Abstract
Aims
Idiopathic membranous nephropathy (IMN) is a common cause of adult nephrotic syndrome. Currently, the diagnosis of IMN mainly depends on renal biopsy, which is invasive. What’s more, markers already known for the clinical diagnosis of IMN are not sensitive enough. The present study aims to investigate the profiling of urinary exosomal circular RNAs (circRNAs) of IMN, and to look for a potential biomarker for diagnosis of IMN.
Methods
Urine exosomes were collected from patients with IMN and idiopathic nephrotic syndrome (INS), as well as healthy controls (HCs) by ultracentrifuge. A pairwise comparison between 5 IMN and 5 HC was performed by high-throughput sequencing. Enrichment analysis were performed to explore the potential functions of differentially expressed circRNAs in IMN. Among three differentially expressed circRNAs which may be involved in signaling pathways of pathogenesis of IMN and matched conserved mouse circRNAs, hsa_circ_0001250 was selected as the target circRNA after quantitative polymerase chain reaction among 23 IMN, 19 INS and 23HC. Sanger sequencing and RNase R digestion assay were performed to validated the ring-structure and sequence of hsa_circ_0001250. ROC (Receiver Operating Characteristic) curve correlation analysis was used to further validate the potential utility of hsa_circ_0001250 as a diagnostic biomarker of IMN. A circRNA-miRNA-mRNA network was constructed to reflect the relationship between hsa_circ_0001250 and its target miRNAs and mRNAs.
Results
766 up-regulated and 283 down-regulated circRNAs were identified in IMN patients. Kyoto Encyclopedia of Genes and Genomes pathway analysis revealed signaling pathways of pathogenesis of IMN which the different expressed circRNAs may participate in. The ring-structure and the sequence of hsa_circ_0001250 were confirmed, the expression of hsa_circ_0001250 was validated significantly increased in IMN, relevant with high level of proteinuria. A circRNA-miRNA-mRNA network reflected that hsa_circ_0001250 may play a role in the pathogenesis of IMN by target hsa-miR-639 and hsa-miR-4449.
Conclusion
We revealed the expression and functional profile of differentially expressed urinary exosomal circRNAs of IMN patients. Urinary exosomal hsa_circ_0001250 was tested as a potential biomarker of IMN and a predicted circRNA-miRNA-mRNA network was constructed.
Similar content being viewed by others
Introduction
Membranous nephropathy (MN), which is characterized by the extensively thickened glomerular basement membrane (GBM), is a common cause of adult nephrotic syndrome, accounting for 20–30% of glomerular disease cases and some cases inevitably progressed to end-stage renal disease [1, 2]. MN can be classified as idiopathic membranous nephropathy (IMN) and secondary MN. It is widely accepted that IMN is an autoimmune disease mediated by the accumulation of antigen–antibody complexes outside the glomerular basement membrane, which leads to the activation of complement system and the changes in the podocyte morphology, causing proteinuria and further damage to the kidney [3]. However, the specific molecular pathogenesis is unclear. At present, the diagnosis of IMN mainly depends on renal biopsy [4], which is invasive. What’s more, there is still a lack of targeted treatment options. Therefore, the most urgent needs are to find non-invasive diagnosis markers and specific therapeutic targets of IMN.
Recently, researchers have studied the diagnostic capabilities of non-coding RNA in exosomes [5, 6]. Exosomes are extracellular vesicles with a diameter of 40–160 nm (average ~ 100 nm) originating from the endosomal pathway by a process including endocytosis, merging and release. Thus, many constituents of a cell, including DNAs, RNAs, lipids, metabolites, and cytosolic and cell-surface proteins can be contained [7]. The double-layer membrane structure of exosomes can protect the substance, so exosomes can be good carrier of molecular markers. Limited by the detection technology, it is only in recent years that researchers gradually recognize what exosome really is. Studies suggest that exosomes play a regulatory role that selectively encapsulate specific RNA molecules, deliver them to nearby or distant target cells, which is an important way of intercellular communication, effectively regulate the biological functions of target cells and closely related to the physiological and pathological processes of many diseases [8]. Exosomes were detected in a variety of body fluids [9], among which urinary exosomes have been proved to be a kind of marker of many kidney diseases, such as chronic kidney disease [10],diabetic kidney disease [11, 12], autosomal dominant polycystic kidney disease [13], clear cell renal cell carcinoma [14], renal fibrosis [15, 16], etc.
Among these non-coding RNAs, circular RNAs (circRNAs), different from traditional linear RNAs, are identified by the special covalently closed loop structure, for which they are more resistance to RNase R and thus have a longer half-life [17, 18]. A large number of studies have shown that circRNAs can be potential diagnostic marker and therapeutic target for they have a unique expression profile and important biological functions in a variety of diseases, such as tumor [19], cardiovascular disease [20], neurological disease [21] and autoimmune disease [22]. Studies have found that circRNAs can enter the extracellular space through exosomes, which suggests that they may be signal molecule for cell communication, and also shows the potentiality as a diagnostic marker [9].
Compared with other body fluids, urine, which is produced by kidney, seems to convey more information about kidney damage and diseases of the urinary system. Thus, the present study aims to look for a target urinary exosomal circRNA as a potential biomarker for diagnosis of IMN patients. In this study, we investigated the difference expression profile of circRNAs in urinary exosomes between patients with IMN and healthy controls by high-throughput sequencing and bioinformatics analysis. And we finally found that hsa_circ_0001250 have relevance in IMN and may be the potential biomarker for IMN.
Materials and methods
Clinical specimens and collection
A total of 47 age- and sex-matched patients with IMN and INS treated at China-Japan Union Hospital of Jilin University and 28 HCs were enrolled in this study. Among which, urinary samples from 5 IMN patients and 5 HCs were collected for RNA high throughput sequencing and bioinformatics analysis, then 23 patients with IMN, 23 HCs and 19 patients with INS as disease controls were enrolled for confirming the RNA sequencing results by qPCR assay and subsequent statistical analysis. Inclusive criteria of IMN or INS group were: diagnosed with IMN or INS by renal biopsy, didn’t receive any immunosuppressive treatment. Exclusive criteria were: patients with other diseases which can cause renal damage such as autoimmune disease, diabetes, hypertension or hepatitis B, or patients suffered diseases with aberrant circRNA expression such as infection, tumor, cardiovascular disease, neurological disease. Samples of blood and urine from these participants were collected at the nephrology department before renal biopsy. Their second-morning urine (approximately 100 ml) were collected into a sterilized centrifuge tube and then stored at -80℃. This study was approved by the Research Ethics Committee of China-Japan Union Hospital of Jilin University (No.2021-KYLL-060002). Written informed consent was obtained from all participants.
Isolation of urinary exosomes
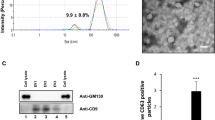
The urine samples were quickly melted at 37 °C in a water-bath and then centrifuged for 30 min at 2000 g, 4 °C. Afterwards, each tube of supernatant was carefully transferred to a new centrifuge tube and centrifuged for 45 min at 10,000g, 4 °C to remove larger vesicles. The supernatant was then filtered through a 0.45 μm filter and the filtrate were collected and then ultracentrifuged at 100,000g for 70 min at 4 °C. The sediment was resuspend with 10 mL pre-cooled 1 × PBS and were ultracentrifuged again at 4 °C, 100,000 g for 70 min. The sediment was resuspended with 100 μL of pre-chilled 1 × PBS, from which we took 5 μL for electron microscope, 50 μL for protein extraction, and 20 μL for RNA extraction.
Detection of exosomes
Transmission electron microscopy (TEM)
In order to observe the morphology of exosomes, isolated exosomes were diluted with PBS buffer and then dropped onto a carbon-coated formvar grids for 1 min and stained with phosphotungstic acid for 1 min. Afterwards, the grid was dried at room temperature for several minutes and subsequently visualized on a transmission electron microscope (TEM; Hitachi, HT7700) at 100 kV.
Western blot assay
Western blot (WB) was used to test the specific exosomal markers as protein CD9, CD63, and calnexin was detected as a negative control. The whole proteins were lysed by RIPA Lysis Buffer. The protein concentration was measured by BCA method. After purified and electrophoretically separated, proteins were transferred to the polyvinylidene fluoride (PVDF) membrane. The membranes were blocked by 5% nonfat milk for 1 h and then incubated overnight at 4 °C with each primary antibodies, and subsequently incubated with HPR-labeled secondary antibodies at room temperature for 1 h. Finally, the enhanced chemiluminescence system (CLINX) was applied for signal visualization.
Total RNA extraction and quality control
Total RNA in the urine was extracted using TRIzol reagent. The concentrations of the RNA were measured using a NanoDrop ND-1000 (Thermo Fisher Scientific, Waltham, MA, USA), the ratio OD260/OD280 is used to estimate the purity of obtained RNAs. OD260/280 ratio around 1.8–1.9 showed the high purity of the RNAs, demonstrated the good integrity of the obtained RNAs that could be used for later experiments.
RNA library preparation, sequencing of circRNAs and bioinformatics analysis
RNA high throughput sequencing was performed by Cloud-Seq Biotech (Shanghai, China). The rRNAs were removed from total RNA with NEBNext rRNA Depletion Kit (New England Biolabs, Inc., Massachusetts, USA). After constructed by NEBNext® Ultra™ II Directional RNA Library Prep Kit (New England Biolabs, Inc., Massachusetts, USA), the RNA libraries were quality controlled and quantified with the BioAnalyzer 2100 system (Agilent Technologies, Inc., USA). Then library sequencing was performed on an illumina Hiseq instrument with 150 bp paired end reads harvested from Illumina HiSeq 4000 sequencer, and were quality controlled by Q30. The cutadapt [23] software (v1.9.3) was used to remove 3’ adaptor-trimming and low quality reads. The high quality trimmed reads were aligned to the reference genome/transcriptome with STAR software [24] (v2.5.1b) and circRNAs were detected and identified with DCC [25] software (v0.4.4). The identified circRNAs were annotated with circBase database [26] and Circ2Traits [27]. Then edgeR software [28] (v3.16.5) was used to normalize the data and perform differentially expressed circRNA analysis. The edgeR software was used to calculate log2FC value and P value of IMN and HC groups for the volcano plot, logCPM for the scatter plot, which were drawed with Python language and matplotlib package. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (https://www.genome.jp/kegg/) is a database resource for understanding high-level functions and utilities of the biological system [29]. After the different expressed circRNAs were obtained, the Fisher’s test was performed for enrichment analysis according to the annotation of the KEGG pathway of each gene, screened the pathways with a P value < 0.05. The obtained results are drawn using the ggplot2 package for R. A map of ten miRNAs and their predicted target mRNAs was constructed of underlying molecular mechanisms through specific base pairing to demonstrate the circRNA-miRNA-mRNA network of hsa_circ_0001250 based on TargetScan (v7.0) [30] and miRanda (v3.3a) [31] software. The significant pairs in network were constructed using Cytoscape software (v3.1.0) [32].
Validation of expression of circRNAs using quantitative reverse transcription PCR (RT-qPCR)
Total RNA was reverse transcribed to synthesize cDNA using a Prime Script RT Reagent Kit (Perfect Real Time; TaKaRa, Osaka, Japan). Then quantitative polymerase chain reaction (qPCR) assay was conducted in triplicate with specific primers of different expressed circRNAs. To find biomarkers for IMN, we targeted the top 50 up-regulated circRNAs according to the value of log2 fold change of the sequencing results. As shown in Fig. 1, to carry on in vivo experiments on mice in the further study, we screened those circRNAs matched conserved mouse circRNAs with circBank database (http://www.circbank.cn/). Finally, we screened out the top 3 circRNAs, the original genes of which may be associated with renal diseases: hsa_circ_0004771, hsa_circ_0000896 and hsa_circ_0001250. The primers were chemically synthesized and validated in RiboBio Company (RiboBio, Guangdong, China), ACTB was used as an internal reference of exosomal circRNAs in urine. PCR primers are listed as follows: hsa_circ_0004771: F: CCGGATGACATCAGAGCTACT R: GTGCATCTTCTGGCTGTGTT hsa_circ_0000896: F: CACCGAGATGCCGACTGATA R: TGCTTGGAGATGCTGGTACT hsa_circ_0001250 F: GGTCACCTGGAAATTGCCTT R: CCGCTAGATCGAGGAAGTCA. ACTB F: AAGGTGACAGCAGTCGGTT R: TGTGTGGACTTGGGAGAGG. qPCR assay was conducted on ABI 7500 real-time PCR system (Applied Biosystems, CA, USA) used SYBR Green (Takara, China). All procedures were performed as follows: 95 °C, 10 min, 40 PCR cycles (95 °C, 15 s; 60 °C, 30 s, 72 °C, 20 s (fluorescence collection)). After the amplification reaction was finished, the procedure was performed as follows: 95 °C, 10 s; 60 °C, 60 s; and 95 °C, 15 s. Then the temperature was slowly increased from 60 to 99 °C (Automatic instrument, Ramp Rate 0.05 °C/s) to establish the melting curve. The relative expression levels of circRNAs were calculated by the 2−ΔΔCq method.
Detection of hsa_circ_0001250
Sanger sequencing
Sanger sequencing covering the backsplicing junction sequence was performed using the qPCR product of hsa_circ_0001250 with the process mentioned above. Divergent primers are used to amplify the full-length of hsa_circ_0001250, qRT-PCR assay was carried out using the following primers: F:GGTCACCTGGAAATTGCCTT R:TTTGATCTCATTCAGTCGAT. Sanger sequecing was performed by Sangon Biotech (Shanghai, China).
RNase R digestion assay
Total RNA was handled with RNase R (Geneseed Biotech Co., Ltd., Guangzhou, China) to confirm the circular structure of hsa_circ_0001250. Total RNA (2 μg) was incubated for 30 min at 37 °C with or without 3 U/μg RNase R. After RNase R treatment, PCR product agarose gel electrophoresis were performed to detect cDNA samples. PCR assay was performed using Easy-Load™ PCR Master Mix (GREEN, 2X). The PCR amplicons were visualized under a Molecular Imager®Gel Doc™ XR + System (Bio-Rad, USA) after electrophoresis in a 1.5% agarose gel electrophoresis with TAE buffer using a 100 bp DNA ladder (Takara, China).
Statistical analysis
Statistical analysis was performed by SPSS 26.0 (Statistical Package for the Social Sciences, IBM Corp., Armonk, NY, USA) and GraphPad Prism (version 8.0.1 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com). Student's t-test was employed to analyse two-group comparisons. One-way ANOVA or Kruskal–Wallis multiple comparison test were used to compare measurement data among multiple groups. ROC curve analysis was performed to explore the diagnostic potential of hsa_circ_0001250. Pearson correlation analysis was used to analyze the relationship between the expression levels of hsa_circ_0001250 and the clinical features. Continuous variables with normal distributions are expressed as the mean ± standard deviation, otherwise are expressed as medians with interquartile ranges. A value of P < 0.05 was considered statistically significant.
Results
Clinical characteristics of the patients
The basic characteristics of 5 IMN patients and 5 HCs enrolled for RNA high throughput sequencing are summarized in Table 1. The basic characteristics of 23 IMN patients, 19 INS patients and 23 HCs enrolled in qPCR assay are listed in Table 2. There were no statistically significant differences among IMN, INS and HC groups with regarded to age, sex or BMI distribution (P > 0.05). The clinical characteristics including serum creatinine and eGFR didn’t show statistical differences (P > 0.05) while serum albumin, serum cholesterol and are significantly different between IMN and HC groups (P < 0.05).
Characterization of urinary exosomes
As shown in Fig. 2, using a TEM, the characteristic cup-shaped morphology of the exosomes was observed with diameters about 40 ~ 160 nm (Fig. 2A). Exosomal protein markers including CD9, CD63 were detected by Western blot analysis. Results showed that CD9, CD63 were all highly enriched in the isolated exosomes, besides, calnexin as a negative control was not enriched (Fig. 2B).
Profiling of differentially expressed urinary exosomal circRNAs of IMN and HC patients
After RNA high throughput sequencing, a total of 19,730 transcripts of circRNAs were identified in all urinary exosome samples. As shown in Fig. 3, the results of the sequencing showed that there were 1049 differentially expressed circRNAs based on the threshold: fold change ≥ 2.0; P < 0.05. Compared with HC group, 766 transcripts were up regulated and 283 transcripts were down regulated in IMN group. Of the 1049 circRNAs, 461 were found for the first time and 588 have been found in previous studies (Fig. 3A). Most of the circRNAs are less than 2000 nt in length (Fig. 3B). These circRNAs were distributed on all chromosomes, including autosomal, sex chromosomes and some down regulated circRNAs were from mitochondrial genes (Fig. 3C). Among the 1049 circRNAs, exonic, intronic and other sources were accounted for 57.3% (601/1049), 17.3% (181/1049) and 25.4% (267/1049) respectively (Fig. 3D). Volcano plots (Fig. 3E) and scatter plot (Fig. 3F) displayed the differentially expressed circRNAs (FC ≥ 2.0; P < 0.05), most of which were up-regulated. Heat map and hierarchical clustering indicate that circRNAs were obviously different between IMN and HC groups (Fig. 3G).
Profiling of differentially expressed urinary exosomal circRNAs of IMN and HC. A Percentage of novel and known differentially expressed circRNAs. B The counts of differentially expressed circRNAs based on their length. C The location of differentially expressed circRNAs on human chromosomes. D The distribution of differentially expressed circRNAs based on their categories. E Volcano plot of differentially expressed circRNAs between IMN and HC groups. Red dots represent circRNAs that are significantly differentially expressed. Outside the two vertical lines are the circRNAs with a Fold Change more than 2, the horizontal line indicates a p-value of 0.05. F Scatter plot of differentially expressed circRNAs in IMN and HC groups. Red and green dots indicate significant up-regulated and down-regulated circRNAs, respectively (with a fold change (FC) > 2.0 and P < 0.05). G Heatmap illustrating differentially expressed circRNAs between IMN and HC groups. The color key indicates the expression levels of circRNAs ranging from low (green) to high (red)
KEGG pathway analysis for the biological function of differentially-expressed circRNAs
We investigated the potential functions of differentially expressed circular RNAs using KEGG pathway analyse with the down-regulated and up-regulated differently expressed circRNAs, respectively. KEGG pathway analysis revealed that the up-regulated circRNAs participated in several key pathways such as Endocytosis and Protein processing in endoplasmic reticulum in the top 10 KEGG pathways enriched (Fig. 4A). Additionally, the down-regulated circRNAs were involved in Proximal tubule bicarbonate reclamation, Notch signaling pathway, Sphingolipid signaling pathway, AMPK signaling pathway, RIG-l-like receptor signaling pathway which may associated with pathogenesis of IMN (Fig. 4B).
Validation of the high expression of hsa_circ_0001250 by qPCR
To explore urinary exosomal circRNAs as biomarkers for IMN, we performed qRT-PCR assay among 23 IMN patients, 19 INS patients, and 23 HCs. The results of qRT-PCR showed that the expression of urinary exosomal hsa_circ_0001250 was significantly increased in IMN group than in the other two groups, while hsa_circ_0004771 and hsa_circ_0000896 didn’t show statistical difference among these 3 groups. This suggested that urinary exosomal hsa_circ_0001250 might be a novel potential diagnostic biomarker for IMN (Fig. 5).
A Comparison of the expression level of following circRNAs between IMN, INS and HC groups by RT-qPCR: hsa_circ_0004771, hsa_circ_0000896 and hsa_circ_0001250, the expression of hsa_circ_0001250 was significantly increased in IMN. B Expression levels of hsa_circ_0001250 in urinary exosomes of IMN, HC and INS groups, showing the concrete distribution of hsa_circ_0001250 expression in the three groups. Data are presented as 2−ΔΔCq relative to ACTB expression (mean ± standard deviation). **P < 0.01
Detection of hsa_circ_0001250 by RNase R digestion and Sanger sequencing
The identified circRNAs of RNA sequencing were annotated with circBase database[30] and Circ2Traits[31]. After RNase R digestion, PCR product agarose gel electrophoresis and qPCR assay were performed to detect cDNA samples. Both of the results showed that the relative expression of linear ACTB and GAPDH in the RNase R + group was greatly reduced, while hsa_circ_0001250 was only slightly reduced (Fig. 6A, B). Compared with the linear ACTB and GAPDH, circRNA showed more tolerance, indicating that hsa_circ_0001250 is more resistant to digestion than linear RNA for the ring structure. These results of Sanger sequencing for fragment contains the splicing junction further proved the ring-structure of hsa_circ_0001250 (Fig. 6C). Sanger sequencing for full-length of hsa_circ_0001250 suggested that it was derived from exon 2 and exon 3 of the GRAMD4 gene, which was consistent with the hsa_circ_0001250 data from circBase. In addition, the sequence of the full length of hsa_circ_0001250 matched the data, as well (Fig. 6D).
A, B PCR product agarose gel electrophoresis and qPCR indicated that the relative expression of ACTB and GAPDH in the RNase R + group was greatly reduced, while hsa_circ_0001250 was only slightly reduced. C Sanger sequencing for fragment contains the splicing junction further proved the ring structure of hsa_circ_0001250. D Sanger sequencing for full-length of hsa_circ_0001250 suggested that hsa_circ_0001250 was derived from exon 2 and exon 3 of the GRAMD4 gene and the sequence was consistent with data from circBase
Diagnostic value of urinary exosomal hsa_circ_0001250 of IMN
ROC curve analysis was used to further validate the potential utility of hsa_circ_0001250 as a diagnostic biomarker of IMN. As shown in Fig. 7, ROC curve analysis showed an association between hsa_circ_0001250 expression and idiopathic membranous nephropathy diagnosis. The area under the curve (AUC) to discriminate IMN from HC group was 0.8034 (95% confidence interval, 95%CI: 0.6667–0.9401) for hsa_circ_0001250, suggesting the high diagnostic potential of hsa_circ_0001250 in IMN patients (Fig. 7A). The AUC to discriminate IMN from INS group was 0.7872 (95% confidence interval, 95%CI: 0.6448–0.9296), suggesting hsa_circ_0001250 help differentiate between IMN and INS (Fig. 7B). Pearson's correlation coefficient indicated in the IMN group, high expression of hsa_circ_0001250 in IMN patients was significantly more relevant with high proteinuria level (r = 0.6402, P < 0.01) (Fig. 7C), while didn’t show obvious correlation with other clinical features (P > 0.05), as shown in Table 3.
A ROC curves with a large AUC for hsa_circ_0001250 indicated its potential as a biomarker for IMN (AUC = 0.8034). AUC, area under roc curve; ROC, receiver-operating characteristic. B ROC curves showing the discriminatory ability of hsa_circ_0001250 to distinguish between IMN and INS (AUC = 0.7872). C Correlation Between hsa_circ_0001250 Expression and serum albumin level in patients with IMN. (r = 0.6402, **P < 0.01)
Prediction and annotation of hsa_circ_0001250 using a circRNA-miRNA-mRNA net work
To further determine the biological function and potential connections of hsa_circ_0001250, a circRNA-miRNA mRNA network was constructed. The top 10 miRNAs and top 5 mRNAs were theoretically predicted from hsa_circ_0001250(Fig. 8). We speculate that circRNAs may bind these miRNAs, after which the suppressive effect of miRNAs on the predicted target mRNAs would be abolished. Among the top ten corresponding miRNAs of hsa_circ_0001250, hsa-miR-639 and hsa-miR-4449 have been found to play a role in some pathogenesis, which may be involved in the pathogenesis and development of kidney disease. Among the predicted mRNAs, S1PR5, STAT3, TBR1, and BMPR1B were found to be related to some kidney diseases, as well. We have predicted downstream biological signaling in detail in the discussion part.
circRNA-miRNA-mRNA interaction network of hsa_circ_0001250. The association between the top 10 targeted miRNAs of hsa_circ_0001250 and their top 5 corresponding mRNAs. The red circles indicate miRNAs which may be more associated with IMN. The blue squares represent mRNAs which may participate the pathogenesis of IMN
Discussion
Various studies have focused on exosomal circRNAs for their stability and the function of transferring substances between cells. circRNAs derived from urinary exosomes contain pathophysiological information of a variety of kidney diseases, which can provide a diagnostic basis for kidney diseases [33, 34]. However, only a few researchers focus on the potentiality of exosomal circRNAs as diagnostic markers for idiopathic membranous nephropathy.
In this study, we used the second morning urine as sample material since it is less influenced by diet compared to the first morning urine [35]. What’s more, exosomes derived from the second morning urine were less affected by exosomes secreted by bladder epithelial cells and erythrocytes in some patients. At the same time, it also avoids the influence of changes in physical and chemical factors caused by staying in the bladder for too long. So we think second morning urine is most suitable for valid analysis of biomarkers in urine. We extracted total RNA from urinary exosomes and RNA high throughput sequencing analysis was performed between IMN group and HC group. We identified 1049 significantly differentially expressed circular RNAs (766 up-regulated and 283 down-regulated) in urinary exosomes RNA, among which 461 novel circRNAs were identified. The up-regulated circRNAs accounted for the majority of the known circRNAs, however, the down-regulated circRNAs account for half of all the novel circRNAs. The results suggested that there are many down-regulated circRNAs to be explored, and more functional experiments about them need to be carried out, as well. Additionally, these different expressed circRNAs were distributed on all chromosomes, interestingly, we found that most down-regulated circRNAs were derived from mitochondria. Studies have shown that mitochondria-derived circRNAs regulate the entry of proteins into mitochondrias and affect the function of mitochondrias [36]. Mitochondrial damage is closely related to kidney damage. Wang et al. have found that the traditional Chinese medicine Jianpi Qushi Recipe can delay renal pathological damage and inhibit the occurrence and development of membranous nephropathy by inhibiting cell apoptosis and up-regulating mitophagy through the PINK1/Parkin pathway [37]. Therefore, further researches on down-regulated urinary exosomal circRNAs in IMN could be focused on the above aspects. KEGG pathway analyse were used to explore the functions of these circRNAs. Results revealed they may participate in Endocytosis, Notch signaling pathway, Sphingolipid signaling pathway, AMPK signaling pathway, RIG-I-like receptor signaling pathway. Related researches could focus on these pathways.
Among differentially expressed circRNAs which may be involved in signaling pathways of pathogenesis of IMN and matched conserved mouse circRNAs, hsa_circ_0001250 was finally selected as the target circRNA, verified with qRT-PCR among IMN, INS and HC groups. To our knowledge, hsa_circ_0001250 is the first qPCR-tested urinary exomal circRNA after a RNA-squencing in IMN, the ring-structure of hsa_circ_0001250 was also confirmed in our research. What’s more, we text the results of RNA-sequecing by qPCR assay. Besides, a circRNA-miRNA-mRNA network is predicted for further understanding of functions of hsa_circ_0001250, leading to a more convincing conclusion.
The circRNAs identified through next generation sequencing and bioinformatics analysis needs to be further verified through experiments for their existence and biological functions. With the gradual deepening of circRNA research, the sequence of circRNAs were found occasionally different from original genes [38], so the identification and analysis of the full-length circRNA sequence becomes more and more important. Sanger sequencing for the full-length of hsa_circ_0001250 were performed to confirm the sequence of hsa_circ_0001250. Sanger sequencing for the fragment contains the splicing junction of hsa_circ_0001250 and RNase R digestion assay followed by PCR product agarose gel electrophoresis and qRT-PCR assay were carried to confirm the ring-structure of hsa_circ_0001250, which are necessary for further research.
Moreover, through accuracy prediction estimation (ROC curve analysis), urinary exosomal hsa_circ_0001250 was tested to serve as a diagnostic biomarker to predict IMN. At the same time, we were able to differentiate between INS and IMN, further supporting that the up-regulated expression of hsa_circ_0001250 are specific to IMN. Pearson's correlation coefficient showed that the expression of hsa_circ_0001250 in IMN patients is positively correlated with the level of proteinuria. We know that high level of proteinuria means progression of IMN, so we speculate that hsa_circ_0001250 may promote the progression of IMN, which requires further experimental verification. However, we didn’t find the relevance between hsa_circ_0001250 and other clinical features, so more samples are required in future experiments to verify our conclusion.
Previous studies speculated that exon-derived circRNAs may perform regulatory functions through a circRNA-miRNA-mRNA network in the cytoplasm, while intron-derived circRNAs seem to play regulatory roles in the nucleus [39]. Through RNA sequencing, we confirmed that hsa_circ_0001250 was derived from exon2 and exon3 of GRAMD4, so it was hypothesized that hsa_circ_0001250 may also regulate a circRNA-miRNA-mRNA network through a competitive endogenous RNA mechanism. A gene network is a group of genes that operate in a coordinated manner to control common functions [40], which mainly lies in gene-to-gene regulation, while circRNA-miRNA-mRNA is a network reflecting the regulation between non-coding RNAs and their target genes. At the molecular level, the sequences of circRNAs contain a large number of miRNA binding sites. When miRNAs were binded with circRNAs,the suppressive effect of miRNAs on target mRNAs would be abolished, resulting in a negative effect. These RNA transcripts are called endogenous competing RNAs (ceRNAs), and this new model of post-transcriptional regulation is called the ceRNA hypothesis [41]. In our research, a circRNA–miRNA–mRNA network was constructed to further understand the potential biological functions of hsa_circ_0001250. In the circRNA-miRNA-mRNA network, we can see among the top 10 miRNAs which may be regulated by hsa_circ_0001250, hsa-miR-639 was found associated with cell proliferation and cell cycle by targeting CDKN1A [42], TGFβ-induced EMT by targeting FOXC1 [43], proliferation and migration through the KAT7/Wnt/β-Catenin Pathway [44] in other diseases. In addition, hsa-miR-4449 was found regulating IL-1β and IL-18 expressions, the level of ROS, and pyroptosis in DKD pathogenesis [45]. So we supposed that hsa_circ_0001250 may also play a role in the pathogenesis IMN by target these miRNAs, which need to be proved in further research. Moreover, among the predicted targeted mRNAs, S1PR5, STAT3, TBR1 and BMPR1B were found to be related to some kidney diseases. The expression of S1PR5 was found up-regulated by TGF-β2 in a time- and concentration-dependent manner, which is a necessary condition for TGF-β to induce profibrotic of CTGF. S1PR5 may be a therapeutic target to treat renal fibrosis [46]. S1PR5 has also been found to be involved in the regulation of immune response [47]. In addition, STAT3 phosphorylation was found reduced by the overexpressed of circ_0007059, protecting cell viability and reducing inflammation in a nephritis cell model [48]. Moreover, Methylation of TBR1 is associated with the progression of renal cell carcinoma [49]. BMPR1B was up-regulated by the loss of oncogenic miR-1274a, reducing cancer cell proliferation and inducing apoptosis in clear cell renal cell carcinoma [50]. Therefore, further study on the mechanistic studies on hsa_circ_0001250 could be focused on these mRNAs. In addition, we didn’t find relevant articles confirming that these miRNAs or mRNAs are decreased or increased in IMN, therefore, follow-up experiments are needed to verify the circRNA-miRNA mRNA network.
However, as urinary exosomes may originate from a variety of cells, such as juxtaglomerular cells, podocytes, parietal cells, proximal tubular epithelial cells, and so on [51], the specific cell source of hsa_circ_0001250 remains unknown, we speculate that in IMN patients, the release of hsa_circ_0001250 may be caused by the damage of podocytes, which needs further experimental confirmation, as well.
Conclusion
In conclusion, we identify a series of differentially expressed circRNAs in urinary exosomes collected from IMN patients and HCs. The identified circRNAs are involved in the biological processes associated with IMN. Moreover, we propose urinary exosomal hsa_circ_0001250 to be a potential candidate biomarker of IMN and predict a potential circRNA-miRNA-mRNA network. To our knowledge, this is the first time the correlation between hsa_circ_0001250 and diseases that has been studied. These findings may point out the direction for further research on molecular pathogenesis of IMN.
Availability of data and materials
Raw reads of the sequencing project have been successfully deposited to SRA database with BioProject ID: PRJNA793462.
Abbreviations
- IMN:
-
Idiopathic membranous nephropathy
- INS:
-
Idiopathic nephrotic syndrome
- HC:
-
Health control
- qPCR:
-
Quantitative polymerase chain reaction
- ROC:
-
Receiver operating characteristic
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- circRNA:
-
Circular RNA
- MN:
-
Membranous nephropathy
- TEM:
-
Transmission Electron Microscopy
- WB:
-
Western blot
- ROC:
-
Receiver operating characteristic
- BMI:
-
Body Mass Index
- eGFR:
-
Estimated Glomerular Filtration Rate
- BP:
-
Biological process
References
Canetta P, Troost J, Mahoney S, Kogon A, Carlozzi N, Bartosh S, et al. Health-related quality of life in glomerular disease. Kidney Int. 2019;95(5):1209–24.
Liu W, Gao C, Liu Z, Dai H, Feng Z, Dong Z, et al. Idiopathic membranous nephropathy: glomerular pathological pattern caused by extrarenal immunity activity. Front Immunol. 2020;11:1846.
Ronco P, Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient’s care. Lancet. 2015;385(9981):1983–92.
KDIGO. Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021. https://doi.org/10.1016/j.kint.2021.05.021.
Thietart S, Rautou PE. Extracellular vesicles as biomarkers in liver diseases: a clinician’s point of view. J Hepatol. 2020;73(6):1507–25.
Li L, Zuo X, Liu D, Luo H, Zhang H, Peng Q, et al. Plasma exosomal RNAs has potential as both clinical biomarkers and therapeutic targets of dermatomyositis. Rheumatology (Oxford). 2021. https://doi.org/10.1093/rheumatology/keab753.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020. https://doi.org/10.1126/science.aau6977.
Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–88.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19(1):47.
Khurana R, Ranches G, Schafferer S, Lukasser M, Rudnicki M, Mayer G, et al. Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA. 2017;23(2):142–52.
Zhao Y, Shen A, Guo F, Song Y, Jing N, Ding X, et al. Urinary exosomal MiRNA-4534 as a novel diagnostic biomarker for diabetic kidney disease. Front Endocrinol (Lausanne). 2020;11:590.
Eissa S, Matboli M, Bekhet MM. Clinical verification of a novel urinary microRNA panal: 133b, -342 and -30 as biomarkers for diabetic nephropathy identified by bioinformatics analysis. Biomed Pharmacother. 2016;83:92–9.
Magayr TA, Song X, Streets AJ, Vergoz L, Chang L, Valluru MK, et al. Global microRNA profiling in human urinary exosomes reveals novel disease biomarkers and cellular pathways for autosomal dominant polycystic kidney disease. Kidney Int. 2020;98(2):420–35.
Song S, Long M, Yu G, Cheng Y, Yang Q, Liu J, et al. Urinary exosome miR-30c-5p as a biomarker of clear cell renal cell carcinoma that inhibits progression by targeting HSPA5. J Cell Mol Med. 2019;23(10):6755–65.
Yu Y, Bai F, Qin N, Liu W, Sun Q, Zhou Y, et al. Non-proximal renal tubule-derived urinary exosomal miR-200b as a biomarker of renal fibrosis. Nephron. 2018;139(3):269–82.
Lv LL, Cao YH, Ni HF, Xu M, Liu D, Liu H, et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305(8):F1220–7.
Zhou WY, Cai ZR, Liu J, Wang DS, Ju HQ, Xu RH. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. 2020;19(1):172.
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–91.
Goodall GJ, Wickramasinghe VO. RNA in cancer. Nat Rev Cancer. 2021;21(1):22–36.
Aufiero S, Reckman YJ, Pinto YM, Creemers EE. Circular RNAs open a new chapter in cardiovascular biology. Nat Rev Cardiol. 2019;16(8):503–14.
Mehta SL, Dempsey RJ, Vemuganti R. Role of circular RNAs in brain development and CNS diseases. Prog Neurobiol. 2020;186: 101746.
Khan AQ, Ahmad F, Raza SS, Zarif L, Siveen KS, Sher G, et al. Role of non-coding RNAs in the progression and resistance of cutaneous malignancies and autoimmune diseases. Semin Cancer Biol. 2020. https://doi.org/10.1016/j.semcancer.2020.07.003.
Martin MJ, Martin M. Cut adapt removes adapter sequences from high-throughput sequencing reads EMBnet. EMBnet. 2011;17(1):10–2.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics (Oxford, England). 2013;29(1):15–21.
Cheng J, Metge F, Dieterich C. Specific identification and quantification of circular RNAs from sequencing data. Bioinformatics (Oxford, England). 2016;32(7):1094–6.
Glazar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20(11):1666–70.
Ghosal S, Das S, Sen R, Basak P, Chakrabarti J. Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits. Front Genet. 2013;4:283.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (Oxford, England). 2010;26(1):139–40.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015. https://doi.org/10.7554/eLife.05005.
Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5(1):R1.
Kohl M, Wiese S, Warscheid BJ. Cytoscape: software for visualization and analysis of biological network. Methods Mol Biol. 2011;696:291–303.
Kurahashi R, Kadomatsu T, Baba M, Hara C, Itoh H, Miyata K, et al. MicroRNA-204-5p: A novel candidate urinary biomarker of Xp11.2 translocation renal cell carcinoma. Cancer Sci. 2019;110(6):1897–908.
Luan R, Tian G, Ci X, Zheng Q, Wu L, Lu X. Differential expression analysis of urinary exosomal circular RNAs in patients with IgA nephropathy. Nephrology (Carlton). 2021;26(5):432–41.
Liu X, Yin P, Shao Y, Wang Z, Wang B, Lehmann R, et al. Which is the urine sample material of choice for metabolomics-driven biomarker studies? Anal Chim Acta. 2020;1105:120–7.
Liu X, Wang X, Li J, Hu S, Deng Y, Yin H, et al. Identification of mecciRNAs and their roles in the mitochondrial entry of proteins. Science China Life sciences. 2020;63(10):1429–49.
Wang XH, Lang R, Zeng Q, Liang Y, Chen N, Ma ZZ, et al. Jianpi Qushi Heluo Formula alleviates renal damages in Passive Hemann nephritis in rats by upregulating Parkin-mediated mitochondrial autophagy. Sci Rep. 2021;11(1):18338.
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F, et al. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J Natl Cancer Inst. 2018;110(3):304–15.
Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, et al. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365(2):141–8.
Kolchanov NA, Anan’ko EA, Kolpakov FA, Podkolodnaia OA, Ignat’eva EV, Goriachkovskaia TN, et al. Gene networks. Mol Biol. 2000;34(4):533–44.
Su Q, Lv X. Revealing new landscape of cardiovascular disease through circular RNA-miRNA-mRNA axis. Genomics. 2020;112(2):1680–5.
Lei ST, Shen F, Chen JW, Feng JH, Cai WS, Shen L, et al. MiR-639 promoted cell proliferation and cell cycle in human thyroid cancer by suppressing CDKN1A expression. Biomed Pharmacother. 2016;84:1834–40.
Lin Z, Sun L, Chen W, Liu B, Wang Y, Fan S, et al. miR-639 regulates transforming growth factor beta-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting FOXC1. Cancer Sci. 2014;105(10):1288–98.
Bai Z, Xia X, Lu J. MicroRNA-639 is down-regulated in hepatocellular carcinoma tumor tissue and inhibits proliferation and migration of human hepatocellular carcinoma cells through the KAT7/Wnt/β-catenin pathway. Med Sci Monit. 2020;26:e919241.
Gao C, Wang B, Chen Q, Wang M, Fei X, Zhao N. Serum exosomes from diabetic kidney disease patients promote pyroptosis and oxidative stress through the miR-4449/HIC1 pathway. Nutr Diabetes. 2021;11(1):33.
Wünsche C, Koch A, Goldschmeding R, Schwalm S, Meyer Zu Heringdorf D, Huwiler A, et al. Transforming growth factor β2 (TGF-β2)-induced connective tissue growth factor (CTGF) expression requires sphingosine 1-phosphate receptor 5 (S1P5) in human mesangial cells. Biochim Biophys Acta. 2015;1851(5):519–26.
van Doorn R, Lopes Pinheiro MA, Kooij G, Lakeman K, van het Hof B, van der Pol SM, et al. Sphingosine 1-phosphate receptor 5 mediates the immune quiescence of the human brain endothelial barrier. J Neuroinflammation. 2012;9:133.
Guo PW, Huang HT, Ma J, Zuo Y, Huang D, He LL, et al. Circular RNA-0007059 protects cell viability and reduces inflammation in a nephritis cell model by inhibiting microRNA-1278/SHP-1/STAT3 signaling. Mol Med (Cambridge, Mass). 2021;27(1):113.
Serth J, Peters I, Dubrowinskaja N, Reese C, Albrecht K, Klintschar M, et al. Age-, tumor-, and metastatic tissue-associated DNA hypermethylation of a T-box brain 1 locus in human kidney tissue. Clin Epigenetics. 2020;12(1):33.
Yoshino H, Yonezawa T, Yonemori M, Miyamoto K, Sakaguchi T, Sugita S, et al. Downregulation of microRNA-1274a induces cell apoptosis through regulation of BMPR1B in clear cell renal cell carcinoma. Oncol Rep. 2018;39(1):173–81.
Turco AE, Lam W, Rule AD, Denic A, Lieske JC, Miller VM, et al. Specific renal parenchymal-derived urinary extracellular vesicles identify age-associated structural changes in living donor kidneys. J Extracell Vesicles. 2016;5:29642.
Acknowledgements
We thank CloudSeq Biotech Inc. (Shanghai, China) for their sequencing support.
Funding
This research was funded by Science and Technology Plan Projects of Jilin Province (20190201247JC and 20200201539JC); Jilin University Project (2019YX396).
Author information
Authors and Affiliations
Contributions
QL reviewed the literature, performed experiment, and wrote the manuscript. MX aided in collecting clinical samples, ZZ and MY performed the statistical analysis, YZ wrote and reviewed the manuscript critically. YZ and FL reviewed and supervised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was supported by the Research Ethics Committee of China-Japan Union Hospital of Jilin University (No.2021-KYLL-060002).
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Q., Xu, M., Zhang, Z. et al. Urinary exosomal hsa_circ_0001250 as a novel diagnostic biomarker of idiopathic membranous nephropathy. J Transl Med 20, 607 (2022). https://doi.org/10.1186/s12967-022-03784-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03784-y