Abstract
Target cancer therapy has been developed for clinical cancer treatment based on the discovery of CRISPR (clustered regularly interspaced short palindromic repeat) -Cas system. This forefront and cutting-edge scientific technique improves the cancer research into molecular level and is currently widely utilized in genetic investigation and clinical precision cancer therapy. In this review, we summarized the genetic modification by CRISPR/Cas and CRISPR screening system, discussed key components for successful CRISPR screening, including Cas enzymes, guide RNA (gRNA) libraries, target cells or organs. Furthermore, we focused on the application for CAR-T cell therapy, drug target, drug screening, or drug selection in both ex vivo and in vivo with CRISPR screening system. In addition, we elucidated the advantages and potential obstacles of CRISPR system in precision clinical medicine and described the prospects for future genetic therapy.
In summary, we provide a comprehensive and practical perspective on the development of CRISPR/Cas and CRISPR screening system for the treatment of cancer defects, aiming to further improve the precision and accuracy for clinical treatment and individualized gene therapy.
Similar content being viewed by others
Introduction
Cancer therapy has been developed from the very initial surgical removal in the ancient to currently precision minimally invasive surgery; from the chemotherapy, radiotherapy to the targeted therapy and precision individualized immunotherapy, under the progress of precise and granular molecular characterization at present [1]. The newly discovered genome editing tool CRISPR (clustered regularly interspaced short palindromic repeat) /Cas system provides a powerful method for the investigation of cancer therapy [2,3,4]. It was described initially in bacteria as a primitive immune system to fight against viral infections and was universally recognized as a genomic modification system in the past decade [5, 6]. In Prokaryotes, the short DNA repeats CRISPR exist between regular spacing units, and are recognized as intervening sequences derived from preexisting fragment of bacteriophages and conjugative plasmids, contributing to bacteria immune system [7]. The genetic sequences of the viral invaders or plasmid challengers are captured and aligned as spacer segments in the CRISPR region in bacteria or archaea [8, 9], comprising the CRISPR-mediated adaptive immunity system [10]. Two classes of CRISPR-Cas systems have been described in prokaryotes based on their effector modules [11,12,13,14], characterized into 6 types, and 33 subtypes described in 2020 [15]. The Class 2 CRISPR-Cas system composed only 10% percentage but has expanded biotechnology toolbox for genome editing with 190,000 shares worldwide from 640 labs [16, 17]. It consists of three types of effectors: type II, type V and type VI, with several widely recognized genetic editing enzymes, being Cas9 in type II, Cas12a (Cpf1), Cas12b (C2c1), Cas12c (C2c3), Cas14 subgroup in type V [18], Cas13a (C2c2), Cas13b (C2c6) and Cas13c (C2c7) in type VI [14, 19]. Schematic representation of two classes of CRISPR/Cas systems were depicted in Fig. 1.
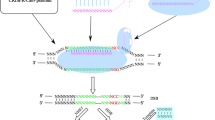
Schematic representative of CRISPR/Cas loci in Class 1 and Class 2 system. Class 1 system show multi-component effectors, while the Class 2 system have one effector. Three subgroups of Class 2 CRISPR systems are presented. Representative Type II-A CRISPR protein contains: Streptococcus pyogenes Cas9 (SpCas9), Staphylococcus aureus Cas9 (SaCas9) and Streptococcus thermophilus Cas9 (StrCas9), all of which have the tracrRNA sequences. Type V CRISPR, which comprises Cas12a, Cas12b and Cas12c, exhibits distinct genome structures. Cas12b has the tracrRNA structure, while Cas12c only has one assistant protein cas1 for genome editing. Cas14 subgroup is not depicted in this figure. Type VI CRISPR systems show few assistant proteins to identify RNA virus, however, type VI-B has csx27 and csx28 proteins to regulate nuclease activity. Illustrated according to Ref [14, 16, 20, 21].
CRISPR/Cas system has been utilized for cellular genetic modification [22, 23] and the generation of animal models for cancer research [24, 25]. Furthermore, the CRISPR/Cas-based genetic screening system was developed for cellular investigation [26,27,28], as well as in tumor studies [25, 29]. In addition, high throughput gRNA libraries have been established to enable efficient genetic screening, specially facilitating personalized treatment strategies for cancer patients individually [30]. In this review, we provide a comprehensive overview of the CRISPR/Cas system and essential elements for successful CRISPR screening system, including gRNA libraries, gRNA validation, and clinical application for cancer research. Furthermore, we explored the application of the CRISPR screening system in cancer therapy from both ex vivo and in vivo investigation, aiming to elucidate the inherent advantages and potential obstacles for clinical precision medicine.
The application of Class 2 CRISPR-Cas effectors and genome modification in cancer therapy
Type II effector Cas9 in cancer research
Both Streptococcus pyogenes Cas9 (SpCas9) and Staphylococcus aureus Cas9 (SaCas9), classified as the type II-A effectors, showed comparable genome editing efficiency for in vitro and in vivo study [21, 31,32,33]. These effectors enable rapid modification of cellular or animal models for transcriptional modulation via CRISPR knockout/knockin or high throughput genomic screening [23, 34]. The compact size of SaCas9 renders it an optimal enzyme for in vivo AAV application. However, SpCas9, one of the pioneering Cas9 proteins, has been extensively investigated and utilized in CRISPR gene editing. Three variants of SpCas9 have been developed, the wild-type Cas9, nickase Cas9 (nCas9), and dead Cas9 (dCas9).
Cas9 mediated DNA cleavage with the two distinct active sites RuvC and HNH, under the assistance of CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA) ribonucleoprotein complex [8]. The dual-tracrRNA: crRNA chimera single guide RNA (sgRNA) was created and directed Cas9 nuclease to the potential target loci for site-specific DNA cleavage, initiating the genome editing system in vitro [35]. The binding of Cas9 to the adjacent sequence of three nucleotides, known as protospacer adjacent motif (PAM), triggers DNA cleavage by inducing double-strand breaks with its scissor-like activity [36]. The recently used Cas9-gRNA ribonucleoprotein (RNP) complexes remarkably increase fidelity and efficacy for double-strand DNA breaks with minimized cell mortality [37]. It also combined with repair donor to achieve site-specific correction of cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in epithelial organoids [38]. Cre-dependent Cas9 knockin mouse was generated, and KRAS, p53, and LKB1 depletion resulted in carcinoma formation in these transgenic mice, providing a robust cancer model for research [24].
One mutation in D10A of Cas9 protein makes a nCas9, which improves genome editing specificity [39]. The combination of sgRNA pairs with nCas9 significantly enhances cutting specificity by 50-1000 folds in cell lines and mouse zygotes [40]. CRISPR-Cas base editing using nCas9 enables precise incorporation of point mutations in genomic DNA without inducing double-strand breaks, demonstrating its potential in treating genetic diseases caused by base-pair alterations through adenine base editors (ABEs) or cytosine base editors (CBEs) [41]. In addition, DNA base editors combining with the leading platform adeno-associated virus (AAV) vector for viral delivery expanded the CRISPR-base-edit toolkit for Prime-editing (PE) [42]. Meanwhile, the recently developed genome editing technique known as NICER utilizes Cas9D10A nickase to correct heterozygous mutations. It generates multiple DNA nicks and triggers gene correction via interhomolog homologous recombination (IH-HR) which rarely induces genomic alterations, making it a precise strategy to restore genetic diseases or single nucleotide mutations [43]. Except the precise single nucleotide restoration, cancer translocations were generated by double strand breaks and paired nicks with either Cas9 or nCas9, creating endogenous chromosomal translocations cell model for investigating tumor driving genes [44].
Catalytically inactive Cas9, a ‘dead’ protein (dCas9) with both mutations in D10A and H840A of RuvC and HNH domains, showed its popularity in gene regulation with inhibition, activation, and cell imaging and labeling [45]. Genome-scale screenings utilizing CRISPR inhibition (CRISPRi) and CRISPR activation (CRISPRa) have been employed to identify both known and novel genes involved in controlling cell growth and sensitivity to toxins [46]. Precise inducible gene knockdown or overexpression can be supported using dCas9-KRAB (Krüppel-associated box) or Cas9 combined with Tetracycline Inducible Expression promoter (TetO) [47]. Firstly, the fusion of dCas9 with transcriptional repressor produces the CRISPRi genetic tool [48]. The dCas9-BFP-KRAB repressor domain enables the suppression of gene expression [49]. Second, fusing dCas9 with RNA polymerase (RNAP) omega subunit upregulates gene expression [50], and dCas9-VP64 was used for transcriptional activation [51]. In addition, dCas9 protein serves as a valuable tool for labeling of endogenous genomic loci in living cells. By employing an optimized sgRNA fused with EGFP-tagged dCas9, repetitive elements in telomeres and various other regions can be robustly labeled [52]. A double-color CRISPR labeling method was established by incorporating MS2 or PP7 RNA aptamers into the sgRNA, fused with the catalytically inactive Cas9 (dCas9) for direct visualization [53]. Finally, dCas9 can be employed for in vivo imaging of chromosomal dynamics and genome organization dimensions [47], allowing systematic fluorescent labeling of up to 10 proteins [48]. Summary of the type II Cas9 enzymes was depicted in Fig. 2.
Summary of Cas9 proteins and modified nCas9 and dCas9 genome editing tools. (A) PAM for SpCas9 is NGG, while PAM for SaCas9 is NNGRRT with the ability to cut DNA double helix. (B) Mutation of D10A leads to the formation of nCas9 while both mutations generate dCas9 protein. (C) nCas9 can be applied for base editing such as CBE and ABE, also for Base editor and developed as NICER to repair heterogenous mutation. (D) dCas9 was modified to generate CRISPRi, CRISPRa and CRISPR labeling tools. dCas9: dead Cas9. nCas9: nickase Cas9. CBE: Cytosine Base Editor, ABE: Adenine Base Editor. RT: reverse transcriptase. pegRNA: prime editing guide RNA.
Type V and type VI effectors in cancer research
Mainly three subtypes of type V effectors were investigated for gene editing, named as type V-A, V-B and V-C. The type V-A effector Cpf1 (CRISPR from Prevotella and Francisella 1), exhibits enhanced genome editing specificity attributed to a T-rich PAM (-5′TTTV) [54], resulting in a staggered DNA double stranded break [55]. Two candidate Cpf1 (Cas12a) enzymes, AsCpf1 from Acidominococcus sp. BV3L6 and LbCpf1 from Lachnospiraceae bacterium ND2006, show a robust genome editing ability in human cells compared to that of Cas9 [56]. Furthermore, successful generation of gene knockout transgenic mice was achieved using both AsCpf1 (40.7%) and LbCpf1 (28.6%), providing a wonderful animal model for research [57, 58]. Multiplex genome editing was conducted using Cpf1 from Aspergillus aculeatus strain TBRC277 [59] and AsCpf1 was engineered with adeno-associated viral vectors (AAVs) for multiplex genome editing of mouse brain in vivo [60]. One-step generation of homology-directed repair (HDR) and checkpoint knockout CAR-T (KIKO CAR-T) was achieved with the adeno-associated virus and CRISPR/Cpf1 system, establishing an efficient AAV-Cpf1 double knockin system and opening new possibilities for cancer research [61]. The type V-B CRISPR effector Cas12b (C2c1) discovered in Bacillus hisashii (BhCas12b) showed a nickase effect at 37 °C for human gene editing, while BhCas12b v4, containing K846R/S893R/E837G mutants, demonstrated strong genome editing ability in human cells comparable to SpCas9 [62]. While the type V-C CRISPR effector Cas12c (C2c3) is a site-specific ribonuclease generating mature crRNAs for DNA targeting, crRNAs direct DNA binding by Cas12c without DNA cutting, providing a DNase-free pathway for transient antiviral immunity [63].
While both type II and type V are effective for DNA targeting in the genome level, the type VI effector Cas13 exhibits efficacy in treating genetic diseases and rescuing diseased sequences at the RNA level. They provide valuable genetic tools for diagnosis and degradation of viruses such as HIV and HPV [64, 65]. Several Cas13 proteins were characterized, such as Cas13a, Cas13b, Cas13bt and Cas13d, showed the efficiency to cleave single stranded RNAs [66,67,68]. Of which Cas13a based SHERLOCK (Specific High-Sensitivity Enzymatic Reporter UnLOCKing) system can detect Zika or Dengue Virus as well as somatic mutations in cell free DNA (cfDNA) samples such as serine/threonine kinase (BRAF) V600E cancer mutation [69]. Shortened detection time and high sensitivity were applied for virus detection via SHERLPCKv2 system [70, 71].
SHERLOCK enables to identify EGFR-T790M mutation in patient DNA with high efficiency by detecting 0.6% mutant ratio samples [72], this system was also used for DNA and RNA detection with single-base specificity and attomolar sensitivity in cancer patients samples [73]. Cas13b was used to fight RNA viruses such as porcine reproductive and respiratory syndrome virus (PRRSV) [74], chikungunya (CHIKV) and dengue in mosquito cells [75] as well as SARS-CoV-2 resistance [76, 77]. Since Cas13b targets RNA without interfering genome sequence of the targeted gene, it provides a potential safer alternative to Cas9 enzymes. Catalytically inactive Cas13b (dCas13b) was engineered to direct adenosine-to-inosine deaminase for precise base editing, enabling the Programmable A to I Replacement (REPAIR) RNA editing platform. This platform can be utilized in transcriptome engineering of advanced leukemias, as well as head, liver, and breast cancers, thereby demonstrating a feasible strategy for investigating gene function in cancer at the RNA level [78, 79]. The RNA-targeting CRISPR-Cas13 system showed promising roles in cancer diagnosis, therapy, and research; with the ability for early detection of cancer markers in liquid biopsy samples, degradation and manipulation of cancer-related mutant transcripts, as well as identification of novel therapeutic drug targets described in the recent review [80].
Altogether, the class 2 effectors expanded the current CRISPR/Cas toolkit. Cas9 possesses recognition ability of specific target sequences, and has the genomic editing ability for precision cancer treatment and mutation detection [2]. Meanwhile, the recently discovered Cas12 and Cas13 expand RNA editing tool, providing novel genetic methods for cancer diagnosis and molecular examination of cancer research [3].
The application of CRISPR screening system in cancer
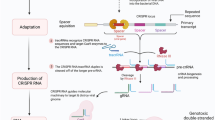
The development of CRISPR/Cas system and high-throughput sequencing makes genetic screening easily accessible in basic biology, drug discovery, and personalized medicine for cancer therapy [3]. Cas9 nuclease is a preferred choice for genetic screening, and has been used for genomic modification in multiple researches [26, 28, 81, 82]. One-step generation of multiplex genome mutations via CRISPR/Cas9 system was successfully achieved in mice, facilitating in vivo functional analysis of redundant genes [83]. CRISPR screening system was developed based on CRISPR/Cas combined with thousands of gRNAs integrated into viral vectors [81, 84]. These libraries harbor gRNAs targeting various genes, and have received up to 1000 annual requests globally, enabling unbiased, phenotypic forward genetic screening [17]. The first whole genomic gRNA libraries for both mouse and human were generated with mouse lentiviral gRNA library containing 87,897 gRNAs for 19,150 coding genes, naming as (GeCKOv1), and was established to screen out unknown genes for Clostridium septicum alpha-toxin or 6-thioguanine (6TG) drug resistance [81]. However, low viral titer of the lentiviral delivery systems in GeCKOv1 limited the usage for biological screening, and genome-scale CRISPR knockout v2 (GeCKOv2), contained 123,411 unique sgRNAs targeting 19,050 annotated protein-coding genes and 1000 control sgRNAs (sg-NTCs), resulting in a 10-fold increase for viral generation [84]. Optimized mouse gRNA libraries targeting 20,611 genes with 130,209 gRNAs were also established with 100-fold increase of functional viral titer [84]. Innovative strategies of CRISPR-Cas9 system have been developed for large-scale genome knockout and transcriptional activation [85], as well as combinatorial genetic screening [27]. Processes for gRNA library generation and amplification were illustrated as depicted in the following Fig. 3.
Schematic representation of gRNA library construction and virus production. (A) Oligoes synthesis and vector construction for gRNA library. (B) Amplification of gRNA library by bacterial culture, collection, and plasmid extraction. (C) PCR examination and sequence confirmation for library coverage. (D) Plasmids transfection and virus production with a certain gRNA library.
gRNA libraries for cancer research
Various of genome-scale gRNA libraries were established for CRISPR screening, and some gRNA libraries for specific selected genes were also established with small capacity. Established gRNA libraries of genome wide and specific selected targets for cancer research were summarized in the Table 1.
Human lentiviral GeCKOv1 library (lentiCRISPRv1) was established for high throughput gene targeting of 18,080 genes, with 64,751 unique gRNAs total, and was used for cell viability-related gene screening in cancer. It was also examined for resistance to a therapeutic RAF inhibitor, vemurafenib, in a A375 melanoma model, leading to the discovery of novel genes sensitive to drug treatment [28]. GeCKOv2 library was also used to identify responsible genes related to EGF-induced apoptosis [86]. Genome-wide sgRNA library (mGeCKOa) transfection in non-metastatic mouse non-small cell lung cancer with 67,405 sgRNAs targeting 20,611 protein-coding genes. Cells were treated and transplanted into immunocompromised Nu/Nu mice, and tumor growth and migration were evaluated in vivo [25]. The pooled lentiviral sgRNA library with 73,151 gRNAs targeting 7114 gene and 100 non-targeting controls were used to screen the resistant genes for nucleotide analog 6TG treatment in human leukemic cell lines, screening resistance genes toward chemotherapeutic etoposide [26]. Patient-derived glioblastoma cell line (GBM), retinal epithelial cells (RPE1), colorectal carcinoma (HCT116 and DLD1), cervical carcinoma (Hela) and melanoma (A375) cells were subjected into genetic screening with the “90k library” containing 17,232 targeting genes and 91,320 gRNA sequences. Subsequentially, the supplemental library naming 176,500 TKO (Toronto KnockOut) library targeting 17,661 protein-coding genes were used to identify fitness genes in cancer cell lines [87]. Lentiviral vectors with genome-scale sgRNA library consisting of 70,290 guides (3 sgRNAs for each transcription start site (TSS)) were used for synthetic activation mediator (SAM)-based screening to target 200 bp upstream of the TSS and confer resistance to a BRAF inhibitor in melanoma cell line A375 and patient derived samples [51].
Although genome scale gRNA libraries are widely used in cancer research, its complexity and transcript isoform variance as well as difficulty in viral vectors cloning limited its usage. Other specific gRNA libraries for certain signal pathways or gene functions were established according to screening purpose for modulating endogenous genes. Total 5920 candidate enhancers were perturbed by the dCas9-KRAB enzyme, establishing the multiplex, expression quantitative trait locus (eQTL) framework, and total 664 cis enhancer-gene pairs were identified and enriched based on 254,974 single-cell transcriptomes in K562 derived from a chronic myologenous leukemia patient [49]. Undescribed immunotherapy targets for transplantable melanoma tumors in mice were explored with the 9992 sgRNAs targeting 2368 genes selected from transduced cells, establishing the in vivo genetic screen tumor models [88]. Recurrently mutated genes derived from pan-cancer The Cancer Genome Atlas datasets were recognized as well-known tumor suppressors genes (TSGs) or oncogenes. Total 49 orthologs of human TSGs were found in mouse genome, and the mouse TSG library containing 280 sgRNAs targeting 56 different genes (7 housekeeping genes) were used for tumor metastasis analysis [89].
The improvements of specificity and validation methods for gRNA Library
The procedure to perform pooled genome-editing experiments was clearly described, and successful CRISPR/Cas9 screening needs the specific and efficient gRNA sequence with proper quality and low off-target effect [91]. Off-target predictions calculated by algorithms indicating false positives and quantified error rates were developed by Bowtie and BWA sequencing methods, or considered by MIT-Broad score and the CFD score as summarized in previous reviews [92]. Computational tools for sgRNA designing with low off-target and high on-target efficacy and specificity have been developed and summarized in 2018 [93]. Several methods have been built for eliminating off-target results such as the utilization of high-efficiency delivery RNP tool, modification of the gRNA sequence, and improvement the specificity of Cas9 Enzymes [94]. The computational tool CRISPOR established high-quality gRNA libraries by selection according to off-target and on-target predictions, it also helps with vector cloning, gRNA validation and expression with primer designing and restriction enzymes depiction [95]. Optimized on-target efficiency prediction model was generated to illustrate the cleavage ability of gRNA sequence (http://crispor.org) [96]. Meanwhile, CRISPResso provides a robust and user-friendly computational pipeline to evaluate effects of coding and noncoding sequences and select off-target sites [97]. For precise gene selection analysis, the Model-based Analysis of Genome-wide CRISPR/Cas9 Knockout (MAGeCK) is the optimized method for both positive and negative selection, which offers high sensitivity and low FDR regardless of sequencing depth or sgRNA numbers for a single gene [98]. Besides that, intergration deficient lentiviral (IDLV) capture [99], and high-throughput genome-wide translocation sequencing (HTGTS) [100] are other methods for off-target detection.
Analysis of gRNAs abundance in pooled libraries plays an important role in targeting efficiency and screening accuracy and specificity. PCR products of gRNA library vectors can be sequenced on Hiseq 2500 and aligned to sgRNAs by Bowtie, an ultrafast, efficient program for aligning short DNA sequence to large genomes [101]. Rigorous analytical methods mitigate the false discovery rates generated by CRISPR screens via a Bayesian classifier of gene essentiality [102]. Sequence quality control can also be carried out under the guide of GPP Pooled Screen Analysis (https://portals.broadinstitute.org/gpp/broad/), and statistical enrichment and gene depletion were calculated by hit calling algorithm STARS (http://www.broadinstitute.org/rnai/public/software/index) based on normalized fold changes [103]. High-content downstream gRNA library sequence validation in tumor immunology were summarized in the recent review [29]. Generally speaking, breaks labeling, enrichment on streptavidin and next-generation sequencing (BLESS) [104], genome-wide unbiased identification of DSBs enabled by sequencing (GUIDE-seq) [105, 106] and discovery of in situ Cas off-targets and verification by sequencing (DISCOVER-seq) [107,108,109] were used as cell based methods with direct sequencing. More sensitive biochemical methods such as digested genome sequencing (Digenome-seq) [110,111,112], selective enrichment and identification of adapter-tagged DNA ends by sequencing (SITE-Seq) [113], circularization for in vitro reporting of cleavage effects by sequencing (CIRCLE-seq) [114, 115] and circularization for high-throughput analysis of nuclease genome-wide effects by sequencing (CHANGE-seq) [116] were developed for accurate sequence confirmation.
CRISPR screening application in cancer therapy
The application of the CRISPR/Cas system for cancer therapy has been investigated using viral vectors including lentivirus, adenovirus, and AAV vectors, as well as non-viral vectors such as polymer nanoparticles, golden nanoparticles, or lipid nanoparticles in both ex vivo and in vivo circumstances as described in recent reviews [117, 118]. Various cancer cell lines [2, 4, 87, 119, 120], T-cells via chimeric antigen receptor (CAR) integration or CAR-T system [90, 121, 122], and organoids derived from patient samples [123] have been explored for cancer therapy research. However, because of manipulation limitations in highly differentiated cells, in vivo clinical precision therapy involving modified cells with AAV vector delivery for the CRISPR modification system is widely used for a broad range of human diseases [118]. In this part, we mainly focus on the application of CRISPR screening system for cancer therapy, including ex vivo and in vivo approaches. Schematic representation of CRISPR screening applications for cancer research is summarized in Fig. 4.
CRISPR screening and its applications in ex vivo and in vivo for cancer therapy. (A) CRISPR screening application in cultured cells. (B) CRISPR screening in vivo application in mouse with direct injection to organs and indirect injection in abdominal and tail vein. (C) Schematic representation of CRISPR screening applications for human cancers; Created with BioRender.com
CRISPR screening in vitro for cancer therapy
CRISPR screening has several potential applications in cancer therapy, including modified T cells and Chimeric antigen receptor CAR-T cancer treatment, novel target identification, drug resistance, drug selection exploration and so on [4, 29]. The CRISPR screening system has been employed to investigate various cancer cell types originating from diverse organs including lymphatic system, esophagus, stomach, intestines, lungs, nervous system, skin, liver, blood cells as well as reproductive organs. CRISPR screening applications in Cancer therapy were summarized in Table 2.
Modified T cell and CAR-T therapy for cancer therapy
Immune system is the most important defender to fight off cancer. Immunotherapy strategy is to make better immune cells such as tumor-infiltrating lymphocytes (TIL) or CAR-T cells to attack cancer via T-cell transfer. TIL therapy uses patient’s own lymphocytes to kill tumor, whereas CAR-T means modified T cells with specific proteins from surface of cancer cells, thus having the ability to attack tumors. In addition to Cas9 utilization, conjugated Cas12 (cCas12a) can be used for CAR-T cell generation. Using an AAV vector, Cas12a-crRNA complex showed robust efficiency to generate site-specific and precisely targeted CAR-T cells [149].
Recent review showed the importance of gamma retroviral or lentiviral vectors for CAR-T cell generation to target B-cell lymphomas and leukemias, although with complex manufacturing procedure, providing a promising “off-the-shelf” products for cancer treatment [150]. Whole-genome CRISPR/Cas9 screening was performed in CAR-T cells and co-cultured with Glioblastoma (GBM) stem cells (GSCs) to explore the PD-1 dependence genes such as TLE4 and IKZF2 for cancer treatment. Meanwhile, transduced GSCs were subjected to CAR-T challenge in order to identify enriched and depleted genes for cancer cell apoptosis [124]. Until 2021, total 3 FDA approved CAR-T therapies have been described as tisagenlecleucel, axicabtagene ciloleurel, and brexucabtagene autoleucel based one CD19-mediated CAR-T cells [151]. Although CAR-T is efficient in blood cancers, its efficiency loss impedes the treatment efficiency. To overcome refractory of B-cell malignancies, genome-scale CRISPR-Cas9 loss-of-function screens were performed, and revealed the crucial role of FADD and TNFRSF10B (TRAIL-R2) in mediating CAR-T cell cytotoxicity [125]. Except for precision CAR-T treatment, multiplexed CRISPR-Cas9 editing applications have been used to generate universal CAR-T products, with the aim of enhancing antitumor efficacy and improving safety of cell-based therapies [152].
Novel targets identification using CRISPR/Cas9 screening in cancer research
The invasion and metastasis of cancers make it more difficult to treat, and new targets should be identified for complete cure. Using genome-wide CRISPR/Cas9 screening, key drivers for invasion and metastasis of esophageal squamous cell carcinoma (ESCC) were identified by gain- and loss-of-function experiments, demonstrating that high expression of Mesoderm Specific Transcript (MEST), interacting with purine rich element binding Protein A, is associated with poor patient survival via activating SRCIN1/RASAL1-ERK-snail signaling [126]. Synergistical effect of genetic deletion and pharmacologic inhibition to increase cytotoxicity of MEK signaling inhibitors in pancreatic ductal adenocarcinoma cells was also investigated by CRISPR knockout screening [127]. Genome wide CRISPR/Cas9 knockout screening identified Zinc finger protein (ZNF) family member ZNF319 as a potent suppressor responsible for metastasis of breast cancer in an orthotopic murine model [153]. In hepatocellular carcinoma (HCC), CRISPR/Cas9 knockout library screening revealed the crucial role of pyruvate metabolism in HCC treatment, particularly when combined with a glutamine-deficient diet, showing the targetable metabolic vulnerabilities of pyruvate dehydrogenase α(PDHA), pyruvate dehydrogenase β(PDHB), and pyruvate carboxylase (PC) [128]. CRISPR-Cas9 knockout mutagenesis to exons encoding functional protein domains was performed to screen drug targets and dependencies, providing a comprehensive identification of protein domains for cancer cell sustainment [120]. In epithelial ovarian cancer (EOC), CRISPR-Cas9 screening combined with olaparib treatment successfully identified five genes, ATM, NBN, MUS81, RAD51B, and BRCA2, as predictive markers for olaparib response. Additionally, CDK12 emerged as a promising therapeutic target for EOC without compromising the efficacy of Olaparib response [129]. The whole-genome CRISPR screening in Guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) mutant uveal melanoma (UM) cells showed that a member of Gα protein family Gαq promoted PI3K/AKT signaling pathway through focal adhesion kinase (FAK) for cell growth and survival [130].
Combinatorial CRISPR screening with scRNA-seq showed that driver gene alterations influenced TSGs, and triggered tumorigenesis in human mammary epithelial cells, indicating the impact of transcriptional epistasis on oncogenic mediators and potential therapeutic targets, including CDK4, SRPK1, and DNMT1 [131]. By analyzing the CRISPR-Cas9 screening data from Depmap (Cancer Dependency Map) and TCGA data of differentially expressed genes, the cell cycle pathway was identified as a key pathway of cell viability regulation in breast cancer patients [154]. The CRISPR/Cas9 screening in chemo-resistant small-cell lung cancer (SCLC) identified serine/threonine kinase cell division cycle 7 (CDC7) as a potential synergistic target. Combination of CDC7 inhibitor XL413 and chemotherapy led to apoptosis of chemo-sensitive SCLC in xenograft tumor [132]. Acute Myeloid Leukemia (AML) cell lines such as MOLM-13, MV4-11, HL-60, OCI-AML2, OCI-AML3 were examined for therapeutic targets via genome-wide CRISPR screening, indicating KAT2A inhibition as a therapeutic strategy in AML [133].
Hepatocellular carcinoma (HCC) was examined via CRISPRa for growth and metastasis driver genes. High MYADML2 protein level reduced sensitivity to chemotherapeutic drugs and led to worse survival [134]. Essential single nucleotide polymorphisms (SNPs) for PrCa proliferation were explored via dCas9-KRAB negative screening with 2166 candidate SNP sites in 9133 gRNAs. RIGOR program analysis identified 117 SNPs which tended to reside near 5 kb flanking the transcription start sites. SNP (rs60464856) site targeting in stable dCas9 expressing cell line showed significant down regulation of RUVBL1 gene, and further validation showed that RUVBL1 was associated with tumorigenesis [135]. dCas9-KRAB perturbation genome screening identified 470 high-confidence cis enhancer-gene pairs in 5920 enhancers in chronic myelogenous leukemia cell K562, facilitating the large-scale mapping of enhancer-gene regulatory interaction network [49].
The utilization of CRISPR-Cas9 in investigating drug resistance against tumors
Resistance to nucleotide analog 6- thioguanine was examined by genome-scale knockout screen in two human cell lines, identified DNA mismatch repair pathway, DNA topoisomerase II (TOP2A) and cyclin-dependent kinase 6, (CDK) for DNA topoisomerase II (TOP2A) poison etoposide, demonstrating Cas9/ sgRNA screens as a powerful tool for systematic genetic analysis in mammalian cells [26]. CRISPR knockout screening in human A549 lung adenocarcinoma cells identified 5 EGF-resistance genes, and further RNAi validation showed DUSP1 increased survival of EGF treated cells, providing a novel target for EGFR-overexpressing cancers [86]. Genome-wide knockout screening using CRISPR-Cas9 was also carried out in respiratory cancers, including Nasopharyngeal carcinoma (NPC) and lung cancer (LC). Nine genes were found to be associated with radiosensitivity of NPC cells (C666-1R, 6-8FR). Fanconi anemia pathway and the TGF-β signaling pathway were reported to be important contributors for radiosensitivity [136]. In the nervous system, neuroblastoma tumorigenesis was investigated via CRISPR genome-wide knockout screening, showed that ubiquitin-specific proteases (USPs) stabilize and increase half-life of repressor element-1 silencing transcription factor (REST), indicating its critical role in neuroblastoma generation [137]. As for reproductive cancers, drug resistance genes as well as lethal genes for cancer cell were identified. Genome-scale screening in ovarian cancer cell lines with the GeCKO library identified one previously validated gene SULF1 and a novel gene ZNF587B responsible for cisplatin resistance [138]. Cervical cancer cell lines such as Hela and Siha were incubated with cisplatin or paclitaxel, respectively, and screened by genome-scale CRISPR/Cas knockout library and ninety-seven genes were identified to be associated with drug resistance [139]. Prostate cancer (PrCa) is one of the most lethal causes of cancer-related death in males. Resistance to Enzalutamide, docetaxel, and Cabazitaxel in metastatic castration-resistant prostate cancer (mCRPC) is a big obstacle for cancer treatment of male patients. Whole-genome CRISPR/Cas9 knockout screening in mCRPC cell line C4 dissected the potential genes responsible for drug resistance. Two genes (IP6K2, XPO4) were validated after the screening process via bioinformatic prediction, highlighting the necessity to perform individualized validation [140].
Phase III clinical trial for Aurora-A (AURKA) inhibitor alisertib (MLN8237) in breast cancer failed to prolong patients’ survival. Rational drug combinations for better therapeutic outcome were carried out based on CRISPR/Cas9 knockout screening of 507 kinases, identifying synthetic lethality interactions with MLN8237 and Haspin (GSG2). The combination of MLN8237 and Haspin inhibitor CHR-6494 reduced tumor growth both in vitro and in vivo [141]. CRISPR screening for 656 E3 ubiquitin ligases in PrCa cells identified 51 genes as tumor repressors. The novel oncodriver Ring Finger Protein 19 A (RNF19A) was frequently amplified and highly expressed in PrCa. It correlated with castration resistance and ubiquitylated Thyroid Hormone Receptor Interactor 13 (TRIP13) and was activated by androgen receptor (AR), and Hypoxia Inducible Factor 1 Subunit Alpha (HIF1A), indicating AR/HIF1A-RNF19A-TRIP13 signaling axis for PrCa therapy [142].
Colorectal cancer (CRC) was examined for drug resistance to oxaliplatin and screened by CRISPR/Cas9 genome-wide library knockdown system. It found that low expression of mitochondrial elongation factor 2 (MIEF2) contributed to oxaliplatin drug resistance by reducing mitochondrial stability and inhibiting apoptosis via decreased cytochrome C release [143]. The CRISPRa system was employed to investigate genes associated with resistance to lymphoma radiotherapy, and a total of 8 genes were screened and subsequently validated, demonstrating a significant correlation with radiotherapy resistance [144]. Patients with Cisplatin-resistant Testicular Germ Cell Tumors (TGCTs) have poor prognosis, and developments of novel therapeutic strategies are critical. CRISPRa system revealed that NEDD8-activating enzyme E1 (NAE1) was highly expressed in drug-resistant colonies of TGCT cells, and indicated that neddylation inhibitor (MLN4924) combined with cisplatin as a novel treatment option for TGCTs [145].
Utilizing CRISPR/Cas9 screening for personalized drug selection through patient-derived organoids
Organoids derived from both healthy and diseased tissues offer a valuable resource for biological or pathological investigations. Although CRISPR screening showed powerful manipulation in cancer cells lines, it is also employed for tumor organoids derived from diverse cancer patients for personalized drug selection. Suspension culture increases efficiency of culturing cancer organoids for genome-wide CRISPR-Cas9 screening and large-scale perturbation screens [146]. Human fetal hepatocyte organoids were generated to model nonalcoholic fatty liver disease (NAFLD), and CRISPR screening was utilized to identify steatosis modulators in APOB−/− and MTTP−/− organoids [147]. CRISPR-Cas9 genetic intervention and high-throughput drug screening have been applied in digestive organoids for personalized disease modeling and therapy [155]. Human Pancreatic cancer organoid biobank established from 31 distinct tumor lines was used for CRISPR/Cas9 genome editing and drug screening, indicated increased sensitivity of kinase inhibitors dasatinib and VE-821 with driver gene ARID1A mutation [148]. Drug response evaluation by in vivo CRISPR screening (DREBIC) method was used in pancreatic ductal adenocarcinoma organoid [127].
CRISPR screening in vivo for cancer therapy
In 2022, FDA approved a total of five CAR-T cell products for the treatment of B cell acute lymphoblastic leukemia or high-grade lymphomas, as well as multiple myeloma using lentiviral or γ-retroviral approaches [156]. Notably, two clinical trials (NCT05143307/NCT03872479) employed AAV as the delivery method in their studies on cancer therapy in vivo based on CAR-T cells and CRISPR/Cas system [117]. CRISPR screening system provides a robust genetic tool for in vivo elucidation of CAR-T resistance mechanisms. Loss-of-function genetic screens in an immunocompetent murine model with B-cell acute lymphoblastic leukemia (B-ALL) identified the IFNR/JAK/STAT signaling and antigen processing and presentation pathway as key factors for CAR-T resistance in vivo. In addition, natural killer (NK) cells also engage in the resistance progress [157]. Gain-of-function CRISPR activation screen in primary CD8 + T cells identified a key factor PRODH2 for improving the in vivo efficacy of CAR-T based cell killing. Augmentation of PRODH2 enhanced metabolic function of CAR-T cells as an immune booster [158].
CRISPR screening was also utilized for in vivo investigation to elucidate gene function within a whole organism or the context of complex biological systems, using lentiviral or AAV mediated sgRNA transfection in living organisms. AAV was the widely used vector for in vivo genetic therapy due to its low immunogenicity and non-pathogenic character [118]. The limitation of AAV’s vector capacity has been addressed through the recent development of a two-split intern vectors system [159], while smaller SpCas9 orthologues such as SaCas9 have demonstrated comparable editing efficiency to that of SpCas9, rendering them suitable for AAV-SaCas9 mediated in vivo genome editing [21]. Additionally, Cre-dependent and constitutive Cas9 expressing transgenic mice were established with EGFP labeling, which provides an animal model for genome-wide targeting and contributes to in vivo investigation [24].
In vivo screens were performed in mouse brain, liver, pancreases, lung and so on. The application of SpCas9 and gRNAs using AAV vectors enabled multiple gene modifications in the adult mouse brain, demonstrating its potential for genetic regulation [33]. Gliomagenesis suppressors were investigated by in vivo stereotaxic injection of AAV carrier sgRNA library in conditional-Cas9 mouse brain [160]. Autochthonous invasion of AAV-mTSGs library in Cre-inducible Cas9 mice liver led to cancer development in situ, and the mice died within 4 months [89]. NIT1 cells (a non-obese-diabetic-derived mouse beta cell line) mutated with GeCKO-v2 were subcutaneously transplanted into type 1 diabetes mouse model to identify genes contributing to autoimmune killing resistance [161]. With the AAV9-LPL gene delivery into the lung, multiple mutations of KRASG12D, p53 and LKB1 were obtained to induce macroscopic tumors. In vivo screening for lung cancer TSGs through CRISPR/Cas9 genome-wide knockout showed that ZNF24 contributed to P65 suppression via NF-κB pathway. Combinational inhibition of KRAS, NF-κB, and PD-1 effectively shrank autochthonous KrasG12D/ZNF24−/− lung cancers in mouse [162].
Examination of immunotherapy-treated normal and Tcra-/- mice in vivo by CRISPR screening showed the loss of CD47 caused resistance to immunotherapy. Deletion of protein tyrosine phosphatase (PTPN2) increased immunotherapy efficacy [88]. CRISPR screening identified PD-1, Tim-3, and RNA helicase Dhx37 as regulators of tumor infiltration and degranulation. Depletion of Dhx37 improved CD8 T cells efficacy towards triple-negative breast cancer in vivo, and the NF-kB signal pathway was involved in the process [163]. In vivo applications of CRISPR screening system were summarized in the following Table 3.
Limitations and prospection
The advances of CRISPR/Cas technology and screening strategies have revolutionized genetic identification, enabling the dissection of functional genes in specific biological processes and diseases, facilitating drug selection and individualized therapy. CRISPR screening has demonstrated great potential in cancer therapy by offering methods to combat drug resistance and aggressive behaviors, as well as identifying possible gene targets for novel approaches to treat cancers. However, there are still several obstacles for CRISPR/Cas application in clinical cancer treatment, including delivery of CRISPR/Cas9 system, Off-target effect, PAM limitation, as well as multiple gene-editing [117]. In this part, we paid more attention on limitations of CRISPR screening system and CAR-T cell therapy for cancers.
Limitations of CRISPR screening system
CRISPR screening delivery primarily relies on lentiviral and AAV vectors, which are crucial tools for either ex vivo or in vivo investigation. Of which, AAV vector has the advantages with mildly immunogenic and long-term transgene expression in post-mitotic cells, making it a leading platform for in vivo cancer therapy [164]. However, AAV vector showed some drawbacks in manufacturing, packaging size limitation, vector quality control and editing specificity, as described in the recent review [118].
Except the delivery limitations, the occurrence of off-target effects and unintended mutations induced by CRISPR technology are barriers to its application in clinical therapy. SpCas9 protein showed the ability to identify PAM sequence and cut specific DNA region in the CRISPR system. Due to the tolerance of gRNA recognition and nucleotide indels in the target region, even a single guide can generate thousands of off-targets as detected by sensitive high-throughput sequencing methods such as GUIDE-Seq and CIRCLE-seq [138, 143]. This raises concerns regarding the application of CRISPR technology in gene therapy [165]. The reason of the off-target effect is the conformational states of HNH domain. The activated conformation of HNH increases DNA cleavage efficiency for DNA double-strand break formation, leading to both on- and off-target effects [166]. To minimize the probability of off-target mutagenesis, other high-fidelity nucleases such as SpCas9-HF1, eSpCas9 and HypaCas9 were developed [167, 168]. In addition, PAM sequence limitation for Cas9 has been broadened by the identification of KKH SaCas9 variant, which exhibits robust genome editing activities with the PAM (NNNRRT) while maintaining comparable levels of off-target effects [169].
Anti-CRISPR is another obstacle to overcome because of the restriction of targeting specificity and activities. The VI-CRISPR inhibitors acrVIA1-7 from phage exhibit the ability to block Cas13a RNA targeting and dCas13a-mediated single nucleic acid editing. Specifically, AcrVIA1, 4, 5 and 6 bind to LwaCas13a, while AcrVIA2 and 3 interact with LwaCas13-crRNA complex [170].
Limitations of CAR-T cancer therapy
Although CAR-T showed success of B-cell malignance treatment, its usage in solid tumors still have some limitations such as T-cell exhaustion, lack of CAR-T cell persistence, and cytokine-related toxicities. To address these challenges, CRISPR technology has been used to generate safe and potent allogeneic universal CAR-T cell products for cancer immunotherapy [152]. However, hurdles remain for solid tumor CAR-T therapy due to target antigen heterogeneity, unable to pass through vascular endothelium to target tumor cells, and the immunosuppressive tumor microenvironments [171]. As viral vectors are commonly used for delivering CAR-T cells, safety concerns have arisen. To address this issue, virus-free CRISPR-CAR (VFC-CAR) T cells were generated [172]. Virus-free CAR-T cells (PD1-19bbz) were generated and a clinical trial was performed and registered at www.clinicaltrials.gov (NCT04213469) [173].
Future perspectives
Given the capacity of CRISPR to precisely modify the human genome in cells, ethical considerations have emerged as a pivotal factor for its application in genetic manipulation [174,175,176]. The challenges posed by off-target effects and unintended mutations serve as barriers to the clinical implementation of CRISPR technology. However, extensive efforts have been made to mitigate these concerns through the development of novel strategies, rendering CRISPR technologies indispensable tools for elucidating gene functions and noncoding elements involved in tumorigenesis, as well as facilitating the creation of next-generation cancer immunotherapies. In summary, CRISPR/Cas system continues to play an essential role in advancing human cancer research and clinical therapy.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- Cas9:
-
CRISPR-associated protein 9
- gRNA:
-
Guide RNA
- SpCas9:
-
Streptococcus pyogenes Cas9
- SaCas9:
-
Staphylococcus aureus Cas9
- StrCas9:
-
Streptococcus thermophilus Cas9
- Cpf1:
-
CRISPR from Prevotella and Francisella 1
- AAV:
-
Adeno-associated vector
- crRNA:
-
CRISPR RNA
- tracrRNA:
-
Trans-activating crRNA
- PAM:
-
Protospacer adjacent motif
- RNP:
-
Ribonucleoprotein
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- nCas9:
-
Cas9 nickase
- ABEs:
-
Adenine base-editors
- CBEs:
-
Cytosine base-editors
- IH-HR:
-
Interhomolog homologous recombination
- dCas9:
-
Dead Cas9
- RuvC:
-
CDD conserved protein domain family
- HNH:
-
Conserved Protein Domain Family HNH, His-Asn-His (HNH)
- CRISPRi:
-
CRISPR inhibition
- CRISPRa:
-
CRISPR activation
- KRAB:
-
Krüppel-associated box
- TetO:
-
Tetracycline Inducible Expression promoter
- RNAP:
-
RNA polymerase
- VP64:
-
Transcriptional activator consists of four copies of VP16
- AsCpf1:
-
Cpf1 enzyme from Acidominococcus sp. BV3L6
- LbCpf1:
-
Cpf1 enzyme from Lachnospiraceae bacterium ND2006
- HDR:
-
Homology-directed repair
- BhCas12b:
-
Cas12b enzyme from Bacillus hisashii
- SHERLOCK:
-
Specific High-Sensitivity Enzymatic Reporter UnLOCKing
- SARS-CoV-2:
-
SARS-coronavirus-2
- ORF:
-
Open reading frame
- cfDNA:
-
Cell free DNA
- PRRSV:
-
porcine reproductive and respiratory syndrome virus
- CHIKV:
-
RNA viruses’ chikungunya
- dCas13b:
-
Catalytically inactive Cas13b
- REPAIR:
-
Programmable A to I Replacement
- TKO:
-
Toronto KnockOut
- GeCKOv1:
-
Genome-scale CRISPR-Cas9 knockout version 1
- MAGeCK:
-
Model-based Analysis of Genome-wide CRISPR/Cas9 Knockout
- SAM:
-
Synthetic activation mediator
- TSS:
-
Transcription start site
- eQTL:
-
Expression quantitative trait locus
- TCGA:
-
The Cancer Genome Atlas
- MAGeCK:
-
Genome-wide CRISPR/Cas9 Knockout
- IDLV:
-
Integration deficient lentiviral
- HTGTS:
-
High-throughput genome-wide translocation sequencing
- BLESS:
-
Breaks labeling, enrichment on streptavidin and next-generation sequencing
- GUIDE-seq:
-
Genome-wide unbiased identification of DSBs enabled by sequencing
- DISCOVER-seq:
-
Discovery of in situ Cas off-targets and verification by sequencing
- Digenome-seq:
-
Digested genome sequencing
- SITE-Seq:
-
Selective enrichment and identification of adapter-tagged DNA ends by sequencing
- CIRCLE-seq:
-
Circularization for in vitro reporting of cleavage effects by sequencing
- CHANGE-seq:
-
Circularization for high-throughput analysis of nuclease genome-wide ffects by sequencing
- CAR:
-
Chimeric antigen receptor
- TIL:
-
Tumor-infiltrating lymphocytes
- GSCs:
-
Glioblastoma (GBM) stem cells
- PD-1:
-
Programmed Cell Death Ligand 1
- ESCC:
-
Esophageal squamous cell carcinoma
- MEST:
-
Mesoderm Specific Transcript
- ZNF:
-
Zinc finger protein
- HCC:
-
Hepatocellular carcinoma
- PC:
-
Pyruvate carboxylase
- EOC:
-
Epithelial ovarian cancer
- GNAQ:
-
Guanine nucleotide-binding protein G(q) subunit alpha
- UM:
-
Uveal melanoma
- FAK:
-
Focal adhesion kinase
- TSGs:
-
Tumor suppressor genes
- Depmap:
-
Cancer Dependency Map
- SCLC:
-
Chemo-resistant small-cell lung cancer
- CDC7:
-
Serine/threonine kinase cell division cycle 7
- AML:
-
Acute Myeloid Leukemia
- HCC:
-
Hepatocellular carcinoma
- SNPs:
-
Single nucleotide polymorphisms
- TOP2A:
-
DNA topoisomerase II
- CDK6:
-
Cyclin-dependent kinase 6
- TOP2A:
-
DNA topoisomerase II
- NPC:
-
Nasopharyngeal carcinoma
- LC:
-
Lung cancer
- USPs:
-
Ubiquitin-specific proteases
- REST:
-
Repressor element-1 silencing transcription factor
- PrCa:
-
Prostate cancer
- CRC:
-
Colorectal cancer
- MIEF2:
-
Mitochondrial elongation factor 2
- NAE1:
-
NEDD8-activating enzyme E1
- NAFLD:
-
Nonalcoholic fatty liver disease
- B-ALL:
-
B-cell acute lymphoblastic leukemia
- PTPN2:
-
Protein tyrosine phosphatase
References
Advancing Cancer Therapy. Nat Cancer. 2021;2:245–6. https://doi.org/10.1038/s43018-021-00192-x.
Huang CH, Lee KC, Doudna JA. Applications of CRISPR-Cas enzymes in Cancer therapeutics and detection. Trends Cancer. 2018;4:499–512. https://doi.org/10.1016/j.trecan.2018.05.006.
Wang M, Chen M, Wu X, Huang X, Yu B. CRISPR applications in cancer diagnosis and treatment. Cell Mol Biol Lett. 2023;28:73. https://doi.org/10.1186/s11658-023-00483-4.
Yang X, Zhang B. A review on CRISPR/Cas: a versatile tool for cancer screening, diagnosis, and clinic treatment. Funct Integr Genomics. 2023;23:182. https://doi.org/10.1007/s10142-023-01117-w.
Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science. 2010;329:1355–8. https://doi.org/10.1126/science.1192272.
Wiedenheft B, Zhou K, Jinek M, Coyle SM, Ma W, Doudna JA. Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense. Cell. 2009;17:904–12. https://doi.org/10.1016/j.str.2009.03.019.
Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174–82. https://doi.org/10.1007/s00239-004-0046-3.
Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012;109:E2579–2586. https://doi.org/10.1073/pnas.1208507109.
Jore MM, Lundgren M, van Duijn E, Bultema JB, Westra ER, Waghmare SP, Wiedenheft B, Pul U, Wurm R, Wagner R, et al. Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nat Struct Mol Biol. 2011;18:529–36. https://doi.org/10.1038/nsmb.2019.
Sternberg SH, Haurwitz RE, Doudna JA. Mechanism of substrate selection by a highly specific CRISPR endoribonuclease. RNA. 2012;18:661–72. https://doi.org/10.1261/rna.030882.111.
Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH, et al. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015;13:722–36. https://doi.org/10.1038/nrmicro3569.
Koonin EV, Makarova KS, Zhang F. Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol. 2017;37:67–78. https://doi.org/10.1016/j.mib.2017.05.008.
Makarova KS, Zhang F, Koonin EV. SnapShot: class 1 CRISPR-Cas systems. Cell. 2017;168:946–946. https://doi.org/10.1016/j.cell.2017.02.018.
Makarova KS, Zhang F, Koonin EV. SnapShot: class 2 CRISPR-Cas systems. Cell. 2017;168:328–328. https://doi.org/10.1016/j.cell.2016.12.038.
Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ, Charpentier E, Cheng D, Haft DH, Horvath P, et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol. 2020;18:67–83. https://doi.org/10.1038/s41579-019-0299-x.
Tang Y, Fu Y. Class 2 CRISPR/Cas: an expanding biotechnology toolbox for and beyond genome editing. Cell Biosci. 2018;8:59. https://doi.org/10.1186/s13578-018-0255-x.
LaManna CM, Pyhtila B, Barrangou R. Sharing the CRISPR Toolbox with an Expanding Community. CRISPR J. 2020;3:248–52. https://doi.org/10.1089/crispr.2020.0075.
Harrington LB, Burstein D, Chen JS, Paez-Espino D, Ma E, Witte IP, Cofsky JC, Kyrpides NC, Banfield JF, Doudna JA. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science. 2018;362:839–42. https://doi.org/10.1126/science.aav4294.
Shmakov S, Smargon A, Scott D, Cox D, Pyzocha N, Yan W, Abudayyeh OO, Gootenberg JS, Makarova KS, Wolf YI, et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat Rev Microbiol. 2017;15:169–82. https://doi.org/10.1038/nrmicro.2016.184.
Liu TY, Doudna JA. Chemistry of Class 1 CRISPR-Cas effectors: binding, editing, and regulation. J Biol Chem. 2020;295:14473–87. https://doi.org/10.1074/jbc.REV120.007034.
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–91. https://doi.org/10.1038/nature14299.
Cong L, Zhang F. Genome engineering using CRISPR-Cas9 system. Methods Mol Biol. 2015;1239:197–217. https://doi.org/10.1007/978-1-4939-1862-1_10.
Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23:R40–46. https://doi.org/10.1093/hmg/ddu125.
Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159:440–55. https://doi.org/10.1016/j.cell.2014.09.014.
Chen S, Sanjana NE, Zheng K, Shalem O, Lee K, Shi X, Scott DA, Song J, Pan JQ, Weissleder R, et al. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell. 2015;160:1246–60. https://doi.org/10.1016/j.cell.2015.02.038.
Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343:80–4. https://doi.org/10.1126/science.1246981.
Joung J, Konermann S, Gootenberg JS, Abudayyeh OO, Platt RJ, Brigham MD, Sanjana NE, Zhang F. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat Protoc. 2017;12:828–63. https://doi.org/10.1038/nprot.2017.016.
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7. https://doi.org/10.1126/science.1247005.
Holcomb EA, Pearson AN, Jungles KM, Tate A, James J, Jiang L, Huber AK, Green MD. High-content CRISPR screening in tumor immunology. Front Immunol. 2022;13:1041451. https://doi.org/10.3389/fimmu.2022.1041451.
Henkel L, Rauscher B, Schmitt B, Winter J, Boutros M. Genome-scale CRISPR screening at high sensitivity with an empirically designed sgRNA library. BMC Biol. 2020;18:174. https://doi.org/10.1186/s12915-020-00905-1.
Kumar N, Stanford W, de Solis C, Aradhana, Abraham ND, Dao TJ, Thaseen S, Sairavi A, Gonzalez CU, Ploski JE. The development of an AAV-Based CRISPR SaCas9 genome editing system that can be delivered to neurons in vivo and regulated via doxycycline and cre-recombinase. Front Mol Neurosci. 2018;11:413. https://doi.org/10.3389/fnmol.2018.00413.
Liao HK, Hatanaka F, Araoka T, Reddy P, Wu MZ, Sui Y, Yamauchi T, Sakurai M, O’Keefe DD, Nunez-Delicado E et al. In vivo target gene activation via CRISPR/Cas9-Mediated trans-epigenetic modulation. Cell 2017, 171:1495–507 https://doi.org/10.1016/j.cell.2017.10.025.
Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015;33:102–6. https://doi.org/10.1038/nbt.3055.
Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–78. https://doi.org/10.1016/j.cell.2014.05.010.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21. https://doi.org/10.1126/science.1225829.
Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–7. https://doi.org/10.1038/nature13011.
Lin S, Staahl BT, Alla RK, Doudna JA. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife. 2014;3:e04766. https://doi.org/10.7554/eLife.04766.
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–8. https://doi.org/10.1016/j.stem.2013.11.002.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23. https://doi.org/10.1126/science.1231143.
Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–9. https://doi.org/10.1016/j.cell.2013.08.021.
Dickson KA, Field N, Blackman T, Ma Y, Xie T, Kurangil E, Idrees S, Rathnayake SNH, Mahbub RM, Faiz A, Marsh DJ. CRISPR single base-editing: in silico predictions to variant clonal cell lines. Hum Mol Genet. 2023;32:2704–16. https://doi.org/10.1093/hmg/ddad105.
Kantor A, McClements ME, MacLaren RE. CRISPR-Cas9 DNA base-editing and prime-editing. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21176240. 21.
Tomita A, Sasanuma H, Owa T, Nakazawa Y, Shimada M, Fukuoka T, Ogi T, Nakada S. Inducing multiple nicks promotes interhomolog homologous recombination to correct heterozygous mutations in somatic cells. Nat Commun. 2023;14:5607. https://doi.org/10.1038/s41467-023-41048-5.
Renouf B, Piganeau M, Ghezraoui H, Jasin M, Brunet E. Creating cancer translocations in human cells using Cas9 DSBs and nCas9 paired nicks. Methods Enzymol. 2014;546:251–71. https://doi.org/10.1016/B978-0-12-801185-0.00012-X.
Martin Jinek KC, Ines Fonfara M, Hauer JA. Doudna,Emmanuelle Charpentier: a programmable dual RNA guided DNA endonuclease in adaptive bacterial immunity. Science. 2012. https://doi.org/10.1126/science.1225829.
Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, et al. Genome-scale CRISPR-Mediated control of gene repression and activation. Cell. 2014;159:647–61. https://doi.org/10.1016/j.cell.2014.09.029.
Mandegar MA, Huebsch N, Frolov EB, Shin E, Truong A, Olvera MP, Chan AH, Miyaoka Y, Holmes K, Spencer CI, et al. CRISPR Interference efficiently induces specific and reversible gene silencing in human iPSCs. Cell Stem Cell. 2016;18:541–53. https://doi.org/10.1016/j.stem.2016.01.022.
Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–51. https://doi.org/10.1016/j.cell.2013.06.044.
Gasperini M, Hill AJ, McFaline-Figueroa JL, Martin B, Kim S, Zhang MD, Jackson D, Leith A, Schreiber J, Noble WS, et al. A genome-wide Framework for Mapping Gene Regulation via Cellular Genetic screens. Cell. 2019;176:377–90. https://doi.org/10.1016/j.cell.2018.11.029.
Bikard D, Jiang W, Samai P, Hochschild A, Zhang F, Marraffini LA. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013;41:7429–37. https://doi.org/10.1093/nar/gkt520.
Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–8. https://doi.org/10.1038/nature14136.
Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li GW, Park J, Blackburn EH, Weissman JS, Qi LS, Huang B. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155:1479–91. https://doi.org/10.1016/j.cell.2013.12.001.
Wang S, Su JH, Zhang F, Zhuang X. An RNA-aptamer-based two-color CRISPR labeling system. Sci Rep. 2016;6:26857. https://doi.org/10.1038/srep26857.
Kim HK, Song M, Lee J, Menon AV, Jung S, Kang YM, Choi JW, Woo E, Koh HC, Nam JW, Kim H. In vivo high-throughput profiling of CRISPR-Cpf1 activity. Nat Methods. 2017;14:153–9. https://doi.org/10.1038/nmeth.4104.
Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–71. https://doi.org/10.1016/j.cell.2015.09.038.
Kleinstiver BP, Tsai SQ, Prew MS, Nguyen NT, Welch MM, Lopez JM, McCaw ZR, Aryee MJ, Joung JK. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat Biotechnol. 2016;34:869–74. https://doi.org/10.1038/nbt.3620.
Kim Y, Cheong SA, Lee JG, Lee SW, Lee MS, Baek IJ, Sung YH. Generation of knockout mice by Cpf1-mediated gene targeting. Nat Biotechnol. 2016;34:808–10. https://doi.org/10.1038/nbt.3614.
Hur JK, Kim K, Been KW, Baek G, Ye S, Hur JW, Ryu SM, Lee YS, Kim JS. Targeted mutagenesis in mice by electroporation of Cpf1 ribonucleoproteins. Nat Biotechnol. 2016;34:807–8. https://doi.org/10.1038/nbt.3596.
Abdulrachman D, Champreda V, Eurwilaichitr L, Chantasingh D, Pootanakit K. Efficient multiplex CRISPR/Cpf1 (Cas12a) genome editing system in Aspergillus Aculeatus TBRC 277. J Biotechnol. 2022;355:53–64. https://doi.org/10.1016/j.jbiotec.2022.06.011.
Zetsche B, Heidenreich M, Mohanraju P, Fedorova I, Kneppers J, DeGennaro EM, Winblad N, Choudhury SR, Abudayyeh OO, Gootenberg JS, et al. Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat Biotechnol. 2017;35:31–4. https://doi.org/10.1038/nbt.3737.
Dai X, Park JJ, Du Y, Kim HR, Wang G, Errami Y, Chen S. One-step generation of modular CAR-T cells with AAV-Cpf1. Nat Methods. 2019;16:247–54. https://doi.org/10.1038/s41592-019-0329-7.
Strecker J, Jones S, Koopal B, Schmid-Burgk J, Zetsche B, Gao L, Makarova KS, Koonin EV, Zhang F. Engineering of CRISPR-Cas12b for human genome editing. Nat Commun. 2019;10:212. https://doi.org/10.1038/s41467-018-08224-4.
Huang CJ, Adler BA, Doudna JA. A naturally DNase-free CRISPR-Cas12c enzyme silences gene expression. Mol Cell. 2022;82:2148–60. https://doi.org/10.1016/j.molcel.2022.04.020.
Yin L, Zhao F, Sun H, Wang Z, Huang Y, Zhu W, Xu F, Mei S, Liu X, Zhang D, et al. CRISPR-Cas13a inhibits HIV-1 infection. Mol Ther Nucleic Acids. 2020;21:147–55. https://doi.org/10.1016/j.omtn.2020.05.030.
Zheng X, Li Y, Yuan M, Shen Y, Chen S, Duan G. Rapid detection of HPV16/18 based on a CRISPR-Cas13a/Cas12a dual-channel system. Anal Methods. 2022;14:5065–75. https://doi.org/10.1039/d2ay01536f.
Xu C, Zhou Y, Xiao Q, He B, Geng G, Wang Z, Cao B, Dong X, Bai W, Wang Y, et al. Programmable RNA editing with compact CRISPR-Cas13 systems from uncultivated microbes. Nat Methods. 2021;18:499–506. https://doi.org/10.1038/s41592-021-01124-4.
Zhang C, Konermann S, Brideau NJ, Lotfy P, Wu X, Novick SJ, Strutzenberg T, Griffin PR, Hsu PD, Lyumkis D. Structural basis for the RNA-Guided ribonuclease activity of CRISPR-Cas13d. Cell. 2018;175:212–23. https://doi.org/10.1016/j.cell.2018.09.001. e217.
Tong H, Huang J, Xiao Q, He B, Dong X, Liu Y, Yang X, Han D, Wang Z, Wang X, et al. High-fidelity Cas13 variants for targeted RNA degradation with minimal collateral effects. Nat Biotechnol. 2023;41:108–19. https://doi.org/10.1038/s41587-022-01419-7.
Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer NM, Freije CA, et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438–42. https://doi.org/10.1126/science.aam9321.
Fozouni P, Son S, Diaz de Leon Derby M, Knott GJ, Gray CN, D’Ambrosio MV, Zhao C, Switz NA, Kumar GR, Stephens SI, et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell. 2020. https://doi.org/10.1016/j.cell.2020.12.001.
Yang L, Zhang Y, Yi W, Dong X, Niu M, Song Y, Han Y, Li H, Sun Y. A rapid and efficient platform for antiviral crRNA screening using CRISPR-Cas13a-based nucleic acid detection. Front Immunol. 2023;141116230. https://doi.org/10.3389/fimmu.2023.1116230.
Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360:439–44. https://doi.org/10.1126/science.aaq0179.
Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc. 2019;14:2986–3012. https://doi.org/10.1038/s41596-019-0210-2.
Cui J, Techakriengkrai N, Nedumpun T, Suradhat S. Abrogation of PRRSV infectivity by CRISPR-Cas13b-mediated viral RNA cleavage in mammalian cells. Sci Rep. 2020;10:9617. https://doi.org/10.1038/s41598-020-66775-3.
Tng PYL, Carabajal Paladino L, Verkuijl SAN, Purcell J, Merits A, Leftwich PT, Fragkoudis R, Noad R, Alphey L. Cas13b-dependent and Cas13b-independent RNA knockdown of viral sequences in mosquito cells following guide RNA expression. Commun Biol. 2020;3413. https://doi.org/10.1038/s42003-020-01142-6.
Fareh M, Zhao W, Hu W, Casan JML, Kumar A, Symons J, Zerbato JM, Fong D, Voskoboinik I, Ekert PG, et al. Reprogrammed CRISPR-Cas13b suppresses SARS-CoV-2 replication and circumvents its mutational escape through mismatch tolerance. Nat Commun. 2021;12:4270. https://doi.org/10.1038/s41467-021-24577-9.
Yu D, Han HJ, Yu J, Kim J, Lee GH, Yang JH, Song BM, Tark D, Choi BS, Kang SM, Heo WD. Pseudoknot-targeting Cas13b combats SARS-CoV-2 infection by suppressing viral replication. Mol Ther. 2023;31:1675–87. https://doi.org/10.1016/j.ymthe.2023.03.018.
Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, Zhang F. RNA editing with CRISPR-Cas13. Science. 2017;358:1019–27. https://doi.org/10.1126/science.aaq0180.
Granados-Riveron JT, Aquino-Jarquin G. CRISPR-Cas13 Precision Transcriptome Engineering in Cancer. Cancer Res. 2018;78:4107–13. https://doi.org/10.1158/0008-5472.CAN-18-0785.
Palaz F, Kalkan AK, Can O, Demir AN, Tozluyurt A, Ozcan A, Ozsoz M. CRISPR-Cas13 System as a Promising and Versatile Tool for Cancer diagnosis, therapy, and Research. ACS Synth Biol. 2021;10:1245–67. https://doi.org/10.1021/acssynbio.1c00107.
Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera Mdel C, Yusa K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol. 2014;32:267–73. https://doi.org/10.1038/nbt.2800.
Shalem O, Sanjana NE, Zhang F. High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet. 2015;16:299–311. https://doi.org/10.1038/nrg3899.
Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–8. https://doi.org/10.1016/j.cell.2013.04.025.
Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–4. https://doi.org/10.1038/nmeth.3047.
Najm FJ, Strand C, Donovan KF, Hegde M, Sanson KR, Vaimberg EW, Sullender ME, Hartenian E, Kalani Z, Fusi N, et al. Orthologous CRISPR-Cas9 enzymes for combinatorial genetic screens. Nat Biotechnol. 2018;36:179–89. https://doi.org/10.1038/nbt.4048.
Kim JS, Lee JH, Jeon SR, Kim Y, Jeon SH, Wu HG. Identification of genes involved in EGF-induced apoptosis using CRISPR/Cas9 knockout screening: implications for Novel therapeutic targets in EGFR-Overexpressing cancers. Cancer Res Treat. 2023. https://doi.org/10.4143/crt.2022.1414.
Hart T, Chandrashekhar M, Aregger M, Steinhart Z, Brown KR, MacLeod G, Mis M, Zimmermann M, Fradet-Turcotte A, Sun S, et al. High-resolution CRISPR screens Reveal Fitness genes and genotype-specific Cancer liabilities. Cell. 2015;163:1515–26. https://doi.org/10.1016/j.cell.2015.11.015.
Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547:413–8. https://doi.org/10.1038/nature23270.
Wang G, Chow RD, Ye L, Guzman CD, Dai X, Dong MB, Zhang F, Sharp PA, Platt RJ, Chen S. Mapping a functional cancer genome atlas of tumor suppressors in mouse liver using AAV-CRISPR-mediated direct in vivo screening. Sci Adv. 2018;4:eaao5508. https://doi.org/10.1126/sciadv.aao5508.
Parnas O, Jovanovic M, Eisenhaure TM, Herbst RH, Dixit A, Ye CJ, Przybylski D, Platt RJ, Tirosh I, Sanjana NE, et al. A genome-wide CRISPR screen in primary Immune cells to Dissect Regulatory Networks. Cell. 2015;162:675–86. https://doi.org/10.1016/j.cell.2015.06.059.
Canver MC, Haeussler M, Bauer DE, Orkin SH, Sanjana NE, Shalem O, Yuan GC, Zhang F, Concordet JP, Pinello L. Integrated design, execution, and analysis of arrayed and pooled CRISPR genome-editing experiments. Nat Protoc. 2018;13:946–86. https://doi.org/10.1038/nprot.2018.005.
Wilson LOW, O’Brien AR, Bauer DC. The current state and future of CRISPR-Cas9 gRNA Design Tools. Front Pharmacol. 2018;9:749. https://doi.org/10.3389/fphar.2018.00749.
Cui Y, Xu J, Cheng M, Liao X, Peng S. Review of CRISPR/Cas9 sgRNA design tools. Interdiscip Sci. 2018;10:455–65. https://doi.org/10.1007/s12539-018-0298-z.
Vakulskas CA, Behlke MA. Evaluation and reduction of CRISPR off-target cleavage events. Nucleic Acid Ther. 2019;29:167–74. https://doi.org/10.1089/nat.2019.0790.
Concordet JP, Haeussler M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018;46:W242–5. https://doi.org/10.1093/nar/gky354.
Haeussler M, Schonig K, Eckert H, Eschstruth A, Mianne J, Renaud JB, Schneider-Maunoury S, Shkumatava A, Teboul L, Kent J, et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016;17148. https://doi.org/10.1186/s13059-016-1012-2.
Pinello L, Canver MC, Hoban MD, Orkin SH, Kohn DB, Bauer DE, Yuan GC. Analyzing CRISPR genome-editing experiments with CRISPResso. Nat Biotechnol. 2016;34:695–7. https://doi.org/10.1038/nbt.3583.
Li W, Xu H, Xiao T, Cong L, Love MI, Zhang F, Irizarry RA, Liu JS, Brown M, Liu XS. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 2014;15:554. https://doi.org/10.1186/s13059-014-0554-4.
Wang X, Wang Y, Wu X, Wang J, Wang Y, Qiu Z, Chang T, Huang H, Lin RJ, Yee JK. Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. Nat Biotechnol. 2015;33:175–8. https://doi.org/10.1038/nbt.3127.
Frock RL, Hu J, Meyers RM, Ho YJ, Kii E, Alt FW. Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat Biotechnol. 2015;33:179–86. https://doi.org/10.1038/nbt.3101.
Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. https://doi.org/10.1186/gb-2009-10-3-r25.
Hart T, Brown KR, Sircoulomb F, Rottapel R, Moffat J. Measuring error rates in genomic perturbation screens: gold standards for human functional genomics. Mol Syst Biol. 2014;10:733. https://doi.org/10.15252/msb.20145216.
Lane-Reticker SK, Kessler EA, Muscato AJ, Kim SY, Doench JG, Yates KB, Manguso RT, Dubrot J. Protocol for in vivo CRISPR screening using selective CRISPR antigen removal lentiviral vectors. STAR Protoc. 2023;4:102082. https://doi.org/10.1016/j.xpro.2023.102082.
Crosetto N, Mitra A, Silva MJ, Bienko M, Dojer N, Wang Q, Karaca E, Chiarle R, Skrzypczak M, Ginalski K, et al. Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat Methods. 2013;10:361–5. https://doi.org/10.1038/nmeth.2408.
Tsai SQ, Zheng Z, Nguyen NT, Liebers M, Topkar VV, Thapar V, Wyvekens N, Khayter C, Iafrate AJ, Le LP, et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol. 2015;33:187–97. https://doi.org/10.1038/nbt.3117.
Malinin NL, Lee G, Lazzarotto CR, Li Y, Zheng Z, Nguyen NT, Liebers M, Topkar VV, Iafrate AJ, Le LP, et al. Defining genome-wide CRISPR-Cas genome-editing nuclease activity with GUIDE-seq. Nat Protoc. 2021;16:5592–615. https://doi.org/10.1038/s41596-021-00626-x.
Wienert B, Wyman SK, Richardson CD, Yeh CD, Akcakaya P, Porritt MJ, Morlock M, Vu JT, Kazane KR, Watry HL, et al. Unbiased detection of CRISPR off-targets in vivo using DISCOVER-Seq. Science. 2019;364:286–9. https://doi.org/10.1126/science.aav9023.
Wienert B, Wyman SK, Yeh CD, Conklin BR, Corn JE. CRISPR off-target detection with DISCOVER-seq. Nat Protoc. 2020;15:1775–99. https://doi.org/10.1038/s41596-020-0309-5.
Zou RS, Liu Y, Gaido OER, Konig MF, Mog BJ, Shen LL, Aviles-Vazquez F, Marin-Gonzalez A, Ha T. Improving the sensitivity of in vivo CRISPR off-target detection with DISCOVER-Seq. Nat Methods. 2023;20:706–13. https://doi.org/10.1038/s41592-023-01840-z.
Kim D, Bae S, Park J, Kim E, Kim S, Yu HR, Hwang J, Kim JI, Kim JS. Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat Methods. 2015;12:237–43. https://doi.org/10.1038/nmeth.3284.
Kim D, Kang BC, Kim JS. Identifying genome-wide off-target sites of CRISPR RNA-guided nucleases and deaminases with Digenome-Seq. Nat Protoc. 2021;16:1170–92. https://doi.org/10.1038/s41596-020-00453-6.
Park J, Childs L, Kim D, Hwang GH, Kim S, Kim ST, Kim JS, Bae S. Digenome-Seq web tool for profiling CRISPR specificity. Nat Methods. 2017;14:548–9. https://doi.org/10.1038/nmeth.4262.
Cameron P, Fuller CK, Donohoue PD, Jones BN, Thompson MS, Carter MM, Gradia S, Vidal B, Garner E, Slorach EM, et al. Mapping the genomic landscape of CRISPR-Cas9 cleavage. Nat Methods. 2017;14:600–6. https://doi.org/10.1038/nmeth.4284.
Lazzarotto CR, Nguyen NT, Tang X, Malagon-Lopez J, Guo JA, Aryee MJ, Joung JK, Tsai SQ. Defining CRISPR-Cas9 genome-wide nuclease activities with CIRCLE-seq. Nat Protoc. 2018;13:2615–42. https://doi.org/10.1038/s41596-018-0055-0.
Tsai SQ, Nguyen NT, Malagon-Lopez J, Topkar VV, Aryee MJ, Joung JK. CIRCLE-seq: a highly sensitive in vitro screen for genome-wide CRISPR-Cas9 nuclease off-targets. Nat Methods. 2017;14:607–14. https://doi.org/10.1038/nmeth.4278.
Lazzarotto CR, Malinin NL, Li Y, Zhang R, Yang Y, Lee G, Cowley E, He Y, Lan X, Jividen K, et al. CHANGE-seq reveals genetic and epigenetic effects on CRISPR-Cas9 genome-wide activity. Nat Biotechnol. 2020;38:1317–27. https://doi.org/10.1038/s41587-020-0555-7.
Khoshandam M, Soltaninejad H, Mousazadeh M, Hamidieh AA, Hosseinkhani S. Clinical applications of the CRISPR/Cas9 genome-editing system: delivery options and challenges in precision medicine. Genes Dis. 2024;11:268–82. https://doi.org/10.1016/j.gendis.2023.02.027.
Wang D, Zhang F, Gao G. CRISPR-Based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020;181:136–50. https://doi.org/10.1016/j.cell.2020.03.023.
Proietti L, Manhart G, Heyes E, Troester S, Grebien F. Arrayed CRISPR/Cas9 screening for the functional validation of Cancer Genetic dependencies. Bio Protoc. 2022. https://doi.org/10.21769/BioProtoc.4577.
Shi J, Wang E, Milazzo JP, Wang Z, Kinney JB, Vakoc CR. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat Biotechnol. 2015;33:661–7. https://doi.org/10.1038/nbt.3235.
Schmidt R, Steinhart Z, Layeghi M, Freimer JW, Bueno R, Nguyen VQ, Blaeschke F, Ye CJ, Marson A. CRISPR activation and interference screens decode stimulation responses in primary human T cells. Science. 2022;375:eabj4008. https://doi.org/10.1126/science.abj4008.
Shifrut E, Carnevale J, Tobin V, Roth TL, Woo JM, Bui CT, Li PJ, Diolaiti ME, Ashworth A, Marson A. Genome-wide CRISPR Screens in Primary Human T Cells Reveal Key Regulators of Immune Function. Cell 2018, 175:1958–1971 e1915.https://doi.org/10.1016/j.cell.2018.10.024.
Geurts MH, Clevers H. CRISPR engineering in organoids for gene repair and disease modelling. Nat Reviews Bioeng. 2023;1:32–45. https://doi.org/10.1038/s44222-022-00013-5.
Wang D, Prager BC, Gimple RC, Aguilar B, Alizadeh D, Tang H, Lv D, Starr R, Brito A, Wu Q, et al. CRISPR Screening of CAR T cells and Cancer stem cells reveals critical dependencies for cell-based therapies. Cancer Discov. 2021;11:1192–211. https://doi.org/10.1158/2159-8290.CD-20-1243.
Dufva O, Koski J, Maliniemi P, Ianevski A, Klievink J, Leitner J, Polonen P, Hohtari H, Saeed K, Hannunen T, et al. Integrated drug profiling and CRISPR screening identify essential pathways for CAR T-cell cytotoxicity. Blood. 2020;135:597–609. https://doi.org/10.1182/blood.2019002121.
Xu WW, Liao L, Dai W, Zheng CC, Tan XP, He Y, Zhang QH, Huang ZH, Chen WY, Qin YR, et al. Genome-wide CRISPR/Cas9 screening identifies a targetable MEST-PURA interaction in cancer metastasis. EBioMedicine. 2023;92104587. https://doi.org/10.1016/j.ebiom.2023.104587.
Szlachta K, Kuscu C, Tufan T, Adair SJ, Shang S, Michaels AD, Mullen MG, Fischer NL, Yang J, Liu L, et al. CRISPR knockout screening identifies combinatorial drug targets in pancreatic cancer and models cellular drug response. Nat Commun. 2018;94275. https://doi.org/10.1038/s41467-018-06676-2.
Yang C, Lee D, Zhang MS, Tse AP, Wei L, Bao MH, Wong BP, Chan CY, Yuen VW, Chen Y, Wong CC. Genome-wide CRISPR/Cas9 Library Screening revealed Dietary Restriction of glutamine in combination with inhibition of pyruvate metabolism as effective Liver Cancer Treatment. Adv Sci (Weinh). 2022;9:e2202104. https://doi.org/10.1002/advs.202202104.
Coelho R, Tozzi A, Disler M, Lombardo F, Fedier A, Lopez MN, Freuler F, Jacob F, Heinzelmann-Schwarz V. Overlapping gene dependencies for PARP inhibitors and carboplatin response identified by functional CRISPR-Cas9 screening in ovarian cancer. Cell Death Dis. 2022;13909. https://doi.org/10.1038/s41419-022-05347-x.
Arang N, Lubrano S, Rigiracciolo DC, Nachmanson D, Lippman SM, Mali P, Harismendy O, Gutkind JS. Whole-genome CRISPR screening identifies PI3K/AKT as a downstream component of the oncogenic GNAQ-focal adhesion kinase signaling circuitry. J Biol Chem. 2023;299102866. https://doi.org/10.1016/j.jbc.2022.102866.
Zhao X, Li J, Liu Z, Powers S. Combinatorial CRISPR/Cas9 screening reveals epistatic networks of interacting tumor suppressor genes and therapeutic targets in human breast Cancer. Cancer Res. 2021;81:6090–105. https://doi.org/10.1158/0008-5472.CAN-21-2555.
Deng L, Yang L, Zhu S, Li M, Wang Y, Cao X, Wang Q, Guo L. Identifying CDC7 as a synergistic target of chemotherapy in resistant small-cell lung cancer via CRISPR/Cas9 screening. Cell Death Discov. 2023;9:40. https://doi.org/10.1038/s41420-023-01315-2.
Tzelepis K, Koike-Yusa H, De Braekeleer E, Li Y, Metzakopian E, Dovey OM, Mupo A, Grinkevich V, Li M, Mazan M, et al. A CRISPR dropout screen identifies genetic vulnerabilities and therapeutic targets in Acute myeloid leukemia. Cell Rep. 2016;17:1193–205. https://doi.org/10.1016/j.celrep.2016.09.079.
Zhang B, Ren Z, Zheng H, Lin M, Chen G, Luo OJ, Zhu G. CRISPR activation screening in a mouse model for drivers of hepatocellular carcinoma growth and metastasis. iScience. 2023;26:106099. https://doi.org/10.1016/j.isci.2023.106099.
Tian Y, Dong D, Wu L, Park JY, Wei GH, Wang L, consortium PE. Combined CRISPR and proteomics screening reveal a cohesin-CTCF-bound allele contributing to increased expression of RUVBL1 and prostate cancer progression. bioRxiv 2023.https://doi.org/10.1101/2023.01.18.524405.
Zhou Z, Chen G, Shen M, Li J, Liu K, Liu M, Shi S, Yang D, Chen W, Chen S, et al. Genome-scale CRISPR-Cas9 knockout screening in nasopharyngeal carcinoma for radiosensitive and radioresistant genes. Transl Oncol. 2023;30101625. https://doi.org/10.1016/j.tranon.2023.101625.
Karapurkar JK, Kim MS, Colaco JC, Suresh B, Sarodaya N, Kim DH, Park CH, Hong SH, Kim KS, Ramakrishna S. CRISPR/Cas9-based genome-wide screening of the deubiquitinase subfamily identifies USP3 as a protein stabilizer of REST blocking neuronal differentiation and promotes neuroblastoma tumorigenesis. J Exp Clin Cancer Res. 2023;42121. https://doi.org/10.1186/s13046-023-02694-1.
Ouyang Q, Liu Y, Tan J, Li J, Yang D, Zeng F, Huang W, Kong Y, Liu Z, Zhou H, Liu Y. Loss of ZNF587B and SULF1 contributed to cisplatin resistance in ovarian cancer cell lines based on genome-scale CRISPR/Cas9 screening. Am J Cancer Res. 2019;9:988–98.
Cao C, Liu T, Zhang Q, Li R, Zeng Z, Cui Z, Wang X, Gong D, Tian X, Hu Z. Somatic mutations and CRISPR/Cas9 library screening integrated analysis identifies cervical cancer drug-resistant pathways. Clin Transl Med. 2021;11e632. https://doi.org/10.1002/ctm2.632.
Haldrup J, Weiss S, Schmidt L, Sorensen KD. Investigation of enzalutamide, docetaxel, and cabazitaxel resistance in the castration resistant prostate cancer cell line C4 using genome-wide CRISPR/Cas9 screening. Sci Rep. 2023;13:9043. https://doi.org/10.1038/s41598-023-35950-7.
Chen A, Wen S, Liu F, Zhang Z, Liu M, Wu Y, He B, Yan M, Kang T, Lam EW, et al. CRISPR/Cas9 screening identifies a kinetochore-microtubule dependent mechanism for Aurora-A inhibitor resistance in breast cancer. Cancer Commun (Lond). 2021;41:121–39. https://doi.org/10.1002/cac2.12125.
Zhang N, Huang D, Ruan X, Ng AT, Tsu JH, Jiang G, Huang J, Zhan Y, Na R. CRISPR screening reveals gleason score and castration resistance related oncodriver ring finger protein 19 A (RNF19A) in prostate cancer. Drug Resist Updat. 2023;67100912. https://doi.org/10.1016/j.drup.2022.100912.
Xie C, Li K, Li Y, Peng X, Teng B, He K, Jin A, Wang W, Wei Z. CRISPR-based knockout screening identifies the loss of MIEF2 to enhance oxaliplatin resistance in colorectal cancer through inhibiting the mitochondrial apoptosis pathway. Front Oncol. 2022;12881487. https://doi.org/10.3389/fonc.2022.881487.
Luo BH, Huang JQ, Huang CY, Tian P, Chen AZ, Wu WH, Ma XM, Yuan YX, Yu L. Screening of Lymphoma Radiotherapy-resistant genes with CRISPR Activation Library. Pharmgenomics Pers Med. 2023;16:67–80. https://doi.org/10.2147/PGPM.S386085.
Funke K, Einsfelder U, Hansen A, Arevalo L, Schneider S, Nettersheim D, Stein V, Schorle H. Genome-scale CRISPR screen reveals neddylation to contribute to cisplatin resistance of testicular germ cell tumours. Br J Cancer. 2023;128:2270–82. https://doi.org/10.1038/s41416-023-02247-5.
Price S, Bhosle S, Goncalves E, Li X, McClurg DP, Barthorpe S, Beck A, Hall C, Lightfoot H, Farrow L, et al. A suspension technique for efficient large-scale cancer organoid culturing and perturbation screens. Sci Rep. 2022;12:5571. https://doi.org/10.1038/s41598-022-09508-y.
Hendriks D, Brouwers JF, Hamer K, Geurts MH, Luciana L, Massalini S, Lopez-Iglesias C, Peters PJ, Rodriguez-Colman MJ, Chuva de Sousa Lopes S, et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nat Biotechnol. 2023. https://doi.org/10.1038/s41587-023-01680-4.
Hirt CK, Booij TH, Grob L, Simmler P, Toussaint NC, Keller D, Taube D, Ludwig V, Goryachkin A, Pauli C, et al. Drug screening and genome editing in human pancreatic cancer organoids identifies drug-gene interactions and candidates for off-label treatment. Cell Genom. 2022;2100095. https://doi.org/10.1016/j.xgen.2022.100095.
Ling X, Chang L, Chen H, Liu T. Efficient generation of locus-specific human CAR-T cells with CRISPR/cCas12a. STAR Protoc. 2022;3:101321. https://doi.org/10.1016/j.xpro.2022.101321.
Wagner DL, Koehl U, Chmielewski M, Scheid C, Stripecke R. Review: sustainable clinical development of CAR-T cells - switching from viral transduction towards CRISPR-Cas Gene Editing. Front Immunol. 2022;13:865424. https://doi.org/10.3389/fimmu.2022.865424.
Sochacka-Cwikla A, Maczynski M, Regiec A. FDA-Approved drugs for hematological malignancies-the last Decade Review. Cancers (Basel). 2021. https://doi.org/10.3390/cancers14010087. 14.
Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol Cancer. 2022;21:78. https://doi.org/10.1186/s12943-022-01559-z.
Wang L, Zhou L, Li M, Zhao J, Liu Y, Chen Y, Qin X, Wang S, Chen H, Piao Y, et al. Genome-wide CRISPR/Cas9 knockout screening uncovers ZNF319 as a novel tumor suppressor critical for breast cancer metastasis. Biochem Biophys Res Commun. 2022;589:107–15. https://doi.org/10.1016/j.bbrc.2021.12.023.
Sun X, Wang Z, Chen X, Shen K. CRISPR-cas9 screening identified Lethal genes enriched in cell cycle pathway and of prognosis significance in breast Cancer. Front Cell Dev Biol. 2021;9:646774. https://doi.org/10.3389/fcell.2021.646774.
Wang Q, Guo F, Jin Y, Ma Y. Applications of human organoids in the personalized treatment for digestive diseases. Signal Transduct Target Ther. 2022;7:336. https://doi.org/10.1038/s41392-022-01194-6.
Cortese MJ, Sauter C. A CRISPR non-viral manufacturing approach for CAR T cell therapies. Mol Ther. 2022;30:3338–40. https://doi.org/10.1016/j.ymthe.2022.09.022.
Ramos A, Koch CE, Liu-Lupo Y, Hellinger RD, Kyung T, Abbott KL, Frose J, Goulet D, Gordon KS, Eidell KP, et al. Leukemia-intrinsic determinants of CAR-T response revealed by iterative in vivo genome-wide CRISPR screening. Nat Commun. 2023;148048. https://doi.org/10.1038/s41467-023-43790-2.
Ye L, Park JJ, Peng L, Yang Q, Chow RD, Dong MB, Lam SZ, Guo J, Tang E, Zhang Y, et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab. 2022;34:595–614. https://doi.org/10.1016/j.cmet.2022.02.009.
Madigan V, Zhang F, Dahlman JE. Drug delivery systems for CRISPR-based genome editors. Nat Rev Drug Discov. 2023;22:875–94. https://doi.org/10.1038/s41573-023-00762-x.
Chow RD, Guzman CD, Wang G, Schmidt F, Youngblood MW, Ye L, Errami Y, Dong MB, Martinez MA, Zhang S, et al. AAV-mediated direct in vivo CRISPR screen identifies functional suppressors in glioblastoma. Nat Neurosci. 2017;20:1329–41. https://doi.org/10.1038/nn.4620.
Li J, Lee YC, Iessi IL, Wu C, Yi P, Cai EP. Protocol for genome-scale in vivo CRISPR screening to study protection of beta cells under autoimmunity in a type 1 diabetes mouse model. STAR Protoc. 2023;4:102155. https://doi.org/10.1016/j.xpro.2023.102155.
Liu L, Lei Y, Chen W, Zhou Q, Zheng Z, Zeng G, Liu W, Feng P, Zhang Z, Yu L, Chen L. In vivo genome-wide CRISPR screening identifies ZNF24 as a negative NF-kappaB modulator in lung cancer. Cell Biosci. 2022;12193. https://doi.org/10.1186/s13578-022-00933-0.
Dong MB, Wang G, Chow RD, Ye L, Zhu L, Dai X, Park JJ, Kim HR, Errami Y, Guzman CD, et al. Systematic Immunotherapy Target Discovery using genome-scale in vivo CRISPR screens in CD8 T cells. Cell. 2019;178:1189–204. https://doi.org/10.1016/j.cell.2019.07.044.
Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358–78. https://doi.org/10.1038/s41573-019-0012-9.
Haeussler M. CRISPR off-targets: a question of context. Cell Biol Toxicol. 2020;36:5–9. https://doi.org/10.1007/s10565-019-09497-1.
Sternberg SH, LaFrance B, Kaplan M, Doudna JA. Conformational control of DNA target cleavage by CRISPR-Cas9. Nature. 2015;527:110–3. https://doi.org/10.1038/nature15544.
Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351:84–8. https://doi.org/10.1126/science.aad5227.
Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, Sternberg SH, Joung JK, Yildiz A, Doudna JA. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature. 2017;550:407–10. https://doi.org/10.1038/nature24268.
Kleinstiver BP, Prew MS, Tsai SQ, Nguyen NT, Topkar VV, Zheng Z, Joung JK. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat Biotechnol. 2015;33:1293–8. https://doi.org/10.1038/nbt.3404.
Lin P, Qin S, Pu Q, Wang Z, Wu Q, Gao P, Schettler J, Guo K, Li R, Li G, et al. CRISPR-Cas13 inhibitors Block RNA editing in Bacteria and mammalian cells. Mol Cell. 2020;78:850–61. https://doi.org/10.1016/j.molcel.2020.03.033.
Safarzadeh Kozani P, Safarzadeh Kozani P, Ahmadi Najafabadi M, Yousefi F, Mirarefin SMJ, Rahbarizadeh F. Recent advances in solid tumor CAR-T cell therapy: driving Tumor cells from Hero to zero? Front Immunol. 2022;13:795164. https://doi.org/10.3389/fimmu.2022.795164.
Mueller KP, Piscopo NJ, Forsberg MH, Saraspe LA, Das A, Russell B, Smerchansky M, Cappabianca D, Shi L, Shankar K, et al. Production and characterization of virus-free, CRISPR-CAR T cells capable of inducing solid tumor regression. J Immunother Cancer. 2022. https://doi.org/10.1136/jitc-2021-004446.
Hu Y, Zu C, Zhang M, Wei G, Li W, Fu S, Hong R, Zhou L, Wu W, Cui J, et al. Safety and efficacy of CRISPR-based non-viral PD1 locus specifically integrated anti-CD19 CAR-T cells in patients with relapsed or refractory Non-hodgkin’s lymphoma: a first-in-human phase I study. EClinicalMedicine. 2023;60:102010. https://doi.org/10.1016/j.eclinm.2023.102010.
Johnson WG, Bowman DM. Inherited regulation for advanced ARTs: comparing jurisdictions’ applications of existing governance regimes to emerging reproductive technologies. J Law Biosci. 2022;9:lsab034. https://doi.org/10.1093/jlb/lsab034.
Cyranoski D. China set to introduce gene-editing regulation following CRISPR-baby furore. Nature. 2019. https://doi.org/10.1038/d41586-019-01580-1.
Scott CT, Selin C. What to expect when expecting CRISPR Baby Number Four. Am J Bioeth. 2019;19:7–9. https://doi.org/10.1080/15265161.2018.1562793.
Acknowledgements
The authors would like to thank Prof. Guokai Chen and Dr. Weiwei Liu from University of Macau, Dr. Carlos Godoy-Parejo from Icahn School of Medicine at Mount Sinai, Ms Qinru Li from University of Toronto for critical comments and wonderful advises on the manuscript. We also thank other laboratory members for helpful discussions of our review.
Funding
This work was funded by Guangdong Basic and Applied Basic Research Foundation (File No.2020A1515110045), by Zhuhai High-level Health Personnel Team Project (File No. Zhuhai HLHPTP201702), and by Zhuhai People’s Hospital (File No. 2020XSYC-12).
Author information
Authors and Affiliations
Contributions
MQ and CD are responsible for the manuscript drafting and revision. LW is contributed to manuscript revision. GL is responsible for manuscript revision and financial support. YM is contributed to manuscript revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qin, M., Deng, C., Wen, L. et al. CRISPR-Cas and CRISPR-based screening system for precise gene editing and targeted cancer therapy. J Transl Med 22, 516 (2024). https://doi.org/10.1186/s12967-024-05235-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05235-2