Abstract
Background
Multiple preclinical studies have reported a beneficial effect of extracellular vesicles (EVs), especially mesenchymal stem cells derived EVs (MSC-EVs), in the treatment of sepsis. However, the therapeutic effect of EVs is still not universally recognized. Therefore, we conducted this meta-analysis by summarizing data from all published studies that met certain criteria to systematically review the association between EVs treatment and mortality in animal models of sepsis.
Methods
Systematic retrieval of all studies in PubMed, Cochrane and Web of Science that reported the effects of EVs on sepsis models up to September 2022. The primary outcome was animal mortality. After screening the eligible articles according to inclusion and exclusion criteria, the inverse variance method of fixed effect model was used to calculate the joint odds ratio (OR) and 95% confidence interval (CI). Meta-analysis was performed by RevMan version 5.4.
Results
In total, 17 studies met the inclusion criteria. Meta-analysis of those studies showed that EVs treatment was associated with reduced mortality in animal models of sepsis (OR 0.17 95% CI: 0.11,0.26, P < 0.001). Further subgroup analysis showed that the mode of sepsis induction, the source, dose, time and method of injection, and the species and gender of mice had no significant effect on the therapeutic effect of EVs.
Conclusion
This meta-analysis showed that MSC-EVs treatment may be associated with lower mortality in animal models of sepsis. Subsequent preclinical studies will need to address the standardization of dose, source, and timing of EVs to provide comparable data. In addition, the effectiveness of EVs in treating sepsis must be studied in large animal studies to provide important clues for human clinical trials.
Similar content being viewed by others
Background
Sepsis, caused by an abnormal host response to infection, is a life-threatening organ dysfunction [1]. According to an epidemiological analysis by Kristina E et al., there were 48.9 million cases of sepsis worldwide in 2017, of which 11 million resulted in patient deaths, accounting for about 20% of total global deaths [2]. The treatment of sepsis mainly involves eliminating the source of infection, suppressing the inflammatory response, and following with corresponding symptomatic and supportive treatment [3]. A potential treatment that can help reduce the massive inflammatory process, tissue damage is still lacking. Therefore, it is urgent to find new therapeutic methods to improve the clinical outcome of patients with sepsis.
Mesenchymal stem cells (MSCs) have attracted interest in the past few years based on advantages such as differentiation potential, self-renewal and self-secretion [4]. MSCs has been clinically studied as a therapeutic agent in a variety of diseases such as diabetes, Alzheimer's disease and Osteoarthritis[5,6,7]. However, there are some challenges with the use of MSC, including low survival rate and difficult to reach injury [8]. In recent years, many preclinical studies have introduced MSC-derived extracellular vesicles into the treatment of various diseases [9,10,11]. Extracellular vesicles (EVs) can be divided into exosomes, microvesicles, and apoptotic bodies according to their diameters and can come from almost all cells, especially stem cells [12]. EVs can carry and protect specific subsets of proteins, lipids, and genetic material such as mRNA, miRNA, and DNA from the extracellular environment [4, 13]. Compared with cell therapy, EVs have the advantages of low immunogenicity, low toxicity and relative stability in the blood [14].
To date, several studies have focused on EVs for the treatment of sepsis in animals [15,16,17]. Most of these studies demonstrated that EVs treatment reduced mortality in animal models of sepsis [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]; however, individual studies have come to different conclusions [34]. In addition, there is no uniform conclusion regarding EVs with different cell sources, and varying injection doses, routes of administration, and duration of treatment have been used. Therefore, this meta-analysis was conducted to explore the efficacy of EVs in treating sepsis in animal models and to provide the latest evidence support for clinical studies.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria was used in this meta-analysis [35]. Ethics approval is not required to analyze published articles. The article provides all supporting data, and additional information is supplemented online.
Data sources and search strategies
A systematic literature review was conducted completely using three databases, including PubMed, Cochrane, and Web of Science, to screen for any in vivo studies investigating the use of EVs for sepsis. Detailed search strategies are shown in Additional file 1. The database was last supplemented on September 23, 2022. Only publications whose language is English are included.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) to evaluate the therapeutic effect of EVs on sepsis animal model; (2) the protective effect of EVs or EVs-derived molecules was the main focus of research; (3) the study involved an animal model of sepsis or endotoxemia; (4) the study reported mortality rates; and (5) The language of the research is English.
The exclusion criteria were as follows: (1) extracellular vesicles were not directly used as therapy; (2) extracellular vesicles were genetically modified; (3) There were other complications in the animal model; (4) no sepsis occurred; (5) lack of end points of interest in the study data; (6) in vitro study; (7) the study was duplicated; (8) studies published in a non-English language; and (9) no original research was performed (e.g., book chapters, reviews, editorials, meta-analysis, etc.)
Data extraction
Two independent reviewers, YS and ZK, conducted data extraction, and the differences encountered were resolved through discussion between them. The following data were collected: first author, country or region, year of publication, animal type, sex, and number, sepsis model type, origin of EVs cells, dose, injection method, injection time, observation time after EVs administration, and indicators related to the primary outcome. For studies that did not provide the required results, Engauge Digitizer version 10.8 software was used to extract data from accompanying graphs [36].
Risk of bias
Risk of bias was assessed according to the Systematic Review Center for Laboratory Animal Experimentation (SYRLE) [37]. SYRCLE includes: selection bias, performance bias, detection bias, attrition bias, reporting bias and others. The risk of bias was assessed carefully by two independent reviewers as low risk, high risk, or unclear risk based on the content of the article. Any disputes encountered during the evaluation process were resolved through discussion.
Statistical analysis
The effect size of this meta-analysis was mortality. The OR values of the EVs treatment group and control group were calculated to determine the combined effect size. The I2 statistic was used to analyze heterogeneity. I2 > 50% indicated significant heterogeneity [38]. The effect model of meta-analysis was selected according to whether the heterogeneity was significant [39]. All statistical analyses in this manuscript were performed by using fixed-effects model according to the heterogeneity test results. Visual inspection of the funnel plot was used to test whether publication bias existed [40]. Sensitivity analyses were performed by removing each study individually from the results of the meta-analysis. All statistical analyses were performed using RevMan version 5.4. A difference of P < 0.05 (two-sided) was considered statistically significant.
Results
Study inclusion
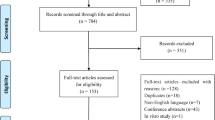
A total of 850 articles were retrieved from the three databases according to the search strategy. After the elimination of 167 duplicate articles, 553 of 683 articles were initially screened and excluded according to the title and abstract. The full text of the remaining 130 articles was reviewed, and 17 articles were selected to be included in this meta-analysis according to the inclusion and exclusion criteria [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The specific filtering steps are shown in Fig. 1.
Study characteristics
This meta-analysis ultimately included 17 articles, the basic characteristics of which are shown in Table 1. A total of 395 rodents (rats and mice) were included. Sepsis models were induced mainly by cecal ligation and puncture (CLP) or by intraperitoneal injection of lipopolysaccharide (LPS). EVs were mainly derived from human or mouse bone marrow, adipose mesenchymal tissue, or human umbilical cord blood mesenchymal tissue. Cecal ligation and puncture (CLP) or intraperitoneal injection of lipopolysaccharide (LPS) were used in the sepsis model. The EVs used to treat sepsis are derived mainly from stem cells derived from human cord blood mesenchymal tissue or stem cells from different parts of the mouse body, such as bone marrow or adipose mesenchymal tissue. EVs were mainly injected intravenously or intratracheally into mice within 6 h after induction. The injection dose ranged from 0.05 to1000μg. The follow-up time was between 2 to 7 days.
Risk of bias assessment
The risk of bias among included studies was assessed by SYRCLE's RoB tool (Table 2). All of the studies were considered to have RoB risks. Although 10 studies (58%) reported randomization of animals, none showed how random sequences were generated or whether assignments were adequately concealed. Therefore, the RoB scores in the selection bias component were "unclear" for all studies. None of the studies mentioned whether the animals were raised and evaluated randomly, or whether the researchers were blind to the animal intervention program. Five studies [18, 24,25,26, 32] mentioned that assessors were blinded to the animal interventions. Three studies [24, 28, 30] did not have sufficient outcome data. All studies had a low risk of reporting bias. One study [23] may have had a problem with the experimental design, which resulted in a RoB score of "high risk". Otherwise, no significant issues concerning bias were identified that were not covered in the SYRCLE’s RoB tool.
Effects of extracellular vesicles on sepsis
A total of 17 reports related to EVs treatment of sepsis were included in this study, all of which reported mortality. Mortality at the end point was 146 of 195 (74.9%) in the control group and 70 of 201 (34.8%) in the EVs-treated group. As shown in Fig. 2, results analysis showed that EVs treatment significantly reduced sepsis mortality (OR 0.17, 95% CI: 0.11, 0.26, P < 0.001). Sensitivity analysis was conducted by excluding each study from the results of meta-analysis. The results showed that reducing any one study did not make a significant difference to the results.
Subgroup analysis
Subgroup analyses were performed to evaluate the efficacy of EVs in the treatment of sepsis, taking into account the generality and reproducibility of treatment outcomes across different experimental conditions. The use of EVs in rats (OR = 0.37, 95% CI: 0.22–0.62, P < 0.001) was more effective than in mice (OR = 0.49, 95% CI: 0.39–0.61, P < 0.001) (Additional file 1: Fig. S1). Animals that did not limit their sex (OR = 0.19, 95% CI: 0.11–0.33, P < 0.001) had higher survival rates than those that used only male animals (OR = 0.14, 95% CI: 0.06–0.29, P < 0.001) (Additional file 1: Fig. S2). Compared with the CLP model (OR = 0.51, 95% CI: 0.41–0.64, P < 0.001), EVs had better therapeutic outcomes in non-CLP models (OR = 0.32, 95% CI: 0.20–0.53, P < 0.001) than in CLP models (Additional file 1: Fig. S3).
Among the 17 studies, EVs in 13 of them were derived from MSCs (OR = 0.15, 95% CI: 0.09–0.26, P < 0.001), which had a better therapeutic effect on sepsis compared with EVs derived from other cells (OR = 0.21, 95% CI: 0.08–0.55, P < 0.001) (Additional file 1: Fig. S4). The most common method of administration of EVs was intravenous injection (N = 12; 70.6%). Subgroup analysis showed that intratracheal administration (OR = 0.08, 95% CI: 0.02–0.33, P < 0.001), in comparison with intravenous injection (OR = 0.19, 95% CI: 0.11–0.32, P < 0.001), was more likely to improve survival (Additional file 1: Fig. S5). Doses and units of injected EVs varied widely according to factors such as particle number, absolute protein mass, and animal weight. Four studies used the number of EVs particles as a unit of treatment. The remaining 13 studies were divided into < 100 μg, 100 μg, and > 100 μg groups according to the injection volume for subgroup analysis. Injection doses of 100 μg (OR = 0.13, 95% CI: 0.06–0.30, P < 0.001) were found to better improve the survival rate of septic animal models (Additional file 1: Fig. S6). Additionally, EVs that were xenogenic (OR = 0.18, 95% CI: 0.09–0.36, P < 0.001) were found to be less effective than those that were allogeneic (OR = 0.15, 95% CI: 0.08–0.28, P < 0.001) (Additional file 1: Fig. S7). Compared to EVs treatment with fewer than five days of observation (OR = 0.13, 95% CI: 0.04–0.44, P < 0.001), EVs treatment with observation exceeding five days (OR = 0.12, 95% CI: 0.07–0.23, P < 0.001) could also improve animal survival. The survival rate of septic animals treated with EVs was higher when observation time exceeded 5 days (OR = 0.12, 95% CI: 0.07–0.23, P < 0.001) (Additional file 1: Fig. S8). Subgroup analysis also showed that antibiotic rehydration (OR = 0.13, 95% CI: 0.04–0.44, P < 0.001) after the establishment of sepsis models improved animal survival (Additional file 1: Fig. S9).
Publication bias
Funnel plots were used to assess potential bias among the studies included in the meta-analysis. Additional file 1: Fig. S10 shows that no publication bias was found.
Discussion
In this manuscript, existing preclinical EVs research was summarized and analyzed by applying meta‐research methods to summarize the efficacy of EVs in the treatment of sepsis in animals. Comprehensive analysis confirmed that MSC-EVs treatment may improve the survival rate of sepsis animal models, which provides important clues for human clinical trials. However, more studies are needed to confirm the optimal therapeutic effect of EVs on sepsis.
The SYRCLE tool was used to assess the methodological quality of the 17 studies included in this meta-analysis. In the evaluation process, a lack of clear method description was noted in the methods of many studies, as well as potential bias due to insufficient randomization and lack of blinding. Issues such as these suggest that detailed and specific method description is crucial when conducting experiments. In addition, this review suggests an urgent need for more standardized EVs characterization in preclinical studies of EVs. Providing more comprehensive experimental details will be more conducive to subsequent related research.
In recent years, EVs have gradually become a safe, feasible alternative for cell therapy due to advantages such as high stability, good permeability, and low immunogenicity and cytotoxicity [41]. EVs are thought to be released from any cell and are involved in the intercellular transmission of information in physiological and pathological processes by transferring their components (such as proteins, miRNAs, mRNAs, and mitochondria) [42, 43]. Some recent studies have also shown that EVs can inhibit the inflammatory response in the pathological process of sepsis by delivering miRNA, lncRNA or cytokines. For example, Guang et al. [44] found that the functional delivery of endothelial progenitor cell-derived extracellular vesicles to lncRNA TUG1 could improve sepsis induced by bacterial outer membrane vesicles by endowing anti-inflammatory macrophages with polarization by impeding miR-9-5p targeting SIRT1 inhibition. Another study found that mesenchymal stromal cell-derived EVs inhibits cytokine release and inflammatory responses during sepsis [17]. However, Yuhan et al. showed that erythrocyte-derived EVs aggravate inflammation, which may be a potential risk factor for transfusion-related immune regulation [34]. So far, there is no consensus on the therapeutic effect of EVs.
This article is the first meta-analysis to summarize the efficacy of EVs in treating sepsis. Animal species, models and interventions were classified for subgroup analysis. Our results show that rats treated with EVs without restriction of animal sex are more effective, while Xue-Yi et al. 's meta-analysis showed that MSCs from male mice had a better effect on sepsis [45]. This may be related to differences in the treatment of MSCs and EVs. More experiments are needed to determine why.
Subgroup analysis also showed that EVs derived from various stem cells were superior to EVs derived from other cells, which may be related to their lower immunogenicity and higher immunomedulatory capacity [46]. From the analysis, we can see that different studies used different EVs doses and dose units. Even if the meta-analysis results suggest that 100 μg may have a better therapeutic effect, there is an urgent need to compare the efficacy of different doses of EVs in the treatment of sepsis. EVs of the same species are more effective than xenografts, which is related to the highly acute rejection caused by natural antibodies and complements [47]. However, the results of subgroup analysis are not yet illustrative, as some subgroups have insufficient references. In addition, although the included studies all referred to injecting EVs into animals after re-suspending EVs with a solution of PBS, none of the studies evaluated the delivery efficiency of EVs, which is an important part of assessing the effectiveness of EVs in the treatment of sepsis. Therefore, future research on EVs treatment needs to standardize the description of EVs in more details for further summary.
Limitations
Several potential limitations of this meta-analysis should be considered. Firstly, the current animal models of EVs in the treatment of sepsis mainly use small animals such as rats and mice, which may overestimate the effect of EVs in the treatment of sepsis. Secondly, studies with positive results are more likely to be published leading to publication bias. Third, the sample size of the included studies is all small, and larger sample size studies are needed to prove the therapeutic effect of EVs on sepsis. Finally, the use of Engauge software to extract data from the survival curve of the article may cause deviations from the original data.
Conclusion
This meta-analysis showed that MSC-EVs treatment may be associated with lower mortality in animal models of sepsis, setting important future directions for EVs treatment of sepsis. Subsequent preclinical studies will need to address the standardization of dose, source, and timing of EVs to provide comparable data. In addition, the effectiveness of EVs in treating sepsis must be studied in large animal studies to provide important clues for human clinical trials.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- EVs:
-
Extracellular vesicles
- MSCs:
-
Mesenchymal stem cells
- SYRCLE:
-
Systematic Review Centre for Laboratory Animal Experimentation
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama-J Am Med Assoc. 2016;315:801–10.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–11.
Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. J Am Med Assoc. 2018;319:62–75.
Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63.
Lv Z, Cai X, Bian Y, Wei Z, Zhu W, Zhao X, et al. Advances in mesenchymal stem cell therapy for osteoarthritis: from preclinical and clinical perspectives. Bioeng -Basel. 2023;10:195.
Hu Q, Chen Y, Deng X, Li Y, Ma X, Zeng J, et al. Diabetic nephropathy: focusing on pathological signals, clinical treatment, and dietary regulation. Biomed Pharmacother. 2023;159: 114252.
Gonçalves R, Vasques JF, Da SA, Gubert F, Mendez-Otero R. Mesenchymal stem cell- and extracellular vesicle-based therapies for Alzheimer’s disease: progress, advantages, and challenges. Neural Regen Res. 2023;18:1645–51.
Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells-Basel. 2019;8:1605.
Rayamajhi S, Nguyen T, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–94.
Cone AS, Yuan X, Sun L, Duke LC, Vreones MP, Carrier AN, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics. 2021;11:8129–42.
Dong L, Pu Y, Chen X, Qi X, Zhang L, Xu L, et al. hUCMSC-extracellular vesicles downregulated hepatic stellate cell activation and reduced liver injury in S. japonicum-infected mice. Stem Cell Res Ther. 2020;11:21.
Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36:301–12.
Schulz-Siegmund M, Aigner A. Nucleic acid delivery with extracellular vesicles. Adv Drug Deliver Rev. 2021;173:89–111.
Khosrojerdi A, Soudi S, Hosseini AZ, Eshghi F, Shafiee A, Hashemi SM. Immunomodulatory and therapeutic effects of mesenchymal stem cells on organ dysfunction in sepsis. Shock. 2021;55:423–40.
Dos SC, Amatullah H, Vaswani CM, Maron-Gutierrez T, Kim M, Mei S, et al. Mesenchymal stromal (stem) cell therapy modulates miR-193b-5p expression to attenuate sepsis-induced acute lung injury. Eur Respir J. 2022;59:2004216.
Zhou Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Chang E, et al. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit Care. 2019;23:44.
Park KS, Svennerholm K, Shelke GV, Bandeira E, Lässer C, Jang SC, et al. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res Ther. 2019;10:231.
Chang CL, Sung PH, Chen KH, Shao PL, Yang CC, Cheng BC, et al. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am J Transl Res. 2018;10:1053–70.
Deng H, Wu L, Liu M, Zhu L, Chen Y, Zhou H, et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate LPS-Induced ARDS by modulating macrophage polarization through inhibiting glycolysis in macrophages. Shock. 2020;54:828–43.
Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 2006;25:586–93.
Deng H, Zhu L, Zhang Y, Zheng L, Hu S, Zhou W, et al. Differential lung protective capacity of exosomes derived from human adipose tissue, bone marrow, and umbilical cord mesenchymal stem cells in sepsis-induced acute lung injury. Oxid Med Cell Longev. 2022;2022:7837837.
Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K, et al. Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci Rep-Uk. 2015;5:13721.
Wang X, Liu D, Zhang X, Yang L, Xia Z, Zhang Q. Exosomes from adipose-derived mesenchymal stem cells alleviate sepsis-induced lung injury in mice by inhibiting the secretion of IL-27 in macrophages. Cell Death Discov. 2022;8:18.
Zhou Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Chang E, et al. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol Ther. 2018;26:1375–84.
Chi D, Chen Y, Xiang C, Yao W, Wang H, Zheng X, et al. Human amnion epithelial cells and their derived exosomes alleviate sepsis-associated acute kidney injury via mitigating endothelial dysfunction. Front Med-Lausanne. 2022;9: 829606.
Chen J, Li C, Liang Z, Li C, Li Y, Zhao Z, et al. Human mesenchymal stromal cells small extracellular vesicles attenuate sepsis-induced acute lung injury in a mouse model: the role of oxidative stress and the mitogen-activated protein kinase/nuclear factor kappa B pathway. Cytotherapy. 2021;23:918–30.
Zhang R, Zhu Y, Li Y, Liu W, Yin L, Yin S, et al. Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury via regulating microRNA-146b expression. Biotechnol Lett. 2020;42:669–79.
Zheng DY, Zhou HN, Wang HC, Zhu Y, Wu Y, Li QH, et al. Mesenchymal stem cell-derived microvesicles improve intestinal barrier function by restoring mitochondrial dynamic balance in sepsis rats. Stem Cell Res Ther. 2021. https://doi.org/10.1186/s13287-021-02363-0.
Su Y, Song XX, Teng JL, Zhou XB, Dong ZH, Li P, et al. Mesenchymal stem cells-derived extracellular vesicles carrying microRNA-17 inhibits macrophage apoptosis in lipopolysaccharide-induced sepsis. Int Immunopharmacol. 2021;95:107408.
Sun J, Sun X, Chen J, Liao X, He Y, Wang J, et al. microRNA-27b shuttled by mesenchymal stem cell-derived exosomes prevents sepsis by targeting JMJD3 and downregulating NF-κB signaling pathway. Stem Cell Res Ther. 2021;12:14.
Zhou Q, Xie M, Zhu J, Yi Q, Tan B, Li Y, et al. PINK1 contained in huMSC-derived exosomes prevents cardiomyocyte mitochondrial calcium overload in sepsis via recovery of mitochondrial Ca(2+) efflux. Stem Cell Res Ther. 2021;12:269.
Gao F, Zuo BJ, Wang YP, Li SL, Yang JP, Sun D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020. https://doi.org/10.1016/j.lfs.2020.117719.
Akhavan RM, Soufi ZM, Abroun S, Atashi A. The effect of exosomes derived from unrestricted somatic stem cells on murine model of sepsis. Cells Tissues Organs. 2021. https://doi.org/10.1159/000520639.
Gao Y, Jin H, Tan H, Cai X, Sun Y. Erythrocyte-derived extracellular vesicles aggravate inflammation by promoting the proinflammatory macrophage phenotype through TLR4-MyD88-NF-κB-MAPK pathway. J Leukocyte Biol. 2022. https://doi.org/10.1002/JLB.3A0821-451RR.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Bmj-Brit Med J. 2021;372: n160.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. Bmc Med Res Methodol. 2014;14:43.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj-Brit Med J. 2003;327:557–60.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111.
Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj-Brit Med J. 2006;333:597–600.
Baek G, Choi H, Kim Y, Lee HC, Choi C. Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cell Transl Med. 2019;8:880–6.
Gupta D, Zickler AM, El AS. Dosing extracellular vesicles. Adv Drug Deliver Rev. 2021;178: 113961.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Bio. 2018;19:213–28.
Ma W, Zhang W, Cui B, Gao J, Liu Q, Yao M, et al. Functional delivery of lncRNA TUG1 by endothelial progenitor cells derived extracellular vesicles confers anti-inflammatory macrophage polarization in epsis via impairing miR-9-5p-targeted SIRT1 inhibition. Cell Death Dis. 2021;12:1056.
Sun XY, Ding XF, Liang HY, Zhang XJ, Liu SH, Bing-Han, et al. Efficacy of mesenchymal stem cell therapy for sepsis: a meta-analysis of preclinical studies. Stem Cell Res Ther. 2020;11:214.
Lombardo E, van der Poll T, DelaRosa O, Dalemans W. Mesenchymal stem cells as a therapeutic tool to treat sepsis. World J Stem Cells. 2015;7:368–79.
Maeda A, Kogata S, Toyama C, Lo PC, Okamatsu C, Yamamoto R, et al. The innate cellular immune response in xenotransplantation. Front Immunol. 2022;13: 858604.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81070125, 81270213, 81670306); the Science and Technology Foundation in Guangdong Province (No. 2010B031600032, 2014A020211002); the National Natural Science Foundation of Guangdong Province (No. 2017A030313503); the Science and Technology Foundation in Guangzhou City (No. 201806020084); the Fundamental Research Funds for the Central Universities (No. 13ykzd16, 17ykjc18); the Futian District Health and Public Welfare Research Project of Shenzhen City (No. FTWS2019001, FTWS2021016, FTWS2022026); and the Shenzhen Fundamental Research Program (No. JCYJ20190808101405466, JCYJ20210324115003008, JCYJ20220530144404009).
Author information
Authors and Affiliations
Contributions
All authors contributed extensively to the work of this paper. TW provided research ideas. SJY conducted a literature analysis search and analyzed the data to compile this article. KLZ, JYH, XL and DSX contributed key data interpretations. XXC, SML, YHH, CQZ and HW contributed to the study protocol and wrote the article. GHZ, CTZ and HDW resolved any differences through discussions. JYF revised the article. The corresponding author had full access to all of the data and the final responsibility to submit the article for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The detailed search strategy. Figure S1. Forest plot summarizing the association between animal species (rat vs. mouse) and mortality in an EVs-treated sepsis model. Figure S2. Forest plot summarizing the association between animal gender and mortality in an EVs-treated sepsis model. Figure S3. Forest plot summarizing the association between sepsis models (CLP and non-CLP) and mortality after EVs treatment. Figure S4. The forest plot summarizes the relationship between EVs sources and mortality in the sepsis model. Figure S5. The forest plot summarizes the relationship between the route of EVs administration (intravenous and intratracheal) and mortality in a sepsis model. Figure S6. Forest plot summarizes the relationship between EVs dose and mortality in a sepsis model. Figure S7. Forest plot summarizing the association between EVs species (allogeneic and xenogenic) and mortality in sepsis models. Figure S8. Forest plot summarizing the relationship between observation days and mortality in a sepsis model treated with EVs. Figure S9. Forest plot to summarizing the relationship between fluid rehydration and mortality in a sepsis model treated with EVs. Figure S10. Forest maps test for publication bias.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, S., Zhang, K., Hou, J. et al. Protective properties of extracellular vesicles in sepsis models: a systematic review and meta-analysis of preclinical studies. J Transl Med 21, 262 (2023). https://doi.org/10.1186/s12967-023-04121-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04121-7