Abstract
Background
Tumor mutational burden (TMB) has been demonstrated to predict the response to immune checkpoint inhibitors (ICIs) in various cancers. However, the role of TMB in head and neck squamous cell carcinoma (HNSCC) has not yet been specifically addressed. Since HNSCC patients exhibit a rather limited response to ICIs, there is an unmet need to develop predictive biomarkers to improve patient selection criteria and the clinical benefit of ICI treatment.
Methods
We conducted a systematic review and meta-analysis according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. HNSCC cohort studies were selected when TMB prior to ICI treatment was evaluated, TMB cutoff value was available, and the prognostic value of TMB was evaluated by time-to-event survival analysis. A total of 11 out of 1960 articles were analyzed, including 1200 HNSCC patients.
Results
The results showed that those patients harboring high TMB exhibited a significantly superior overall response rate (OR = 2.62; 95% CI 1.74–3.94; p < 0.0001) and a survival advantage (HR = 0.53; 95% CI 0.39–0.71; p < 0.0001) after ICI treatment.
Conclusion
This is the first meta-analysis to demonstrate a higher response and clinical benefit from ICI therapy in HNSCC patients with high TMB.
Graphical Abstract

Similar content being viewed by others
Background
Head and neck squamous cell carcinoma (HNSCC) is the predominant cancer in the head and neck area, and the sixth most frequently malignancy worldwide [1]. In recent decades, the survival rates for HNSCC patients have only modestly improved due to combined treatment modalities, which are mainly surgery, radiotherapy, and chemotherapy. Therefore, there is an imperative need to enhance the prognosis prediction ability for HNSCC patients, to tailor effective individualized therapeutic strategies and to acquire insight into the underlying mechanisms that contribute to treatment response/resistance and therapeutic failure [2].
The intricate interplay between the tumor and the surrounding stroma, the so-called jointly tumor microenvironment (TME), plays a central role in tumor progression and treatment response. The TME encompasses diverse cellular components, such as stromal fibroblasts, endothelial cells, blood vessels, lymph vessels, and immune cells, among others [3]. Immune evasion has been recognized as an emerging hallmark of cancer, and in recent years numerous mechanisms have been identified as promising targets for anti-cancer immunotherapy [4].
Immune checkpoint inhibitors (ICIs) have emerged as a novel promising class of anticancer agents and a central pillar in the treatment of advanced cancers. There is substantial evidence of long-lasting response to ICIs and survival advantage in platinum-pretreated recurrent and metastatic (R/M) HNSCC [5,6,7,8]. However, the overall response rates (ORRs) to these agents in platinum-refractory recurrent and metastatic (R/M) HNSCC are as of yet rather modest, ranging from 13 to 18% [5, 9, 10]. Given the significant cost and potential immune-related toxicities associated to ICI therapy, it poses a crucial challenge to identify and validate reliable predictive biomarkers of ICI efficacy to guide patient selection and clinical decision-making. Among the biomarkers for consideration are PD-L1 protein expression, intratumoral immune cell infiltration, immune-gene expression profiling, and tumor mutational burden (TMB). However, none of these biomarkers have been validated in the context of HNSCC to date [10, 11].
The TMB refers to the number of non-synonymous mutations per megabase detected in a tumor exome by next-generation sequencing (NGS). An elevated TMB corresponds to increased neoantigen production by tumor cells, which enhances potential recognition by the immune system. Consequently, ICI-induced antitumor immune responses may facilitate the elimination of tumor cells [12, 13]. A comprehensive analysis integrating 45 clinical studies and data from 103,078 cancer patients revealed that high TMB may serve as an unfavorable prognostic indicator for patients undergoing non-immunotherapy treatment. By contrast, in the subset of patients who received immunotherapy, high TMB was globally found to associate significantly with improved survival and treatment efficacy irrespective of the cancer type [14].
The predictive and prognostic value of TMB in cancer immunotherapy has been validated in certain specific cancer types, such as non-small cell lung cancer, melanoma, and colorectal cancer [13, 15,16,17]. However, the impact of TMB in HNSCC patients has not been specifically and properly addressed. Even though it has been reported that HNSCC patients with elevated TMB who received immunotherapy showed higher response rates and better prognoses, the studies published to date present several methodological limitations. Overall, the small sample size and lack of consensus on the optimal TMB cutoff value complicate a possible implementation in routine clinical practice [18,19,20,21,22,23,24,25,26,27,28]. This prompted us to undertake the first meta-analysis aimed at evaluating the prognostic and predictive significance of TMB in R/M HNSCC patients treated with ICIs.
Materials and methods
Search strategy
We conducted a systematic review through the examination of the current existing literature according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [29]. The PRISMA Checklist is included as Additional file 1: Table S1. The overall goal of this search strategy was to identify and include all relevant articles specifically focused on assessing the impact of TMB in HNSCC patient cohorts. This systematic review is registered in Open Science Framework (identifier: https://osf.io/7gmdc).
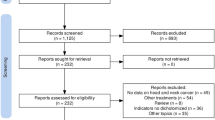
An updated PubMed, Embase, Web of Science and Scopus internet search was performed on December 26, 2023, encompassing English language publications from 2011 to 2023, the time period since the first ICI agent was approved by the FDA. The search criteria included the following terms in the title or abstract: "Tumor Mutational Burden" combined with "Head and Neck Cancer" OR "Oral cancer" OR "Oropharyngeal cancer" OR "Laryngeal cancer" OR "Hypopharyngeal cancer" (Additional file 1: Table S2). Two independent researchers (JPR and MS-C) reviewed the search results to identify potentially eligible studies. In those studies where the abstract mentioned follow-up data and outcomes related to TMB in HNSCC, the full-text article was retrieved and reviewed. Additionally, all review articles were thoroughly examined. The references of the retrieved full-text articles were cross-checked to ensure appropriate inclusion in this review (Fig. 1). Any disagreements regarding the eligibility of an article were resolved through consensus.
Selection criteria
Studies were included in the analysis if they met the following inclusion criteria: (1) evaluation of the predictive effect of TMB in the outcome of ICIs in HNSCC patients; (2) TMB assessment before treatment; (3) TMB cutoff value was provided; (4) data availability of TMB-related hazard ratio (HR) and its corresponding 95% confidence interval (95% CI) for overall survival (OS), as well as the odds ratio (OR) and its corresponding 95% CI for the objective response rate (ORR) to ICIs; and (5) original articles published in English between January 2011 and December 2023.
The following exclusion criteria were also applied to the selected studies: (1) insufficient or lacking information related to TMB prognostic accuracy, including HRs or ORs with 95% CI and (2) article type classified as a letter, case report, non-clinical study, or conference abstract.
Data extraction
The relevant information from each selected paper was independently extracted by two reviewers (JPR and MS-C), and any disagreements were resolved through consensus. The predetermined data from each article were documented as follows: Name of first author, year of publication, country, study population (number of patients with high or low TMB), study design, median age, median follow-up, treatment method, clinical stage, sample source (tumor or blood), sequencing method (NGS or WES), covariant, cut-off value of TMB, method of cut-off value determination, median TMB value, and survival analysis.
Quality assessment
Two authors (JPR and MS-C) independently assessed the quality of the eligible studies by Newcastle–Ottawa Scale (NOS), using three parameters with a maximum of 9 points: comparability (0–4 points), selection (0–2 points), and outcome confirmation (0–3 points) [30]. A score greater than 6 points indicated a high-quality article, while a score of 6 points or lower indicated a low-quality article.
Statistical analysis
Meta-analysis was performed using Review Manager (RevMan) version 5.4 and package meta from the software environment R (www.r-project.ort). Significant results were defined as those having a p-value below 0.05. To assess the predictive efficacy of TMB in HNSCC patients treated with ICIs, the OS and ORR were compared between the high and low TMB patient subgroups using HRs and ORs. Forest plots were used to visualize the overall effect. A Dersimonian-Laird random-effect model was employed in order to deal with potential heterogeneity among the included studies. Statistical heterogeneity was assessed through visual examination of the forest plots, and its magnitude was quantified using the I-square and Chi-Square tests. Subgroup analysis was performed based on the sequencing method (NGS or WES), cut-off value (≥ 10 or < 10), and TMB quantification method (muts/Mb or muts/exome) in order to detect sources of heterogeneity and to analyze the results of populations with different characteristics. The leave-one-out procedure was used to complement the subgroup analysis for evaluating the sensitivity of the obtained results. Finally, publication bias was assessed by visual inspection of funnel plots and Egger’s test [31].
Results
Search results
Our search strategy led to a total of 2985 studies retrieved from PubMed, Web of Science, EMBASE and Scopus databases. However, 1960 of these studies were screened after removal of duplicates. We excluded animal studies, reviews, comments, case reports, studies unrelated to the topic and non-English language studies through title and abstract screening. This resulted in 49 studies that were further reviewed in detail. Following a thorough assessment by full-text review, we removed studies that were not clinical trials or cohort studies, as well as those lacking extractable data or utilizing different analytic parameters. Finally, 11 studies published between 2018 and 2023 were included in this meta-analysis (Fig. 1) [18,19,20,21,22,23,24,25,26,27,28].
Study characteristics
See Additional file 1: Table S3 presents study characteristics, while Additional file 1: Table S4 provides a summary of the key findings. This meta-analysis included a total of 1200 patients, with each study ranging from 10 to 257 patients, and two studies [25, 27] including two different cohorts. Among the 11 included studies, seven studies were conducted in the USA, while single studies were performed in Japan, Italy and China, and another across multiple geographic areas.
This analysis consisted of four prospective studies and seven retrospective cohort studies. In terms of treatment approaches, all studies included ICI treatment (monotherapy or in combination). The survival data were reported as OS in four out of 11 studies, six studies presented data as ORR, and the remaining study reported both OS and ORR.
Quality assessment
Based on NOS assessment, three studies achieved a score of 7, indicating intermediate-quality, while eight studies obtained scores ranging from 8 to 9, indicating high-quality (Additional file 1: Table S5).
TMB and method of obtaining the cut-off value
TMB was determined through NGS/WES sequencing analyses of tumor samples in all the studies, except one study [20] that also assessed peripheral blood samples (Additional file 1: Table S3).
Different methods were used to establish the cut-off values for high and low TMB. The cut-off of muts/Mb values ranged from 2.54 to 20.0, with a mean of 8.14 and the muts/exome values ranged from 86.0 to 175.0, with a mean of 130.5. Data were reported in mutations per megabase (muts/Mb) in nine studies, and mutations per exome (mut/exome) in two studies [21, 26]. The median muts/Mb values ranged from 3.04 to 7.6, with a mean of 5.47. The muts/exome values were not available. One study defined a high TMB value of 175.0 muts/exome based on a literature review [21], and two studies used a cut-off of 10.0 and 15.0 muts/Mb [20, 28]. Three studies established cut-off values based on the median TMB, with calculated values of 5.0 [18], 6.71 [22], and 7.6 [23]. Another study determined the optimal value using the Cox proportional hazards model, setting 10 muts/Mb as a cut-off [25], while another one employed R language survival package analysis to determine a cut-off value of 2.54 [24]. In addition, one study determined the optimal cut-point using the Youden Index, setting it at 86.0 muts/exome [26], in another a threshold for TMB was chosen to attain the best performance in predicting progression free survival (PFS) in a univariable survival model, setting it at 3.34 muts/Mb [27], whereas in the remaining study the method used to obtain the cut-off value was not specified [19].
Association between TMB and objective response rate after ICI treatment
The pooled ORR for ICI treatment was evaluated across seven studies (eight cohorts) involving 623 patients, using a random-effects model [18,19,20,21,22, 26, 27]. The group of patients with high TMB exhibited a superior ORR (OR = 2.62, 95% CI 1.74–3.94, p < 0.0001; Fig. 2A). Pooled data were homogenous under a random-effects model (p = 0.83, I2 = 0%).
Association between TMB and overall survival in ICI-treated HNSCC patients
The impact of TMB on HNSCC survival after ICI treatment was assessed in five studies (six cohorts) involving 596 patients, using a random-effects model [18, 23,24,25, 28]. Results from these studies consistently showed a better survival in patients harboring high TMB. The group of patients with high TMB exhibited a survival advantage (HR = 0.53; 95% CI 0.39–0.71, p < 0.0001; Fig. 2B). Pooled data were homogenous under a random-effects model (p = 0.19, I2 = 33%).
Heterogeneity and sensitivity analysis
Subgroup analysis was conducted to investigate the factors contributing to heterogeneity. No substantial alteration in heterogeneity was observed when subgroup analyses were performed based on sequencing method (NGS or WES), cut-off value (≥ 10 or < 10 muts/Mb), or TMB quantification (muts/Mb or muts/exome). These data are shown in Additional file 1: Table S6.
In addition, a sensitivity analysis was performed using the leave-one-out method to explore potential sources of heterogeneity among the 11 included studies and the robustness of the results. This analysis revealed that the exclusion of any single study did not lead to a significant alteration in the overall prevalence or heterogeneity. These results are included in Additional file 1: Figure S7.
Published status bias analysis
Publication bias in literature was assessed by funnel plot (Fig. 3) and Egger’s test. There was no evidence of publication bias for ORR after ICI-treated patients (Egger’s test: p = 0.9782). In the OS in ICI-treated patients we detect some publication bias (Egger´s test: p = 0.0461). This bias was mostly caused by Xu et al. [24], a small study with the largest effect. It is noteworthy that when this study was removed, the effect changed from 0.53 to 0.56 (Additional file 1: Figure S7). In addition, if we symmetrize the data (i.e. by including an artificial study with the same weight that Xu et al. [24] mirrored respect the average effect size), the obtained random-effect HR would be 0.55 [0.38–0.80] (Additional file 1: Figure S8).
Discussion
This is the first meta-analysis specifically evaluating the prognostic and predictive significance of TMB in R/M HNSCC after ICI treatment. It includes a substantial number of patients (n = 1200) across 11 studies to consistently demonstrate that patients with a high TMB had a significant survival advantage, both in terms of OS and ORR. In addition, an elevated TMB was associated with a better response to ICI therapy. Thus, the risk of death was reduced by 47% in patients harboring high TMB, and the ORR was 2.62 times higher compared to the low TMB subgroup.
In the last few decades, TMB has been gradually gaining prominence as a prospective biomarker for immunotherapy response. Earlier research indicated a positive correlation between elevated TMB and increased tumor neoantigens displayed by major histocompatibility complex class (MHC) molecules. This, in turn, plays a role in promoting immune detection and enhancing the response to anti-tumor immunotherapies [32]. Numerous studies have demonstrated that TMB has the capacity to predict ICI efficacy across different tumor types [12,13,14,15,16,17, 33, 42] and also some meta-analyses that included few (< 3) HNSCC studies [34, 35]. A portion of TMB has the potential to induce the formation of neoantigens during tumor progression. As the tumor's TMB increases, the likelihood of neoantigen formation also rises, thus triggering the recognition and activation of T cells [32, 36]. This is exemplified by the direct correlation between the mean TMB and the objective response rate observed across 27 tumor types [37]. By contrast, the presence of numerous tumor subclones may lead to neoantigen heterogeneity and cause host immune invalidation despite harboring a high mutational load [38]. This could explain why some patients with high TMB do not respond to ICI therapy.
Based on our meta-analysis it seems that the detection of TMB could be helpful to improve the selection of HNSCC patients who may benefit from ICI therapy. However, there are still several challenges related to the clinical interpretation of TMB testing results, as highlighted by Ma et al. [39]. At present, there are no clear consensus methods for optimal cut-off determination of high TMB. This is evident in this meta-analysis since each of the included studies employed a different method to calculate the cut-off values for TMB. In addition, the number of mutations obtained in each study was variable and the cut-off of muts/Mb values ranged from 2.54 to 20.0, with a mean of 8.14, and the muts/exome values ranged from 86.0 to 175.0, with a mean of 130.5. The TMB values are heterogeneous among different cancer types, and an appropriate high TMB value has not been established for all solid tumors nor for each specific tumor subtype. Finally, the TMB testing method varied; most studies used NGS, but four studies used WES. Even though initial investigations into TMB relied on the WES approach, its practical use in clinical settings is limited by its high cost, long detection time, complex data interpretation, and the need for fresh samples. The rapid advancement of NGS technologies has enabled the acceleration of whole genome sequencing, due to its ultrahigh throughput, scalability, and speed. Several studies have also highlighted a significant correlation between TMB measured through WES and targeted panel sequencing [13, 40, 41]. However, differences in the targeted exome panels, sequencing depth, and bioinformatics algorithms could lead to heterogeneous results. Uniform industry standards for TMB testing by NGS are needed for clinical use [39].
These difficulties in defining the cut-off values and inconsistent detection platforms for TMB prompted the study by Oiu et al. [42] to search for an alternative biomarker. These authors found that comutation of the Spliceosome (Sp) pathway and Hedgehog (He) signaling pathway (defined as SpHe-comut +) was associated with increased TMB and neoantigen load, as well as increased levels of immune-related signatures. Furthermore, this study also revealed SpHe-comut + as an effective predictor of immunotherapeutic benefit, with an OR of 1.74 [1.74–2.15] for ORR and a HR of 0.76 [0.64–0.91] for OS after ICI treatment, which is similar to our reported results in ORR and OS. Therefore, SpHe-comut + has emerged as an optional and cost-effective approach to identify potential immunotherapy responders, and hence to improve patient stratification and treatment decision-making [42].
Another relevant issue that influences TMB detection is the origin of samples. Tissue-based TMB (tTMB) assessment is predominant in clinical settings. However, when tissue samples are insufficient, inadequate and/or clinically unfeasible for TMB measurement, blood-based TMB (bTMB) could be instead used to evaluate immunotherapy efficacy [13]. All but one study included in this meta-analysis used tissue-based TMB assessment, whereas the study performed by Noji et al. [20] included both tTMB and bTMB. Another important issue to consider for prediction of ICI effectiveness is the source of the tumor sample analyzed, due to the inconsistent status of various biomarkers in primary tumors and paired metastasis [43]. Notably, TMB and microsatellite instability status were less prone to change between primary tumors and their corresponding metastases. In marked contrast, PD-L1, PD-1, PD-L2, and tumor-infiltrating lymphocyte (TIL) density led to a higher frequency of discordance [43].
Our meta-analysis demonstrates the impact of TMB on the effectiveness of ICI treatment in R/M HNSCC patients. Nevertheless, there are still several limitations, such as the potential existence of publication bias suggested by our funnel plots, or unreported data, that cannot be ruled out. Moreover, some selected studies adopted a retrospective, non-randomized approach that could potentially amplify the impact of confounding factors. Furthermore, the potential application of TMB as a biomarker in clinical settings continues to be a subject of debate. Challenges persist in standardizing uniform TMB assessment procedures. Further prospective investigations are warranted to validate the optimal selection of TMB assessment platforms and targeted sequencing panels. The existing research on TMB as a predictive biomarker in HNSCC immunotherapy remains insufficient. Possible correlations between TMB and other predictive biomarkers require validation through large scale randomized trials, and establishment of the most effective predictive combination. Recent advancements in machine learning techniques, particularly artificial intelligence-based predictive analysis, offer a tremendous potential for the identification of TME-related biomarkers in HNSCC patients. This progress enables the analysis of extensive multi-omics datasets into transformative solutions to improve clinical decision-making and implement fundamental changes in the tumor treatment paradigm of patients with locally advanced or R/M HNSCC [44].
Conclusion
The results of this unprecedented meta-analysis serve to demonstrate that HNSCC patients with high TMB exhibit a higher benefit from ICI-based therapy compared to the low TMB subgroup, hence suggesting that tumor mutation load could be a useful biomarker to predict ICI response and treatment efficacy in these patients. Together these findings should encourage further investigation of TMB assessment prior to immunotherapy in R/M HNSCC as a standardized and validated biomarker in prospective clinical trials.
Availability of data and materials
The data collected during the systematic review are available on request.
Abbreviations
- bTMB:
-
Blood-based TMB
- HR:
-
Hazard ratio
- HNSCC:
-
Head and neck squamous cell carcinoma
- ICIs:
-
Immune checkpoint inhibitors
- MHC:
-
Major histocompatibility complex class
- Muts/exome:
-
Mutations per exome
- Muts/Mb:
-
Mutations per megabase
- NGS:
-
Next-generation sequencing
- NOS:
-
Newcastle–Ottawa scale
- ORR:
-
Objective response rate
- OR:
-
Odds ratio
- OS:
-
Overall survival
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- PFS:
-
Progression free survival
- R/M:
-
Recurrent and metastatic
- tTMB:
-
Tissue-based TMB
- TME:
-
Tumor microenvironment
- TMB:
-
Tumor mutational burden
- WES:
-
Whole-exome sequencing
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. https://doi.org/10.3322/caac.21262.
Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. https://doi.org/10.1038/nrc2982.
Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The immunoscore: colon cancer and beyond. Clin Cancer Res. 2020;26(2):332–9. https://doi.org/10.1158/1078-0432.ccr-18-1851.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. https://doi.org/10.1158/2159-8290.cd-21-1059.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–67. https://doi.org/10.1056/nejmoa1602252.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE, Even C, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. https://doi.org/10.1016/j.oraloncology.2018.04.008.
Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, Berger R, Eder JP, Burtness B, Lee SH, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838–45. https://doi.org/10.1200/jco.2016.68.1478.
Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: recent advances and future directions. Oral Oncol. 2019;99:104460. https://doi.org/10.1016/j.oraloncology.2019.104460.
Larkins E, Blumenthal GM, Yuan W, He K, Sridhara R, Subramaniam S, Zhao H, Liu C, Yu J, Goldberg KB, et al. FDA approval summary: pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum-containing chemotherapy. Oncologist. 2017;22(7):873–8. https://doi.org/10.1634/theoncologist.2016-0496.
Oliva M, Spreafico A, Taberna M, Alemany L, Coburn B, Mesia R, Siu LL. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann Oncol. 2019;30(1):57–67. https://doi.org/10.1093/annonc/mdy507.
Zou W, Wolchok JD, Chen L. PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. https://doi.org/10.1126/scitranslmed.aad7118.
Klempner SJ, Fabrizio D, Bane S, Reinhart M, Peoples T, Ali SM, Sokol ES, Frampton G, Schrock AB, Anhorn R, et al. Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: a review of current evidence. Oncologist. 2020;25(1):e147–59. https://doi.org/10.1634/theoncologist.2019-0244.
Li Y, Ma Y, Wu Z, Zeng F, Song B, Zhang Y, Li J, Lui S, Wu M. Tumor mutational burden predicting the efficacy of immune checkpoint inhibitors in colorectal cancer: a systematic review and meta-analysis. Front Immunol. 2021;12:751407. https://doi.org/10.3389/fimmu.2021.751407.
Cao D, Xu H, Xu X, Guo T, Ge W. High tumor mutation burden predicts better efficacy of immunotherapy: a pooled analysis of 103078 cancer patients. Oncoimmunology. 2019;8(9):e1629258. https://doi.org/10.1080/2162402x.2019.1629258.
Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G, Malboeuf C, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–8. https://doi.org/10.1038/s41591-018-0134-3.
Yusko E, Vignali M, Wilson RK, Mardis ER, Hodi FS, Horak C, Chang H, Woods DM, Robins H, Weber J. Association of tumor microenvironment T-cell repertoire and mutational load with clinical outcome after sequential checkpoint blockade in melanoma. Cancer Immunol Res. 2019;7(3):458–65. https://doi.org/10.1158/2326-6066.cir-18-0226.
Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, Zhu B, Wang S, Zhuo M, Sun J, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 2019;5(5):696–702. https://doi.org/10.1001/jamaoncol.2018.7098.
Burcher KM, Lantz JW, Gavrila E, Abreu A, Burcher JT, Faucheux AT, Xie A, Jackson C, Song AH, Hughes RT, et al. Relationship between tumor mutational burden, PD-L1, patient characteristics, and response to immune checkpoint inhibitors in head and neck squamous cell carcinoma. Cancers (Basel). 2021;13(22):5733. https://doi.org/10.3390/cancers13225733.
Mezi S, Pomati G, Zizzari IG, Di Filippo A, Cerbelli B, Cirillo A, Fiscon G, Amirhassankhani S, Valentini V, De Vincentiis M, et al. Genomic and immune approach in platinum refractory HPV-negative head and neck squamous cell carcinoma patients treated with immunotherapy: a novel combined profile. Biomedicines. 2022;10(11):2732. https://doi.org/10.3390/biomedicines10112732.
Noji R, Tohyama K, Kugimoto T, Kuroshima T, Hirai H, Tomioka H, Michi Y, Tasaki A, Ohno K, Ariizumi Y, et al. Comprehensive genomic profiling reveals clinical associations in response to immune therapy in head and neck cancer. Cancers (Basel). 2022;14(14):3476. https://doi.org/10.3390/cancers14143476.
Pfister DG, Haddad RI, Worden FP, Weiss J, Mehra R, Chow LQM, Liu SV, Kang H, Saba NF, Wirth LJ, et al. Biomarkers predictive of response to pembrolizumab in head and neck cancer. Cancer Med. 2023;12(6):6603–14. https://doi.org/10.1002/cam4.5434.
Saba NF, Steuer CE, Ekpenyong A, McCook-Veal A, Magliocca K, Patel M, Schmitt NC, Stokes W, Bates JE, Rudra S, et al. Pembrolizumab and cabozantinib in recurrent metastatic head and neck squamous cell carcinoma: a phase 2 trial. Nat Med. 2023;29(4):880–7. https://doi.org/10.1038/s41591-023-02275-x.
Hanna GJ, Lizotte P, Cavanaugh M, Kuo FC, Shivdasani P, Frieden A, Chau NG, Schoenfeld JD, Lorch JH, Uppaluri R, et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight. 2018;3(4):e98811. https://doi.org/10.1172/jci.insight.98811.
Xu X, Li R, Zhang L, Zhu G, Ren D, Wu L, Gong X. Identification of factors related to immunotherapy efficacy and prognosis in patients with advanced head and neck squamous cell carcinoma. Diagn Pathol. 2021;16(1):110. https://doi.org/10.1186/s13000-021-01147-7.
Wildsmith S, Li W, Wu S, Stewart R, Morsli N, Raja R, Zhang Q, Ye J, He P, Shetty J, et al. Tumor mutational burden as a predictor of survival with durvalumab and/or tremelimumab treatment in recurrent or metastatic head and neck squamous cell carcinoma. Clin Cancer Res. 2023. https://doi.org/10.1158/1078-0432.ccr-22-2765.
Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, Sher X, Liu XQ, Lu H, Nebozhyn M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593. https://doi.org/10.1126/science.aar3593.
Valero C, Golkaram M, Vos JL, Xu B, Fitzgerald C, Lee M, Kaplan SK, Han CY, Pei X, Sarkar R, et al. Clinical-genomic determinants of immune checkpoint blockade response in head and neck squamous cell carcinoma. J Clin Invest. 2023. https://doi.org/10.1172/jci169823.
Scobie MR, Zhou KI, Ahmed S, Kelley MJ. Utility of tumor mutational burden as a biomarker for response to immune checkpoint inhibition in the VA population. JCO Precis Oncol. 2023;7:e2300176. https://doi.org/10.1200/po.23.00176.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372):n71. https://doi.org/10.1136/bmj.n71.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. https://doi.org/10.1007/s10654-010-9491-z.
Deeks JJ, HJ, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. Cochrane handbook for systematic reviews of interventions version 6.3 (updated Feb 2022). 2022. https://training.cochrane.org/handbook/current/chapter-10#section-10-10-2. Accessed 29 Dec 2023.
Verdegaal EM, de Miranda NF, Visser M, Harryvan T, van Buuren MM, Andersen RS, Hadrup SR, van der Minne CE, Schotte R, Spits H, et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;536(7614):91–5. https://doi.org/10.1038/nature18945.
Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–6. https://doi.org/10.1038/s41588-018-0312-8.
Lu S, Stein JE, Rimm DL, Wang DW, Bell JM, Johnson DB, Sosman JA, Schalper KA, Anders RA, Wang H, Hoyt C, Pardoll DM, Danilova L, Taube JM. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol. 2019;5(8):1195–204.
Osipov A, Lim SJ, Popovic A, Azad NS, Laheru DA, Zheng L, Jaffee EM, Wang H, Yarchoan M. Tumor mutational burden, toxicity, and response of immune checkpoint inhibitors targeting PD(L)1, CTLA-4, and combination: a meta-regression analysis. Clin Cancer Res. 2020;26(18):4842–51. https://doi.org/10.1158/1078-0432.ccr-20-0458.
Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. https://doi.org/10.1126/science.aaa4971.
Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–1. https://doi.org/10.1056/nejmc1713444.
McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–9. https://doi.org/10.1126/science.aaf1490.
Ma HH, Chen DS, Li S, Xiao MZ, Qi C. The value and inadequacy of tumor mutation burden on efficacy of immune checkpoint inhibitors in head and neck cancers. Oral Oncol. 2021;117:105179. https://doi.org/10.1016/j.oraloncology.2021.105179.
Johnson DB, Frampton GM, Rioth MJ, Yusko E, Xu Y, Guo X, Ennis RC, Fabrizio D, Chalmers ZR, Greenbowe J, et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol Res. 2016;4(11):959–67. https://doi.org/10.1158/2326-6066.cir-16-0143.
Campesato LF, Barroso-Sousa R, Jimenez L, Correa BR, Sabbaga J, Hoff PM, Reis LF, Galante PA, Camargo AA. Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget. 2015;6(33):34221–7. https://doi.org/10.18632/oncotarget.5950.
Qiu J, Li X, He Y, Wang Q, Li J, Wu J, Jiang Y, Han J. Identification of comutation in signaling pathways to predict the clinical outcomes of immunotherapy. J Transl Med. 2022;20(1):613. https://doi.org/10.1186/s12967-022-03836-343.
Zou Y, Hu X, Zheng S, Yang A, Li X, Tang H, Kong Y, Xie X. Discordance of immunotherapy response predictive biomarkers between primary lesions and paired metastases in tumours: a systematic review and meta-analysis. EBioMedicine. 2021;63:103137. https://doi.org/10.1016/j.ebiom.2020.103137.
Van den Bossche V, Zaryouh H, Vara-Messler M, Vignau J, Machiels JP, Wouters A, Schmitz S, Corbet C. Microenvironment-driven intratumoral heterogeneity in head and neck cancers: clinical challenges and opportunities for precision medicine. Drug Resist Updat. 2022;60:100806. https://doi.org/10.1016/j.drup.2022.100806.
Acknowledgements
We thank Norman Feltz for proofreading the language in this manuscript.
Funding
This study was supported by the Instituto de Salud Carlos III (ISCIII) through the project grants PI19/00560, PI22/00167 and CIBERONC (CB16/12/00390) and co-funded by the European Union, the Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Fundación Bancaria Cajastur-IUOPA, and Universidad de Oviedo. Additional funding was provided through the grant “Ayudas para Grupos de Investigación de Organismos del Principado de Asturias 2021–2023” (IDI/2021/000079), funded by Principado de Asturias through FICYT and the FEDER Funding Program from the European Union. FH-P is recipient of a Maria Zambrano grant at the University of Oviedo, and MO-R is recipient of a PFIS predoctoral fellowship from ISCIII (FI23/00037).
Author information
Authors and Affiliations
Contributions
JPR and MS-C contributed to the conception and design. MS-C drafted the main manuscript text. JPR, MS-C, MO-R, FH-P, PM-C and JMG-P performed analysis and data interpretation, data presentation and figures. JPR, JMG-P and PM-C reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to publish.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
PRISMA 2020 Checklist. Table S2. Search strategy in PubMed. Table S3. Main features of the selected studies. Table S4. Key findings of the selected studies. Table S5. The Newcastle–Ottawa Scale (NOS) for assessing the quality of studies in meta-analyses. Table S6. Subgroup analyses of ORR and OS in HNSCC patients treated with ICIs. Figure S7. Leave-one-out sensitivity analysis of ORR and OS in HNSCC patients treated with ICIs. Figure S8. Forest plot of OS after symmetrizing the data in HNSCC patients treated with ICIs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rodrigo, J.P., Sánchez-Canteli, M., Otero-Rosales, M. et al. Tumor mutational burden predictability in head and neck squamous cell carcinoma patients treated with immunotherapy: systematic review and meta-analysis. J Transl Med 22, 135 (2024). https://doi.org/10.1186/s12967-024-04937-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-04937-x