Abstract
Integrins, which consist of two non-covalently linked α and β subunits, play a crucial role in cell–cell adhesion and cell-extracellular matrix (ECM) interactions. Among them, integrin β1 is the most common subunit and has emerged as a key mediator in cancer, influencing various aspects of cancer progression, including cell motility, adhesion, migration, proliferation, differentiation and chemotherapy resistance. However, given the complexity and sometimes contradictory characteristics, targeting integrin β1 for therapeutics has been a challenge. The emerging understanding of the mechanisms regulating by integrin β1 may guide the development of new strategies for anti-cancer therapy. In this review, we summarize the multiple functions of integrin β1 and signaling pathways which underlie the involvement of integrin β1 in several malignant cancers. Our review suggests the possibility of using integrin β1 as a therapeutic target and highlights the need for patient stratification based on expression of different integrin receptors in future clinical studies.
Similar content being viewed by others
Background
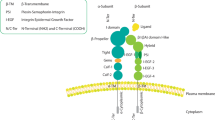
Integrins, comprised of α and β subunits non-covalently bound together, form heterodimeric complexes found in endothelial cells, pericytes, fibroblasts, and tumor cells. In mammals, there are a total of 18 α subunits and 8 β subunits. Through their mutual combinations, at least 24 αβ integrin heterodimers are formed. Of these, half contain the β1 subunit [1]. The β subunit consists of a plexin-semaphorin-integrin domain, a hybrid domain, an I-like domain which is inserted in the hybrid domain and is homologous to the αI-domain of the α subunit, and also EGF1-4 and β tail domains. The α subunit is composed of an extracellular domain consisting of a seven-bladed β-propeller head domain, a thigh domain and two calf domains (calf 1 and calf 2). The αI domain, containing approximately 200 amino acids, is inserted between β propeller blades 2 and 3. The αI-domain contains a metal ion-dependent adhesion site, which participates in ligand binding [2]. Both α and β subunits have large extracellular domains, enabling them to sense and respond to stimuli from extracellular matrix (ECM) components such as collagen, fibronectin, fibrinogen, laminin and vitronectin. Furthermore, research has revealed that integrins contain a transmembrane domain and a short cytoplasmic domain which play a central role in signal transduction involving FAK, AKT, MAPK, and Src family kinases, thus regulating cell survival, migration, immune escape, and resistance to radiotherapy and chemotherapy [3].
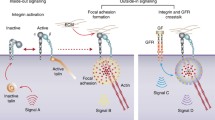
The expression and function of the major integrins and their relationship to tumor types and metastatic sites are different. For example, the progression of liver and endometrial cancer is mainly related to integrin αvβ6, while thyroid cancer is associated with integrin α6β4. Integrin αvβ3 plays a vital role in cervical cancer and bone metastasis of tumors, as does integrin αvβ6 [4]. In addition, integrin β1, also recognized as CD29, which is one of the most common subunits in the integrin family and is composed of a β1 subunit and different α subunits, plays a non-negligible role in crucial developmental pathways. Integrin β1 is a human protein-coding gene with a total length of 58048 bp, located on human chromosome 10p11.2 and consisting of 18 exons. Moreover, its mRNA encodes approximately 798 amino acids, with a molecular weight ranging from 100 to 132 kDa [5]. This gene has three transcript variants, including transcript variants 1A, 1E, and 1D. Transcript variant 1A has a full length of 3735 bp, contains 16 exons, and encodes a protein of 798 amino acids; transcript variant 1E has a full length of 3794 bp and encodes a protein of 798 amino acids; transcript variant 1D has a full length of 3739 bp and encodes a protein of 801 amino acids [6]. The primary function of integrin β1 is to facilitate adhesion between cancer cells and the ECM, forming the basis for cancer cell survival. It is closely associated with cancer cell metastasis, radiotherapy, chemotherapy, and targeted therapy, among other activities [7]. When cancer cells adhere to the ECM, two types of cellular signaling are triggered by integrin β1 activity: an “inside-out” signal in which signals from inside the cell activate the integrin for binding to extracellular ligands, and an “outside-in” signal in which the extracellular ligand interacts with the integrin receptor, causing the integrin cytoplasmic domain to separate and thereby activate the integrin receptor and trigger intracellular signaling molecules (Fig. 1). Herein, we provide a systematic and complete review of integrin β1-mediated signal transduction and its role in tumor drug resistance, and highlight ongoing efforts to develop new therapies from bench to clinic.
Framework diagram of cellular signaling triggered by integrin β1 in tumor microenvironment. During the cell adherence to ECM, integrin β1 activity undergoes conformational changes that induce cellular signaling including “inside-out” signal in which signals from inside the cell activate the integrin for binding to the extracellular ligands and “outside-in” signal in which extracellular ligand interaction activates integrin receptor by separating the integrin cytoplasmic domain triggering the intracellular signaling molecules
Function of the integrin β1 family
Each heterodimer of integrin β1 binds to a specific molecule and follows a unique signaling pathway activation pattern (Table 1). Based on their affinity for ligands, integrins can be categorized into four groups, each with distinct receptors; namely, arginine-glycine-aspartate (RGD)-binding receptors (αvβ1, αvβ3, αvβ5, αvβ6, αvβ8, α5β1, α8β1, and αIIbβ3), leukocyte-specific receptors (the β2 subfamily plus α4β1, α9β1, α4β7, and αEβ7), laminin-binding receptors (α3β1, α7β1, α6β1, and α6β4), as well as collagen-binding receptors (α1β1, α2β1, α10β1, and α11β1) [5, 23]. The expression and functions of integrin β1 in various cancer types were summarized in Table 2. Among these, the intriguing roles of integrin β1, in combination with distinct α subunits, are clarified as follows.
Integrins α5β1, α8β1, and αvβ1 in RGD receptors
The role of integrins α5β1, α8β1, and αvβ1 in tumor progression
RGD receptors which recognize the triplet sequence RGD motif, are found in many ECM proteins such as fibronectin, collagen, vitronectin, osteopontin and thrombospondin [33]. The RGD-binding subfamily members play an important role in angiogenesis and thrombosis and are considered the most essential integrin targets in drug discovery [34]. Currently, anti-integrin drugs designed to block the interaction between integrins and ECM have been developed for the prevention and treatment of various diseases [35, 36]. Moreover, integrins binding to RGD receptors regulate cell proliferation and survival signals, as well as the localization and activation of transforming growth factor-β (TGF-β), supporting angiogenesis [37, 38]. The expression level of integrin α5β1 is higher in liver cancer tissues than in paired adjacent tissues, and the interactions between integrin α5β1 and fibronectin promotes tumor growth and angiogenesis [39]. Immunohistochemistry analyses have confirmed that integrin α5β1 is overexpressed in esophageal squamous cell cancer, with high expression being linked to a poor prognosis and potentially serving as an independent prognostic factor [40]. Immunoprecipitation and mass spectrometry have revealed that all monoclonal antibodies recognized integrin α5β1 and blocking α5 in diffuse-type gastric cancer cells or fibronectin deposited on cancer-associated fibroblasts abrogate the heterocellular interaction [41]. In lung cancer, the expression of α8 subunit is downregulated, and patients with high expression exhibit a favorable prognosis, which is closely linked with the immune microenvironment, tumor heterogeneity, and cancer cell stemness [30]. Simultaneously, the low expression of α8 subunit is correlated with poor disease-free survival in renal cell carcinoma patients [31]. Reports indicate that the overexpression of the α8 subunit induces endothelial-mesenchymal transition (EMT) and enhances cell migration and invasion in early relapsed multiple myeloma patients [9]. Accordingly, the expression of the α8 subunit is closely linked to the occurrence of colorectal cancer [42]. Integrin αvβ1 is enriched in extracellular vesicles of metastatic breast cancer cells mediated by galectin-3, and integrin αvβ1 is important for extracellular vesicle retention in ECM [43, 44].
Signaling pathways mediated by integrins α5β1, α8β1, and αvβ1
It has been reported that chenodeoxycholic acid attenuate lung cancer pathogenesis via the integrin α5β1/FAK/p53 axis [8]. Ryu et al. indicated that the α8 subunit may regulate CXCR4/SDF-1α signaling, causing multiple myeloma cells to migrate, and also found the crosstalk between the α8 subunit and PDGF receptor may mediate multiple myeloma pathogenesis [9]. Increased levels of integrin αvβ1 heterodimers induced by tenascin-C activated the TGF-β signaling cascade, resulting in the transformation of highly contractile myofibroblasts in breast cancer [10].
Integrins α4β1 and α9β1 in leukocyte-specific receptors
The role of integrins α4β1 and α9β1in tumor progression
Leukocyte-specific receptors are crucial for host defense. Their most prevalent function is to facilitate the recruitment of neutrophils to inflamed tissues and promote phagocytosis of pathogens. Recent data likewise indicate that they play a role in regulating neutrophil apoptosis. Neutrophils are terminally differentiated cells that undergo constitutive apoptosis, and their apoptosis and clearance are essential for inflammation resolution [45]. For example, integrin α4β1, also recognized as very late antigen-4, is a heterodimeric cell surface receptor expressed on most white blood cells, forming the foundation for leukocyte homing, migration, differentiation, activation, and survival [46]. In bone marrow samples from patients with primary acute myeloid leukemia, CD44 engagement by hyaluronan is involved in inducing the inside-out activation of integrin α4β1, thereby enhancing leukemia cell adhesion to vascular cell adhesion molecule-1 (VCAM-1) [11]. Integrin α4β1 is also a major adhesion receptor mediating multiple myeloma cell-stromal interactions, and its expression and function are downregulated by bortezomib, an anti-multiple myeloma agent, leading to inhibition of cell adhesion-mediated drug resistance and cell apoptosis [47]. In addition, integrin α4β1 plays a significant role in controlling the positioning of both healthy and malignant B cells within tissues, thereby determining the pattern of organ infiltration [48]. In chronic lymphocytic leukemia, the level of integrin α4β1 was determined by measuring the expression of the CD49d chain by flow cytometry. The results illustrated that higher levels of integrin α4β1 were associated with a worse prognosis, consistent with its crucial role as a key molecule facilitating protective niche formation of lymphocytic leukemia cells in the bone marrow and lymph nodes [49]. The α9 subunit used to be known as ITGA4L (integrin-α4-like), because the α9 and α4 subunits show peptide sequence similarities and share several common ligands. However, the α9 and α4 subunits exert distinct as well as similar physiological functions [50]. It has been demonstrated that integrin α9β1 functioned as an active heterodimer on the plasma membrane of endometrial stromal, endometrial epithelial, and porcine spermatogonial stem cells in an undifferentiated state [51, 52]. Varney et al. reported the critical importance of integrin α9β1 loss in epidermal tumor cells for maintaining persistent stromal vessel density [53]. Additionally, fully activated integrin α9β1 has been correlated with less migratory behavior in melanoma cells [54]. Moreover, there has been a suggestion of a potential role for integrin α9β1 expressed in neutrophils in cases of aspiration pneumonia [55, 56]. Results indicated that integrin α9β1, when in a high activation state, can induce and localize to focal adhesions, but in its intermediate activity state, it typically supports melanoma cell adhesion consistent with migration [57]. Functional studies strongly support the role of integrin α9β1 in the adhesion and differentiation of hematopoietic stem and progenitor cells in the endosteal stem cell niche [58]. Furthermore, it has been proposed that α9 subunit may function as a tumor suppressor gene in nasopharyngeal cancer, influencing tumor cell biology [59]. In various reports, integrin α9β1 has been shown to enhance malignant tumor growth and metastasis, with its expression being increased in highly metastatic triple-negative breast cancer cells [13].
Signaling pathways mediated by integrins α4β1 and α9β1
The interaction between integrin α4β1 and VCAM-1 promotes the activation of AKT, MAPK, NF-κB, and mTOR signals, leading to reduced apoptosis in acute myeloid leukemia cells [35]. Moreover, the α9 subunit was observed to suppress hepatoma cell migration and invasiveness through FAK/Src-Rac1/RhoA signaling [12]. α9 subunit depletion, on the other hand, was determined to suppress triple-negative breast cancer growth and metastasis by promoting β-catenin degradation through the ILK/PKA/GSK3 pathway [13].
Integrins α6β1, α3β1, and α7β1 in laminin-binding receptors
The role of integrins α6β1, α3β1, and α7β1 in tumor progression
Laminins are one of major components of the ECM, consisting of glycoproteins with relatively high molecular weights (400–900 kDa) that are typically found in the basement membranes of various epithelial tissues and take the form of a cross or T made up of three interlaced chains (α, β, and γ) [60, 61]. Integrin α6β1 expression in cancer cells has been reported, and it has been argued that it facilitates tumor invasion, angiogenesis, and cancer progression [62]. Laminin-511 and laminin-521 preserve the pluripotency of pluripotent stem cells and human embryonic stem cells via the integrin α6β1/αvβ1 pathways [63]. Integrin α6β1 is highly expressed in metastatic and androgen receptor-positive prostate cancer [14]. Accordingly, integrin α3β1 promotes angiogenesis of glioblastoma-associated endothelial cells through calcium-mediated exocytosis of macropinosomes and lysosomes [64]. Numerous studies have demonstrated that integrin α3β1 supported the motility and invasion of thyroid papillary cancer cells and was involved in tumor progression [65]. Moreover, integrin α3β1 is implicated in regulating tumor-derived proteases bone morphogenetic protein 1, matrix metalloproteinase-9, and matrix metalloproteinase-3 in the secretome of epidermal tumors, making it a potential therapeutic target [66]. Additionally, integrin α3β1 on keratinocytes facilitates the secretion of IL-1α and exerts paracrine regulation of fibroblast gene expression and differentiation [67]. Integrin α3β1 has also been found to induce the Brn-2 transcription factor, thereby promoting invasion and metastatic properties in breast cancer cells [68, 69]. Aberrantly glycosylated integrin α3β1 is a unique urinary biomarker for the diagnosis of bladder cancer [70]. Polymersomal docetaxel targeting integrin α3β1 has emerged as an advanced nanotherapeutic for non-small cell cancer treatment [71]. Meanwhile, the α7 subunit was reported to be overexpressed in clear cell renal cell cancer, correlating with higher pathological grade, increased T stage, advanced TNM stage, and worse survival [72]. Additionally, the α7 subunit was associated with worse clinical features and prognosis. In tongue squamous cell cancer, its knockdown inhibited cell proliferation and stemness [73]. Similarly, in non-small-cell lung cancer, the α7 subunit promoted proliferation, apoptosis and stemness [74]. In esophageal squamous cell cancer, the α7 subunit has also served as a functional cancer stem cell surface marker [16].
Signaling pathways mediated by integrins α6β1, α3β1, and α7β1
It has been reported that integrin α6β1 was highly expressed in metastatic and androgen receptor-positive prostate cancer and promoted survival and resistance through PI3K and NF-κB signal pathways [14]. Multiple data demonstrate that integrin α3β1, in conjunction with CD151, governs the signaling pathways responsible for the viability of differentiating keratinocytes. Integrin α3β1 also plays a crucial function as a regulator of pro-tumorigenic pathways in skin carcinogenesis [15]. Furthermore, the α7 subunit regulates stem cell properties through the activation of the FAK-mediated signal pathways in esophageal squamous cell cancer [16].
Integrins α10β1, α1β1, α2β1, and α11β1 in collagen-binding receptors
The role of integrins α10β1, α1β1, α2β1, and α11β1 in tumor progression
Collagen is as the most abundant component of the ECM, and its structure and function vary according to tissue types. Similar to other integrins, collagen-binding integrins act as bidirectional signaling receptors upon biochemical or mechanical activation [75]. Among them, integrin α10β1 is the most prevalent collagen-binding integrin in cartilage tissue, exhibiting distinct expression patterns compared to other collagen-binding integrins. Research has shown that targeting the α10 subunit with antibodies effectively inhibits adhesion, migration, proliferation and sphere formation of glioblastoma cells, providing a promising therapeutic approach for glioblastoma treatment [76, 77]. Studies have also revealed that α10 subunit expression is upregulated in malignant melanoma cells compared to primary melanocytes [78]. Integrin α10β1 promotes angiogenesis and aggregation of stromal cells, which in turn secrete tumor-promoting factors, thereby fostering ovarian tumor growth [17]. Specific inhibitors of integrin α1β1 can reduce collagen V-driven invasion and suppress ECM-driven cancer cell invasion through paclitaxel, suggesting that integrin α1β1 also contributes to the progression of colon cancer [19]. It was suggested that integrin α1β1 also contributes to colon cancer progression [79]. Notably, both collagen-binding integrin α1β1 and integrin α2β1, as well as laminin-binding integrin α3β1, are involved in regulating tumor cell proliferation, survival and EMT processes. It was shown that cell proliferation was suppressed in the presence of the α2β1 inhibitor [80]. Buddlejasaponin IV induced anoikis by inhibiting integrin α2β1-mediated cell adhesion and signaling and inhibited lung metastasis of colon cancer cells [81]. In primary ovarian cancer, integrin α2β1 serves as a prognostic and predictive marker. progression-free survival was shorter in patients with a high integrin α2β1 expression [82]. This investigation also provided evidence that integrin α2β1-collagen interaction activated pathways relevant to mitotic hepatoma carcinoma progression. After binding to collagen, integrin α2β1 was shown to activate the pro-oncogenic YAP in hepatoma cells, which correlated well with tumor progression and outcome in patients [83]. Alternagin-C is a substance that binds to integrin α2β1 and can weaken the adhesion of triple-negative breast cancer cells to collagen matrix while stimulating the expression of transfer inhibitory factor 1 [84]. It has been revealed that integrin α2β1 is involved in protecting tumor cells from aging, and reducing the expression of integrin α2β1 triggers an atypical signaling mechanism based on AKT, resulting in the process of cellular aging [85]. Integrin α2β1 inhibition attenuated prostate cancer cell proliferation by cell cycle arrest, promoted apoptosis and reduced EMT [86]. It has been hypothesized that integrin α11β1 promoted cutaneous squamous cell cancer by regulating ECM synthesis and collagen organization within a highly dynamic and interactive tumor microenvironment (TME) [87]. It has also been found that integrin α11β1 promoted tumorigenicity and metastasis in non-small cell lung cancer and controlled the stiffness of the cancer stroma [88].
Signaling pathways mediated by integrins α10β1, α1β1, α2β1, and α11β1
Integrin α10β1 functions as a receptor for the HU177 epitope, expressing α-smooth muscle actin in stromal cells, thereby regulating ERK-dependent migration [17]. Activation of the TRIO–RAC–RICTOR–mTOR signaling by the α10 subunit promotes tumor cell survival, and inhibitors of RAC and mTOR have shown anti-tumor effects in vivo, providing a potential therapeutic strategy for high-risk leiomyosarcoma patients [18]. Reports indicate that collagen V directly signals through integrin α1β1, driving cell migration. Additionally, collagen V increases invasion in triple-negative breast cancer cells through α1β1-mediated ERK1/2 signaling. The use of integrin α1β1 specific inhibitors suppresses paclitaxel-induced ECM-driven cancer cell invasion [19]. In colon cancer cells, another significant role of integrin α1β1 in tumorigenesis has been demonstrated through its interaction with talin and paxillin, activating FAK/Src and leading to focal adhesion clustering and activation of the p130Cas/JNK, thus promoting cancer cell invasion [20]. Research suggests that collagen I mediates osteosarcoma development through the integrin α2β1/JAK/STAT3 signaling pathway. Blockade of integrin α2β1 efficiently improved the outcome of chemotherapy and radiotherapy, which suggests new approaches for eradicating tumors in the clinic [21]. The integrin α11β1-Src-YAP1 signaling pathway is involved in resistance of melanoma to MAPK and PI3K/mTOR dual-targeted therapy [22].
Clinical significance of integrin β1
Integrin β1 has emerged as an essential mediator in several cancers in recent years. The expression of integrin β1 in multiple cancer types is shown in Fig. 2, which indicates the applicability of integrin β1 as a therapeutic target and underlines the requirement for patient stratification in future clinical studies. For example, in esophageal cancer, high expression of integrin β1 is related to worse overall survival, and targeting integrin β1 alleviates tumor metastasis and chemotherapy resistance of patients [89, 90]. Combined inhibition of the integrin β1 and the stress-mediator JNK induces radiosensitization, which is caused by defective DNA repair associated with chromatin changes, enhanced ataxia-telangiectasia mutated phosphorylation and prolonged G2/M cell cycle arrest in glioblastoma [91]. Eke et al. have reported that compared with EGFR single inhibition, the combination of integrin β1 and EGFR targeting resulted in enhanced cytotoxicity and radiosensitization of head and neck cancer cells, which responded with FAK dephosphorylation [92]. In addition, the combination of gemcitabine and hERG1/integrin β1 complex antibody reduced the volume of tumor masses and produced an increase in survival without significant toxic side effects in pancreatic cancer [93]. However, in melanomas, although the combination of MAPK and PI3K/AKT inhibitors was successfully used in preclinical experiments and early clinical trials, dual-drug resistance was inevitably observed. Co-targeting MAPK/PI3K pathway with integrin β1 synergistically inhibited the proliferation of melanoma cells [22]. Moreover, stabilizing the expression of integrin β1 on the surface of gastric cancer cells led to drug resistance through activation of the FAK-YAP1 signaling pathway. This finding provides a potential avenue for gastric cancer chemotherapeutics [94].
The gene expression profile across all tumor samples and paired normal tissues. Data for ITGB1 encoding integrin β1 across human cancers were collected with GEPIA. ACC adrenocortical cancer, BLCA bladder urothelial cancer, BRCA breast invasive cancer, CESC cervical squamous cell cancer and endocervical adenocarcinoma, CHOL cholangiocarcinoma, COAD colon adenocarcinoma, ESCA esophageal cancer, GBM glioblastoma multiforme, HNSC head and neck squamous cell cancer, KICH kidney chromophobe, KIRC kidney renal clear cell cancer, KIRP kidney renal papillary cell cancer, LIHC liver hepatocellular cancer, LUAD lung adenocarcinoma; LUSC lung squamous cell cancer, OV ovarian serous cystadenocarcinoma, PAAD pancreatic adenocarcinoma, PCPG pheochromocytoma and paraganglioma, PRAD, prostate adenocarcinoma, READ rectum adenocarcinoma, SARC sarcoma, SKCM skin cutaneous melanoma, STAD stomach adenocarcinoma, THCA thyroid cancer, UCEC uterine corpus endometrial cancer, UCS uterine carcinosarcoma.*, P < 0.05
Nevertheless, the relationship between integrin β1 and clinical characteristics of patients is controversial and the prognostic significance of increased integrin β1 expression also varies depending on the type of cancer (Table 3). It has been reported that integrin β1 exerts an influence on prognosis in periampullary cancer but not in ductal pancreatic cancer [95]. Other studies have demonstrated that integrin β1 was strongly associated with a shorter survival time of gastric cancer patients [96]. Sun et al. have proved that high expression of integrin β1 was linked to poorer overall survival in lung cancer [97]. Immunohistochemistry analyses have revealed that the highest integrin β1 intensity score was associated with significantly decreased 10-year overall survival and disease-free survival in invasive breast cancer [98]. In addition, univariate and multivariate analysis has indicated that lack of integrin β1 expression was associated with biochemical recurrence and time to recurrence after radical prostatectomy [99]. Lu et al. have shown that the low expression of the α8 subunit was associated with poor prognosis for overall survival and disease-free survival in clear cell renal cell cancer patients [100]. Moreover, studies have reported that integrin β1 overexpression in colorectal tumors was associated with poor prognosis, as well as aggressive clinicopathological features [101].
Integrin β1 and therapy
The above results all shed light on the importance of the integrin β1 molecule in tumor growth, metastasis and drug resistance and highlight the potential of integrin β1 in personalized cancer therapy. The potential clinical applications of integrin β1 as a target for cancer therapy have generated great interest and shown theoretical potential as novel drugs for anti-tumor therapy, and indeed multiple antagonists and agonists of the integrin β1 signaling pathway provide the rationale for clinical development. Integrin β1 has historically been a promising yet challenging target for the treatment of multiple cancers. For example, integrin α5β1 has been used as a targeting strategy in clinical trials for non-small cell lung cancer, pancreatic cancer, epithelial ovarian cancer, primary peritoneal carcinoma, renal cell carcinoma and melanoma. In addition, targeting integrin α4β1 was also effective in the treatment of acute myeloid leukemia and solid tumors. The ongoing clinical studies of integrin β1-targeting drugs currently being tested as disease therapies are summarized in Table 4.
Discussion
In this review, we elucidate our understanding of the characteristics, ligands, signaling pathways and biological functions of integrin β1, which can be classified into four receptors; namely, the RGD-binding receptors, leukocyte-specific receptors, laminin-binding receptors and collagen binding receptors according to the specificity of the ligands [102]. The current investigation provides evidence that the integrin β1–ECM interaction activates FAK, MAPK, PI3K-AKT, and other pathways for tumor growth, metastasis, invasion and angiogenesis [103]. Moreover, integrin β1 also confers tumor cell chemoresistance, radioresistance, and immunoresistance [104]. Binding of integrin β1 to collagen I induces breast cancer cell insensitivity to cisplatin, doxorubicin, and mitoxantrone cytotoxicity [105]. Integrin β1 molecules promote radiotherapy resistance by repairing DNA double-strand breaks and induce pro-survival signaling through the engagement of FAK and JNK signal pathways in head and neck cancer [106, 107]. The c-Met/integrin β1 complex is formed during the metastasis and invasion of glioblastoma, liver cancer and breast cancer, and its decoupling helps to alleviate drug resistance [108]. Xu et al. have reported that higher expression of integrin β1 was associated with worse pathological G-staging and tumor T-staging, which was positively correlated with CD8+ T cells in gastric cancer [109]. Therefore, targeting integrin β1 provide therapeutic benefit to overcome multiple drug resistance. The expression of integrins varies greatly between normal and tumor tissues and is related to the type of cancer. In addition, different α subunits combining with the same β subunit may play very different roles. For instance, integrin α10β1 plays an important role in the progression of melanoma, while integrin α9β1 is strongly related to breast cancer, ovarian cancer and colon cancer [4]. Hence, it is critical for different tumor types to be considered in personalized targeted therapy. Currently, there are about 90 kinds of integrin-based therapeutic drugs or imaging agents which have been applied in clinical research, including small molecules, antibodies, synthetic mimic peptides, antibody–drug conjugates, chimeric antigen receptor T-cell therapy and imaging agents, among others [4].
Conclusions
Considering the potential function of integrin inhibition in overcoming acquired resistance to chemotherapy, radiotherapy and immunotherapy, combination therapy of anti-tumor drugs with integrin antagonists is expected to overcome the current difficulty of drug resistance in tumors. Also, this study indicates the applicability of integrin β1 as a therapeutic target and highlights the need for patient stratification according to expression of different integrin receptors in future clinical studies.
Availability of data and materials
Not applicable.
References
Justo BL, Jasiulionis MG. Characteristics of TIMP1, CD63, and β1-integrin and the functional impact of their interaction in cancer. Int J Mol Sci. 2021;22(17):9319. https://doi.org/10.3390/ijms22179319.
Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87(4):733–43. https://doi.org/10.1016/s0092-8674(00)81392-6.
Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92(13):6161–5. https://doi.org/10.1073/pnas.92.13.6161.
Pang X, He X, Qiu Z, Zhang H, Xie R, Liu Z, et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct Target Ther. 2023;8(1):1. https://doi.org/10.1038/s41392-022-01259-6.
Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339(1):269–80. https://doi.org/10.1007/s00441-009-0834-6.
Li J, Peng L, Chen Q, Ye Z, Zhao T, Hou S, et al. Integrin β1 in pancreatic cancer: expressions, functions, and clinical implications. Cancers. 2022;14(14):3377. https://doi.org/10.3390/cancers14143377.
Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18(9):533–48. https://doi.org/10.1038/s41568-018-0038-z.
Shen D, Zeng Y, Zhang W, Li Y, Zhu J, Liu Z, et al. Chenodeoxycholic acid inhibits lung adenocarcinoma progression via the integrin α5β1/FAK/p53 signaling pathway. Eur J Pharmacol. 2022;923: 174925. https://doi.org/10.1016/j.ejphar.2022.174925.
Ryu J, Koh Y, Park H, Kim DY, Kim DC, Byun JM, et al. Highly expressed Integrin-α8 Induces epithelial to mesenchymal transition-like features in multiple myeloma with early relapse. Mol Cells. 2016;39(12):898–908. https://doi.org/10.14348/molcells.2016.0210.
Katoh D, Kozuka Y, Noro A, Ogawa T, Imanaka-Yoshida K, Yoshida T. Tenascin-C induces phenotypic changes in fibroblasts to myofibroblasts with high contractility through the Integrin αvβ1/transforming growth factor β/SMAD signaling axis in human breast cancer. Am J Pathol. 2020;190(10):2123–35. https://doi.org/10.1016/j.ajpath.2020.06.008.
Gutjahr JC, Bayer E, Yu X, Laufer JM, Höpner JP, Tesanovic S, et al. CD44 engagement enhances acute myeloid leukemia cell adhesion to the bone marrow microenvironment by increasing VLA-4 avidity. Haematologica. 2021;106(8):2102–13. https://doi.org/10.3324/haematol.2019.231944.
Zhang YL, Xing X, Cai LB, Zhu L, Yang XM, Wang YH, et al. Integrin α9 suppresses hepatocellular carcinoma metastasis by Rho GTPase Signaling. J Immunol Res. 2018;2018:4602570. https://doi.org/10.1155/2018/4602570.
Wang Z, Li Y, Xiao Y, Lin HP, Yang P, Humphries B, et al. Integrin α9 depletion promotes β-catenin degradation to suppress triple-negative breast cancer tumor growth and metastasis. Int J Cancer. 2019;145(10):2767–80. https://doi.org/10.1002/ijc.32359.
Nollet EA, Cardo-Vila M, Ganguly SS, Tran JD, Schulz VV, Cress A, et al. Androgen receptor-induced integrin α6β1 and Bnip3 promote survival and resistance to PI3K inhibitors in castration-resistant prostate cancer. Oncogene. 2020;39(31):5390–404. https://doi.org/10.1038/s41388-020-1370-9.
Ramovs V, Krotenberg Garcia A, Kreft M, Sonnenberg A. Integrin α3β1 is a key regulator of several protumorigenic pathways during skin carcinogenesis. J Invest Dermatol. 2021;141(4):732-41.e6. https://doi.org/10.1016/j.jid.2020.07.024.
Ming XY, Fu L, Zhang LY, Qin YR, Cao TT, Chan KW, et al. Integrin α7 is a functional cancer stem cell surface marker in oesophageal squamous cell carcinoma. Nat Commun. 2016;7:13568. https://doi.org/10.1038/ncomms13568.
Caron JM, Ames JJ, Contois L, Liebes L, Friesel R, Muggia F, et al. Inhibition of ovarian tumor growth by targeting the HU177 cryptic collagen epitope. Am J Pathol. 2016;186(6):1649–61. https://doi.org/10.1016/j.ajpath.2016.01.015.
Okada T, Lee AY, Qin LX, Agaram N, Mimae T, Shen Y, et al. Integrin-α10 dependency Identifies RAC and RICTOR as therapeutic targets in high-grade myxofibrosarcoma. Cancer Discov. 2016;6(10):1148–65. https://doi.org/10.1158/2159-8290.CD-15-1481.
Guarin JR, Fatherree JP, Oudin MJ. Chemotherapy treatment induces pro-invasive changes in liver ECM composition. Matrix Biol. 2022;112:20–38. https://doi.org/10.1016/j.matbio.2022.08.002.
Boudjadi S, Carrier JC, Groulx JF, Beaulieu JF. Integrin α1β1 expression is controlled by c-MYC in colorectal cancer cells. Oncogene. 2016;35(13):1671–8. https://doi.org/10.1038/onc.2015.231.
Wei D, Li C, Ye J, Xiang F, Liu J. Extracellular collagen mediates osteosarcoma progression through an integrin α2β1/JAK/STAT3 Signaling Pathway. Cancer Manag Res. 2020;12:12067–75. https://doi.org/10.2147/CMAR.S273466.
Yu C, Zhang M, Song J, Zheng X, Xu G, Bao Y, et al. Integrin-Src-YAP1 signaling mediates the melanoma acquired resistance to MAPK and PI3K/mTOR dual targeted therapy. Mol Biomed. 2020;1(1):12. https://doi.org/10.1186/s43556-020-00013-0.
Ou Z, Dolmatova E, Lassègue B, Griendling KK. β1- and β2-integrins: central players in regulating vascular permeability and leukocyte recruitment during acute inflammation. Am J Physiol Heart Circ Physiol. 2021;320(2):H734–9. https://doi.org/10.1152/ajpheart.00518.2020.
Ganguly K, Cox JL, Ghersi D, Grandgenett PM, Hollingsworth MA, Jain M, et al. Mucin 5AC-Mediated CD44/ITGB1 clustering mobilizes adipose-derived mesenchymal stem cells to modulate pancreatic cancer stromal heterogeneity. Gastroenterology. 2022;162(7):2032-46.e12. https://doi.org/10.1053/j.gastro.2022.02.032.
Estrugo D, Fischer A, Hess F, Scherthan H, Belka C, Cordes N. Ligand bound beta1 integrins inhibit procaspase-8 for mediating cell adhesion-mediated drug and radiation resistance in human leukemia cells. PLoS ONE. 2007;2(3): e269. https://doi.org/10.1371/journal.pone.0000269.
Xie J, Guo T, Zhong Z, Wang N, Liang Y, Zeng W, et al. ITGB1 drives hepatocellular carcinoma progression by modulating cell cycle process Through PXN/YWHAZ/AKT Pathways. Front Cell Dev Biol. 2021;9: 711149. https://doi.org/10.3389/fcell.2021.711149.
Scalici JM, Harrer C, Allen A, Jazaeri A, Atkins KA, McLachlan KR, et al. Inhibition of α4β1 integrin increases ovarian cancer response to carboplatin. Gynecol Oncol. 2014;132(2):455–61. https://doi.org/10.1016/j.ygyno.2013.12.031.
Yousefi H, Vatanmakanian M, Mahdiannasser M, Mashouri L, Alahari NV, Monjezi MR, et al. Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene. 2021;40(6):1043–63. https://doi.org/10.1038/s41388-020-01588-2.
Gupta SK, Oommen S, Aubry MC, Williams BP, Vlahakis NE. Integrin α9β1 promotes malignant tumor growth and metastasis by potentiating epithelial-mesenchymal transition. Oncogene. 2013;32(2):141–50. https://doi.org/10.1038/onc.2012.41.
Li X, Zhu G, Li Y, Huang H, Chen C, Wu D, et al. LINC01798/miR-17-5p axis regulates ITGA8 and causes changes in tumor microenvironment and stemness in lung adenocarcinoma. Front Immunol. 2023;14:1096818. https://doi.org/10.3389/fimmu.2023.1096818.
Bartolomé RA, Barderas R, Torres S, Fernandez-Aceñero MJ, Mendes M, García-Foncillas J, et al. Cadherin-17 interacts with α2β1 integrin to regulate cell proliferation and adhesion in colorectal cancer cells causing liver metastasis. Oncogene. 2014;33(13):1658–69. https://doi.org/10.1038/onc.2013.117.
Sun G, Cao Y, Guo J, Li M, Dai Y. Heat Shock Cognate Protein 70 Enhanced Integrin β1 Mediated Invasion in Cancer Cells. Cancer Manag Res. 2020;12:981–91. https://doi.org/10.2147/CMAR.S235791.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. https://doi.org/10.1016/s0092-8674(02)00971-6.
Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8(8):604–17. https://doi.org/10.1038/nrc2353.
Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016;15(3):173–83. https://doi.org/10.1038/nrd.2015.10.
Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9(10):804–20. https://doi.org/10.1038/nrd3266.
Worthington JJ, Klementowicz JE, Travis MA. TGFb: a sleeping giant awoken by integrins. Trends Biochem Sci. 2011;36(1):47–54. https://doi.org/10.1016/j.tibs.2010.08.002.
Ozawa A, Sato Y, Imabayashi T, Uemura T, Takagi J, Sekiguchi K. Molecular basis of the ligand-binding specificity of αvβ8 integrin. J Biol Chem. 2016;291(22):11551–65. https://doi.org/10.1074/jbc.M116.719138.
Peng Z, Hao M, Tong H, Yang H, Huang B, Zhang Z, et al. The interactions between integrin α5β1 of liver cancer cells and fibronectin of fibroblasts promote tumor growth and angiogenesis. Int J Biol Sci. 2022;18(13):5019–37. https://doi.org/10.7150/ijbs.72367.
Xie YH, Ran LQ, Wu ZY, Sun C, Xu XE, Zou HY, et al. Role of Integrin β1 in the progression and chemo-resistance of esophageal squamous cell carcinoma. J Cancer. 2022;13(7):2074–85. https://doi.org/10.7150/jca.68647.
Miyamoto S, Nagano Y, Miyazaki M, Nagamura Y, Sasaki K, Kawamura T, et al. Integrin α5 mediates cancer cell-fibroblast adhesion and peritoneal dissemination of diffuse-type gastric carcinoma. Cancer Lett. 2022;526:335–45. https://doi.org/10.1016/j.canlet.2021.11.008.
Kok-Sin T, Mokhtar NM, Ali Hassan NZ, Sagap I, Mohamed Rose I, Harun R, et al. Identification of diagnostic markers in colorectal cancer via integrative epigenomics and genomics data. Oncol Rep. 2015;34(1):22–32. https://doi.org/10.3892/or.2015.3993.
Zhang DX, Dang XTT, Vu LT, Lim CMH, Yeo EYM, Lam BWS, et al. αvβ1 integrin is enriched in extracellular vesicles of metastatic breast cancer cells: a mechanism mediated by galectin-3. J Extracell Vesicles. 2022;11(8): e12234. https://doi.org/10.1002/jev2.12234.
Sedlář A, Trávníčková M, Bojarová P, Vlachová M, Slámová K, Křen V, et al. Interaction between Galectin-3 and integrins mediates cell-matrix adhesion in endothelial cells and mesenchymal stem cells. Int J Mol Sci. 2021;22(10):5144. https://doi.org/10.3390/ijms22105144.
Mayadas TN, Cullere X. Neutrophil beta2 integrins: moderators of life or death decisions. Trends Immunol. 2005;26(7):388–95. https://doi.org/10.1016/j.it.2005.05.002.
Anselmi M, Baiula M, Spampinato S, Artali R, He T, Gentilucci L. Design and pharmacological characterization of α4β1 integrin cyclopeptide agonists: computational investigation of ligand determinants for agonism versus antagonism. J Med Chem. 2023;66(7):5021–40. https://doi.org/10.1021/acs.jmedchem.2c02098.
Sevilla-Movilla S, Arellano-Sánchez N, Martínez-Moreno M, Gajate C, Sánchez-Vencells A, Valcárcel LV, et al. Upregulated expression and function of the α4β1 integrin in multiple myeloma cells resistant to bortezomib. J Pathol. 2020;252(1):29–40. https://doi.org/10.1002/path.5480.
Härzschel A, Zucchetto A, Gattei V, Hartmann TN. VLA-4 expression and activation in B cell malignancies: functional and clinical aspects. Int J Mol Sci. 2020;21(6):2206. https://doi.org/10.3390/ijms21062206.
Zucchetto A, Tissino E, Chigaev A, Hartmann TN, Gattei V. Methods for Investigating VLA-4 (CD49d/CD29) expression and activation in chronic lymphocytic leukemia and its clinical applications. Methods Mol Biol. 2019;1881:101–12. https://doi.org/10.1007/978-1-4939-8876-1_8.
Høye AM, Couchman JR, Wewer UM, Fukami K, Yoneda A. The newcomer in the integrin family: integrin α9 in biology and cancer. Adv Biol Regul. 2012;52(2):326–39. https://doi.org/10.1016/j.jbior.2012.03.004.
Park HJ, Yun JI, Lee ST. Localization of integrin heterodimer α9β1 on the surface of uterine endometrial stromal and epithelial cells in mice. Anim Cells Syst. 2020;24(4):228–32. https://doi.org/10.1080/19768354.2020.1804446.
Park MH, Yun JI, Lee E, Lee ST. Integrin heterodimer α9β1 is localized on the surface of porcine spermatogonial stem cells in the undifferentiated state. Reprod Domest Anim. 2019;54(11):1497–500. https://doi.org/10.1111/rda.13555.
Varney SD, Wu L, Longmate WM, DiPersio CM, Van De Water L. Loss of Integrin α9β1 on tumor keratinocytes enhances the stromal vasculature and growth of cutaneous tumors. J Invest Dermatol. 2022;142(7):1966-75.e8. https://doi.org/10.1016/j.jid.2021.11.020.
Høye AM, Couchman JR, Wewer UM, Yoneda A. The phosphorylation and distribution of cortactin downstream of integrin α9β1 affects cancer cell behaviour. Sci Rep. 2016;6:28529. https://doi.org/10.1038/srep28529.
Taooka Y, Ohe M, Chen L, Sutani A, Higashi Y, Isobe T. Increased expression levels of integrin α9β1 and CD11b on circulating neutrophils and elevated serum IL-17A in elderly aspiration pneumonia. Respiration. 2013;86(5):367–75. https://doi.org/10.1159/000345390.
Saldanha-Gama RF, Moraes JA, Mariano-Oliveira A, Coelho AL, Walsh EM, Marcinkiewicz C, et al. alpha(9)beta(1) integrin engagement inhibits neutrophil spontaneous apoptosis: involvement of Bcl-2 family members. Biochim Biophys Acta. 2010;1803(7):848–57. https://doi.org/10.1016/j.bbamcr.2010.03.012.
Lydolph MC, Morgan-Fisher M, Høye AM, Couchman JR, Wewer UM, Yoneda A. Alpha9beta1 integrin in melanoma cells can signal different adhesion states for migration and anchorage. Exp Cell Res. 2009;315(19):3312–24. https://doi.org/10.1016/j.yexcr.2009.09.022.
Schreiber TD, Steinl C, Essl M, Abele H, Geiger K, Müller CA, et al. The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica. 2009;94(11):1493–501. https://doi.org/10.3324/haematol.2009.006072.
Nawaz I, Hu LF, Du ZM, Moumad K, Ignatyev I, Pavlova TV, et al. Integrin α9 gene promoter is hypermethylated and downregulated in nasopharyngeal carcinoma. Oncotarget. 2015;6(31):31493–507. https://doi.org/10.18632/oncotarget.5154.
Yasuda H, Nakagawa M, Kiyokawa H, Yoshida E, Yoshimura T, Koshikawa N, et al. Unique Biological Activity and Potential Role of Monomeric Laminin-γ2 as a Novel Biomarker for Hepatocellular Carcinoma: A Review. Int J Mol Sci. 2019;20(1):226. https://doi.org/10.3390/ijms20010226.
Moon YW, Rao G, Kim JJ, Shim HS, Park KS, An SS, et al. LAMC2 enhances the metastatic potential of lung adenocarcinoma. Cell Death Differ. 2015;22(8):1341–52. https://doi.org/10.1038/cdd.2014.228.
Liu Y, Wang CL, Pang ZQ, Gao K, Shen LK, Xu WH, et al. Endostatin 33 peptide is a deintegrin α6β1 agent that exerts antitumor activity by inhibiting the PI3K-Akt signaling pathway in prostate cancer. J Clin Med. 2023;12(5):1861. https://doi.org/10.3390/jcm12051861.
Chen C, Jiang Z, Yang G. Laminins in osteogenic differentiation and pluripotency maintenance. Differentiation. 2020;114:13–9. https://doi.org/10.1016/j.diff.2020.05.002.
Bae E, Huang P, Müller-Greven G, Hambardzumyan D, Sloan AE, Nowacki AS, et al. Integrin α3β1 promotes vessel formation of glioblastoma-associated endothelial cells through calcium-mediated macropinocytosis and lysosomal exocytosis. Nat Commun. 2022;13(1):4268. https://doi.org/10.1038/s41467-022-31981-2.
Mautone L, Ferravante C, Tortora A, Tarallo R, Giurato G, Weisz A, et al. Higher integrin alpha 3 Beta1 expression in papillary thyroid cancer is associated with worst outcome. Cancers. 2021;13(12):2937. https://doi.org/10.3390/cancers13122937.
Longmate WM, Miskin RP, Van De Water L, DiPersio CM. epidermal integrin α3β1 REGULATES TUMOR-DERIVED PROTEASES BMP-1, matrix metalloprotease-9, and matrix metalloprotease-3. JID Innov. 2021;1(2): 100017. https://doi.org/10.1016/j.xjidi.2021.100017.
Zheng R, Longmate WM, DeFreest L, Varney S, Wu L, DiPersio CM, et al. Keratinocyte integrin α3β1 promotes secretion of IL-1α to effect paracrine regulation of fibroblast gene expression and differentiation. J Invest Dermatol. 2019;139(9):2029-38.e3. https://doi.org/10.1016/j.jid.2019.02.025.
Miskin RP, Warren JSA, Ndoye A, Wu L, Lamar JM, DiPersio CM. Integrin α3β1 promotes invasive and metastatic properties of breast cancer cells through induction of the Brn-2 transcription factor. Cancers. 2021;13(3):480. https://doi.org/10.3390/cancers13030480.
Ndoye A, Miskin RP, DiPersio CM. Integrin α3β1 represses reelin expression in breast cancer cells to promote invasion. Cancers. 2021;13(2):344. https://doi.org/10.3390/cancers13020344.
Jin D, Zhang R, Chen H, Li C. Aberrantly glycosylated integrin α3β1 is a unique urinary biomarker for the diagnosis of bladder cancer. Aging (Albany NY). 2020;12(11):10844–62. https://doi.org/10.18632/aging.103297.
Zou Y, Sun Y, Guo B, Wei Y, Xia Y, Huangfu Z, et al. α3β1 integrin-targeting polymersomal docetaxel as an advanced nanotherapeutic for nonsmall cell lung cancer treatment. ACS Appl Mater Interfaces. 2020;12(13):14905–13. https://doi.org/10.1021/acsami.0c01069.
Chen Y, Wang Y, Cai Z, Fan X, Zhang Y. Integrin α7 is overexpressed and correlates with higher pathological grade, increased T stage, advanced TNM stage as well as worse survival in clear cell renal cell carcinoma patients: a retrospective study. J Clin Lab Anal. 2020;34(1): e23034. https://doi.org/10.1002/jcla.23034.
Lv Z, Yang Y, Yang C. Integrin α7 correlates with worse clinical features and prognosis, and its knockdown inhibits cell proliferation and stemness in tongue squamous cell carcinoma. Int J Oncol. 2020;56(1):69–84. https://doi.org/10.3892/ijo.2019.4927.
Xia D, Chen B, Yang X. Correlation of integrin alpha 7 with clinicopathological characteristics and survival profiles, as well as its regulatory role in cell proliferation, apoptosis, and stemness in non-small-cell lung cancer. J Clin Lab Anal. 2019;33(8): e22973. https://doi.org/10.1002/jcla.22973.
Bourgot I, Primac I, Louis T, Noël A, Maquoi E. Reciprocal interplay between fibrillar collagens and collagen-binding integrins: implications in cancer progression and metastasis. Front Oncol. 2020;10:1488. https://doi.org/10.3389/fonc.2020.01488.
Masoumi KC, Huang X, Sime W, Mirkov A, Munksgaard Thorén M, Massoumi R, et al. Integrin α10-antibodies reduce glioblastoma tumor growth and cell migration. Cancers. 2021;13(5):1184. https://doi.org/10.3390/cancers13051184.
Munksgaard Thorén M, Chmielarska Masoumi K, Krona C, Huang X, Kundu S, Schmidt L, et al. Integrin α10, a Novel therapeutic target in glioblastoma, regulates cell migration, proliferation, and survival. Cancers. 2019;11(4):587. https://doi.org/10.3390/cancers11040587.
Wenke AK, Kjellman C, Lundgren-Akerlund E, Uhlmann C, Haass NK, Herlyn M, et al. Expression of integrin alpha10 is induced in malignant melanoma. Cell Oncol. 2007;29(5):373–86. https://doi.org/10.1155/2007/601497.
Boudjadi S, Bernatchez G, Sénicourt B, Beauséjour M, Vachon PH, Carrier JC, et al. Involvement of the integrin α1β1 in the progression of colorectal cancer. Cancers. 2017;9(8):96. https://doi.org/10.3390/cancers9080096.
Zhang Y, Cheng K, Xu B, Shi J, Qiang J, Shi S, et al. Epigenetic input dictates the threshold of targeting of the integrin-dependent pathway in non-small cell lung cancer. Front Cell Dev Biol. 2020;8:652. https://doi.org/10.3389/fcell.2020.00652.
Kim JE, Lee SK, Park J, Jung MJ, An SE, Yang HJ, et al. Buddlejasaponin IV induces apoptotic cell death by activating the mitochondrial-dependent apoptotic pathway and reducing α2β1 integrin-mediated adhesion in HT-29 human colorectal cancer cells. Oncol Rep. 2023;49(3):58. https://doi.org/10.3892/or.2023.8495.
Dötzer K, Schlüter F, Koch FEV, Brambs CE, Anthuber S, Frangini S, et al. Integrin α2β1 represents a prognostic and predictive biomarker in primary ovarian cancer. Biomedicines. 2021;9(3):289. https://doi.org/10.3390/biomedicines9030289.
Juratli MA, Zhou H, Oppermann E, Bechstein WO, Pascher A, Chun FK, et al. Integrin α2 and β1 cross-communication with mTOR/AKT and the CDK-Cyclin Axis in hepatocellular carcinoma cells. Cancers. 2022;14(10):2430. https://doi.org/10.3390/cancers14102430.
Moritz MNO, Casali BC, Stotzer US, Karina Dos Santos P, Selistre-de-Araujo HS. Alternagin-C, an alpha2beta1 integrin ligand, attenuates collagen-based adhesion, stimulating the metastasis suppressor 1 expression in triple-negative breast tumor cells. Toxicon. 2022;210:1–10. https://doi.org/10.1016/j.toxicon.2022.02.001.
Kozlova NI, Morozevich GE, Berman AE. Implication of integrin α2β1 in senescence of SK-Mel-147 human melanoma cells. Aging (Albany NY). 2021;13(14):18006–17. https://doi.org/10.18632/aging.203309.
Salemi Z, Azizi R, Fallahian F, Aghaei M. Integrin α2β1 inhibition attenuates prostate cancer cell proliferation by cell cycle arrest, promoting apoptosis and reducing epithelial-mesenchymal transition. J Cell Physiol. 2021;236(7):4954–65. https://doi.org/10.1002/jcp.30202.
Martínez-Nieto GA, Teppo HR, Petrelius N, Izzi V, Devarajan R, Petäistö T, et al. Upregulated integrin α11 in the stroma of cutaneous squamous cell carcinoma promotes skin carcinogenesis. Front Oncol. 2022;12: 981009. https://doi.org/10.3389/fonc.2022.981009.
Navab R, Strumpf D, To C, Pasko E, Kim KS, Park CJ, et al. Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer. Oncogene. 2016;35(15):1899–908. https://doi.org/10.1038/onc.2015.254.
Xu Z, Zou L, Ma G, Wu X, Huang F, Feng T, et al. Integrin β1 is a critical effector in promoting metastasis and chemo-resistance of esophageal squamous cell carcinoma. Am J Cancer Res. 2017;7(3):531–42.
Zhang Y, Chen X, Qiao Y, Yang S, Wang Z, Ji M, et al. DNA aptamer selected against esophageal squamous cell carcinoma for tissue imaging and targeted therapy with integrin β1 as a molecular target. Anal Chem. 2022;94(49):17212–22. https://doi.org/10.1021/acs.analchem.2c03863.
Vehlow A, Klapproth E, Storch K, Dickreuter E, Seifert M, Dietrich A, et al. Adhesion- and stress-related adaptation of glioma radiochemoresistance is circumvented by β1 integrin/JNK co-targeting. Oncotarget. 2017;8(30):49224–37. https://doi.org/10.18632/oncotarget.17480.
Eke I, Zscheppang K, Dickreuter E, Hickmann L, Mazzeo E, Unger K, et al. Simultaneous β1 integrin-EGFR targeting and radiosensitization of human head and neck cancer. J Natl Cancer Inst. 2015;107(2):419. https://doi.org/10.1093/jnci/dju419.
Lottini T, Duranti C, Iorio J, Martinelli M, Colasurdo R, D’Alessandro FN, et al. Combination therapy with a bispecific antibody targeting the hERG1/β1 integrin complex and gemcitabine in pancreatic ductal adenocarcinoma. Cancers. 2023;15(7):2013. https://doi.org/10.3390/cancers15072013.
Uchihara T, Miyake K, Yonemura A, Komohara Y, Itoyama R, Koiwa M, et al. Extracellular vesicles from cancer-associated fibroblasts containing annexin A6 induces FAK-YAP activation by stabilizing β1 integrin. Enhancing Drug Resist Cancer Res. 2020;80(16):3222–35. https://doi.org/10.1158/0008-5472.CAN-19-3803.
Böttger TC, Maschek H, Lobo M, Gottwohl RG, Brenner W, Junginger T. Prognostic value of immunohistochemical expression of beta-1 integrin in pancreatic carcinoma. Oncology. 1999;56(4):308–13. https://doi.org/10.1159/000011984.
Hu C, Ni Z, Li BS, Yong X, Yang X, Zhang JW, et al. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut. 2017;66(1):31–42. https://doi.org/10.1136/gutjnl-2015-309322.
Sun Q, Zhou C, Ma R, Guo Q, Huang H, Hao J, et al. Prognostic value of increased integrin-beta 1 expression in solid cancers: a meta-analysis. Onco Targets Ther. 2018;11:1787–99. https://doi.org/10.2147/OTT.S155279.
Yao ES, Zhang H, Chen YY, Lee B, Chew K, Moore D, et al. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007;67(2):659–64. https://doi.org/10.1158/0008-5472.
Pontes-Júnior J, Reis ST, Bernardes FS, Oliveira LC, Barros ÉA, Dall’Oglio MF, et al. Correlation between beta1 integrin expression and prognosis in clinically localized prostate cancer. Int Braz J Urol. 2013;39(3):335–42. https://doi.org/10.1590/S1677-5538.IBJU.2013.03.06.
Lu X, Wan F, Zhang H, Shi G, Ye D. ITGA2B and ITGA8 are predictive of prognosis in clear cell renal cell carcinoma patients. Tumour Biol. 2016;37(1):253–62. https://doi.org/10.1007/s13277-015-3792-5.
Liu QZ, Gao XH, Chang WJ, Gong HF, Fu CG, Zhang W, et al. Expression of ITGB1 predicts prognosis in colorectal cancer: a large prospective study based on tissue microarray. Int J Clin Exp Pathol. 2015;8(10):12802–10.
Li S, Sampson C, Liu C, Piao HL, Liu HX. Integrin signaling in cancer: bidirectional mechanisms and therapeutic opportunities. Cell Commun Signal. 2023;21(1):266. https://doi.org/10.1186/s12964-023-01264-4.
Bou Antoun N, Chioni AM. Dysregulated signalling pathways driving anticancer drug resistance. Int J Mol Sci. 2023;24(15):12222. https://doi.org/10.3390/ijms241512222.
Dzobo K, Dandara C. The extracellular matrix: its composition, function, remodeling, and role in tumorigenesis. Biomimetics. 2023;8(2):146. https://doi.org/10.3390/biomimetics8020146.
Baltes F, Pfeifer V, Silbermann K, Caspers J, Wantoch von Rekowski K, Schlesinger M, Bendas G. β1-Integrin binding to collagen type 1 transmits breast cancer cells into chemoresistance by activating ABC efflux transporters. Biochim Biophys Acta Mol Cell Res. 2020;1867(5):118663. https://doi.org/10.1016/j.bbamcr.2020.118663.
Dickreuter E, Eke I, Krause M, Borgmann K, van Vugt MA, Cordes N. Targeting of β1 integrins impairs DNA repair for radiosensitization of head and neck cancer cells. Oncogene. 2016;35(11):1353–62. https://doi.org/10.1038/onc.2015.212.
Eke I, Deuse Y, Hehlgans S, Gurtner K, Krause M, Baumann M, et al. β1 Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J Clin Invest. 2012;122(4):1529–40. https://doi.org/10.1172/JCI61350.
Jahangiri A, Nguyen A, Chandra A, Sidorov MK, Yagnik G, Rick J, et al. Cross-activating c-Met/β1 integrin complex drives metastasis and invasive resistance in cancer. Proc Natl Acad Sci USA. 2017;114(41):E8685–94. https://doi.org/10.1073/pnas.1701821114.
Xu C, Xie XL, Kang N, Jiang HQ. Evaluation of ITGB1 expression as a predictor of the therapeutic effects of immune checkpoint inhibitors in gastric cancer. BMC Gastroenterol. 2023;23(1):298. https://doi.org/10.1186/s12876-023-02930-0.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China (82203547), Natural Science Foundation of Jiangsu Province (BK20210136) and China Postdoctoral Science Foundation (2022M721433).
Author information
Authors and Affiliations
Contributions
LS and SG wrote the manuscript. YX was responsible for the graph draft. YY designed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, L., Guo, S., Xie, Y. et al. The characteristics and the multiple functions of integrin β1 in human cancers. J Transl Med 21, 787 (2023). https://doi.org/10.1186/s12967-023-04696-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04696-1