve analysis was used to determine optimal cut-off values. Survival curves were established using the Kaplan–Meier method and compared using the log-rank test. Hazard ratio (HR) and corresponding 95% confidence interval (CI) were calculated by the univariate Cox proportional hazards regression analysis. Variables with p < 0.1 in the univariate Cox regression analysis were included in the stepwise multivariate Cox regression analysis to identify independent factors. Inter- and intraobserver reproducibility of fast LA-LAS was evaluated by calculating the coefficient of variation (COV) and intraclass correlation coefficient (ICC). Two-tailed p < 0.05 indicated statistical significance. Statistical analysis was performed using MedCalc (version13.0, Ostend, Belgium) and SPSS (version17.0, Statistical Package for the Social Sciences, International Business Machines, Inc., Armonk, New York, USA).
Results
Baseline clinical characteristics
A total of 386 HCM patients were enrolled. After excluding 27 patients for poor cine images or arrhythmia, 359 patients with HCM and 100 healthy controls were included in the analysis. Baseline clinical and CMR data of patients with HCM and healthy controls are summarized in Tables 1 and 2. In comparison with healthy controls, HCM patients had larger LV volume, lower LV ejection fraction (LVEF), larger LV mass index (LVMI), lower right ventricular (RV) end-systolic volume index (RVESVI), larger LAV, and lower LAEF.
Fast LA strain measurements
The mean values of fast LA-LAS (εs, εe, and εa) in HCM patients were significantly lower than those of healthy controls (Table 3, all p < 0.01). Patients with the combined outcome showed significantly lower LA εs and LA εe than those without the combined outcome (Table 3, all p < 0.01).
Association among fast LA-LAS, LGE, and volumetric measurements
Fast LA-LAS showed strong correlation with LAEF (r: 0.79 to 0.67; Table 4, all p < 0.05), moderate correlation with LA diameter and LAV index (LAVI) (r: − 0.53 to − 0.32), and weak association with the extent of LGE ( r: − 0.17 to − 0.11). Using the upper limit for the LA anteroposterior dimension in the Chinese population of 41 mm as the LA diameter cutoff [19], the increased LA diameter group showed significantly decreased LA εs, LA εe, and LA εa (Table 3, all p < 0.01). Compared with healthy controls, fast LA-LAS (εs, εe, and εa) in patients with normal LA size (≤ 41 mm) was also significantly impaired (Fig. 2, all p < 0.01).
Outcome analysis
Fifty-nine patients achieved the combined outcome during an average follow-up of 40.9 ± 19.8 months, including 11 (18.6%) cardiovascular deaths, 2 (3.4%) SCD aborted by appropriate ICD discharge, 5 (8.5%) resuscitations after syncope, and 41 (69.5%) hospital admissions related to HF. In comparison to patients without the combined outcome, those with the combined outcome were older, had more obstructive HCM, lower LVEF, lower LV end-diastolic volume index (LVEDVI), lower LV end-systolic volume index (LVESVI), lower LVMI, higher LGE extent, larger LAV, and lower LAEF (all p < 0.05).
Based on receiver operating curve analysis, the best cutoff values for fast LA εs, εe, and εa to predict the combined outcome were 19.5% (AUC = 0.70), 8.1% (AUC = 0.72), and 10.6% (AUC = 0.59), respectively (all p < 0.05). Kaplan–Meier curves for the combined outcome indicated that patients with impaired LA εs, LA εe, and LA εa achieved a significantly higher rate of the combined outcome (p < 0.05, Fig. 3). Figure 4 shows comparisons of the combined outcome among patients with impaired LA εs, LA εe, and LA εa stratified based on the presence of LGE. Impaired LA εs, LA εe, and LA εa were associated with the combined outcome in patients with positive LGE (all p < 0.05). In the subgroup of LGE negative patients, those with impaired LA εs and LA εe also had a significantly higher rate of the combined outcome (all p < 0.05), but there was no significant difference in the survival rate in those LGE negative patients with LA εa > 10.6% and those with LA εa ≤ 10.6% (p = 0.93). Figure 5 demonstrates the subgroup survival analysis based on LA diameter. Impaired LA εs and LA εe significantly increased the risk of the combined outcome irrespective of LA diameter (all p < 0.05); however, no significant differences were found between patients with impaired LA εa or preserved LA εa in this subgroup (p = 0.17). We selected the upper limit of LAVI max and LAVI min as reference (59.1 ml/m2, 27.8 ml/m2, respectively) [19]. Figure 6 demonstrated that impaired LA εs and LA εe were significantly associated with a higher risk of the combined outcome in patients with LAVI max > 59.1 ml/m2, and LAVI min > 27.8 ml/m2 (Fig. 6a–d, p < 0.05), and in patients with LAVI max ≤ 59.1 ml/m2 and LAVI min ≤ 27.8 ml/m2 (Fig. 6g, h, all p < 0.05).
Graphs demonstrating cumulative event-free survival according to left atrial longitudinal strains in HCM patients with LA diameter > 41.0 mm (a–c) and LA diameter ≤ 41.0 mm (d–f). a, d grouped according to the cut-off value of εs; B and E, grouped according to the cut-off value of εe; and c, d grouped according to the cut-off value of εa. LA left atrial, εs reservoir strain, εe conduit strain, εa active strain
Graphs demonstrating cumulative event-free survival according to left atrial longitudinal strains in HCM patients with LAVI max > 59.1 ml/m2 (a, c), LAVI min > 27.8 ml/m2 (b, d), LAVI max ≤ 59.1 ml/m2 (e, g) and LAVI min ≤ 27.8 ml/m2 (f, h). a, b, e, f grouped according to the cut-off value of εs; c, d, g, h grouped according to the cut-off value of εe. LA left atrial, εs reservoir strain, εe conduit strain, LAVI max maximal left atrial volume index, LAVI min minimal left atrial volume index
Cox regression analysis
The results of univariate Cox regression analyses are summarized in Table 5. Fast LA εs and LA εe emerged as predictors of the combined outcome. The results of stepwise multivariate proportional hazard analyses for the combined outcome are summarized in Tables 6 and 7. When fast LA-LAS was not included as a covariate, age (HR, 1.05), LVMI (HR, 1.01), LGE extent (HR, 1.03), and LAVI min (HR, 1.01) were associated with the combined outcome (Model 1, all p < 0.05). When LA εs was included as a covariable to adjust Model 1, age (HR, 1.04), LVMI (HR, 1.01), LGE extent (HR, 1.03), and LA εs (HR, 0.94) were identified to be independent predictors of the combined outcome (Model 2, all p < 0.05). When LA εe was included as a covariable to adjust Model 1, age (HR, 1.03), LVEDVi (HR, 1.01), and LA εe (HR, 0.89) were associated with the combined outcome (Model 3, all p < 0.05). When all parameters of LA-LAS were included as covariables, age, LVEDVI and εe continued to be independent predictors of the combined outcome (Model 4, all p < 0.05). However, when LA εa was included as a covariable to adjust Model 1, εa was not an independent predictor of the combined outcome in the stepwise multivariate Cox regression analysis (Table 7).
Reproducibility of fast LA-LAS
Table 8 presents the results of the reproducibility of fast LA-LAS. Intra- and interobserver reproducibility of fast LA εs and LA εe were excellent (ICC: 0.95−0.96; COV: 7.2%–9.6%). ICC of fast LA εa was 0.87 and 0.88, respectively, and COV of fast LA εa was 9.8% and 10.5%, respectively.
Discussion
In this study, we report the prognostic value of semi-automated fast LA-LAS using standard cine-CMR images in HCM. First, we found that the mean values of fast LA εs, LA εe, and LA εa were impaired in patients with HCM compared to healthy controls; moreover, the values were more impaired in HCM patients with LA enlargement than in those with normal LA size. Second, fast LA εs, LA εe, and LA εa were found to be significantly associated with LA diameter, corresponding LAEF, and LAVI. Third, fast LA εs and εe were independently associated with adverse clinical outcomes in HCM patients, irrespective of the presence of LGE and presence of LA enlargement.
LA morphology and function are important prognostic parameters in HCM. In 2014, the European Society of Cardiology guidelines proposed that LA size should be considered as a risk factor to calculate the 5-year risk of SCD [1]. A number of studies have reported the relationship between LAV and function and long-term outcomes in patients with HCM [11, 20, 21]. In this study, we found a significant association between LA strain and LA diameter, corresponding LAEF, and LAVI.
Leng et al.demonstrated that LA εs, LA εe, and LA εa were impaired in patients with HCM [13], which is consistent with our results. Further, Kaplan–Meier survival analysis revealed that HCM patients with impaired LA εs, LA εe and LA εa were at an increased risk of adverse clinical outcomes. Vasquez et al. retrospectively studied 94 patients with HCM based on echocardiography and found that low LA reservoir and conduit strain were associated with adverse cardiovascular outcomes of HF, stroke, and death during a follow-up of 5.8 ± 3.3 years; however, they did not find a significant relationship between LA contractile strain and adverse cardiovascular outcomes [7]. Recently, Hinojar et al.conducted a CMR-FT study involving 75 patients with HCM and demonstrated that LA strain was associated with all-cause mortality, cardiovascular death, hospital admission related to HF, and lethal ventricular arrhythmias during a mean follow-up of 3.3 ± 1.2 years [11]. Our findings corroborate with those findings on the prognostic value of LA strain. Univariate and multivariate Cox regression analysis showed that fast LA εs and LA εe were significantly associated with adverse clinical outcomes of cardiovascular death, resuscitated cardiac arrest, SCD aborted by appropriate implantable cardioverter-defibrillator discharge, and hospital admission related to HF in patients with HCM (Table 6, Model 2, Model 3). However, we did not find a significant correlation between εa and adverse clinical outcomes. An increase in LA pressure due to pathophysiological changes mainly affected LA εs and εe in patients with HCM (Table 3). LA εa is the intrinsic function of the LA and mainly determined by LA intrinsic contractility [22]. The lower reproducibility of LA εa might be due to smaller amplitude of the strain values and therefore lower signal-to-noise.
LV LGE and LA size have been significantly correlated with adverse clinical outcomes in patients with HCM [9, 23,24,25]. Therefore, we performed subgroup survival analyses based on the presence of LV LGE and LA size. HCM patients with impaired εs and εe reached a significantly higher rate of the combined outcome, regardless of the presence of LV LGE. Irrespective of LA size, impaired εs and εe also significantly increased the risk of adverse clinical outcomes. However, the prognostic value of LA εa was not found in HCM patients with negative LGE and normal LA size. Moreover, Previous studies have demonstrated that LAVI min is associated with poor clinical prognoses [20, 26]. This finding is consistent with our results (Table 6, Model 1); however, the multivariate Cox regression model established after adding fast LA-LAS did not include LAVI min. The results of subgroup survival analyses based on LAVI showed that irrespective of whether LAVI max and LAVI min were normal or not, impaired LA εs and LA εe were significantly associated with a higher risk of the combined outcome. These results suggested that fast LA εs and LA εe are stronger prognostic factors than LA size, LAVI min, LAVI max, and the presence of LV LGE. Yang et al.have shown that patients with non-obstructive HCM have LA reservoir and conduit dysfunction before LA enlargement [18]. In our research, the LA-LAS also was impaired in HCM patients with normal LA size, and it can detect early changes of LA function in HCM. Therefore, fast LA εs and LA εe provided added prognostic value over LA size and LAV in HCM.
Limitations
This study has several limitations. First, this was a single-center study. Second, we did not include LA strain rate and diffuse LV tissue characteristics, such as native T1 and extracellular volume fraction in the multiparametric models. Thus, further studies are warranted to completely understand atrial pathophysiology and its interaction with ventricular tissue characteristics to validate our findings in a larger population of patients with HCM.
Conclusions
Semi-automated fast LA reservoir and conduit strains are independently associated with adverse clinical outcomes in HCM, irrespective of the presence of LGE and LA enlargement.
Availability of data and materials
The data will be available from the corresponding author on reasonable request.
Abbreviations
- εs:
-
Reservoir strain
- εe:
-
Conduit strain
- εa:
-
Active (Booster) strain
- AV:
-
Atrioventricular
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- bSSFP:
-
Balanced steady state free precession
- CMR:
-
Cardiovascular magnetic resonance
- CAD:
-
Coronary artery disease
- COV:
-
Coefficients of variation
- CI:
-
Confidence interval
- ECV:
-
Extracellular volume
- FT:
-
Feature tracking
- HCM:
-
Hypertrophic cardiomyopathy
- HF:
-
Heart failure
- HR:
-
Hazard ratio
- ICC:
-
Intra-class correlation coefficient
- ICD:
-
Implantable cardioverter-defibrillator
- LA:
-
Left atrium/left atrial
- LA-LAS:
-
Left atrial long-axis strain
- LAEF:
-
Left atrial emptying fraction
- LAV:
-
Left atrial volume
- LAVI:
-
Left atrial volume index
- LAVImin :
-
Minimum left atrial volume
- LAVImax :
-
Maximal left atrial volume index
- LAVI p-ac:
-
Left atrial volume index prior to atrial contraction
- LAVI min:
-
Minimal left atrial volume index
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle/left ventricular
- LVEDVI:
-
Left ventricular end-diastolic volume index
- LVEF:
-
Left ventricular ejection fraction
- LVESVI:
-
Left ventricular end-systolic volume index
- LVMI:
-
Left ventricular mass index
- LVOT:
-
LV outflow tract
- RV:
-
Right ventricular
- RVEDVI:
-
Right ventricular end-diastolic volume
- RVESVI:
-
Right ventricular end-systolic volume index
- SCD:
-
Sudden cardiac death
- SD:
-
Standard derivation
- WT:
-
Wall thickness
References
Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–79.
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2703–38.
Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–9.
Lorenzini M, Anastasiou Z, O’Mahony C, Guttman OP, Gimeno JR, Monserrat L, et al. Mortality among referral patients with hypertrophic cardiomyopathy vs the general European population. JAMA Cardiol. 2019. https://doi.org/10.1001/jamacardio.2019.4534.
Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–20.
Geske JB, Sorajja P, Nishimura RA, Ommen SR. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007;116:2702–8.
Vasquez N, Ostrander BT, Lu DY, Ventoulis I, Haileselassie B, Goyal S, et al. Low left atrial strain is associated with adverse outcomes in hypertrophic cardiomyopathy patients. J Am Soc Echocardiogr. 2019;32(593–603):e1.
Badran HM, Soltan G, Almeleigi R, Faheem N, Yacoub MH. Prognostic significance of left ventricular end diastolic pressure using E/E’ in patients with hypertrophic cardiomyopathy. Echocardiography. 2019. https://doi.org/10.1111/echo.14539.
Nistri S, Olivotto I, Betocchi S, Losi MA, Valsecchi G, Pinamonti B, et al. Prognostic significance of left atrial size in patients with hypertrophic cardiomyopathy (from the Italian Registry for Hypertrophic Cardiomyopathy). Am J Cardiol. 2006;98:960–5.
Yang YX, Yin G, Jiang Y, Song L, Zhao SH, Lu MJ. Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: a feasibility and reproducibility study. J Cardiovasc Magn Reson. 2020;22(1):1.
Hinojar R, Zamorano JL, Fernández-Méndez M, Esteban A, Plaza-Martin M, González-Gómez A, et al. Prognostic value of left atrial function by cardiovascular magnetic resonance feature tracking in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2019;12:1055–65.
Chirinos JA, Sardana M, Ansari B, Satija V, Kuriakose D, Edelstein I, et al. Left atrial phasic function by cardiac magnetic resonance feature tracking is a strong predictor of incident cardiovascular events. Circ Cardiovasc Imaging. 2018;11:e007512.
Leng S, Tan RS, Zhao X, Allen JC, Koh AS, Zhong L. Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2018;20:71.
Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505.
Leng S, Ge H, He J, Kong LC, Yang YN, Yan FH, et al. Long-term prognostic value of cardiac MRI left atrial strain in ST-segment elevation myocardial infarction. Radiology. 2020;296(2):299–309.
Yang FY, Wang J, Li YC, Li WH, Xu YW, Wan K, et al. The prognostic value of biventricular long axis strain using standard cardiovascular magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2019. https://doi.org/10.1016/j.ijcard.2019.08.010.
Wan K, Sun JY, Yang D, Liu H, Wang J, Cheng W, et al. Left ventricular myocardial deformation on cine MR images: relationship to severity of disease and prognosis in light-chain amyloidosis. Radiology. 2018;288:73–80.
Harrigan CJ, Peters DC, Gibson CM, Maron BJ, Manning WJ, Maron MS, et al. Hypertrophic cardiomyopathy: quantification of late gadolinium enhancement with contrast-enhanced cardiovascular MR imaging. Radiology. 2011;258:128–33.
Li WH, Wan K, Han YC, Liu H, Cheng W, Sun JY, et al. Reference value of left and right atrial size and phasic function by SSFP CMR at 30 T in healthy Chinese adults. Sci Rep. 2017;7:3196.
Shin SH, Jang JH, Baek YS, Kwon SW, Park SD, Woo SI, et al. Prognostic impact of left atrial minimal volume on clinical outcome in patients with non-obstructive hypertrophic cardiomyopathy. Int Heart J. 2018;59:991–5.
Losi MA, Betocchi S, Barbati G, Parisi V, Tocchetti CG, Pastore F, et al. Prognostic significance of left atrial volume dilatation in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2009;22:76–81.
Rosca M, Lancellotti P, Popescu BA, Pierard LA. Left atrial function: pathophysiology, echocardiographic assessment, and clinical applications. Heart. 2011;97:1982–9.
O’Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur Heart J. 2014;35:2010–20.
Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, et al. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9:1392–402.
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–95.
Wu VC, Takeuchi M, Kuwaki H, Iwataki M, Nagata Y, Otani K, et al. Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: comparison with 2D echocardiography. JACC Cardiovasc Imaging. 2013;6:1025–35.
Acknowledgement
Not applicable
Funding
We received funding from the National Natural Science Foundation of China (contract grant no.: 81571638), Key Research and Development Projects in Sichuan Province (2020YFS0123) and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC18013).
Author information
Authors and Affiliations
Contributions
YCC, FYY, and JW designed the study. FYY and JW collected clinical data, performed statistical analysis and drafted the manuscript. LTP, YWX and DY collected the data and performed statistical analysis. WHL and KW collected outcome data. JYS and YJZ contributed to the data analysis. YH made contributions to interpretation of the data and critically edited the manuscript. All author read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Ethics Committee of West China Hospital, Sichuan University. Written informed consent was obtained from all study participants.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, F., Wang, L., Wang, J. et al. Prognostic value of fast semi-automated left atrial long-axis strain analysis in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 23, 36 (2021). https://doi.org/10.1186/s12968-021-00735-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12968-021-00735-2