Abstract
Background
Myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease (ME/CFS/SEID) is a condition diagnosed primarily based on clinical symptoms, including prolonged fatigue and post-exertional malaise; however, there is no specific test for the disease. Additionally, diagnosis can be challenging since healthcare professionals may lack sufficient knowledge about the disease. Prior studies have shown that patients with ME/CFS/SEID have low serum acylcarnitine levels, which may serve as a surrogate test for patients suspected of having this disease. This systematic review and meta-analysis aimed to investigate the differences in serum acylcarnitine levels between patients with ME/CFS/SEID and healthy controls.
Methods
This systematic review was conducted using PubMed and Ichushi-Web databases. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, we included all studies from the databases’ inception until February 17, 2023, that evaluated blood tests in both patients with ME/CFS/SEID and healthy control groups. The primary endpoint was the difference in serum acylcarnitine levels between the two groups.
Results
The electronic search identified 276 studies. Among them, seven met the eligibility criteria. The serum acylcarnitine levels were analyzed in 403 patients with ME/CFS/SEID. The patient group had significantly lower serum acylcarnitine levels when compared with the control group, and the statistical heterogeneity was high.
Conclusion
The patient group had significantly lower serum acylcarnitine levels when compared with the control group. In the future, the measurement of serum acylcarnitine levels, in addition to clinical symptoms, may prove to be a valuable diagnostic tool for this condition.
Similar content being viewed by others
Background
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a chronic disease characterized by a broad range of symptoms, including central nervous system abnormalities, gastrointestinal dysfunction, morbid fatigue, muscle pain, and cognitive impairment that does not improve with adequate rest [1]. Despite extensive research, the pathogenesis of ME/CFS is not yet understood, and there are no specific tests or biological markers for its diagnosis [2]. Currently, the Centers for Disease Control-1994/Fukuda criteria are often used to diagnose ME/CFS [3]. However, approximately 80% of ME/CFS cases are not correctly diagnosed. Patients with ME/CFS are often not diagnosed with ME/CFS and are referred to medical facilities with indefinite complaints [4]. Therefore, it takes longer to identify patients with ME/CFS and their symptoms worsen [5].
Despite some existing research on ME/CFS, many healthcare professionals may still lack awareness of the disease [6]. However, the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has brought greater attention to ME/CFS [7]. Typical acute phase symptoms of COVID-19 include fever, cough, and smell and taste disorders [8]. Notably, many patients experience COVID-19 sequelae long after they have recovered from the peak of the disease than from the acute phase symptoms of COVID-19. Typical sequelae such as post-exertional malaise (PEM), unrefreshing sleep, and orthostatic intolerance are considered the clinical symptoms of ME/CFS [5]. In 2015, the National Academy of Medicine developed diagnostic criteria for ME/CFS and proposed a new umbrella term, systemic exertion intolerance disease (SEID), to replace ME/CFS [5, 9]. The current diagnostic criteria for ME/CFS/SEID are based solely on symptoms. However, the diagnosis of the disease will become less challenging as more specific blood tests and imaging findings for the disease become available in the future.
In this study, we collected and reviewed articles in which serum acylcarnitine, free carnitine, and total carnitine levels were measured in patients with ME/CFS/SEID. In our previous case report on post-COVID-19 ME/CFS/SEID, we speculated that viral infection may have adversely affected energy metabolism in muscular tissue, resulting in decreased serum acylcarnitine levels [10]. Against this background, the measurement of serum acylcarnitine levels may be useful for the diagnosis of ME/CFS/SEID. Therefore, we identified studies that included blood tests for patients diagnosed with ME/CFS/SEID. Next, we analyzed serum acylcarnitine, free carnitine, and total carnitine levels in patients with ME/CFS/SEID and in healthy controls. Finally, we conducted a systematic review and meta-analysis to evaluate their usefulness for future ME/CFS/SEID diagnosis.
Methods
Protocol registration
This systematic review and meta-analysis was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Additional file 1: Table S1) [11]. Our study protocol was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) on March 1, 2023 (UMIN-CTR: UMIN000050465; URL: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000057475).
Eligibility criteria
The inclusion criteria for our systematic review were as follows: (1) systematic reviews and prospective and retrospective studies of patients with ME/CFS/SEID, (2) articles published in Japanese or English, and (3) articles searched using PubMed (MEDLINE) and Ichushi-Web (NPO Japan Medical Abstracts Society) on February 17, 2023.
The exclusion criteria for our systematic review were as follows: (1) guidelines, narrative reviews, case reports, and conference presentations on ME/CFS/SEID, (2) studies that did not measure serum acylcarnitine, free carnitine, or serum total carnitine levels, and (3) articles published in languages other than Japanese or English.
Literature search strategy
This systematic review was conducted using the databases PubMed and Ichushi-Web. The search included articles available from the inception of the databases up to February 17, 2023. The following electronic search terms were used to retrieve the literature in PubMed: (“Carnitine Acyltransferases”[MeSH] OR “acylcarnitine”[tiab] OR “COVID-19”[tiab] OR “COVID-19”[MeSH] OR “COVID-19 Vaccines”[tiab] OR “COVID-19 Vaccines”[MeSH] OR “COVID-19 serotherapy”[tiab] OR “COVID-19 serotherapy”[tiab] OR “COVID-19 Nucleic Acid Testing”[tiab] OR “covid-19 nucleic acid testing”[MeSH] OR “COVID-19 Serological Testing”[tiab] OR “covid-19 serological testing”[MeSH] OR “COVID-19 Testing”[tiab] OR “covid-19 testing”[MeSH] OR “SARS-CoV-2”[tiab] OR “sars-cov-2”[MeSH] OR “Severe Acute Respiratory Syndrome Coronavirus 2”[tiab] OR "NCOV”[tiab] OR “2019 NCOV”[tiab]) AND (“fatigue syndrome, chronic”[MeSH] OR chronic fatigue syndrome[tiab] OR myalgic encephalomyelitis[tiab] OR systemic exertion intolerance disease[tiab]). In addition, an electronic search strategy was created on Ichushi-Web based on the aforementioned search terms.
Literature selection
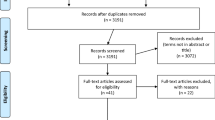
Figure 1 presents a flowchart of the literature collection process. First, we searched the literature on COVID-19, ME/CFS/SEID, and serum acylcarnitine levels, resulting in 272 studies from PubMed and five studies from Ichushi-Web. Out of the 276 articles initially identified, 259 were excluded during primary screening due to unrelated titles and abstracts to ME/CFS/SEID, and one duplicate article was removed. Next, 17 studies were subjected to secondary screening, and a systematic review was conducted on nine datasets extracted from seven studies [12,13,14,15,16,17,18]. None of the studies were extracted from other sources.
Data extraction and quality assessment
A data extraction Excel sheet was created and included the following data: study design, title, first author, publication journal, publication year, number of patients with ME/CFS/SEID, sex, age, and blood test findings such as serum total carnitine levels, free carnitine levels, and acylcarnitine levels. The Newcastle–Ottawa Scale (NOS) was used for quality assessment (Additional file 2: Table S2).
The ME/CFS/SEID diagnostic criteria
The following ME/CFS/SEID diagnostic criteria were used in the seven studies included in this systematic review: the diagnostic criteria proposed by Holmes et al. [19] Kitani et al. [20] Fukuda et al. [3] and the Working Group of the Royal Australasian College of Physicians [21].
Definition of primary and secondary endpoints
The primary endpoint was the difference in serum acylcarnitine levels between the patients with ME/CFS/SEID and healthy controls. As secondary endpoints, differences in serum total and free carnitine levels were examined.
Data synthesis and statistical analysis
We compiled an Excel sheet with the number of participants in each study, the mean and standard deviation (SD) of serum acylcarnitine levels of each study’s patient group, and the mean and SD of the serum acylcarnitine levels of each study’s control group. The differences in serum acylcarnitine levels between the two groups were calculated, and forest plots were generated based on a random-effects model to account for interstudy bias. The results were expressed as 95% confidence intervals (CI) for the difference in serum acylcarnitine levels between the two groups, and the statistical heterogeneity was assessed by Breslow (τ2), Higgins (I2), and Birge’s ratio (H2) indices. The I2 statistics were classified as < 30%, 30–60%, 61–75%, and > 75% with low, moderate, and high heterogeneity, respectively. In our study, the H2 statistics were classified as < 40%, 30–60%, 50–90%, and 75–100%, and were defined as low heterogeneity, moderate heterogeneity, possibly high heterogeneity, and high heterogeneity, respectively. Publication bias was tested by examining funnel plot. Statistical significance was set at p < 0.05. All analyses were performed using STATA® version 17 software (StataCorp, College Station, TX, USA).
Results
Characteristics of this systematic review
The initial search identified 276 studies, excluding one duplicate. Following screening against our inclusion and exclusion criteria, 17 studies remained. Of these, seven were ultimately included in the meta-analysis [12,13,14,15,16,17,18]. Among the seven studies included, one [15] provided information on both Swedish and Japanese patients. Data from Japanese and Swedish patients are reported separately (Table 1). These studies involved a total of 403 patients with ME/CFS/SEID and 893 healthy controls, and were published between 1992 and 2010, with most of them being conducted in Japan. In addition, all seven studies were retrospective in nature. The mean overall quality score on the NOS for the included studies was 6.9 (SD, 0.4) (Additional file 2: Table S2).
Outcomes of differences in serum carnitine levels between the ME/CFS/SEID patient group and the control group
In our study, serum acylcarnitine levels were obtained from 403 patients with ME/CFS/SEID. Among them, we compared the serum acylcarnitine levels of 365 of these patients with those of healthy controls. Table 2 shows the serum total carnitine levels and free carnitine levels between the ME/CFS/SEID patient group and the control group. Nine serum total carnitine and free carnitine datasets were collected from the seven studies included in the meta-analysis [12,13,14,15,16,17,18]. Although some values were missing, the majority of studies did not demonstrate a significant difference in serum total carnitine and free carnitine levels between the two groups. As presented in Table 3, serum acylcarnitine levels from the ME/CFS/SEID patient group and the control group were collected from 11 datasets from the seven studies included in the meta-analysis [12,13,14,15,16,17,18]. Two of these datasets had missing values, and forest plots were created from the remaining nine datasets. Based on the analysis of these nine datasets, our study found that the ME/CFS/SEID patient group had significantly lower serum acylcarnitine levels when compared with the healthy control group (Δ = − 0.69; [95% CI − 1.09, − 0.30]), with a high statistical heterogeneity of 86.5% (Fig. 2).
Forest plot for serum acylcarnitine levels between the ME/CFS/SEID patient group and the control group. The ME/CFS/SEID patient group had significantly lower serum acylcarnitine levels when compared with the control group (Δ = − 0.69; [95% CI − 1.09, − 0.30]), with a high heterogeneity. ME myalgic encephalomyelitis, CFS chronic fatigue syndrome, SEID systemic exertion intolerance disease, CI confidence interval, N number, SD standard deviation
Publication bias
No publication bias was found from the funnel plot of studies comparing serum acylcarnitine levels in the ME/CFS/SEID patient group and healthy control group (Fig. 3).
Discussion
CFS is a syndrome characterized by chronic unexplained fatigue and muscular pain; however, its cause remains unknown. In 1988, Holmes et al. proposed CFS as the new name for chronic Epstein–Barr virus syndrome, [19] which led to further studies of the disease by Kitani et al. [20] Fukuda et al. [3] and the Working Group of the Royal Australasian College of Physicians [21]. However, its diagnosis is difficult because of the variety of clinical symptoms and the lack of specific tests. Moreover, healthcare professionals often have limited knowledge of the disease, as the pathophysiology of ME/CFS/SEID is not typically taught thoroughly in medical education. This lack of knowledge contributes to the low diagnosis rate, with only approximately 20% of patients with this disease being correctly diagnosed [4, 22].
In recent years, this disease has been increasingly reported as a sequela of COVID-19 and is gradually being recognized [8]. We have previously reported a case of the disease after recovery from COVID-19 [10]. In addition to respiratory symptoms, COVID-19 sequelae present with a variety of endocrine and metabolic disorders such as thyroid dysfunction and diabetes mellitus, gastrointestinal symptoms such as abdominal pain and diarrhea, skin symptoms such as alopecia and dermatitis, and extreme muscular fatigue and general malaise related to the disease [23].
In the course of researching mental health problems post-COVID-19, we realized that many patients have psychiatric disorders such as depression and anxiety due to incorrect diagnosis and treatment of ME/CFS/SEID, which led us to this systematic review, in the hope of helping to solve these problems. The cause of this disease has been suggested to be a type of viral infection, and the diagnostic criteria specify unexplained fatigue for more than 6 months, PEM, and unrefreshing sleep as core symptoms [8]. In 2015, the National Academy of Medicine proposed SEID as the new name to replace ME/CFS [5].
After a thorough review of the literature on ME, CFS, and SEID reported to date, we hypothesize that low serum acylcarnitine levels in patients with ME/CFS/SEID may be due to an impaired mitochondrial fatty acid oxidation cascade caused by viral infections, including COVID-19 [24, 25]. Normally, in the mitochondrial matrix, fatty acids taken up by cells undergo β-oxidation to produce ATP. In addition, long-chain fatty acids are converted to acyl-CoA in cells, which reacts with carnitine to produce acylcarnitine [26]. Carnitine is an essential factor in the aforementioned cascade.
Kuratsune et al. studied acylcarnitine and carnitine levels in patients with ME/CFS/SEID. The results showed that acylcarnitine levels were lower in patients with ME/CFS/SEID and that lower acylcarnitine levels may correlate with fatigue levels. It was then considered that patients with ME/CFS/SEID may exhibit symptoms such as general malaise and fatigue due to decreased brain uptake of acylcarnitine and consequently decreased synthesis of neurotransmitters such as gamma-aminobutyric acid and glutamate [12,13,14,15, 25].
We encountered a case in which we were able to accurately diagnose this disease based on literature [10]. At present, the relationship between ME/CFS/SEID and serum acylcarnitine levels remains unclear; however, we hope that the measurement of serum acylcarnitine levels will be useful in the future. Measurement of serum acylcarnitine levels is a minimally invasive and reliable test that can be widely used without radiation exposure or high costs. Based on our systematic review and meta-analysis, ME/CFS/SEID should be suspected in patients presenting with subjective symptoms of long-term unexplained chronic fatigue, PEM, unrefreshing sleep, cognitive impairment, and orthostatic intolerance. Measurement of serum acylcarnitine levels may assist in the diagnosis.
This study had some limitations. First, the ME/CFS/SEID diagnostic criteria were not standardized in each study [3, 19,20,21]. Second, the sample size of each study was limited, and no studies related to this topic has been published in recent years. Third, there was some bias in the nationalities, sex, and body size of the patients with ME/CFS/SEID in the seven retrospective studies included in this meta-analysis. Finally, the method used to measure serum acylcarnitine levels differed slightly between the studies.
Overall, our systematic review and meta-analysis suggest the utility of measuring serum acylcarnitine levels in patients with ME/CFS/SEID. Further studies comparing and examining laboratory findings, including serum acylcarnitine levels, in patients with ME/CFS/SEID and in healthy controls may contribute to more patients being correctly diagnosed with ME/CFS/SEID.
Conclusion
In cases where ME/CFS/SEID is suspected based on clinical symptoms, the measurement of serum acylcarnitine levels may contribute to a more definitive diagnosis of the disease. In addition, the blood test is highly versatile with extremely low invasiveness and may become more widely used in the future.
Data availability statement
The data that support the findings of this study are available from the corresponding authors (RJ) upon reasonable request.
Abbreviations
- ME:
-
Myalgic encephalomyelitis
- CFS:
-
Chronic fatigue syndrome
- PEM:
-
Post-exertional malaise
- SEID:
-
Systemic exertion intolerance disease
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- NOS:
-
Newcastle–ottawa scale
- SD:
-
Standard deviation
- CI:
-
Confidence interval
References
Baraniuk JN, Kern G, Narayan V, Cheema A. Exercise modifies glutamate and other metabolic biomarkers in cerebrospinal fluid from Gulf War illness and myalgic encephalomyelitis/chronic fatigue syndrome. PLoS ONE. 2021;16:e0244116.
Silverman RH, Nguyen C, Weight CJ, Klein EA. The human retrovirus XMRV in prostate cancer and chronic fatigue syndrome. Nat Rev Urol. 2010;7:392–402.
Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International chronic fatigue syndrome study group. Ann Intern Med. 1994;121:953–9.
Bested AC, Marshall LM. Review of myalgic encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30:223–49.
Committee on the diagnostic criteria for myalgic encephalomyelitis/chronic fatigue syndrome, board on the health of select populations, Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. National Academies Press; 2015
Lim EJ, Son CG. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. 2020;18:289.
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33.
Carod-Artal FJ. Post-COVID-19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev Neurol. 2021;72:384–96.
Asprusten TT, Sulheim D, Fagermoen E, Winger A, Skovlund E, Wyller VB. Systemic exertion intolerance disease diagnostic criteria applied on an adolescent chronic fatigue syndrome cohort: evaluation of subgroup differences and prognostic utility. BMJ Paediatr Open. 2018;2:e000233.
Jinushi R, Nishiguchi S, Masuda S, Sasaki A, Koizumi K, Ryozawa S. A case of post-COVID-19 myalgic encephalomyelitis/chronic fatigue syndrome characterized by post-exertional malaise and low serum acylcarnitine level. Clin Case Rep. 2023;11:e6930.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Kuratsune H, Yamaguti K, Hattori H, Tazawa H, Takahashi M, Yamanishi K, et al. Symptoms, signs and laboratory findings in patients with chronic fatigue syndrome. Nihon Rinsho. 1992;50:2665–72.
Kuratsune H, Yamaguti K, Takahashi M, Misaki H, Tagawa S, Kitani T. Acylcarnitine deficiency in chronic fatigue syndrome. Clin Infect Dis. 1994;18(Suppl 1):S62–7.
Plioplys AV, Plioplys S. Serum levels of carnitine in chronic fatigue syndrome: clinical correlates. Neuropsychobiology. 1995;32:132–8.
Kuratsune H, Yamaguti K, Lindh G, Evengard B, Takahashi M, Machii T, et al. Low levels of serum acylcarnitine in chronic fatigue syndrome and chronic hepatitis type C, but not seen in other diseases. Int J Mol Med. 1998;2:51–6.
Soetekouw PM, Wevers RA, Vreken P, Elving LD, Janssen AJ, van der Veen Y, et al. Normal carnitine levels in patients with chronic fatigue syndrome. Neth J Med. 2000;57:20–4.
Jones MG, Goodwin CS, Amjad S, Chalmers RA. Plasma and urinary carnitine and acylcarnitines in chronic fatigue syndrome. Clin Chim Acta. 2005;360:173–7.
Reuter SE, Evans AM. Long-chain acylcarnitine deficiency in patients with chronic fatigue syndrome. Potential involvement of altered carnitine palmitoyltransferase-I activity. J Intern Med. 2011;270:76–84.
Holmes GP, Kaplan JE, Gantz NM, Komaroff AL, Schonberger LB, Straus SE, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108:387–9.
Kitani T, Kuratsune H. Chronic fatigue syndrome. Nippon Naika Gakkai Zasshi. 1992;81:573–82.
Working Group of the Royal Australasian College of Physicians. Chronic fatigue syndrome clinical practice guidelines–2002 clinical practice guidelines. Med J Aust. 2002;176:S17–55.
Solomon L, Reeves WC. Factors influencing the diagnosis of chronic fatigue syndrome. Arch Intern Med. 2004;164:2241–5.
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–15.
Piccoli C, Quarato G, Ripoli M, D’Aprile A, Scrima R, Cela O, et al. HCV infection induces mitochondrial bioenergetic unbalance: causes and effects. Biochim Biophys Acta. 2009;1787:539–46.
De Simone C, Famularo G, Kuratsune H, Yamaguti K, Watanabe Y, Takahashi M, et al. Acylcarnitine and chronic fatigue syndrome. In: De Simone C, Famularo G, editors., et al., Carnitine today. Austin: RG Landes Company; 1997.
Miwa S, Takikawa O. Chronic fatigue syndrome and neurotransmitters. Nihon Rinsho. 2007;65:1005–10.
Acknowledgements
Support for compiling the manuscript based on the authors’ detailed instructions was provided by Editage (www.editage.com), a division of Cactus Communications.
Funding
No financial grants or any other funding were received for this study. We thank the study participants for their invaluable contributions to this project.
Author information
Authors and Affiliations
Contributions
Study conception and design: RJ. Acquisition of data: RJ and SM. Analysis and interpretation of data: RJ. Drafting of the manuscript: RJ. Critical revisions of the manuscript: RJ, SM, YT, SN, KS, RS, KS, TS, RS, AF, MM, and SR.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. PRISMA 2020 statement
Additional file 2
: Table S2. NOS for assessing the quality of retrospective studies included in the systematic review
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jinushi, R., Masuda, S., Tanisaka, Y. et al. Comparison of serum acylcarnitine levels in patients with myalgic encephalomyelitis/chronic fatigue syndrome and healthy controls: a systematic review and meta-analysis. J Transl Med 21, 398 (2023). https://doi.org/10.1186/s12967-023-04226-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04226-z