Abstract
Targeting non-coding RNAs (ncRNAs), including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), has recently emerged as a promising strategy for treating malignancies and other diseases. In recent years, the development of ncRNA-based therapeutics for targeting protein-coding and non-coding genes has also gained momentum. This review systematically examines ongoing and completed clinical trials to provide a comprehensive overview of the emerging landscape of ncRNA-based therapeutics. Significant efforts have been made to advance ncRNA therapeutics to early clinical studies. The most advanced trials have been conducted with small interfering RNAs (siRNAs), miRNA replacement using nanovector-entrapped miRNA mimics, or miRNA silencing by antisense oligonucleotides. While siRNA-based therapeutics have already received FDA approval, miRNA mimics, inhibitors, and lncRNA-based therapeutics are still under evaluation in preclinical and early clinical studies. We critically discuss the rationale and methodologies of ncRNA targeting strategies to illustrate this rapidly evolving field.
Similar content being viewed by others
Background
“Non-coding RNA (ncRNA)-based therapeutics” refer to therapeutic tools that utilize ncRNA molecules as drugs to target protein-coding or non-coding genes [1]. On the other hand, several strategies aim to consider ncRNAs as targets for therapeutic intervention [2]. RNA molecules produced by the non-coding regions constitute most of the genome (about 98%) and, despite being previously considered as non-functional “junk” DNA, are now recognized as crucial regulators of endogenous processes. Their alterations can cause pathological effects, making them suitable for clinical targeting [3].
NcRNAs are conventionally classified based on their length into small ncRNAs (< 200 nucleotides) [4], including the most studied family of microRNAs (miRNAs) [5], or long ncRNAs (lncRNAs) (> 200 nucleotides) [6, 7]. In recent years, the crucial role of various classes of ncRNAs in regulating gene expression and protein functions has emerged [8, 9]. Dysregulation of these molecules has been associated with several diseases, including cancer [10,11,12]. NcRNAs can affect cancer development by regulating key signaling pathways involved in cellular processes such as proliferation, apoptosis, invasion, and metastasis [13, 14]. However, while there is growing evidence on the relevance of ncRNA involvement in cancer onset, progression [15], and treatment sensitivity/response [16,17,18,19,20], the translation of this knowledge into novel therapeutics has been hampered by technical and regulatory constraints [3, 21].
Even though the clinical development of ncRNA therapeutics is still in its infancy, modulation of the ncRNA landscape has emerged as a promising anticancer strategy through different approaches [22,23,24]. These mainly include: i) small interfering RNAs (siRNAs) that can silence specific coding and non-coding genes by targeting and degrading the corresponding mRNA molecules [25, 26], ii) miRNA mimics or inhibitors, depending on the need to replace a tumor-suppressive miRNA or inhibit an oncogenic miRNA (onco-miR) [27, 28], iii) antisense oligonucleotides (ASOs), which are short, synthetic single-stranded nucleic acids that can bind to specific RNA molecules and modulate gene expression by blocking translation or promoting degradation of target RNA [29,30,31], iv) lncRNA targeting [32], for example, via CRISPR-Cas9 editing technology [33] or by small molecules that bind within their 3D shape with stabilizing or destabilizing effects [34,35,36], and v) manipulation of circular RNAs, a class of ncRNAs that forms covalently closed continuous loops [37].
Research in RNA therapeutics is rapidly evolving [38, 39], holding great promise for treating a wide range of diseases [1], including vascular disorders [40, 41], neurodegenerative diseases [42], and cancer [43,44,45,46]. Moreover, miRNAs and lncRNAs are emerging as biomarkers for several cancer types [47,48,49,50,51,52,53,54]. However, challenges such as effective delivery, risk of off-target effects, and safety concerns need to be further addressed for successful translation into more advanced clinical development paths [3].
Here, we review the most recent progress in the use of ncRNA therapeutics and strategies targeting ncRNAs for clinical purposes, including the initial evidence of safety and clinical activity in early clinical trials.
Molecular bases for intervention on non-coding RNAs
The clinical application of ncRNA-based therapeutics remains a significant challenge [55] due to issues with efficacy, specificity, and delivery in humans. These challenges have prompted the medical and scientific community to focus on different strategies to facilitate delivery into cells. These approaches include the use of lipid-based carriers such as lipid nanoparticles or liposomes (vesicles composed of lipid bilayers, designed for optimal encapsulation and protection of bioactive small ncRNAs [56]) as well as the modification of the chemical structure of ncRNAs-based drugs to enhance their stability and bioavailability [57].
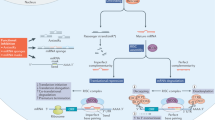
Here, we discuss the chemical and biological features of ncRNA drugs used in clinical trials, including siRNA, saRNA (small-activating RNA), miRNA mimics and inhibitors, and ASO-based strategies (Fig. 1). The rationale underlying the development of specific ncRNA therapeutics depends on the specific target [1]. For example: i) siRNAs are used for targeting cytoplasmic RNAs or triggering transcriptional silencing via histone modification and chromatin remodeling in the nucleus by binding to promoter regions [58], ii) saRNAs are preferred for counteracting the downregulation of silent tumor suppressor genes and triggering gene production by targeting promoter sequences [59], iii) miRNA mimics or inhibitors are administered when multitargeting is required, as they can bind different effectors in key pathways [60], and iv) ASOs efficiently target both nuclear or cytoplasmic mRNAs thanks to the ubiquitous distribution of RNase H1, which promotes ASO-mediated transcript degradation. ASOs may also modulate gene expression via occupancy-mediated mechanisms, alter RNA splicing using splice-switching ASOs to induce exon skipping or inclusion, inhibit miRNA binding to target mRNAs, lead to nonsense-mediated decay, and inhibit or activate translation [61].
Specific features of each strategy adopted in clinical settings are discussed below.
ncRNA therapeutics used in clinical trials (Created with BioRender.com)
Small interference RNA-based therapeutics
SiRNA-based therapeutics involve the use of synthetic siRNAs, which are duplexes of complementary RNA oligonucleotides that specifically target and silence gene expression post-transcriptionally [62]. They are also widely developed for silencing lncRNAs and circRNAs [63]. Due to their highly degradable structure, siRNAs for therapeutic use need to be encapsulated into lipid nanoparticles (LNPs) to achieve specific delivery into cells [64]. Various nanoparticles have been developed with different chemistries and are presently used through different administration routes such as nasal, cutaneous, subcutaneous, and more. Despite numerous siRNAs targeting mRNAs of oncogenes under investigation in cancer, only few LNP-siRNAs have recently received FDA approval [65]. Moreover, only two siRNAs targeting ncRNAs (lncRNAs and circRNAs) have been investigated in preclinical studies but have not yet reached clinical trials.
One example is the siRNA-LNP targeting lncRNA LINC01257, proposed as a novel and safe therapeutic approach for t(8;21) Pediatric Acute Myeloid Leukemia [66]. Another recently approved LNP-siRNA effectively targets Hsa_circ_0136666, identified as a competitive up regulator of the DNA-PK catalytic subunit (PRKDC) expression by sponging miR-375-3p. PRKDC binds and phosphorylates PD-L1, promoting its stability and thus tumor immune escape. The LNP-siRNA effectively induces the knocks down of Hsa_circ_0136666, improving anti-PD-L1 drug efficacy in an in vivo model of gastric cancer [67].
An EphA2-targeting DOPC-encapsulated siRNA (EphA2 siRNA) delivered via neutral liposome (1,2-dioleoyl-sn-glycero-3-phosphatidylcholine, DOPC) is a formulation under evaluation for safety and tolerability (toxicity profile) (NCT01591356). Other technologies include AtuPLEX, based on a siRNA-lipoplex directed against protein kinase N3 (PKN3), NBF-006 (evaluated in NCT03819387), a glutathione S-transferase Pi (GSTP) siRNA encapsulated within a LNP, and TKM-080301, an LNP formulation comprising four lipids and a synthetic, double-stranded siRNA directed against human PLK1 mRNA.
STP705 (NCT04844983) takes advantage of dual-targeted inhibitory properties to directly knock down both TGF-β1 and COX-2 gene expression. Based on polypeptide nanoparticle (PNP)-enhanced delivery and second-generation GalNAc conjugation, this product has received multiple Investigational New Drug (IND) approvals from both the U.S. Food and Drug Administration (FDA) and the Chinese National Medical Products Administration (NMPA), for the treatment of cholangiocarcinoma, non-melanoma skin cancer, and hypertrophic scars. Different pipeline programs for various products prioritized by STP705 are currently ongoing: late-stage clinical development for Squamous Cell Carcinoma in situ (isSCC), completion of phase 2 for Basal Cell Carcinoma (BCC), and phase 1 for fat remodeling. Additionally, STP705 has received Orphan Drug Designation for treating cholangiocarcinoma (CCA) and primary sclerosing cholangitis (PSC).
The approach used for APN401, a first-in-class personalized cellular therapy, involves manufacturing cells derived from patient blood cells where the APN401 siRNA blocks the intracellular master immune checkpoint Cbl-b, to strengthen tumor-specific immune reactivity and killing of tumor cells. The transfected cells are immediately re-infused into patients in an ambulatory setting [68].
siRNAG12D-LODER™ is a proprietary miniature biodegradable polymeric matrix containing siRNAs for the mutated KRAS oncogene, KRAS G12D (siG12D) [69], with potential antitumor activity. Upon intratumoral injection, siG12D is released locally, preventing the translation of KRAS proteins and potentially inhibiting the growth of tumor cells overexpressing KRAS, which is mutated in over 90% of human pancreatic ductal adenocarcinomas and is associated with tumor cell proliferation and reduced survival.
An original approach has been used to deliver RNA interference (RNAi) in GBM cells. NU-0129 is a brain-penetrant RNAi-based spherical nucleic acid (SNA) consisting of gold nanoparticle cores covalently conjugated with radially oriented and densely packed siRNA oligonucleotides specific for the GBM oncogene Bcl2Like1270.
Small activating RNA-based therapeutics
The saRNA MTL-CEBPA is a first-in-class saRNA oligonucleotide drug designed to upregulate the transcription factor C/EBP-α. Encapsulated in SMARTICLES liposomal nanoparticles, this drug acts as a master regulator of hepatic and myeloid functions as well as multiple oncogenic processes [71].
A unique approach has been used to develop TB1-1301. This is a gene-modified T cell product containing a NY-ESO-1 specific T cell receptor (TCR) introduced via the MS3II-NY-ESO1-SiTCR retroviral vector. This vector encodes TCR α and β chains that recognize a NY-ESO-1 derived epitope (amino acids 157–165: SLLMWITQC) presented in the context of HLA-A*02:01 and HLA-A*02:06 molecules. Additionally, the vector encodes siRNAs homologous to the constant region sequence of the endogenous, but not the transduced, TCR α and β chain mRNAs. These siRNAs increase the expression of the transduced TCR [72].
MicroRNA-based therapeutics
MiRNAs are the most extensively studied class of ncRNAs for therapeutic purposes [60, 73, 74]. MiRNA-based therapies can regulate miRNA expression through two distinct strategies: i) replacing downregulated miRNAs and ii) inhibiting upregulated miRNAs to reestablish their basal levels. Unlike siRNAs, which selectively suppress the expression of a specific target mRNA, miRNAs have the ability to target multiple genes, thereby aiding in the regulation of entire pathways [75].
The first strategy involves designing synthetic oligonucleotides that mimic endogenous miRNAs once they reach the cells. This requires optimizing their effectiveness, specificity, and delivery methods. To meet these challenges, various delivery systems have been developed, including polymeric vectors, lipid-based carriers, biomaterials with different charges (positive, negative, or neutral), and inorganic materials.
Several encapsulated miRNAs have progressed in clinical development. In 2017, two phase 1 clinical studies explored the safety of MRX34 and TargomiRs. MRX34, sponsored by Mirna Therapeutics, Inc., is a miR-34a encapsulated in liposomal vesicles. Although the trial was closed early due to serious immune-mediated adverse events, a dose-dependent modulation of relevant target genes was demonstrated [76].
TargomiRs are minicells (EnGeneIC Dream Vectors) loaded with miR-16-based mimics to counteract the loss of miR-15 and miR-16 family miRNAs in mesothelioma cells. These TargomiRs target EGFR, which is expressed in 44–97% of malignant pleural mesothelioma cells [77]. Specifically, bacterial minicells are anucleate nanoparticles produced by inactivating the genes that control normal bacterial cell division, allowing efficient packaging of cytotoxic drugs and internalization into cancer cells [78].
Recently, a phase 1 study evaluated the toxicity and pharmacokinetics of INT-1B3, a LNP-formulated miR-193a-3p, in advanced solid tumors (NCT04675996). These third-generation LNPs were developed by InteRNA Technologies, a company specializing in cancer therapeutics based on a proprietary technology platform for the rapid identification and validation of therapeutic miRNAs.
A significant challenge in using miRNAs as therapeutics has been addressed by introducing multiple chemical modifications to miRNA mimics oligonucleotides, enhancing their stability, delivery, and efficient cellular uptake in vivo. Among synthetic molecules, morpholino-miRNAs have been developed. These are synthetic neutral-charged oligomers with backbones composed of morpholine rings connected by phosphorodiamidate linkages, providing stability and preventing enzymatic degradation. Although morpholino-miRNAs specifically bind to RNA target sequences, extensive exploratory work with these molecules has not yet led to clinical evaluation.
The strategy of inhibiting upregulated miRNAs primarily uses ASOs, which have proven to be a powerful approach for targeting both coding and ncRNAs [79].
Antisense oligonucleotide-based therapeutics
Antisense technology involves identifying Watson-Crick base pairing sites on target RNA to modify gene expression [80]. Synthetic single-strand antisense oligonucleotides (ssASOs) are RNA or DNA molecules, typically 13–22 nucleotides long, which bind to complementary nucleic acid sequences, impairing their function. This emerging class of drugs directly regulates disease-causing genes and their variants.
Like miRNA mimics, translating ASOs into clinical successes requires continuous technological advancements to overcome formulation, pharmacological, and toxicological challenges. Several chemical modifications of the ASO backbone have been implemented, including phosphorothioates (PS), locked nucleic acids (LNA), morpholinos, 2’-O-methoxyethyl (2’-MOE), and peptide nucleic acids (PNAs), to enhance efficient nucleic acid targeting [81].
In this section, we provide an overview of antisense technology used to develop oligonucleotides under clinical trial evaluation.
PS modification involves replacing one of the non-bridging oxygen atoms in the phosphate backbone with a sulfur atom. This modification improves pharmacokinetics (PK) and increases in vivo efficacy. PS-modified ASOs are typically introduced alongside other structural changes, such as LNA. LNA modification involves altering the ribose ring of the nucleotide with a 2’-O,4’-C-methylene bridge, creating a “locked” conformation. This enhances the stability and binding affinity of LNA-oligonucleotides to their complementary RNA or DNA targets.
Recent designs of ASOs include gapmers, which contain a central block of DNA nucleotides flanked by LNA sequences. Gapmers are used for gene silencing by stimulating RNA cleavage through the recruitment of RNase H. The key features of LNA oligonucleotides include enhanced stability, as the locked conformation increases the thermal melting point of the oligonucleotide when bound to its complementary sequence, and high binding affinity, leading to increased specificity. LNA-ASOs with fully PS-modified backbones are particularly suitable for therapeutic use. The increased thermal stability of LNA-oligonucleotide and target duplexes makes them more resistant to nuclease degradation, contributing to a prolonged half-life in biological systems. Additionally, the higher binding affinity and stability translate into increased potency and efficacy of LNA-PS-modified ASOs with efficient and specific target binding, crucial for therapeutic success.
It is notable that LNA modifications provide better discrimination between mismatched and perfectly matched base pairs. This increased specificity is advantageous in preventing off-target effects and minimizing unintended interactions with non-target RNA sequences, an essential consideration for minimizing toxicities in therapeutic applications [82].
The first LNA inhibitor explored in a pilot trial was EZN-2968 (NCT00466583), targeting hypoxia-inducible factor-1 alpha (HIF-1α) mRNA. Later, LNA antisense technology was successfully applied to target ncRNAs beyond cancer applications. Both ssASOs and double-strand ASOs (siRNAs) targeting coding RNA have received clinical approval for various diseases, while ASOs targeting ncRNA candidates are only recently undergoing early clinical trials.
The first LNA inhibitor of an oncogenic ncRNA evaluated in patients with refractory solid tumors was LNA-i-miR-221, a 13-mer LNA inhibitor of miR-221 with a fully PS-modified backbone [83, 84] developed for clinical use. This agent demonstrated anti-tumor activity against human xenografts in mice [85] and favorable toxicokinetics in rats [86] and monkeys [47]. Importantly, the promising biomodulator showed anti-tumor activity in a recently completed first-in-human clinical trial, along with an excellent safety profile in treated cancer patients [87, 88].
ASOs are known to undergo rapid renal clearance, which has delayed their clinical translation. However, the PK properties of LNA-i-miR-221, including binding to plasma proteins and reduced clearance [48, 49], result in favorable PK suitable for multiple allometric interspecies scaling methods successfully used to infer human pharmacokinetics. This early trial has paved the way for a wider clinical evaluation of ncRNA inhibitors in cancer treatment.
Although LNA technology offers significant advantages, it is important to note that the choice of chemical modification depends on the specific requirements of the intended application. Different modifications may be preferred based on factors such as the targeted RNA sequence, delivery method, and therapeutic goals.
Emerging picture of early clinical trials based on ncRNA-therapeutics in cancer
In the timeframe from 2016 to 2023, according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement, a comprehensive search from available online databases (PubMed, Embase, MedLine, ClinicalTrial.gov) was performed, using following keyword: “RNA therapeutics”, “non-coding RNA” OR “ncRNA”, “microRNA” or “miRNA”, “safety”, “activity”, “circulating RNA” OR “circRNA”, “small interference RNA” or “siRNA”, “long non-coding RNA” OR “lncRNA”, “clinical trial”, “refractory”, “advanced solid tumor” OR “advanced solid tumour”, “recurrent”, “dose-escalation” OR “dose escalation”, “first-in-human” OR “first-in-man”, “dose finding”, “phase”, “cancer”, “cell engineering” and others as specified in Fig. 2. Two investigators (AC and FL) independently performed the consultation and records selection, and disagreements were solved after consensus.
Eligibility criteria for studies inclusion were clinical trials enrolling patients with cancer treated with ncRNA therapeutics. Ongoing and completed phase 1, 2 or 3 trials were included. Exclusion criteria were preclinical trials only, non-English reports, no RNA therapeutic involvement, review and/or meta-analysis articles, studies that investigated only diagnostic, prognostic and/or predictive ncRNAs’ role. PICO (patient/population, intervention, comparison and outcomes) design of Inclusion criteria is reported in Table 1.
23 trials emerged from our systematic review (Fig. 2): 7 ongoing and 1 suspended clinical trials, 14 completed clinical trials, 1 clinical trial of unknown status. No additional studies have been recorded on manual search.
Solid and/or hematological malignancies were involved (Fig. 3).
Pie chart showing the status of ncRNA therapeutics – based clinical trial emerged from our systematic review. Histogram bars show the number of studies for each tumor type (Partially created with BioRender.com)
Several studies were observational but, especially among the more recent, there were interventional trials, almost all of phase 1, investigating the therapeutic role of molecules like miRNA mimics, siRNAs, and LNA-ASO. The most common point among these studies was the therapeutic use of ncRNA technology for the treatment of refractory/relapsed solid cancer patients (Table 2).
Completed trials
In a recently published phase 0 clinical trial, Kumthekar et al. demonstrated the efficacy of siBcl2L12-SNA (NU-0129) due to its capability of brain penetration in patients with refractory glioblastoma (GBM) [70]. This trial revealed that siRNA NU-0129 uptake by GBM cells decreases the levels of the oncogene Bcl2L12, demonstrating that nanoconjugates could benefit precision medicine treatments for GBM.
Lavie et al., in a phase 1/2a trial, demonstrated the safety and potential efficacy of BC-819 (H19-DTA) in patients with ovarian or primitive peritoneal cancer with hyperexpression of the riboregulator ncRNA H19, who were considered platinum refractory/resistant [89]. BC-819 is a double-stranded DNA plasmid that drives the expression of the diphtheria toxin A (DT-A) gene under the regulation of the H19 promoter sequence. The use of a ncRNA overexpressed in tumor cells as a regulator of DT-A expression ensures the selective initiation of the toxin as a targeted treatment and allows the stratification of patients that may benefit from the therapy according to H19 expression. Although the best response according to RECIST criteria was stable disease, the median survival was about 6.5 months in the experimental cohort (dose of BC-819 administered 240 mg), which appears promising in this unfavorable setting.
Hong et al., in a dose-escalation phase 1 clinical trial, evaluated the role of a liposomal mimic of miR-34a (MRX34) in patients with refractory solid tumors. This study aimed to determine the recommended phase 2 dose of MRX34 and evaluate its safety in patients with advanced solid tumors, with dexamethasone premedication. The safety profile was considered acceptable, especially with dexamethasone premedication, even if four deaths occurred during the trial. The recommended dose for a phase 2 clinical trial was defined at 70 mg/m² and 93 mg/m² for hepatocellular carcinoma (HCC) and non-HCC solid tumors, respectively [90]. This ncRNA-therapeutic was also tested by Beg et al. in a published phase 1 clinical trial, which recruited patients with refractory solid tumors, including HCC. The maximum tolerated dose (MTD) was 110 mg/m² for non-HCC patients, with good antitumor activity and a safety profile enhanced by dexamethasone premedication. The phase 1 clinical trial has reached completion [76].
Ishihara et al. tested TBI-1301 in patients with refractory solid tumors and enhanced expression of NY-ESO-1. This trial demonstrated a good safety profile, also confirming that the administration of T cells with silenced endogenous TCR and the enhanced affinity of NY-ESO-1 TCR-T cells was safe, with the exception of the incidence of grade 3 lung toxicity [72].
El Dika et al. used TKM-080301, LNP containing a siRNA that targets the PLK1 gene product, in patients with primary or secondary liver cancer in good clinical conditions (Performance Statis, according to ECOG scale, < 2, Child-Pugh class A). The MTD was reached at 0.6 mg/kg with a favorable safety profile, albeit with limited anti-tumor activity [91].
Van Zandwijk et al., in a phase 1 clinical trial, used TargomiRs in weekly administrations in patients with malignant pleural mesothelioma. The safety profile was good. These results suggest a potential role for TargomiRs in combination with chemotherapy or immune checkpoint inhibitors (ICI) [77].
Sarker D. et al. in the phase 1 trial investigates the effect mediated by the saRNA MTL-CEBPA, that upregulates transcription factor C/EBP-α (CCAAT/enhancer-binding protein alpha), alone or in combination with sorafenib in HCC [71].
Tassone et al., in a recent dose-escalation, first-in-human phase 1 clinical trial, investigated both the safety profile and the antitumor activity of LNA-i-miR-221, an original LNA inhibitor of miR-221, a miRNA well known for its oncogenic role, for the first time in cancer patients with advanced refractory solid tumors. The safety profile was excellent, and the MTD was not reached. Interesting data emerged from the evaluation of antitumor activity, with tumor regression and a maintained major response in a colon cancer patient. Favorable pharmacokinetic and pharmacodynamic profiles were also demonstrated, with in vivo modulation of canonical miR-221 targets [87].
Triozzi et al. conducted a phase 1 trial that disclosed the potential role of APN401 (suspension of autologous peripheral blood mononuclear cells transfected with a siRNA that knocks down Cbl-b), with promising feasibility and safety in pancreatic, colorectal, and renal cell cancer [68].
Golan et al., in a phase 1 trial, studied the role of siRNAG12D-LODER™ targeting KRAS G12D in combination with chemotherapy in locally advanced pancreatic cancer. The authors demonstrated a CA 19.9 decrease in 70% of patients, a median overall survival of 15.12 months, and an 18-month survival of 38.5%69.
Schultheis et al. designed a randomized phase 1/2 trial in which siRNA Atu027, which inhibits protein kinase N3 in vascular endothelium, was administered with two different schedules (0.235 mg/kg once or twice weekly), in combination with gemcitabine in pancreatic cancer, showing increased outcomes in metastatic disease [92].
In the trial NCT00466583, the LNA EZN-2968 directed against hypoxia-inducible factor 1α was evaluated in solid tumors and lymphoma. NCT00285103 is a phase 1/2 trial in which the activity of SPC2996, an LNA targeting Bcl-2, was evaluated in chronic lymphocytic leukemia. To our knowledge, no results from these two clinical trials have been published to date, despite the studies concluding in 2011 and 2012, respectively.
Ongoing or suspended trials
From our systematic review, 7 studies emerged as ongoing and 1 as suspended trial. Five of these trials are investigating siRNAs, 3 small activating RNAs (saRNAs) and one miRNA: i) the phase 1 trial NCT01591356 is conducted in various solid neoplasms and investigates the effect of a siRNA, delivered via neutral liposome (1,2-dioleoyl-sn-glycero-3-phosphatidylcholine or DOPC), targeting EphA2, a receptor belonging to ephrins’ family; ii) NCT04675996 is a phase 1 trial in which INT-1B3, a LNP formulated miRNA (miR-193a-3p) mimic, is investigated in advanced solid neoplasms. This trial was suspended for insufficient fundings; iii) NCT05499013 is a phase 1/2 trial investigating SLN124, GaLNAc conjugated 19-mer siRNA that targets tmprss6, in polycythemia vera; iv) the phase 1 trial NCT03608631 evaluates mesenchymal stromal cells-derived exosomes with a siRNA directed against KRAS G12D (iExosomes) in pancreatic cancer; v) NCT03819387 is a phase 1 trial that investigates siRNA NBF-006, targeting Glutathione s-trasìnsferase Pi (GSTP), in pancreatic, colorectal and non-small cell lung cancer; vi) NCT04844983 phase 2 trial focuses on siRNA STP705, directed against transforming growth factor-beta 1 (TGF-beta 1) and cyclooxygenase-2 (COX-2), in squamous cell carcinoma in situ. The treatment with saRNA MTL-CEBPA is under investigation in the phase 1 trial NCT04105335, in combination with pembrolizumab in advanced solid tumors vii), and in the comparative phase 2 trial NCT04710641 in combination with sorafenib versus sorafenib alone in HCC viii).
Trial with unknown status
Among the trials of unknown status, NCT01676259 is a study investigating siG12D-LODER, miniature biodegradable bio polymeric matrix that encompasses a siRNA directed against KRAS G12D, in combination with chemotherapy, in pancreatic cancer.
Conclusions
NcRNA therapeutics require complex interdisciplinary investigation to develop optimal RNA-based agents for clinical evaluation, including assessments of pharmacokinetics, pharmacodynamics, biomodulatory activity, off-target effects, safety, and anti-tumor efficacy. Significant pre-clinical advancements are now being translated into clinical trials, with the success of these translations closely linked to the safety, specificity, and delivery systems of new agents. Early trials in antagonizing miRNAs are encouraging, showing promising results in terms of primary endpoint achievement, including safety, preliminary antitumor activity, and effectiveness in biomodulatory activity. An additional crucial advantage is the affordable scale-up and the potential to rapidly produce enhanced second-generation therapeutics. This opens an exciting scenario for the development of ncRNA therapeutics, either as single agents or in combination with conventional drugs to overcome resistant/refractory disease. We believe our systematic review, provides novel information and a comprehensive view of the current landscape. This can help understand the advantages and limitations of ncRNA therapeutics in clinical use. A major challenge in early experimental therapeutics is the heterogeneity in study design, route of administration, biomonitoring, and endpoint definition, necessitating strong cooperative efforts. Nevertheless, the emerging scenario is highly promising, and significant advancements are expected in the near future across all pioneering areas of RNA therapeutics.
Data availability
N/A.
Abbreviations
- ASO:
-
Antisense oligonucleotide
- CCA:
-
Cholangiocarcinoma
- circRNAs:
-
Circular RNAs
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- GBM:
-
Glioblatoma Multiforme
- HCC:
-
Hepatocellular Carcinoma
- ICI:
-
Immune Checkpoint Inhibitors
- IND:
-
Investigational New Drug
- LNA:
-
Locked Nucleic Acid
- LNP:
-
Lipid Nanoparticle
- lncRNAs:
-
Long Non-Coding RNAs
- miRNA:
-
MicroRNA
- MTD:
-
Maximum Tolerated Dose
- ncRNA:
-
Non-Coding RNA
- NMPA:
-
National Medical Products Administration
- PICO:
-
Patient/population, Intervention, Comparison and Outcomes
- PNA:
-
Peptide Nucleic Acid
- PNP:
-
Polypeptide Nanoparticle
- saRNA:
-
Small Activating RNA
- siRNA:
-
Small Interfering RNAs
- SNA:
-
Spherical Nucleic Acids
- ssASO:
-
Single-Strand ASO
- TCR:
-
T cell receptor
References
Zhu Y, Zhu L, Wang X, Jin H. RNA-based therapeutics: an overview and prospectus. Cell Death Dis. 2022;13:1–15.
Chen BQ et al. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct Target Ther 7, (2022).
Winkle M, El-Daly SM, Fabbri M, Calin GA. Noncoding RNA therapeutics - challenges and potential solutions. Nat Rev Drug Discov. 2021;20:629–51.
Li X, Peng J, Yi C. The epitranscriptome of small non-coding RNAs. Non-coding RNA Res. 2021;6:167–73.
Tomasello L, Distefano R, Nigita G, Croce CM. The MicroRNA Family gets wider: the IsomiRs classification and role. Front cell Dev Biol 9, (2021).
Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118.
Mattick JS, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24:430–47.
Yi Q et al. Recent advances of exosomal circRNAs in cancer and their potential clinical applications. J Transl Med 21, (2023).
Zhang P, Wu W, Chen Q, Chen M. Non-coding RNAs and their Integrated Networks. J Integr Bioinform 16, (2019).
Good DJ. Non-coding RNAs in Human Health and diseases. Genes (Basel). 14, (2023).
Zhang Z, Zhang J, Diao L, Han L. Small non-coding RNAs in human cancer: function, clinical utility, and characterization. Oncogene. 2021;40:1570–7.
Bhatti GK, et al. Emerging role of non-coding RNA in health and disease. Metab Brain Dis. 2021;36:1119–34.
Liao Y et al. Non-coding RNAs in lung cancer: emerging regulators of angiogenesis. J Transl Med 20, (2022).
Parol M, Gzil A, Bodnar M, Grzanka D. Systematic review and meta-analysis of the prognostic significance of microRNAs related to metastatic and EMT process among prostate cancer patients. J Transl Med 19, (2021).
Grillone K et al. Non-coding RNAs in cancer: platforms and strategies for investigating the genomic ‘dark matter’. J Exp Clin Cancer Res 39, (2020).
Di Martino MT et al. miRNAs and lncRNAs as Novel therapeutic targets to Improve Cancer Immunotherapy. Cancers (Basel). 13, (2021).
Caracciolo D, et al. The potential role of miRNAs in multiple myeloma therapy. Expert Rev Hematol. 2018;11:793–803.
Caracciolo D et al. miR-22 modulates Lenalidomide Activity by counteracting MYC addiction in multiple myeloma. Cancers (Basel). 13, (2021).
Fang Y, Zhang XL, Huang HF, Zeng Z. The interplay between noncoding RNAs and drug resistance in hepatocellular carcinoma: the big impact of little things. J Transl Med 21, (2023).
Zhang M et al. Comprehensive characterization of stemness-related lncRNAs in triple-negative breast cancer identified a novel prognostic signature related to treatment outcomes, immune landscape analysis and therapeutic guidance: a silico analysis with in vivo experiments. J Transl Med 22, (2024).
Hueso M et al. ncRNAs in therapeutics: challenges and limitations in Nucleic Acid-based drug delivery. Int J Mol Sci 22, (2021).
Damase TR et al. The limitless future of RNA therapeutics. Front Bioeng Biotechnol 9, (2021).
Kim YK. RNA therapy: rich history, various applications and unlimited future prospects. Exp Mol Med. 2022;54:455–65.
Feng R, Patil S, Zhao X, Miao Z, Qian A. RNA therapeutics - research and clinical advancements. Front Mol Biosci 8, (2021).
Friedrich M, Aigner A. Therapeutic siRNA: state-of-the-art and future perspectives. BioDrugs. 2022;36:549–71.
Chen J, et al. Therapeutic efficacy of a novel self-assembled immunostimulatory siRNA combining apoptosis promotion with RIG-I activation in gliomas. J Transl Med. 2024;22:1–18.
Iacomino G, miRNAs. The Road from Bench to Bedside. Genes (Basel). 14, (2023).
Hanna J, Hossain GS, Kocerha J. The potential for microRNA therapeutics and clinical research. Front Genet 10, (2019).
Bartolucci D, Pession A, Hrelia P, Tonelli R. Precision Anti-Cancer Medicines by Oligonucleotide Therapeutics in Clinical Research Targeting Undruggable Proteins and Non-Coding RNAs. Pharmaceutics 14, (2022).
Quemener AM et al. The powerful world of antisense oligonucleotides: from bench to bedside. Wiley Interdiscip Rev RNA 11, (2020).
Xiong H, Veedu RN, Diermeier SD. Recent advances in Oligonucleotide therapeutics in Oncology. Int J Mol Sci 22, (2021).
Jiang M-C, Ni J-J, Cui W-Y, Wang B-Y, Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9:1354.
Mahato RK, et al. Targeting long non-coding RNAs in cancer therapy using CRISPR-Cas9 technology: a novel paradigm for precision oncology. J Biotechnol. 2024;379:98–119.
Zhao R, Fu J, Zhu L, Chen Y, Liu B. Designing strategies of small-molecule compounds for modulating non-coding RNAs in cancer therapy. J Hematol Oncol 15, (2022).
Rocca R et al. Hit identification of novel small molecules interfering with MALAT1 triplex by a structure-based virtual screening. Arch Pharm (Weinheim). 356, (2023).
Scionti F et al. TERRA G-quadruplex stabilization as a new therapeutic strategy for multiple myeloma. J Exp Clin Cancer Res 42, (2023).
He AT, Liu J, Li F, Yang BB. Targeting circular RNAs as a therapeutic approach: current strategies and challenges. Signal Transduct Target Ther 6, (2021).
Rocca R et al. Targeting non-coding RNAs: perspectives and challenges of in-silico approaches. Eur J Med Chem 261, (2023).
Bayraktar E et al. Targeting miRNAs and other non-coding RNAs as a Therapeutic Approach: an update. Non-coding RNA 9, (2023).
Hung J, Miscianinov V, Sluimer JC, Newby DE, Baker AH. Targeting non-coding RNA in Vascular Biology and Disease. Front Physiol 9, (2018).
Greco S et al. Noncoding RNAs implication in cardiovascular diseases in the COVID-19 era. J Transl Med 18, (2020).
Nguyen LD, Chau RK, Krichevsky AM. Small molecule drugs targeting non-coding RNAs as treatments for Alzheimer’s Disease and related dementias. Genes (Basel). 12, (2021).
Ito M, Miyata Y, Okada M. Current clinical trials with non-coding RNA-based therapeutics in malignant diseases: a systematic review. Transl Oncol 31, (2023).
Ascrizzi S et al. Lynch Syndrome Biopathology and Treatment: the potential role of microRNAs in clinical practice. Cancers (Basel). 15, (2023).
Di Martino MT, et al. miR-221/222 as biomarkers and targets for therapeutic intervention on cancer and other diseases: a systematic review. Mol Ther Nucleic Acids. 2022;27:1191–224.
Li J, He D, Bi Y, Liu S. The emerging roles of exosomal miRNAs in breast Cancer progression and potential clinical applications. Breast cancer (Dove Med Press. 2023;15:825–40.
Cantafio MEG et al. Pharmacokinetics and pharmacodynamics of a 13-mer LNA-inhibitor-miR-221 in mice and non-human Primates. Mol Ther Nucleic Acids 5, (2016).
Franzoni S et al. Development and validation of bioanalytical methods for LNA-i-miR-221 quantification in human plasma and urine by LC-MS/MS. J Pharm Biomed Anal 188, (2020).
Franzoni S, et al. Development and validation of a bioanalytical method for quantification of LNA-i-miR-221, a 13-mer oligonucleotide, in rat plasma using LC-MS/MS. J Pharm Biomed Anal. 2018;150:300–7.
Beylerli O, Gareev I, Sufianov A, Ilyasova T, Guang Y. Long noncoding RNAs as promising biomarkers in cancer. Non-coding RNA Res. 2022;7:66–70.
Sabato C et al. A novel microRNA signature for the detection of melanoma by liquid biopsy. J Transl Med 20, (2022).
Fridrichova I, et al. Mir-497-5p decreased expression Associated with High-Risk Endometrial Cancer. Int J Mol Sci. 2020;22:1–18.
Badowski C, He B, Garmire LX. Blood-derived lncRNAs as biomarkers for cancer diagnosis: the Good, the bad and the Beauty. NPJ Precis Oncol 6, (2022).
Sherif S, et al. Immune-related 3-lncRNA signature with prognostic connotation in a multi-cancer setting. J Transl Med. 2022;20:1–20.
Nemeth K, Bayraktar R, Ferracin M, Calin GA. Non-coding RNAs in disease: from mechanisms to therapeutics. Nat. Rev. Genet 2023;253 25:211–232 (2023).
Zhang X, Hai L, Gao Y, Yu G, Sun Y. Lipid nanomaterials-based RNA therapy and cancer treatment. Acta Pharm Sin B. 2023;13:903–15.
Zhao Y, Shu R, Liu J. The development and improvement of ribonucleic acid therapy strategies. Mol Ther Nucleic Acids. 2021;26:997–1013.
Matzke MA, Birchler JA. RNAi-mediated pathways in the nucleus. Nat Rev Genet. 2005;6:24–35.
Li LC et al. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl. Acad. Sci. U. S. A 2006;103:17337–17342.
Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet. 2022;38:613–26.
Crooke ST, Baker BF, Crooke RM, Liang X. Hai. Antisense technology: an overview and prospectus. Nat Rev Drug Discov. 2021;20:427–53.
Lam JKW, Chow MYT, Zhang Y, Leung SWS. siRNA Versus miRNA as therapeutics for Gene Silencing. Mol Ther Nucleic Acids. 2015;4:e252.
Hattab D, Gazzali AM, Bakhtiar A. Clinical Advances of siRNA-Based Nanotherapeutics for Cancer Treatment. Pharmaceutics 13, (2021).
Goyal R, Chopra H, singh I, Dua K, Gautam RK. Insights on prospects of nano-siRNA based approaches in treatment of Cancer. Front Pharmacol 13, (2022).
Mullard A. 2023 FDA approvals. Nat. Rev. Drug Discov 2024;23:88–95.
Connerty P et al. Development of siRNA-loaded lipid nanoparticles targeting long non-coding RNA LINC01257 as a novel and safe therapeutic approach for t(8;21) pediatric acute myeloid leukemia. Pharmaceutics 13, (2021).
Miao Z et al. Hsa_circ_0136666 stimulates gastric cancer progression and tumor immune escape by regulating the miR-375/PRKDC Axis and PD-L1 phosphorylation. Mol Cancer 22, (2023).
Triozzi P, et al. Phase I clinical trial of adoptive cellular immunotherapy with APN401 in patients with solid tumors. J Immunother Cancer. 2015;3:P175.
Golan T, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6:24560–70.
Kumthekar P et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci Transl Med 13, (2021).
Sarker D, et al. MTL-CEBPA, a small activating RNA therapeutic upregulating C/EBP-α, in patients with Advanced Liver Cancer: A First-in-Human, Multicenter, Open-Label, phase I Trial. Clin Cancer Res. 2020;26:3936–46.
Ishihara M et al. NY-ESO-1-specific redirected T cells with endogenous TCR knockdown mediate tumor response and cytokine release syndrome. J Immunother cancer 10, (2022).
Abd-Aziz N, Kamaruzman NI, Poh CL. Development of MicroRNAs as Potential Therapeutics against Cancer. J. Oncol 2020, (2020).
Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin G. A. microRNA Therapeutics in Cancer - An Emerging Concept. EBioMedicine 2016;12:34–42.
Abba ML, et al. MicroRNAs as novel targets and tools in cancer therapy. Cancer Lett. 2017;387:84–94.
Beg MS, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35:180–8.
van Zandwijk N, et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017;18:1386–96.
MacDiarmid JA, et al. Bacterially derived 400 nm particles for Encapsulation and Cancer Cell Targeting of Chemotherapeutics. Cancer Cell. 2007;11:431–45.
Collotta D, Bertocchi I, Chiapello E, Collino M. Antisense oligonucleotides: a novel Frontier in pharmacological strategy. Front Pharmacol 14, (2023).
Crooke ST, Liang XH, Baker BF, Crooke RM. Antisense technology: a review. J Biol Chem 296, (2021).
Pollak AJ, Zhao L, Crooke ST. Systematic analysis of chemical modifications of phosphorothioate antisense oligonucleotides that modulate their Innate Immune Response. Nucleic Acid Ther. 2023;33:95–107.
Kim Y. Drug Discovery perspectives of antisense oligonucleotides. Biomol Ther (Seoul). 2023;31:241–52.
Di Martino MT, et al. In Vitro and in vivo activity of a Novel locked nucleic acid (LNA)-Inhibitor-miR-221 against multiple myeloma cells. PLoS ONE. 2014;9:e89659.
Santolla MF, et al. MiR-221 stimulates breast cancer cells and cancer-associated fibroblasts (CAFs) through selective interference with the A20/c-Rel/CTGF signaling. J Exp Clin Cancer Res. 2018;37:1–12.
Gullà A, et al. A 13 mer LNA-i-miR-221 inhibitor restores drug sensitivity in Melphalan-Refractory multiple myeloma cells. Clin Cancer Res. 2016;22:1222–33.
Di Martino MT, et al. Dose-finding study and Pharmacokinetics Profile of the Novel 13-Mer antisense miR-221 inhibitor in Sprague-Dawley rats. Mol Ther Nucleic Acids. 2020;20:73–85.
Tassone P et al. Safety and activity of the first-in-class locked nucleic acid (LNA) miR-221 selective inhibitor in refractory advanced cancer patients: a first-in-human, phase 1, open-label, dose-escalation study. J Hematol Oncol 16, (2023).
Ali A et al. LNA-i-miR-221 activity in colorectal cancer: a reverse translational investigation. Mol Ther Nucleic Acids 35, (2024).
Lavie O, et al. A phase 1/2a, dose-escalation, safety, pharmacokinetic, and preliminary efficacy study of intraperitoneal administration of BC-819 (H19-DTA) in subjects with recurrent ovarian/peritoneal cancer. Arch Gynecol Obstet. 2017;295:751–61.
Hong DS, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020;122:1630–7.
El Dika I, et al. An Open-Label, Multicenter, Phase I, dose escalation study with phase II expansion cohort to Determine the Safety, Pharmacokinetics, and preliminary Antitumor activity of intravenous TKM-080301 in subjects with Advanced Hepatocellular Carcinoma. Oncologist. 2019;24:747–e218.
Schultheis B, et al. Safety, Efficacy and Pharcacokinetics of targeted therapy with the liposomal RNA interference therapeutic Atu027 combined with Gemcitabine in patients with pancreatic adenocarcinoma. A randomized phase Ib/IIa study. Cancers (Basel). 2020;12:1–13.
Acknowledgements
N/A.
Funding
This work has been partially supported by the Italian Association for Cancer Research (AIRC) Investigator Grant, Project N. 21588, PI: P. Tassone.
Author information
Authors and Affiliations
Contributions
KG and GC contributed to conception, design and revision of the manuscript sections, wrote sections of the manuscript and created Figs. 2 and 3. FL and AC performed the two independent systematic review, wrote sections of the manuscript and created Fig. 1; Table 1. MTDM wrote sections of the manuscript. PTag. Critically revised the manuscript and the protocol of the systematic review. PTas conceived and supervised the manuscript by providing critical feedback and revisions. The authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable (N/A).
Consent for publication
N/A.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Grillone, K., Caridà, G., Luciano, F. et al. A systematic review of non-coding RNA therapeutics in early clinical trials: a new perspective against cancer. J Transl Med 22, 731 (2024). https://doi.org/10.1186/s12967-024-05554-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05554-4