Abstract
Currently, women around the world are still suffering from various female common diseases with the high incidence, such as ovarian cancer, uterine fibroids and preeclampsia (PE), and some diseases are even with the high mortality rate. As a negative feedback regulator in G Protein-Coupled Receptor signaling (GPCR), the Regulator of G-protein Signaling (RGS) protein family participates in regulating kinds of cell biological functions by destabilizing the enzyme–substrate complex through the transformation of hydrolysis of G Guanosine Triphosphate (GTP). Recent work has indicated that, the Regulator of G-protein Signaling 2 (RGS2), a member belonging to the RGS protein family, is closely associated with the occurrence and development of certain female diseases, providing with the evidence that RGS2 functions in sustaining women’s health. In this review paper, we summarize the current knowledge of RGS2 in female common diseases, and also tap and discuss its therapeutic potential by targeting multiple mechanisms.
Similar content being viewed by others
Background
The Regulator of G Protein Signaling (RGS) protein family is a feedback regulator in GPCR signaling, which functions as a common GTPase activating protein (GAP) for G proteins and usually acts to add the natural guanosine triphosphatase (GTPase) activity of the Gα-subunit. Generally, through the enhancement of the GTPase activity of the Gα-subunit, RGS proteins encourage GTP hydrolysis to guanosine diphosphate (GDP), therefore switching the Gα subunit to its devitalized state and decreasing its signaling ability [1]. The family of RGS protein can be roughly classified into two sub-groups: (1) one group is composed mainly of a classical RGS domain with small extensions at both the amino-terminal (N-terminal) and carboxyl-terminal (C-terminal); (2) and one group that possesses several other domains in addition to the RGS domain.
To date, at least 37 different RGS proteins have been successfully discoveried and defined, with an extra 14 RGS proteins containing the domain that is nominated as the nonfunctional RGS homology domain [2], while the RGS proteins that posses a functional RGS domain can be divided into eight sub-families at a minimum [2]. As is depicted in Fig. 1, there are two kinds of systems for classifying RGS proteins, among which one system sorts sub-families via alphabet A to alphabet F, and the names of subfamily in the other classifying system are derived from the typical RGS member [3, 4]. For instance, the RZ family is nominated by the typical RGSZ protein [3, 5]. Based on the identity, structure and function of amino acid (AA) sequence, each family grouping is sub-sorted. For example, members belonging to the family named C/R7 possess a peculiar Disheveled, EGL-10, Pleckstrin (DEP) domain, which is called the R7H domain, whereas the A/RZ RGS proteins and B/R4 RGS proteins posses little more than a functional RGS domain. As a member in R4 family, RGS2 contains the simplest AA sequence structure [6]. RGS2-like proteins, including RGS1, RGS4, RGS5, RGS10, RGS13, RGS16, RGS18, and RGS-GAIP, belong to the small subfamily, which have little or almost no affinity for Gα-GDP complexes. In contrast, those proteins show high affinity in their binding to Gα-subunit complex particularly with AlF4 and GDP, contributing to a conformation of the Gα-subunit that features the interim condition causing hydrolysis of GTP. In rats and humans, the gene locus of RGS2 is located in chromosome 1 and is consists of five exons. The RGS2 locus, encoding a 212-residue protein, contains an RGS domain with120 AA that is sided by an N-terminal domain with 80-residue and a C-terminal tail of short length [7], which is similar to other RGS proteins in B/R4 class [6]. Of note, RGS2 protein owns the intrinsic GAP activity, which is vigorous and optional for Gq-class Gα-subunits in vitro, whereas other B/R4-family RGS proteins, in contrst to RGS2, possess intrinsic GAP activity for both Gi/o-class and Gq-class Gα-subunits [8, 9].
The classical RGS domain is essential and adequate for Gα-subunits binding. A previous report showed a crystal structure of the RGS domain in the RGS4 complex with Giα1 and GDP-AlF4, supporting the comprehension of how RGS proteins boost GTP hydrolysis [9]. The RGS domain is found to bind to the three switches area in Giα, and those binding-regions receive conformational changes upon their binding with guanine nucleotide and their hydrolysis. As has been proved, the RGS domain is comprised of nine α-helices that are further folded into two sub-domains of small size, and one small sub-domain shapes a structure with right-handed anti-parallel four-helix bundle. In addition, three parts with high conservation located in the RGS domain straightly link to the Giα switch spots, while mutations in these parts greatly interfere with the GAP viability of RGS protein. More recently, the results from structural and biochemical researches additionally reveal that RGS proteins spur Gα GTP hydrolysis through decreasing the energy of transitional state, leading to the destabilization of the enzyme substrate complex [9].
Recently, emerging evidence has suggested that RGS proteins play a fundamental role not only in embryonic development but also in various diseases, including gestational disorders as well as gynecological diseases including gynecology malignancies [11,12,13], such as ovarian carcinoma and hysteromyoma (Fig. 2).
A diagram showing the female reproductive system. In this figure, bilateral ovarian tissue has obvious abnormal differentiation, the right ovarian tissue has obvious enlargement, and the ovarian tissue structure is heterogeneous, indicating the presence of ovarian cancer in this reproductive system. Abnormal spherical tissues of different sizes and degrees appeared in the uterine muscle wall, submucosa and serous membrane, respectively, indicating the presence of uterine fibroids in the uterus
Ovarian carcinoma
As the mortality rate of patients has been over 60%, ovarian carcinoma is considered as one of the fatal gynecological cancers [14]. As is commonly believed, early detection contributes to elevating overall-survival, while only a quarter of ovarian cancers are discovered at stage I [15]. According to the previous researches, the development and existence of tumor cell resistance to chemotherapeutic drugs and the frequent diagnosis of ovarian carcinoma at an advanced stage are the major cause of high mortality rate [16]. Consistently, recent clinical data also indicated that low expression of RGS2 promotes poor prognosis in high-grade serous ovarian cancer (Fig. 3) [17].
A diagram showing the gestational disorders during female pregnancy. Gestational hypertention, PE, postpartum depression are marked, and related diseases, including pulmonary hypoplasia and breast caner are also indicated. The woman in the picture shows signs of depression, which is a possible expression of postpartum depression. An abnormal lump structure in the breast tissue indicates breast cancer. Near the heart, a description of the woman’s abnormal blood pressure suggests that the woman has gestational hypertension. Abnormal proteinuria and other organic changes in the umbilical cord indicate that the woman is in a state of PE. The lung of the fetus in utero is marked with abnormal pulmonary dysplasia and signs of pulmonary fibrosis
GPCRs and epidermal growth factor receptors (EGFRs) are abundently expressed in ovarian carcinoma tissues [18]. G-proteins control GPCR-mediated cell survival signaling, which is opposite to the bioactivity of RGS proteins [19]. By straight association with the activated Gα subunit of G-proteins, RGS proteins can hasten the termination of GPCR signalings to raise hydrolysis of GTP into GDP, which returns G-proteins to a devitalized condition [20, 21]. Therefore, RGS proteins play an important role in cell survival and tumorigenesis. The aberrant expression of RGS2 is related to solid tumor development, and RGS2 expression is down-regulated in various cancers, such as ovarian cancer and prostate cancer [22,23,24]. Nevertheless, the molecular mechanisms underlying RGS2 expression inhibition are not clear yet. Therefore, inhibition of RGS2 may promote chemoresistance by EGFR or GPCR-mediated cell growth and cell survival signaling pathways.
Due to the accumulation of DNA methyltransferase 1 (DNMT1) and class I Histone deacetylases (HDACs) at the RGS2 promoter region, the expression of RGS2 gene is inhibited in ovarian cancer cells. Thus, these findings indicate that the epigenetic changes, including DNA methylation and histone modifications such as deacetylation and acetylation, may lead to the absence of RGS2 expression in the ovarian cancer cells with chemoresistance. A recent research revealed the modulation of RGS2 expression by DNA methylation and histone deacetylation particularly in the chemoresistant ovarian cancer cells, and as a result, the expression of RGS2 is declined in the ovarian cancer cells resistant to drugs [24]. Thus, one conclusion can be made that drug exposure may give rise to the deficiency of RGS2 expression during carcigogenesis in ovarian [25]. In addition, the acetylated histone H3 levels at the RGS2 promoter are obviously reduced in chemoresistant ovarian cancer cells, compared with that in chemosensitive cells. However, the expression of histone H3 in ovarian cancer cells are similar to those in the chemosensitive cells, indicating that the acetylational absence at RGS2 promoter leads to the deficiency of RGS2 expression in ovarian cancer cells with chemoresistance [25]. Therefore, histone acetylation potentiates RGS2 expression, while HDACs antagonize it. On the other hand, class I HDACs can inhibit the expression of RGS2 in chemically blocked ovarian cancer cells [25]. These findings demonstrate that the increased HDACs (including HDAC1-3) binding in chemoresistant ovarian cancer cells corresponds to the reduction of histone acetylation at the RGS2 promoter. Consistently, it has been shown that class I HDACs are abundantly distributed and widely expressed in ovarian cancer tissues, whereas abnormal HDAC expression is conserded to be closely associated with adverse response to chemotherapy [26, 27]. Therefore, HDAC inhibitors can be potentially employed to promote histone acetylation levels and to therefore elevate RGS2 expression levels, providing with the evidence that inhibition of histone deacetylase activity may be a new therapeutic target for epithelial ovarian cancers in clinic, particularly the platinum-resistant ovarian cancer [27].
Upregulation of methylation of tumor suppressor genes is often referred to as a potential biomarker for cancer progression [28, 29], and DNA methylation has also been found to be associated with ovarian cancer chemoresistance [23, 30,31,32]. DNMT1 is essential for maintaining the established DNA methylation patterns, whereas DNMT3a and DNMT3b normally promote the formation of de novo DNA methylation patterns [33]. Therefore, to maintain RGS2 repression during ovarian cancer progression, RGS2 promoter might require the accumulation of DNMT1.
In addition to RGS2, other RGS proteins such as RGS5, RGS10 and RGS17 are also closely related to ovarian cancer [34]. Under certain hypoxic conditions, RGS5 expression is increased in ovarian cancer carcinoma-derived endothelial cells (ODMECs), and the proliferative capacity of ODMECs was significantly increased after targeted decrease of RGS5 expression. This suggests that RGS5 functions as a key mediator in the carcinogenesis of ovarian cancer [35]. Similarly, cellular deficiency in RGS10 promotes chemoresistance in ovarian cancer [35]. RGS10 expression is regulated by DNMT1 and HDAC1, and dysregulation of RGS10 reduces chemoresistance in ovarian cancer cells [25, 34]. RGS17 can affect the occurrence and development of ovarian cancer through the PI3K/AKT cellular survival pathway [36]. Lysophosphatidic acid (LPA) can activate Gαi protein by binding to one of its receptors in an autocrine manner, which phosphorylates and subsequently activates protein kinase B (PKB, also known as AKT) and promotes cell survival consequently. The elevation in RGS17 expression resulted in a decrease in AKT activation followed by LPA treatment, thus illustrating the mechanism of growth arrest of ovarian cancer cells and the relevance of RGS17 expression in ovarian cancer cells.
In summary, since DNA methylation regulation is reversible, DNA demethylating agents may be an effective means of treating cancer in future and modulation of RGS2, RGS10 and RGS17 expression could be a promising approach to enhance the chemotherapeutic drug activity against drug-resistant cancer cells.
Hysteromyoma
Hysteromyoma, known as the most common benign tumor in the female reproductive organs, are derived from the aberrant proliferation of uterine smooth muscle cells, where a small portion of fibrous connective tissue functions as a kind of support “material”. During pregnancy, due to the gestational function, uterus will undergo great changes in the first trimester. For instance, the volume and weight can increase by as much as 20 times compared to those before pregnancy [37]. Among these changes, the myometrium remains relatively static in order to ensure the normal development of the fetus and does not respond to uterine contractions. As a result, the myometrium is less sensitive to oxytocin through the relatively low levels of oxytocin receptor expression [38, 39]. Nevertheless, the levels of GPCR that regulate bradykinin, prostaglandins, angiotensin II, endothelin and hemolysin, and others, are not decreased obviously, suggesting that the myometrium does not perceive of endogenous concentrations of these drugs at the post-receptor level for most of the pregnancy. Part of the research suggests that the reason for non-perception is derived from the RGS2-induced reduction in GTPase activity. Due to the increased G-protein signaling of agonists and the decreased RGS2 mRNA expression, uterine contraindications will take place at the end of pregnancy. Similarly, progesterone, an important progestin, it itself is important in upregulating RGS2 mRNA expression. Therefore, RGS2 mRNA expression can be prolonged by reducing the endogenous levels of progesterone, thereby treating uterine contraindications.
In addition to the above-mentioned regulation of GPCR signaling at the G protein expressing level, receptor insensitivity may be also expressed by means of specific kinases that phosphorylate residues in the carboxy-terminal domain of G protein-coupled receptors. Brenninkmeijer et al. reported that uterine tractions at the end of pregnancy in humans may be regulated by G protein-coupled receptor kinases 2 and 6 [40]. In this process, the uterotonic contractile agent will antagonize the effect of increase in the intracellular cAMP concentration [40]. During pregnancy, the expression of Gs in the myometrium is greatly increased and the potential to produce cAMP is enhanced [41], while the activity of Gs and its coupled adenylyl cyclase is decreased after delivery [42]. Despite that RGS2 indirectly inhibits GTPase activity, it has been reported that RGS2 directly suppresses adenylyl cyclase III activity in olfactory neurons [43]. However, since RGS2 mRNA levels are minimal at term, this finding alone does not support the conclusion that RGS2 causes a decrease in adenylate cyclase activity at term. Given the association of discrete physiological events during pregnancy with changes in RGS2 mRNA expression, it can be proposed that the myometrial RGS2 levels also participate in regulating uterine contractions [44]. However, some studies have indicated that the uterus is more responsive to stimulation by contractile agents [45]. In one study, this situation occurs when RGS2 expression is decreased. Therefore, it is likely that RGS2 exerts effects on regulation of myometrium.
Taken together, RGS2 plays a key role in regulating the myometrial response to progesterone. As uterine fibroids are a hormone-dependent tumor, the high hormone environment during pregnancy is one of the important factors causing uterine fibroids. The treatment of uterine fibroids through the regulation of RGS2 will undoubtedly have an important role in future research progress.
Gestational hypertension
Gestational hypertension is diagnosed as two episodes of systolic blood pressure of 140 mm Hg or more, or diastolic blood pressure of 90 mm Hg or more, or both, at least 4 h apart after 20 weeks of pregnancy in women with normal blood pressure before pregnancy (Fig. 4) [46]. Women with hypertension is usually not accompanied by proteinuria or severe features, and can return to normal maternal blood pressure levels after delivery [46]. The main pathogenic manifestations of gestational hypertension are the systemic vasospasm and decreased multi-organ perfusion [47].
Lately, kinds of RGS B/R4 members have been demonstrated in vascular disorders of pregnancy. The cardiovascular system undergoes great changes during normal pregnancy. Cardiac output is remarkably increased, the renin-angiotensin system is activated, and angiotensin (ANG) is secreted when gestation is in week 24. In addition, arginine vasopressin (AVP) secretion is activated simultaneously in the same week. Both hormones take effect in the elevation of water absorption and retention, and as a result, the retained fluids can be raised to 45%, leading to a subsequent reduction in osmolality. During pregnancy, blood pressure can be also decreased to 5–10 mm Hg, and most of these changes appear early during the 6–8 week-range of gestation [48]. Pregnancy shows a general decrease in blood pressure that is considered to be affected by nitric oxide, progesterone or relaxin instead of the activation by vasoconstrictor hormones [48, 49]. Additionally, there are obviously reduced vasoconstriction responses to ANG during pregnancy [50].
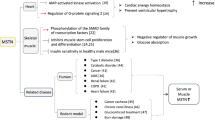
RGS2 is widely distributed and expressed within human body. In addition to placenta, high expression levels of RGS2 are also detected in other tissues, such as brain, kidney and heart, and more recently, RGS2 expression is also found and tested in vascular smooth muscle cells (VSMCs) [51,52,53]. In addition, Rgs2 is found to be expressed in certain regions in mouse brain, including the cortex, the striatum and the hippocampus as well. The expression levels of RGS2 are notably up-regulated by the activation of synaptic bioactivity and is involved in modulating the Gαq and/or Gαi-mediated activation of M2 muscarinic acetylcholine receptor (AChR) [54]. Both heterozygous (Rgs2±) knockout mice and homozygous (Rgs2−/−) knockout mice present a obvious phenotype of hypertension, making it hard to differentiate between the heterozygous (Rgs2±) and homozygous (Rgs2−/−) by simple observation [54]. Furthermore, lack of Rgs2 (Rgs2−/−) leads to the enhancement in the inner thickness of renal arterial walls, which is a trait in humans carrying chronic hypertension [55]. The ratio of blood pressure decrease is significantly lowered in Rgs2 knockout mice (Rgs2−/−) than that of wild-type (Rgs2+/+), with the administration of vasodilator agonists. In addition, treatment with vasoconstrictor in VSMCs where Rgs2 is lost leads to the increase in peak calcium responses but the extended decrease in intracellular calcium levels post challenge [55]. Rgs2 deficiency also mediates hypertension by increasing vascular tone, which is proved by the enhanced shrinkage of mesenteric obstacles and renal interlobar arteries, and by the elevated hypertension responses to ANG as well [55,56,57]. Despite that RGS2 mainly acts to restrain Gαq signaling in VSMCs, RGS2 is found to modulate Gαi/o signaling also in the vascular endothelium. Gαi/o signaling suppresses vasodilation, and absence of Rgs2 leads to the enhancement of Gαi/o signaling and the abnormal vasodilation in exposure to acetylcholine [57]. Additionally, RGS2 is demonstrated to modulate Gαs signaling. Nevertheless, RGS2 fails to interact with Gαs itself. Recently, RGS2 has been shown to restrain the generation of cyclic adenosine monophosphate (cAMP), particularly in olfactory membranes of Gαs, and as a result, RGS2 adjusts adenylyl cyclase activity. Among the multiple adenylyl cyclases, RGS2 is identified to particularly modulate several adenylyl cyclases, including adenylyl cyclases III, adenylyl cyclases V, and adenylyl cyclases VI [58]. On the other hand, RGS2 is found to regulate the AVP-induced V2 receptor (Gαs)-mediated signaling. Within the kidneys, expression distribution of RGS2 is limited to the area of nephron where V2 receptors are expressed, and as the major signaling mediator of V2 receptors, the levels of cAMP are markedly up-regulated in kidneys from Rgs2 knockout mice (Rgs2−/−). Accumulating evidence has additionally shown that AVP [59,60,61], endothelin-1 (ET-1) [62, 63], and ANG II [62, 64, 65] participate in the occurrence and progression of cardiovascular disorders during female gestation such as the hypertension syndrome. The main receptor subtypes that involved in cellular signaling transduction, such as V1A [47, 66,67,68], V2 [69], ETA/ETB [70,71,72] and AT1 receptors [73, 74], can respond to these vascular hormones, respectively, to activate G proteins and subsequently their corresponding second-messenger cascades in target cells and specific tissues. Regulators of G protein signaling or downstream mediators may share a generally common mechanism underlying cardiovascular disorders during gestation and thereby hopefully provide with potential targets for more promising therapy of gestational cardiovascular disorders in clinic.
Preeclampsia (PE)
PE is a common complication in pregnancy, and there is substantial evidence that the reduced uteroplacental blood flow in this condition is due to a toxic combination of hypoxia, inflammation, an imbalance of angiogenic and antiangiogenic factors, and immune disturbances [75, 76]. In addition, women who suffer from PE also have an increased risk in cardiovascular and renal diseases [77, 78].
Recently, RGS2 has been found to be linked to PE and data from several clinical studies revealed that RGS2 expression was dysregulated in pregnant women with PE (Table 1 and Fig. 5) [79,80,81]. Researchers found that hypertension in selected human populations, a single nucleotide polymorphism (SNP, rs4606) located in the 3’-untranslated region (UTR) of RGS2 gene is correlated with PE [77] and the same SNP (rs4606) is closely associated with an increased risk in PE development, particularly in a population of overweight females [82,83,84]. Moreover, women that carry the SNP (rs4606) are at increased odds of having PE and cardiovascular disease later in life [85]. Down-regulated levels of RGS2 transcript expression has been found to be correlated with the SNP in the 3’-UTR of the RGS2 gene [80]. Noticeably, as is discussed previously [82], Rgs2 knockout mice (Rgs2−/−) and Rgs2 heterozygous mice (Rgs2±) both reveal the hypertensive phenotypes, indicating Rgs2 gene may exhibit haplo-insufficiency. Furthermore, both the homozygotes (Rgs2−/−) and heterozygotes (Rgs2±) exhibit alterations in the rs4606 C1114G SNP that are associated with the higher possibilities of PE development in human.
Pregnancy arouses physiological reshape of the cardiovascular system to help the organism keep usual blood pressure and normal organ perfusion, in case of facing the elevated maternal extracellular fluid volume [86, 87]. During pregnancy, notably increased levels of vasoactive hormones, particularly ANG II, are able to increase peripheral resistance, blood pressure, vasoconstriction, and sodium retention. Nevertheless, such effects usually are countered by up-regulating the generation of endothelium-derived relaxing factors, primarily nitric oxide (NO) [88]. Moreover, due to the effects caused by ANG II, the arterial vasculature during gestation turns refractory to vasoconstriction [89], despite the possible molecular mechanisms involved remain elusive yet. Having said that, flaws in such compensatory mechanisms, thus, may result in PE and hypertension during female pregnancy [90, 91].
RGS2 have protective effects on compensatory mechanisms that hold blood pressure steady during gestational period, which could be impeded in PE. By activating the AT1 receptors coupled with Gq, ANG II can trigger vasoconstriction that potentiates the activity or expression of RGS2 or other Gq adjusting proteins offering possibly adaptive mechanisms that usually hold blood pressure steady during pregnancy. The pathophysiology of PE may be derived from the impairment of such adaptive mechanisms. Recent studies have clarified the G protein signaling pathways that are related to blood pressure modulation in preeclamptic females and normotensive pregnant females, and have confirmed that, the Rgs2 SNP (rs4606) located in the 3’-UTR is connected with progression and risk in PE [79]. Further evidence has shown that women carrying this RGS2 SNP and suffering PE have the increased probability of developing hypertension after delivery [92]. More recently, work from our group elucidates that RGS2 regulates trophoblast cells epithelial-mesenchymal transition (EMT) in human placenta, and dysregulation of RGS2 is closely associated with hypoxia that contributes to PE [93, 94]. That being said, in order to discover how G protein signaling is precisely controlled and functionally remodeled during gestation, more work is in need to answer such questions as: 1) whether RGS2 is a significant component of this control mechanism? And: 2) whether RGS2 may provide with a promising therapeutic target for PE in clinic?
Postpartum depression
Postpartum depression (PPD) is a frequent and serious psychological health disease that is associated with pregnant women’s pain and has lots of negative consequences for descendants. The half year after delivery is considered as a high-risk period for depression, with the prevalence rate from 13 to 19% [95]. Current research on antidepressant therapy and pathogenesis of depression is focused on the depression-like behavior phenotype [96], and some researchers have discovered that the mice with depression-like behavior have a high level of Rgs2 expression [97]. In contrast, down-regulation of Rgs2 relieved mouse cognitive impairment, and consistently, siRNA-mediated knockdown of Rgs2 reinforced the 5-hydroxytryptamine (5-HT) levels in hippocampal CA1 neurons. Tryptophan is the source of 5-HT, which is synthesized both centrally and systemically [98], while 5-HT is recognized as one of the significant neurotransmitters in the pathogenesis of postpartum depression. Besides, it is reported that 5-HT receptors are underlying targets for treatment of cognitive impairment in senescence [99], and consistently, activity of cAMP pathway is elevated upon silencing of RGS2 [99]. This mechanism can prevent cognitive impairment in mice with depressive behavior and enhance the regeneration of hippocampal neurons [99]. Consequently, silencing of RGS2 decreases oxidative stress injury and inflammation, but increases 5-HT concentration and cAMP pathway activity, thereby alleviating the cognitive impairment and neuronal damage in mice with depression-like behaviors. From this point of view, it can be suspected that RGS2 may play a role in the pathogenesis of PPD. However, the mechanisms are not fully clear yet, which is worth further investigation.
Breast cancer
Although breast cancer (BC) is not belonging to diseases in gynaecology and obstetrics, it is one the most frequently diagnosed life-threatening cancer in women. We thereby herein also discuss the potential relationship between RGS and BC.
Now, it is well known that maternal BC can have significant effects on both the pregnant woman and the newborn due to the breastfeeding after newborn deliveries. BC is a terrible female disease with the high morbidity and mortality, which seriously affects the quality of women's life. A previous study reported that miR-183-5p could worsen BC progression through regulating RGS2. Moreover, RGS16, belonging to the same family, is indicated to be associated with BC, and RGS16 is considered as a potential susceptibility gene in BC [100]. In a large number of BC, it has been shown that the allelic imbalance rate at the RGS16 locus (1q25.3) is about 50%. Chromosomal breakpoints are predominantly represented by microdeletions in the RGS16 promoter region, and the RGS16 promoter is methylated in 10% of these tumors. In addition, almost two-thirds (∼67%) of the tumors with this mutation lead to the reduction in RGS16 expression [101]. It is therefore very likely that RGS16 is closely related to the occurrence and development of BC during pregnancy and childbirth, with potentially corresponding values in the future.
Pulmonary hypaoplasia
Gestational disorders during pregnancy in maternal body would give rise to neonatal healthy problems, such as pulmonary hypoplasia, and some previous reports have found that RGS2 is also involved in the growth and development of neonatal lung.
To ensure the fetus to develop and grow well outside the body during childbirth, the neonatal lungs must provide sufficient volume and surface area for gas exchange. During all stages of lung growth and development, the dynamic activity of fetal respiratory movement and the accumulation of pulmonary fluid are the top priorities. RGS2 suppresses the signals such as duration and amplitude, which are modulated by Gq-coupled GPCRs [102,103,104]. Intriguingly, some Gq-coupled GPCRs and their corresponding ligands are significantly actuated of pulmonary fibrosis, such as lysophosphatidic acid receptor 1, protease-activated receptor-1 (PAR1), and endothelin receptors [105,106,107,108]. Repression of these signals by RGS2 that is up-regulated by pirfenidone (PFD) thus provides with a potentially mechanistic argument for the useful effects of both PFD and RGS2 in terms of decreasing fibrotic reacts of lung fibroblasts. As a matter of fact, when thrombin is contacted with human fetal lung fibroblast 1 (HFL1) cells, a protease is increased in bronchial alveolar lavage fluid of idiopathic pulmonary fibrosis (IPF) patients [109], while direct up-regulation of RGS2 expression to the levels comparable to those increased by PFD treatment can cause a series of anti-fibrotic responses in human lung HFL1 cells. Therefore, discontinued PFD treatment or RGS2 inhibition can activate the thrombin-induced differentiation and proliferation of HFL1 cells, indicating that HFL1 cells are a key component of IPF [110]. In contrast, PFD treatment or overexpression of RGS2 restrains the thrombin-induced collagen production, connective tissue growth factor (CTGF) expression, and gel shrinkage as well. Moreover, overexpression of RGS2 significantly reduces thrombin-induced intracellular Ca2+ transportation [110], whereas the up-regulated intracellular Ca2+ promotes the proliferation and differentiation of fibroblasts, induces fibroblast-to-myofibroblasts conversion [111], leads to apoptosis of type II lung cells [112], and ultimately gives rise to the formation of pulmonary fibrosis. To sum up, despite that the relevant mechanism is not clear and needs further research and demonstration, it can be naturally speculated that a premature infant may be short of RGS2 expression in pregnant women, leading to the occurrence of pulmonary hypoplasia during pregnancy.
Other diseases associated with women’s health
As an important signaling protein, RGS2 is closely related to the occurrence and development of obstetrics and gynecology diseases. In addition to the research reports on diseases mentioned above (Table 2), RGS2 is also found to play an important role in other systemic diseases, such as prostate cancer and cardiac hypertrophy [113], where RGS2 expression levels are dysregulated. Moreover, up-regulated expression of RGS2 causes reduced insulin signaling in human endothelial cell lines, which is associated with poorly controlled diabetes [114], suggesting RGS2 expression would be also linked to gestational diabetes mellitus, known as one of the most common medical complications of pregnancy. Recent researches have also indicated that gastric cancer and colorectal cancer are related to RGS2. A large amount of RGS2 is deposited in the matrix of gastric cancer [115] and the down-regulation of RGS2 is involved in the metastasis of colorectal cancer [116], indicating the possibility of RGS2 as a therapeutic target in cancer. These abdominal malignant tumors have the potential to metastasize and infiltrate the female reproductive system, thus affecting women’s health consequently. Lately, some studies demonstrate that the dynamic regulation of RGS2 is a potential mechanism for thyroid hormone regulation, which is the main cause of high incidence of thyroid sarcoidosis in women [117, 118], reinforcing the notion that RGS2 expression is strictly and precisely controlled depending on the specific context within human body. Therefore, continuously in-depth work on RGS2 protein is in need to fully figure out the function and molecular mechanisms underlying RGS2 regulation in different diseases by studies as well as experiments in vitro.
Conclusion and perspective
Owing to the efforts in past decades, we have obtained a better understanding on RGS2 and its role in diseases in obstetrics and gynecology. Given that the alterations in RGS2 expression levels and/or functions consequently result in various implications involved in certain diseases, and that some small chemical molecules such as the compound (1-(5-chloro-2-hydroxyphenyl)-3-(4-(trifluoromethyl)phenyl)-1 H-1,2,4-triazol-5(4 H)-one) tested to be a Gαq-RGS2 signaling inhibitor by animal experiments [119], RGS2 is likely to be a promising drug target for human diseases in future. Thus, identifying viable small molecule drugs that target RGS2 would hopefully contribute to the intervention and treatment of related diseases.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- 5-HT:
-
5-Hydroxytryptamine
- AA:
-
Amino acid
- AChR:
-
Acetylcholine receptor
- ANG:
-
Angiotensin
- AVP:
-
Arginine vasopressin
- BC:
-
Breast cancer
- Camp:
-
Cyclic adenosine monophosphate
- CTGF:
-
Connective tissue growth factor
- DBP:
-
Diastolic blood pressure
- DEP:
-
Disheveled, EGL-10, Pleckstrin
- DNA:
-
Deoxyribonucleic acid
- EGFRs:
-
Epidermal growth factor receptors
- EMT:
-
Epithelial-mesenchymal transition
- ET-1:
-
Endothelin-1
- GAP:
-
GTPase activating protein
- GDP:
-
Guanosine diphosphate
- GPCR:
-
G protein-coupled receptor signaling
- GTP:
-
Guanosine triphosphate
- GTPase:
-
Guanosine triphosphatase
- HFL1:
-
Human fetal lung fibroblast 1
- IUGR:
-
Intrauterine growth restriction
- IPF:
-
Idiopathic pulmonary fibrosis
- LPA:
-
Lysophosphatidic acid
- mRNA:
-
Messenger RNA
- NO:
-
Nitric oxide
- ODMECs:
-
Ovarian cancer carcinoma derived endothelial cells
- PAR1:
-
Protease-activated receptor-1
- PE:
-
Preeclampsia
- PFD:
-
Pirfenidone
- PIH:
-
Pregnancy induced hypertension syndrome
- PlGF:
-
Placental growth factor
- PKB:
-
Protein kinase B
- PPD:
-
Postpartum depression
- P/C:
-
Urinary protein/creatatine
- RGS:
-
Regulator of G-protein signaling
- RGS2:
-
Regulator of G-protein signaling 2
- RNA:
-
Ribonucleic Acid
- SBP:
-
Systolic blood pressure
- sFLT-1:
-
Soluble fms-like tyrosine kinase-1
- SNP:
-
Single nucleotide polymorphism
References
Hurst JH, Hooks SB. Regulator of G-protein signaling (RGS) proteins in cancer biology. Biochem Pharmacol. 2009;78(10):1289–97.
Stewart A, Fisher RA. Introduction: G protein-coupled receptors and RGS proteins. Prog Mol Biol Transl Sci. 2015;133:1–11.
Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modula- tors and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–59.
Zheng B, De Vries L, Gist FM. Divergence of RGS proteins: evidence for the existence of six mammalian RGS subfamilies. Trends Biochem Sci. 1999;24:411–4.
Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. https://doi.org/10.1146/annurev.biochem.69.1.795.
Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 2005;1:51–66.
Heximer SP, Lim H, Bernard JL, Blumer KJ. Mechanisms governing subcellular local- ization and function of human RGS2. J Biol Chem. 2001;276:14195–203. https://doi.org/10.1074/jbc.M009942200.
Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc Natl Acad Sci USA. 1997;94:14389–93.
Nance MR, Kreutz B, Tesmer VM, Sterne-Marr R, Kozasa T, Tesmer JJ. Structuraland functional analysis of the regulator of G protein signaling 2-galphaq complex. Structure. 2013;21:438–48.
Zheng M, Mullikin H, Hester A, Czogalla B, Heidegger H, Vilsmaier T, Vattai A, Chelariu-Raicu A, Jeschke U, Trillsch F, Mahner S, Kaltofen T. Development and validation of a Novel 11-gene prognostic model for serous ovarian carcinomas based on lipid metabolism expression profile. Int J Mol Sci. 2020;21(23):9169.
Hu Y, Zheng M, Wang S, Gao L, Gou R, Liu O, Dong H, Li X, Lin B. Identification of a five-gene signature of the RGS gene family with prognostic value in ovarian cancer. Genomics. 2021;113(4):2134–44.
Wu C, Tuo Y, Hu G, Luo J. miR-183-5p aggravates breast cancer development via mediation of RGS2. Comput Math Methods Med. 2021;20(2021):9664195.
Chan KYY, Zhang C, Wong YTS, Zhang XB, Wang CC, Ng WH, Fok SP, Tang PMK, Kang W, Feng B, Poon ENY, Lee KY, Lee CK, Chen C, Leung TY, Ng MHL, To KF, Wang H, Lam HS, Ng PC, Yuen PMP, Li K, Leung AWK, Li CK, Leung KT. R4 RGS proteins suppress engraftment of human hematopoietic stem/progenitor cells by modulating SDF-1/CXCR4 signaling. Blood Adv. 2021;5(21):4380–92.
Terry KL, Schock H, Fortner Y, Husing A, Fichorova RN, Yamamoto HS, et al. A prospective evaluation of early detection biomarkers for ovarian cancer in the European EPIC cohort. Clin Cancer Res. 2016;22:4664–75.
Badgwell D, Bast RC Jr. Early detection of ovarian cancer. Dis Markers. 2007;23:397–410.
Thedrez A, Lavoue V, Dessarthe B, Daniel P, Henno S, Jaffre I, et al. A quantitative deficiency in peripheral blood Vγ9Vδ2 cells is a negative prognostic biomarker in ovarian cancer patients. PLoS ONE. 2013;8: e63322.
Ihlow J, Monjé N, Hoffmann I, Bischoff P, Sinn BV, Schmitt WD, Kunze CA, Darb-Esfahani S, Kulbe H, Braicu EI, Sehouli J, Denkert C, Horst D, Taube ET. Low expression of RGS2 promotes poor prognosis in high-grade serous ovarian cancer. Cancers. 2022;14(19):4620.
Bhola NE, Grandis JR. Crosstalk between G-protein-coupled receptors and Epidermal growth factor receptor in cancer. Front Biosci. 2008;13:1857–65.
Hurst JH, Hooks SB. Regulator of G-protein signaling (RGS) proteins in cancer biology. Biochem Pharmacol. 2009;78:1289–97.
Lambert NA, Johnston CA, Cappell SD, Kuravi S, Kimple AJ, Willard FS, et al. Regulators of G-protein signaling accelerate GPCR signaling kinetics and govern sensitivity solely by accelerating GTPase activity. Proc Nat Acad Sci. 2010;107:7066–71.
Cacan E, Kratzer JT, Cole MF, Gaucher EA. Interchanging functionality among homologous elongation factors using signatures of heterotachy. J Mol Evol. 2013;76:4–12.
Wolff DW, Xie Y, Deng C, Gatalica Z, Yang M, Wang B, et al. Epigenetic repression of regulator of G-protein signaling 2 promotes androgen-independent prostate cancer cell growth. Int J Cancer. 2012;130:1521–31.
Ali MW, Cacan E, Liu Y, Pierce JY, Creasman WT, Murph MM, et al. Transcriptional suppression, DNA methylation, and histone deacetylation of the regulator of G-protein signaling 10 (RGS10) gene in ovarian cancer cells. PLoS ONE. 2013;8: e60185.
Hurst JH, Mendpara N, Hooks SB. Regulator of G-protein signaling expression and function in ovarian cancer cell lines. Cell Mol Biol Lett. 2009;14:153–74.
Chemother J. Epigenetic regulation of RGS2 (Regulator of G-protein signaling 2) in chemoresistant ovarian cancer cells. J Chemother. 2017;29(3):173–8.
Jin KL, Pak JH, Park JY, Choi WH, Lee JY, Kim JH, et al. Expression profile of histone deacetylases 1, 2 and 3 in ovarian cancer tissues. J Gynecol Oncol. 2008;19:185–90.
Pchejetski D, Alfraidi A, Sacco K, Alshaker H, Muhammad A, Monzon L. Histone deacetylases as new therapy targets for platinum-resistant epithelial ovarian cancer. J Cancer Res Clin Oncol. 2015;142:1659–71.
Ozdemir F, Altinisik J, Karateke A, Coksuer H, Buyru N. Methylation of tumor suppressor genes in ovarian cancer. Exp Ther Med. 2012;4:1092–6.
Tian F, Yip SP, Kwong DL, Lin Z, Yang Z, Wu VW. Promoter hypermethylation of tumor suppressor genes in serum as potential biomarker for the diagnosis of nasopharyngeal carcinoma. Cancer Epidemiol. 2013;37:708–13.
Cacan E, Ali MW, Boyd NH, Hooks SB, Greer SF. Inhibition of HDAC1 and DNMT1 modulate RGS10 expression and decrease ovarian cancer chemoresistance. PLoS ONE. 2014;9: e87455.
Cacan E. Histone deacetylase-1-mediated suppression of FAS in chemoresistant ovarian cancer cells. Anticancer Res. 2016;36:2819–26.
Li M, Balch C, Montgomery JS, Jeong M, Chung JH, Yan P, et al. Integrated analysis of DNA methylation and gene expression reveals specific signaling pathways associated with platinum resistance in ovarian cancer. BMC Med Genomics. 2009;2:34.
Jin B, Li Y, Robertson KD. DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer. 2011;2:607–17.
Hooks SB, Callihan P, Altman MK, Hurst JH, Ali MW, Murph MM. Regulators of G-protein signaling RGS10 and RGS17 regulate chemoresistance in ovarian cancer cells. Mol Cancer. 2010;9:289.
Wang D, Xu Y, Feng L, Yin P, Song SS, Wu F, Yan P, Liang Z. RGS5 decreases the proliferation of human ovarian carcinoma-derived primary endothelial cells through the MAPK/ERK signaling pathway in hypoxia. Oncol Rep. 2019;41(1):165–77.
Nilsson UK, Grenegard M, Berg G, Svensson SP. Different pro- liferative responses of Gi/o-protein-coupled receptors in human myometrial smooth muscle cells: a possible role of calcium. J Mol Neurosci. 1998;11:11–21.
Fuchs AR, Periyasamy S, Alexandrova M, Soloff MS. Correlation between oxytocin receptor concentration and responsiveness to oxytocin in pregnant rat myometrium: effects of ovarian steroids. Endocrinology. 1983;113:742–9.
Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734–41.
Brenninkmeijer CB, Price SA, Lopez Bernal A, Phaneuf S. Ex- pression of G-protein-coupled receptor kinases in pregnant term and nonpregnant human myometrium. J Endocrinol. 1999;162:401–8.
Europe-Finner GN, Phaneuf S, Watson SP, LopezBernal A. Identification and expression of G-proteins in human myometrium: up-regulation of Gs in pregnancy. Endocrinology. 1993;132:2484–90.
Europe-Finner GN, Phaneuf S, Tolkovsky AM, Watson SP, Lopez BA. Down-regulation of Gs in human myometrium in term and preterm labor: a mechanism for parturition. J Clin En- docrinol Metab. 1994;79:1835–9.
Sinnarajah S, Dessauer CW, Srikumar D, Chen J, Yuen J, Yilma S, et al. RGS2 regulates signal transduction in olfactory neurons by attenuating activation of adenylyl cyclase III. Nature. 2001;409:1051–5.
Mhaouty-Kodja S, Bouet-Alard R, Limon-Boulez I, Maltier JP, Legrand C. Molecular diversity of adenylyl cyclases in human and rat myometrium: correlation with global adenylyl cyclase activity during mid- and term pregnancy. J Biol Chem. 1997;272:31100–6.
Suarez VR, Park ES, Hankins GD, Soloff MS. Expression of regulator of G protein signaling-2 in rat myometrium during pregnancy and parturition. Am J Obstet Gynecol. 2003;188(4):973–7.
ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):1.
Clark SM, Dunn HE, Hankins GD. A review of oral labetalol and nifedipine in mild to moderate hypertension in pregnancy. Semin Perinatol. 2015;39(7):548–55.
Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, et al. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol. 2005;7(4):405–11.
Kilts JD, Grocott HP, Kwatra MM. G alpha(q)-coupled receptors in human atrium function through protein kinase C epsilon and delta. J Mol Cell Cardiol. 2005;38(2):267–76.
Filmore D. It’s a GPCR world: cell-based screening assays and structural studies are fueling G-protein coupled receptors as one of the most popular classes of investigational drug targets. Mod Drug Discovery. 2004;7(11):24.
Saitoh O, Masuho I, Itoh M, Abe H, Komori K, Odagiri M. Distribution of regulator of G protein signaling 8 (RGS8) protein in the cerebellum. Cerebellum. 2003;2(2):154–60.
Patel J, McNeill E, Douglas G, Hale AB, de Bono J, Lee R, et al. RGS1 regulates myeloid cell accumulation in atherosclerosis and aortic aneurysm rupture through altered chemokine signalling. Nat Commun. 2015;6:6614.
Mittmann C, Chung CH, Hoppner G, Michalek C, Nose M, Schuler C, et al. Expression of ten RGS proteins in human myocardium: functional characterization of an upregulation of RGS4 in heart failure. Cardiovasc Res. 2002;55(4):778–86.
Saitoh O, Murata Y, Odagiri M, Itoh M, Itoh H, Misaka T, et al. Alternative splicing of RGS8 gene determines inhibitory function of receptor type-specific Gq signaling. Proc Natl Acad Sci USA. 2002;99(15):10138–43.
Sayasith K, Sirois J, Lussier JG. Expression and regulation of regulator of G-protein signaling protein-2 (RGS2) in equine and bovine follicles prior to ovulation: molecular characterization of RGS2 transactivation in bovine granulosa cells. Biol Reprod. 2014;91(6):139.
Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc Natl Acad Sci USA. 1997;94(26):14389–93.
Zhou J, Moroi K, Nishiyama M, Usui H, Seki N, Ishida J, et al. Characterization of RGS5 in regulation of G protein-coupled receptor signaling. Life Sci. 2001;68(13):1457–69.
Rodriguez-Lebron E, Liu G, Keiser M, Behlke MA, Davidson BL. Altered Purkinje cell miRNA expression and SCA1 pathogenesis. Neurobiol Dis. 2013;54:456–63.
Hague C, Bernstein LS, Ramineni S, Chen Z, Minneman KP, Hepler JR. Selective inhibition of alpha1A-adrenergic receptor signaling by RGS2 association with the receptor third intracellular loop. J Biol Chem. 2005;280(29):27289–95.
Park SE, Kim JM, Seok OH, Cho H, Wadas B, Kim SY, et al. Control of mammalian G protein signaling by N-terminal acetylation and the N-end rule pathway. Science. 2015;347(6227):1249–52.
Song D, Nishiyama M, Kimura S. Potent inhibition of angiotensin AT1 receptor signaling by RGS8: importance of the C-terminal third exon part of its RGS domain. J Recept Signal Transduct Res. 2016;36(5):478–87.
Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54(3):527–59.
Tikhonova IG, Boulegue C, Langer I, Fourmy D. Modeled structure of the whole regulator G-protein signaling-2. Biochem Biophys Res Commun. 2006;341(3):715–20.
Park IK, Klug CA, Li K, Jerabek L, Li L, Nanamori M, et al. Molecular cloning and characterization of a novel regulator of G-protein signaling from mouse hematopoietic stem cells. J Biol Chem. 2001;276(2):915–23.
Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, et al. CD4+ T- helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57(5):949–55.
Cacan E. Epigenetic regulation of RGS2 (Regulator of G-protein signaling 2) in chemoresistant ovarian cancer cells. J Chemother. 2017;29(3):173–8.
Wang J, Zhou Y, Fei X, Chen X, Zhu Z. Regulator of G-protein signaling 3 targeted by miR-126 correlates with poor prognosis in gastric cancer patients. Anticancer Drugs. 2017;28(2):161–9.
Matsuzaki N, Nishiyama M, Song D, Moroi K, Kimura S. Potent and selective inhibition of angiotensin AT1 receptor signaling by RGS2: roles of its N-terminal domain. Cell Signal. 2011;23(6):1041–9.
Dinh DT, Frauman AG, Johnston CI, Fabiani ME. Angiotensin receptors: distribution, signalling and function. Clin Sci. 2001;100(5):481–92.
Rodriguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincon J, Chavez M, et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 2001;59(6):2222–32.
Sivapalaratnam S, Basart H, Watkins NA, Maiwald S, Rendon A, Krishnan U, et al. Monocyte gene expression signature of patients with early onset coronary artery disease. PLoS ONE. 2012;7(2): e32166.
Tuomi JM, Chidiac P, Jones DL. Evidence for enhanced M3 muscarinic receptor function and sensitivity to atrial arrhythmia in the RGS2-deficient mouse. Am J Physiol Heart Circ Physiol. 2010;298(2):H554–61.
Bondjers C, Kalen M, Hellstrom M, Scheidl SJ, Abramsson A, Renner O, et al. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162(3):721–9.
Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, et al. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101(20):2382–7.
Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):10941112.
Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15(5):275–89.
Kvehaugen AS, Melien O, Holmen OL, Laivuori H, Dechend R, Staff AC. Hypertension after preeclampsia and relation to the C1114G polymorphism (rs4606) in RGS2: data from the Norwegian HUNT2 study. BMC Med Genet. 2014;15:28.
Ramos JGL, Sass N, Costa SHM. Preeclampsia. Rev Bras Ginecol Obstet. 2017;39(9):496–512.
Karppanen T, Kaartokallio T, Klemetti MM, Heinonen S, Kajantie E, Kere J, Kivinen K, Pouta A, Staff AC, Laivuori H. An RGS2 3’UTR polymorphism is associated with preeclampsia in overweight women. BMC Genet. 2016;17(1):121.
Mendelova A, Holubekova V, Grendar M, Zubor P, Svecova I, Loderer D, Snahnicanova Z, Biringer K, Danko J, Lasabova Z. Association between 3’UTR polymorphisms in genes ACVR2A, AGTR1 and RGS2 and preeclampsia. Gen Physiol Biophys. 2018;37(2):185–92.
Perschbacher KJ, Deng G, Sandgren JA, Walsh JW, Witcher PC, Sapouckey SA, Owens CE, Zhang SY, Scroggins SM, Pearson NA, Devor EJ, Sebag JA, Pierce GL, Fisher RA, Kwitek AE, Santillan DA, Gibson-Corley KN, Sigmund CD, Santillan MK, Grobe JL. Reduced mRNA expression of RGS2 (Regulator of G Protein Signaling-2) in the placenta is associated With human preeclampsia and sufficient to cause features of the disorder in mice. Hypertension. 2020;75(2):569–79.
Wong AY, Kulandavelu S, Whiteley KJ, Qu D, Langille BL, Adamson SL. Maternal cardiovascular changes during pregnancy and postpartum in mice. Am J Physiol Heart Circ Physiol. 2002;282:H918–25.
Clapp JF 3rd, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80:1469–73.
Slangen BF, Out IC, Verkeste CM, Peeters LL. Hemodynamic changes in early pregnancy in chronically instrumented, conscious rats. Am J Physiol. 1996;270:H1779–84.
Chu ZM, Beilin LJ. Nitricoxide-mediatedchangesinvascularreactivityinpregnancyin spontaneously hypertensive rats. Br J Pharmacol. 1993;110:1184–8.
Conrad KP, Morganelli PM, Brinck-Johnsen T, Colpoys MC. The renin-angiotensin system during pregnancy in chronically instrumented, conscious rats. Am J Obstet Gynecol. 1989;161:1065–72.
Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am J Physiol Renal Physiol. 2005;288:F614–25.
Friedman SA, Lubarsky SL, Ahokas RA, Nova A, Sibai BM. Preeclampsia and related disorders. Clinical aspects and relevance of endothelin and nitric oxide. Clin Perinatol. 1995;22:343–55.
Kvehaugen AS, Melien O, Holmen OL, et al. Single nucleotide polymorphisms in G protein signaling pathway genes in preeclampsia. Hypertension. 2013;61:655–61.
Paauw ND, Lely AT. Cardiovascular sequels during and after preeclampsia. Adv Exp Med Biol. 2018;1065:455–70.
Filipek A, Jurewicz E. Preeklampsja – choroba kobiet w ciąży [Preeclampsia - a disease of pregnant women]. Postepy Biochem. 2018;64(4):232–229.
Kvehaugen AS, Melien Ø, Holmen OL, Laivuori H, Dechend R, Staff AC. Hypertension after preeclampsia and relation to the C1114G polymorphism (rs4606) in RGS2: data from the Norwegian HUNT2 study. BMC Med Genet. 2014;5(15):28.
Jin M, Xu S, Li J, Yao Y, Tang C. MicroRNA-3935 promotes human trophoblast cell epithelial-mesenchymal transition through tumor necrosis factor receptor-associated factor 6/regulator of G protein signaling 2 axis. Reprod Biol Endocrinol. 2021;19(1):134.
Jin M, Xu S, Cao B, Xu Q, Yan Z, Ren Q, Lin C, Tang C*. Regulator of G protein signaling 2 is inhibited by hypoxia-inducible factor-1α/E1A binding protein P300 complex upon hypoxia in human preeclampsia. Int J Biochem Cell Biol. 2022;147:106211.
O’Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol. 2013;9:379–407.
Huang YL, Zeng NX, Chen J, Niu J, Luo WL, Liu P, Yan C, Wu LL. Dynamic changes of behaviors, dentate gyrus neurogenesis and hippocampal miR- 124 expression in rats with depression induced by chronic unpredictable mild stress. Neural Regener Res. 2020;15:1150–9.
Sahu A, Gopalakrishnan L, Gaur N, Chatterjee O, Mol P, Modi PK, Dagamajalu S, Advani J, et al. The 5-Hydroxytryptamine signaling map: an overview of serotonin-serotonin receptor mediated signaling network. J Cell Commun Signaling. 2018;12:731–5.
Mitchell ES, McDevitt RA, Neumaier JF. Adaptations in 5-HT receptor expression and function: implications for treatment of cognitive impairment in aging. J Neurosci Res. 2009;87:2803–11.
Zhu C, Hui Li, Zheng Ke, Liu L, Liu J, Lv W. Silencing of RGS2 enhances hippocampal neuron regeneration and rescues depression-like behavioral impairments through activation of cAMP pathway. Brain Res. 2020;11(1746):147018.
Wang C, Xue H, Zhao R, Sun Z, Gao X, Qi Y, Wang H, Xu J, Deng L, Li G. RGS16 regulated by let-7c-5p promotes glioma progression by activating PI3K-AKT pathway. Front Med. 2022. https://doi.org/10.1007/s11684-022-0929-y.
Wiechec E, Overgaard J, Kjeldsen E, Hansen LL. Chromosome 1q25.3 copy number alterations in primary breast cancers detected by multiplex ligation-dependent probe amplification and allelic imbalance assays and its comparison with fluorescent in situ hybridization assays. Cell Oncol. 2013;36(2):113–20.
Nakayama S, Mukae H, Sakamoto N, Kakugawa T, Yoshioka S, Soda H, et al. Pirfenidone inhibits the expression of HSP47 in TGF-beta1-stimulated human lung fibroblasts. Life Sci. 2008;82:210–7.
Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gαq function. Proc Natl Acad Sci USA. 2008;94(14389–93):23.
Bernstein LS, Ramineni S, Hague C, Cladman W, Chidiac P, Levey AI, Hepler JR. RGS2 binds directly and selectively to the M1 muscarinic acetylcholine receptor third intracellular loop to modulate Gq/11alpha signaling. J Biol Chem. 2004;279:21248–56.
Shahar I, Fireman E, Topilsky M, Grief J, Schwarz Y, Kivity S, et al. Effect of endothelin-1 on alpha-smooth muscle actin expression and on alveolar fibroblasts proliferation in interstitial lung diseases. Int J Immunopharmacol. 1999;21:759–75.
Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54.
Königshoff M, Dumitrascu R, Udalov S, Amarie OV, Reiter R, Grimminger F, et al. Increased expression of 5-hydroxytryptamine2A/B receptors in idiopathic pulmonary fibrosis: a rationale for therapeutic intervention. Thorax. 2010;65:949–55.
José RJ, Williams AE, Chambers RC. Proteinase-activated receptors in fibroproliferative lung disease. Thorax. 2014;69:190–2.
Kimura M, Tani K, Miyata J, Sato K, Hayashi A, Otsuka S, et al. The significance of cathepsins, thrombin and aminopeptidase in diffuse interstitial lung diseases. J Med Invest. 2005;52:93–100.
Xie Y, Jiang H, Zhang Q, et al. Upregulation of RGS2: a new mechanism for pirfenidone amelioration of pulmonary fibrosis. Respir Res. 2016;17(1):103.
Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, Paya CV. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169(10):5546–54.
Winters CJ, Koval O, Murthy S, Allamargot C, Sebag SC, Paschke JD, et al. CaMKII inhibition in type II pneumocytes protects from bleomycin-induced pulmonary fibrosis by preventing Ca2+-dependent apoptosis. Am J Physiol Lung Cell Mol Physiol. 2016;310(1):L86-94.
Yang S, Sun B, Li W, Yang H, Li N, Zhang X. Fatty acid metabolism is related to the immune microenvironment changes of gastric cancer and RGS2 is a new tumor biomarker. Front Immunol. 2022;14(13):1065927. https://doi.org/10.3389/fimmu.2022.1065927.
Jiang Z, Wang Z, Xu Y, Wang B, Huang W, Cai S. Analysis of RGS2 expression and prognostic significance in stage II and III colorectal cancer. Biosci Rep. 2010;30(6):383–90. https://doi.org/10.1042/BSR20090129.
Miles RR, Sluka JP, Santerre RF, Hale LV, Bloem L, Boguslawski G, Thirunavukkarasu K, Hock JM, Onyia JE. Dynamic regulation of RGS2 in bone: potential new insights into parathyroid hormone signaling mechanisms. Endocrinology. 2000;141(1):28–36. https://doi.org/10.1210/endo.141.1.7229.
Eszlinger M, Holzapfel HP, Voigt C, Arkenau C, Paschke R. RGS 2 expression is regulated by TSH and inhibits TSH receptor signaling. Eur J Endocrinol. 2004;151(3):383–90. https://doi.org/10.1530/eje.0.1510383.
Linder A, Hagberg Thulin M, Damber JE, Welén K. Analysis of regulator of G-protein signalling 2 (RGS2) expression and function during prostate cancer progression. Sci Rep. 2018;8(1):17259.
Vazquez-Jimenez JG, Corpus-Navarro MS, Rodriguez-Chavez JM, Jaramillo-Ramirez HJ, Hernandez-Aranda J, Galindo-Hernandez O, Machado-Contreras JR, Trejo-Trejo M, Guerrero-Hernandez A, Olivares-Reyes JA. The increased expression of regulator of G-protein signaling 2 (RGS2) inhibits insulin-induced Akt phosphorylation and is associated with uncontrolled glycemia in patients with type 2 diabetes. Metabolites. 2021;11(2):91.
Beladiya JV, Chaudagar KK, Mehta AA. Protective effects of Gαq-RGS2 signalling inhibitor in aminophylline induced cardiac arrhythmia. Clin Exp Pharmacol Physiol. 2019;46(11):1037–43.
Fitzgerald K, Tertyshnikova S, Moore L, Bjerke L, Burley B, Cao J, Carroll P, Choy R, Doberstein S, Dubaquie Y, Franke Y, Kopczynski J, Korswagen H, Krystek SR, Lodge NJ, Plasterk R, Starrett J, Stouch T, Thalody G, Wayne H, van der Linden A, Zhang Y, Walker SG, Cockett M, Wardwell-Swanson J, Ross-Macdonald P, Kindt RM. Chemical genetics reveals an RGS/G-protein role in the action of a compound. PLoS Genet. 2006;2(4): e57.
Acknowledgements
Not applicable.
Funding
This work was supported by Foundation for The Top-Notch Youth Talent Cultivation Project of Independent Design Project of National Clinical Research Center for Child Health (No. Q21A0006), the Starting Research Foundation of The Children’s Hospital, Zhejiang University School of Medicine (No. 481), and the National Natural Science Foundation of China (No. 31801207) to C. Tang.
Author information
Authors and Affiliations
Contributions
QX wrote the original manuscript and prepared the figures and table. MY edited the manuscript. CT designed and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The author declares that all work described here has not been published before and that its publication has been approved by all co-authors.
Competing interests
The authors declare no potential conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, Q., Yao, M. & Tang, C. RGS2 and female common diseases: a guard of women’s health. J Transl Med 21, 583 (2023). https://doi.org/10.1186/s12967-023-04462-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04462-3