Abstract
Background
RET fusions are rare oncogenic drivers in non-small cell lung cancer (NSCLC). While activating RET rearrangements are found in NSCLC patients harboring epidermal growth factor receptor (EGFR) genetic alterations at resistance to EGFR inhibitors, the extent to which co-occurring genomic alterations exist and how they might affect prognosis or therapy response is poorly understood.
Methods
Targeted next-generation sequencing (NGS) was used to assess 380 baseline patients with primary RET fusions and 71 EGFR-mutated NSCLC patients who acquired RET fusions after developing resistance to EGFR-tyrosine kinase inhibitors (EGFR-TKIs).
Results
Primary RET fusions were more likely associated with females and younger age, with KIF5B being the predominant fusion partner. In baseline patients, both SMAD4 (5.3% vs. 0.0%, P = 0.044) and MYC copy-number gain variants (6.9% vs. 0.0%, P = 0.009) were more frequently co-mutated with KIF5B-RET than CCDC6-RET. By contrast, CDKN2A (11.3% vs. 2.4%, P = 0.003) mutations were significantly enriched in CCDC6-RET-rearranged baseline patients. A significant increase in the proportion of CCDC6-RET was observed in acquired RET-rearranged patients (47.3% vs. 22.5%, P < 0.001). The median progression-free survival (PFS) of patients harboring RB1 and TP53 double-mutations (5.5 vs. 10.0 months, P = 0.020) or ERBB2 amplification (5.6 vs. 10.0 months, P = 0.041) was significantly shorter than the wild-type counterparts. Moreover, we identified that RET fusions were more likely associated with acquired resistance (AR) to third-generation EGFR-TKIs than previous generations of EGFR-TKIs.
Conclusions
In conclusion, we depicted the mutational profiles of NSCLC patients who harbor RET fusions at baseline or after resistance to EGFR-TKIs. Furthermore, our results suggest that RET fusions mediate secondary resistance to third-generation EGFR-TKIs and might be associated with poor prognosis in patients with NSCLC.
Similar content being viewed by others
Background
Non-small-cell lung cancer (NSCLC) is the leading cause of cancer-related mortality, which accounts for more than 80% of lung cancers, with lung adenocarcinoma (ADC) being the most common histological type. RET (Rearranged during transfection) gene fusions are present in approximately 1–2% of NSCLC and have emerged as a targetable oncogenic driver for NSCLC patients [1,2,3,4].
The fusion of RET with another unrelated gene occurs due to an aberrant DNA repair process [2]. The resulting fusion product activates various downstream signaling pathways that play essential roles in cell proliferation and survival [5]. Previous studies have shown that RET fusion-positive (RET+) NSCLC patients testing negative for EGFR/ALK/BRAF/ROS1 are usually young never-smokers with ADC [6,7,8,9]. While the most common RET fusion partners in NSCLC patients are KIF5B and CCDC6, other reported partners include NCOA4, TRIM33, etc. [10]. The overall survival of CCDC6-RET+ baseline patients was nearly three times longer than those with KIF5B-RET fusions (median: 113.5 vs. 37.7 months, P = 0.009) [11]. Besides, treatment responses of RET inhibitors were heterogeneous among baseline RET+ patients harboring different fusion variants [9, 11,12,13,14], highlighting the importance of fusion partner types in clinical outcomes toward targeted therapy.
More recently, receptor tyrosine kinase (RTK) fusions have emerged as a rare but targetable acquired resistance (AR) mechanism in EGFR-mutated NSCLC patients on EGFR-TKI treatment. Notably, RET fusions are the most commonly reported RTK fusions that mediate AR to EGFR-TKIs [15]. Within secondary RET fusions, CCDC6-RET is the most common fusion variant, followed by NCOA4-RET. Interestingly, NSCLC patients harboring KIF5B-RET fusions showed minimal response after RET TKI (RXDX-105) treatment, whereas the response rate was 67% in non-KIF5B-RET+ NSCLC patients [16]. Dual blockade of EGFR driver mutation and RET fusion, such as CCDC6-RET and NCOA4-RET, demonstrated safety and clinical efficacy in both clinical and preclinical studies [17].
To date, two highly potent RET-specific TKIs, selpercatinib and pralsetinib, have been approved by the US Food and Drug Administration (FDA) for the treatment of advanced or metastatic RET-altered NSCLC and thyroid cancers. Selpercatinib and pralsetinib effectively against RET alterations, including CCDC6-RET and KIF5B-RET fusions, RET activating mutations (C634W and M918T), and RET gatekeeper mutations V804L/M/E [18, 19]. Remarkably, selpercatinib has > 100-times selectivity against VEGFR2, and pralsetinib has 87-times selectivity against VEGFR2 and 20-times selectivity against JAK1 [20]. Findings from the phase I/II LIBRETTO-001 trial (NCT03157128) demonstrated that selpercatinib has an overall response rate (ORR) of 64% in previously treated NSCLC patients and ORR of 85% in treatment-naïve RET-altered NSCLC patients [21]. In addition, the antitumor potential of selpercatinib is irrespective of specific RET fusion types. On the other hand, initial data from the phase I/II ARROW trial (NCT03037385) demonstrated that pralsetinib has a high potency and durable activity and is well-tolerated in adult patients with metastatic RET-altered NSCLC. The ORR in previously treated patients was 61%, and ORR in treatment-naïve patients was 70% [22]. Most treatment-related adverse events (TRAE) of selpercatinib and pralsetinib are mild and controllable, including anemia, elevated alanine aminotransferase and hypertension [5, 20]. However, the safety profiles of these two RET inhibitors need to be further studied, given that some safety warnings have been reported [20,21,22,23,24].
In this study, we delineated the mutational profiles of 380 baseline and 71 EGFR-mutated NSCLC patients who acquired RET fusions after resistance to EGFR-TKIs by targeted NGS and revealed RET fusion partners associated with primary and acquired patients. We also investigated the impact of co-occurring genetic alterations in RET-rearranged NSCLC patients, which might explain the poor prognosis of patients harboring secondary RET fusions.
Methods
Patients and sample collection
Tumor tissue and/or plasma samples were collected from 451 RET+ NSCLC patients admitted to all participating hospitals between June 2015 and June 2021. Specifically, formalin-fixed paraffin-embedded (FFPE) tumor or fresh tumor tissue were confirmed by pathologists from the centralized clinical testing center. 5–10 mL of peripheral blood was collected from each patient in EDTA-coated tubes (BD Biosciences) and shipped to the clinical testing center within 48 h of blood collection for the following tests. Clinical characteristics and treatment history were extracted from medical records. Progression-free survival (PFS) was defined as the time from the initiation of the treatment to disease progression/patient death. Patients who had not progressed were censored at the date of their last follow-up. This study was conducted in accordance with the declaration of Helsinki and was approved by the Ethical Review Board of Fujian Medical University Union Hospital. Informed written consent was obtained from each subject before sample collection.
Targeted next-generation sequencing
DNA extraction, library construction, and targeted NGS were performed as previously described in a Clinical Laboratory Improvement Amendments (CLIA)-certified and College of American Pathologists (CAP)-accredited clinical testing laboratory (Nanjing Geneseeq Technology Inc., Nanjing, China) [25, 26]. FFPE samples were de-paraffinized with xylene followed by genomic DNA extraction using QIAamp DNA FFPE Tissue Kit (Qiagen Cat. No. 56404) according to the manufacturer’s instructions. Genomic DNA from fresh tumor tissue was extracted using the DNeasy Blood and Tissue Kit (Qiagen Cat. No. 69504) according to standard protocols. Peripheral blood samples were centrifuged at 1800g for 10 min, followed by cell-free DNA (cfDNA) extraction and purification using QIAamp Circulating Nucleic Acid Kit (Qiagen Cat. No. 55114). Genomic DNA of white blood cells in sediments was extracted using the DNeasy Blood and Tissue Kit (Qiagen Cat. No. 69504) as normal control. Genomic DNA was qualified using Nanodrop2000 (Thermo Fisher Scientific, Waltham, MA), and cfDNA fragment distribution was analyzed on a Bioanalyzer 2100 using the High Sensitivity DNA Kit (Agilent Technologies, Santa Clara, CA, 5067-4626). DNA quantification was performed using the dsDNA HS assay kit on a Qubit 3.0 fluorometer (Life Technology, US). NGS libraries were prepared using the KAPA Hyper Prep kit (KAPA Biosystems) with an optimized manufacturer’s protocol for different sample types. Targeted capture enrichment was performed as previously described [27]. The target-enriched library was then sequenced on HiSeq4000 or HiSeq4000 NGS platforms (Illumina) according to the manufacturer’s instructions.
Mutation calling
Sequencing data were processed as previously described [25]. In brief, the data was first demultiplexed and subjected to FASTQ file quality control using Trimmomatic [28]. Low-quality data (QC below 15) and N bases were removed. Raw reads were then mapped to the Human Genome (hg19) using Burrows-Wheeler Aligner (BWA-mem, v0.7.12; https://github.com/lh3/bwa/tree/master/bwakit). Genome Analysis Toolkit (GETK 3.4.0; https://software.broadinstitute.org/gatk/) was employed to perform local realignment, base quality score recalibration, and detect germline mutations. Picard was used to remove PCR duplicates. VarScan2 was applied to detect single-nucleotide variations (SNVs) and insertion/deletion mutations. The limit of detection (LOD) of tumor tissues and plasma samples under specific sequencing depths has been tested repetitively in Nanjing Geneseeq Technology Inc. to ensure the mutation calling results are consistent and have optimal performance for identifying genetic alterations. According to its internal specifications, sequencing of tissue and plasma ctDNA via targeted NGS can reach a sensitivity of 98% and a positive predictive value (PPV) of 95% [26, 29, 30]. SNVs were filtered out if the VAF was less than 1% for tumor tissue and 0.3% for plasma samples. Common SNVs were excluded if they were present in > 1% population in the 1000 Genomes Project or the Exome Aggregation Consortium (ExAC) 65,000 exomes database. The resulting mutation list was further filtered by an in-house list of recurrent artifacts based on a normal pool of whole blood samples. Parallel sequencing of matched white blood cells from each patient was performed to remove sequencing artifacts, germline variants, and clonal hematopoiesis. Genomic fusions were identified by FACTERA [31] with default parameters (≥ 2 reads). The fusion reads were manually reviewed and confirmed on Integrative Genomics Viewer (IGV). By definition, RET fusions were annotated as a protein fusion involving: (i) a 5′ non-RET partner gene and (ii) an intact 3′ RET kinase domain (NM_020975: exon 12–18). In this study, RET fusions were classified into canonical (single or compound KIF5B-RET and CCDC6-RET) and noncanonical RET. In this regard, a noncanonical RET fusion includes: (i) a rearrangement with a partner rather than KIF5B and CCDC6; (ii) A rearrangement with a novel partner gene; and (iii) a rearrangement with an intergenic region (IGR). Tumor mutational burden (TMB, mutation per Megabase) was determined based on the number of missense mutations in the targeted regions of the gene panel covering 0.85 Mb of coding genome, excluding known driver mutations as they are over-represented in the panel. Chromosome instability score (CIS) was defined as the proportion of the genome with aberrant (purity-adjusted segment-level copy number ≥ 3 or ≤ 1) segmented copy number [32]. For gene-level analyses in the baseline cohort, genetic alterations with a mutation frequency ≥ 2% were considered frequently mutated. All 139 genes in the PULMOCAN™ gene panel (Geneseeq Technology Inc.) were subjected to pathway enrichment analyses. Specifically, the genetic alterations identified by targeted panel sequencing were first categorized into ten signaling pathways associated with common hallmarks of cancer that control cell-cycle progression, apoptosis, cell proliferation and growth [33]. The proportion of baseline patients with specific RET fusion variants harboring mutation(s) in the relevant pathways was compared to reveal the differences of co-existing mutations in baseline RET+ patients.
Statistical analysis
Fisher’s exact tests were used to compare the categorical variables between groups. Kaplan–Meier curves were used to analyze the PFS of various patient groups, and the statistical difference was analyzed using the log-rank test. A two-sided P value of less than 0.05 was considered significant for all tests unless indicated otherwise (*P < 0.05, 0.01 < **P < 0.05, ***P < 0.001). All statistical analyses were performed using R (version 4.1.2).
Results
Patient overview
After excluding patients with either poor NGS quality samples or incomplete RET kinase domain fusions, 451 patients with NSCLC harboring RET fusions were included in the study cohort (Fig. 1). In particular, 380 patients had baseline RET fusions in EGFR-TKI treatment-naïve tissue samples, whereas 71 patients who carried baseline EGFR mutations acquired RET fusions after resistance to EGFR-TKIs. According to the EGFR-TKI treatment regimens, the 71 acquired RET+ patients were subdivided into three groups: (i) patients treated with first-line (1L) 1st- or 2nd-generation (G) EGFR-TKIs (N = 13), (ii) patients treated with second-line (2L) 3rd-G EGFR-TKIs (N = 51), and (iii) patients treated with 1L 3rd-G EGFR-TKIs (N = 7).
Patient overview. A total of 451 patients were included in the final analysis, of which 380 were baseline RET+ patients and 71 were EGFR-mutated patients who acquired RET fusions after resistance to EGFR-TKIs. Only baseline RET fusion-positive (RET+) patients with available FFPE and/or biopsy tumor samples were qualified for the following tests. Acquired RET+ patients (N = 71) were those who gained RET fusions after EGFR-TKI treatments targeting primary EGFR oncogenic mutations. Samples belonging to this category were divided into three groups depending on when RET fusion was detected. Specifically, group 1 contains 13 patients treated with first-line (1L) 1st- or 2nd-G EGFR-TKIs. Group 2 includes 51 patients who received second-line (2L) 3rd-G EGFR-TKI treatment. Lastly, group 3 consists of 7 patients previously treated with 1L 3rd-G EGFR-TKIs
In the baseline cohort, 58.9% of patients were under 60 years (58.9% vs. 41.1%, P < 0.001), and the proportion of females was significantly higher than males (55.3% vs. 44.7%, P = 0.038, Table 1). Of the 71 patients with acquired RET fusions, a higher proportion of younger (63.4% vs. 36.6%, P = 0.024) and female patients (64.8% vs. 35.2%, P = 0.013) was observed. In both baseline and acquired RET+ patient cohorts, ADC was the predominant histological subtype in NSCLC patients. All 71 patients harbored baseline EGFR mutations, including 47 with exon 19 deletions (19-Del, 66.2%), 23 with substitution mutation L858R (32.4%) and one G719C/S768I double-mutant patient (1.4%).
Distribution of RET fusion partners between primary and acquired RET+ patients
By targeted NGS, a total of 491 RET fusions were identified in 451 RET+ patients (Fig. 2a), of which KIF5B-RET (51.1%) was the most frequently observed RET rearrangement, followed by CCDC6-RET (23.4%). Notably, 39 patients (8.6%) carried more than one RET fusion, of whom 36 were baseline and 3 were acquired RET+ patients. Next, we tried to depict functional domains and breakpoints of canonical RET fusions. In particular, 78% of KIF5B-RET (195/251) fusions contained the kinesin motor domain and coiled coil domain from exons 1–15 of KIF5B and the kinase domain from exons 12–18 of RET (Fig. 2b). In comparison, 78% (90/115) of CCDC6-RET fusions contained a fused coiled coil domain from exon 1 of CCDC6 and the kinase domain from exons 12–18 of RET. These results were consistent with previous findings, showing that most genomic breakpoints are located within intron 11 of RET, while translocation events may also occasionally occur within intron 7 and 10, resulting in the inclusion of the RET transmembrane domain [34].
Distribution of RET fusions. a RET fusions identified by targeted NGS in the total patient cohort (N = 451). b A schematic demonstration of functional domains in KIF5B-RET and CCDC6-RET fusions identified in the study cohort. Descriptions on the right indicate exons in the partner gene and RET gene. KIF5B-RET fusion proteins contain the kinesin motor domain (orange), the coiled coil domain (blue) from KIF5B, and the kinase domain (pink) of RET, or the transmembrane domain (grey) and the kinase domain of RET. CCDC6-RET fusions contain the coiled coil domain (yellow) of CCDC6 and the kinase domain of RET, or the transmembrane domain and the kinase domain of RET. c, d Distribution of RET fusion partner genes in baseline (c) and acquired (d) RET+ patients
The next question we wanted to address was whether the percentage of specific RET fusion variants differs in baseline and acquired RET+ patients. We identified 417 and 74 RET rearrangements in these two cohorts, respectively (Fig. 2c, d). The most commonly observed fusion variants in baseline patients were KIF5B-RET (53.7%), followed by CCDC6-RET (22.5%, Fig. 2c). Surprisingly, KIF5B-RET accounted for only 8.1% of total translocation events in baseline EGFR-mutated NSCLC patients resistant to EGFR-TKIs, whereas CCDC6-RET was identified in 47.3% of translocations events, ranking as the predominant RET fusion variant in acquired RET+ patients (Fig. 2d). The identification of CCDC6-RET was the most common fusion variant, followed by NCOA4-RET, was consistent with previous findings [15]. Furthermore, these results implied that KIF5B-RET and non-KIF5B-RET fusions might have different functionalities in NSCLC. At the same time, noncanonical RET fusions, including those with hitherto unreported partner genes [10], were identified in 23.8% and 44.6% of baseline and secondary patient cohorts, respectively (Additional file 1: Table S1).
As RET fusion variants distributed differentially in baseline and acquired RET+ patients, we investigated whether RET fusion types could be associated with the patient's clinical manifestations. Interestingly, females were more likely associated with KIF5B-RET (P = 0.006) and noncanonical RET fusions (P = 0.015) than CCDC6-RET in baseline patients (Additional file 2: Fig. S1a). However, neither the patient’s age nor cancer stage at diagnosis was directly associated with RET fusion types in baseline or secondary patients (Additional file 2: Fig. S1b-e).
RET fusion as a primary oncogenic driver in NSCLC patients
RET fusions are oncogenic drivers that usually occur mutually exclusive to other driver genes in NSCLC patients. Research to date has not yet addressed why RET-rearranged patients with different fusion partners responded differently to treatments. We hypothesized that this could result from different mutational profiles among baseline RET+ patients. Accordingly, we analyzed the NGS data of 380 baseline RET+ patients and profiled their genomic landscape. Genetic alterations, such as TP53, MDM2, CDKN2A/B, ATM, and RB1, were frequently detected in baseline RET+ patients (Fig. 3a). Notably, although 94.5% (360/380) of our baseline RET+ patients contained only RET fusions as the primary oncogenic driver of NSCLC, 21 patients (5.5%) harbored additional driver gene aberrations, including EGFR (11/21), KRAS (6/21), BRAF (2/21), ERBB2 (1/21) and ROS1 (1/21) (Additional file 3: Fig. S2a). However, there was no significant correlation between the patient number and the patient’s clinical characteristics (Additional file 3: Fig. S2b).
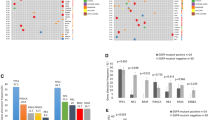
The genomic landscape of baseline RET fusion-positive patients. a Distribution of genetic alterations associated with baseline RET+ patients (N = 380). The distribution of somatic mutations (top) and CNVs (bottom) in baseline patients were assessed by targeted NGS. Each column represents one patient. Clinical characteristics of baseline RET+ patients are shown at the top. The frequency of each gene alteration is listed on the right. b Top frequently mutated gene alterations identified in RET + baseline patients. *P < 0.05, **P < 0.01, ***P < 0.001. c The correlation between signaling pathways in which the concurrent mutations occur and different types of RET fusions. The bar graph illustrates the proportion of baseline RET fusion-positive patients harboring genetic alterations in the relevant pathways. *P < 0.05, **P < 0.01, ***P < 0.001
Our next objective was to investigate whether specific genomic alterations could be associated with baseline patients harboring specific RET fusion variants. Hence, we performed both gene- and pathway-level enrichment analyses using targeted NGS results of tumor tissues collected from 380 baseline RET+ patients. EGFR mutations were more commonly found in patients with CCDC6-RET (5.0% vs. 0.4%, P = 0.014) and noncanonical RET fusions (7.1% vs. 0.4%, P < 0.001) compared to KIF5B-RET fusions (Fig. 3b). Meanwhile, co-existing mutations in PTEN were more frequently associated with noncanonical RET fusions than KIF5B-RET fusions (7.1% vs. 0.4%, P = 0.042). In addition, CDKN2A mutations were more frequently co-existed with CCDC6-RET than KIF5B-RET (11.3% vs. 2.4%, P = 0.003), while SMAD4 mutations (5.3% vs. 0.0%, P = 0.044) and MYC amplification (6.9% vs. 0.0%, P = 0.009) were more frequently found in patients with KIF5B-RET fusions than those with CCDC6-RET fusions. By performing pathway-level analyses, we noticed that noncanonical RET fusions were more likely to be associated with mutations in the PI3K and RAS/RTK pathways (Fig. 3c). In great contrast, the aberrant MYC pathway more frequently co-occurred with KIF5B-RET than CCDC6-RET (7.8% vs. 0.0%, P < 0.001) in baseline RET-rearranged patients with NSCLC. None of the other oncogenic pathways examined showed a significant difference among patients harboring KIF5B-RET or CCDC6-RET fusions.
Lastly, we examined tumor mutational burden (TMB) and chromosomal instability in baseline RET+ patients, where we found no significant difference in TMB, chromosomal instability score (CIS), or arm-level copy number variations (CNVs) among different types of RET fusions (Additional file 4: Fig. S3). From the above analysis, we depicted mutational profiles of RET+ patients and identified concomitant mutations that may contribute to the differential treatment responses in RET-rearranged patients.
RET fusion confers a secondary resistance mechanism to EGFR-TKIs
Despite their rarity, it is clear from previous studies that RTK fusions, such as RET rearrangements, are actionable resistance mechanisms to EGFR-TKIs. We aim to increase awareness of this emerging paradigm by comprehensively profiling baseline EGFR-mutated NSCLC patients who acquired RET fusions after developing resistance to EGFR-TKIs. In acquired RET+ patients, second-site EGFR mutations, such as T790M (50.7%), C797S/G (16.9%) and L718V/Q (5.6%), were among the top frequently mutated gene alterations (Fig. 4a). It was also apparent that cell cycle control pathway gene alterations, such as TP53, RB1, CDKN2A, CDKN2B, CDKN1B, and CDK6, co-existed with acquired RET fusions. In addition, ALK and NTRK1 fusions that belong to the RTK family were identified to co-occur with RET fusion in two patients. These results implied that RTK fusions and other genetic variants could potentially serve as resistance mechanisms to anti-EGFR treatment in NSCLC.
Somatic gene alterations identified in acquired RET+ patients who exhibit resistance to EGFR-TKIs. a Genomic landscape of somatic gene alterations in patients with acquired RET fusions (N = 71). Grouping of patients is based on EGFR-TKI treatment regimens. Each column represents one patient. The clinical characteristics of each patient are shown on the top. The percentage on the right shows the mutation frequency of each gene. Grouping of second-line PFS was in line with the results of the AURA3 study (mPFS duration of 10.1 months) [43]. b Kaplan–Meier estimates of PFS in acquired RET+ patients who previously received 2L 3rd-G EGFR-TKI therapy (N = 49, see Additional file 5: Fig. S4b for patient assortment) with or without bypass pathway mutations (activating mutations in KRAS and PIK3CA, copy-number gain in ERBB2 and MET, fusions in ALK and NTRK). c Kaplan–Meier estimates of PFS comparing patients with or without double-mutated RB1 and TP53 genes. d Kaplan–Meier estimates of PFS comparing patients with or without ERBB2 amplification. e The incidence of RET fusions in EGFR-mutant NSCLC patients previously treated with different EGFR-TKIs
To characterize the AR mechanism through RET fusions in EGFR-mutated NSCLC patients and their survival outcomes, we compared the PFS among patients treated with different EGFR-TKI regimens. However, no significant difference was observed (Additional file 5: Fig. S4a). Alternatively, as 2L 3rd-G EGFR-TKI treatment has been employed as a standard practice for EGFR-mutated NSCLC patients with progressive disease on first-line targeted therapy, we performed integrated survival analyses using group 2 patients. Notably, we excluded one patient with the rare EGFR double-mutants and one with SCC histology, leaving 49 patients with only EGFR 19-Del or L858R mutations in the refined cohort (Additional file 5: Fig. S4b). We first performed a univariate analysis on various demographic and mutational features of refined cohort 2 patients. Interestingly, bypass pathway genetic alterations (KRAS and PIK3CA activation mutations, ERBB2 and MET amplification, ALK and NTRK fusions), RB1 and TP53 co-mutation, and ERBB2 copy-number gain had significant associations with the prognosis of acquired RET+ patients who underwent 2L 3rd-G EGFR-TKI treatment (Additional file 1: Table S2). We then performed multivariate analysis using these three features, and we discovered that they were no longer statistically significant, with bypass mutations and RB1and TP53 co-mutations being close to significance. This result suggested that there might be some interactions or correlations among these prognostic features. Nonetheless, the PFS of patients harboring bypass pathway gene alterations was significantly shorter than wild-type patients (median: 6.7 vs. 12.0 months, P = 0.020, Fig. 4b). In addition, the PFS of patients with TP53 and RB1 co-mutations was significantly shorter than wild-type counterparts (median: 5.5 vs. 10.0 months, P = 0.02, Fig. 4c). ERBB2 gene amplifications that were present in 5.6% of acquired RET+ patients were also associated with poor prognosis in NSCLC patients (median: 5.6 vs. 10.0 months, P = 0.041, Fig. 4d). Notably, although the majority of acquired RET fusions were detected by circulating nucleic acid method using plasma ctDNA, 4 out of 49 patients in the refined cohort contributed only FFPE samples, raising a concern of missing rare fusions compared to tumor-based comprehensive genomic profiling. We, thereby, excluded patients with only tissue samples and re-performed the analysis in Fig. 4 using only ctDNA data (Additional file 6: Fig. S5a). Overall, our results about the co-occurring genetic alterations were consistent when using either all samples (tissue and ctDNA) or only ctDNA (Additional file 6: Fig. S5b-d), suggesting that our conclusions are not likely to be affected by different sample types.
Collectively, comprehensive molecular profiling of acquired RET + patients showed that co-existing genomic alterations, such as TP53 and RB1 co-mutations and ERBB2 amplification, might be associated with a poor prognosis in EGFR-mutated NSCLC patients upon drug resistance.
RET fusions are more frequently associated with third-generation EGFR-TKIs
Previous case reports have linked RET fusions, such as CCDC6-RET, TRIM24-RET, NCOA4-RET and ERC1-RET, to osimertinib resistance in EGFR-mutated NSCLC patients [35,36,37,38,39]. Here, we demonstrated a higher incidence rate of RET fusions in patients who developed AR to 3rd-G EGFR-TKIs from a population perspective. Interestingly, the incidence of RET fusions was highest in patients who underwent 2L 3rd-G EGFR-TKI treatment (1.5%, 51/3330), followed by patients treated with 1L 3rd-G EGFR-TKIs (1.2%, 7/589, Fig. 4e). These numbers were about ten-fold higher than those in patients treated with first- or second-generation EGFR-TKIs at the front line (0.15%, 13/8732). Overall, we demonstrated that RET fusions were more likely associated with EGFR-mutant NSCLC patients who received therapeutic interventions targeting EGFR with third-generation EGFR-TKIs. The complexity of non-RET secondary mutations in acquired RET+ patients might contribute to the differential treatment responses to follow-up therapies.
Discussion
With comprehensive genomic profiling, clinicians can use the knowledge of specific clinical features associated with individual alterations to optimize therapeutic decision-making. Here, we retrospectively analyzed the mutational profiles of 451 patients carrying either baseline or acquired RET fusions to characterize the roles of RET fusions in NSCLC.
As a rare oncogenic driver mutation, RET rearrangement occurs in 1–2% of NSCLC patients [1, 2]. It has been shown that RET-rearranged NSCLC patients are more likely associated with ADC. Consistent with this notion, we found that 76.1% of patients developed ADC as the predominant histological type in the baseline cohort. On the other hand, a much-debated question is whether baseline RET fusions can correlate with the patient’s gender or age [8, 40, 41]. Hence, we compared clinical characteristics of baseline patients with RET fusions, such as gender and age. Our results showed that primary RET fusions were more likely to occur in females than males (55.3% vs. 44.7%, P = 0.038) and were associated with younger patients (58.9% vs. 41.1%, P < 0.001). The controversy could be due to variations in the subject’s ethnicity, genetic background, environmental factors, and even lifestyles. Then, we compared whether the distribution of specific RET fusions differed in baseline and acquired RET+ patients. We found that the predominant fusion type in baseline patients was KIF5B-RET, whereas CCDC6-RET was most frequently identified in patients who acquired RET fusions at resistance to EGFR-TKIs. The proportion of patients harboring NCOA4-RET fusions also increased from 1.0 to 16.2% in acquired RET+ patients. These results were consistent with previous findings, suggesting that KIF5B-RET and non-KIF5B-RET fusions might have different functionalities in EGFR-TKI progression [17, 35]. Lastly, we characterized mutational profiles of the 380 baseline patients and demonstrated that concurrent gene alterations, such as SMAD4 mutations and MYC copy-number gain, were more frequently associated with KIF5B-RET than CCDC6-RET fusions in baseline RET-rearranged NSCLC patients. Our results may provide insights into why NSCLC patients harboring KIF5B-RET fusions have a nearly three times shorter overall survival than those harboring CCDC6-RET fusions.
The diversity and complexity of molecular mechanisms underlying the acquired adaptation of cancer cells to targeted therapies, such as EGFR-TKIs, is an area of active investigation. In this study, we demonstrated that RET fusions, as a rare but actionable AR mechanism to EGFR-TKIs, confer a poor prognosis in EGFR-mutated NSCLC patients. It has been previously suggested that patients harboring TP53 and RB1 co-mutations are at unique risk of histologic transition from ADC to SCC or eventually small cell transformation [42]. Here, our results showed that RET+ patients with co-occurring TP53 and RB1 double-mutations had significantly shorter PFS than wild-type patients, highlighting the role of TP53 and RB1 in controlling cell proliferation and disease progression. In addition, ERBB2 copy-number gain can also present a similar effect, resulting in reduced PFS in patients who acquired RET fusions at resistance to EGFR-TKIs. It should also be noted that prognostic-related factors being examined in the survival analyses, including bypass pathway gene alterations, TP53 and RB1 co-mutations, and ERBB2 copy-number gain, were not completely independent. The limited sample size may have impaired the statistical power to achieve significant results. Further studies using larger cohorts that consider additional variables need to be undertaken to better understand prognostic factors associated with the patient's survival.
In the final part of our study, we compared the incidence of secondary RET fusions in NSCLC patients who progressed on different EGFR-TKI therapies. A ten-fold higher incidence of RET fusions was observed in patients who underwent third-generation EGFR-TKI treatment than those treated with front-line 1st-/2nd-G EGFR-TKIs. This result was consistent with a previous finding which suggested that RET fusions were significantly enriched after 3rd-G EGFR-TKI treatment [15]. As previously mentioned, selpercatinib and pralsetinib have been granted FDA approval, showing equipotent for treating RET-rearranged NSCLC and thyroid cancer with minor and controllable adverse effects. The ORR of previously treated RET+ NSCLC patients on selpercatinib and pralsetinib were 64% and 61%, respectively, while the median PFS of previously treated patients on these two RET inhibitors was 16.5 months and 17.1 months, respectively [21, 22]. Given the high efficacy and mild side effects of the two RET-specific inhibitors, it is worth investigating their clinical utility to treat EGFR-mutated NSCLC patients who acquired RET oncogenic alterations after TKI resistance, especially those receiving 3rd-G EGFR-TKIs.
Despite efforts we made to systemically characterize the mutational profiles of baseline and acquired RET+ patients, there are limitations in our study. We did not validate the authenticity of RET fusions with hitherto unreported partner genes. However, we reasoned that patients without known driver mutations might harbor functional RET fusions since RET fusions usually occur mutually exclusively. Due to the unavailability of RET-specific inhibitors during the patient’s treatment, we could not evaluate the PFS of baseline patients with different RET fusion partners on RET-specific inhibitors or patients with acquired RET fusions after EGFR-TKI resistance. However, dynamic monitoring of these acquired RET+ patients is currently ongoing. We intend to investigate the treatment outcomes for EGFR-mutated NSCLC patients harboring acquired RET fusions on the follow-up RET-specific inhibitor therapy upon obtaining more data on these patients.
Conclusions
In conclusion, we systematically evaluated the mutational profiles of RET+ baseline patients and those who acquired RET fusions at secondary resistance to EGFR-TKIs. We identified unique genetic features explaining the differential treatment responses in NSCLC patients harboring baseline RET fusions. Furthermore, we demonstrated that 3rd-G EGFR-TKIs are more likely associated with secondary RET fusions. The high efficacy and mild side effects of the two RET-specific inhibitors may provide treatment options for EGFR-mutated NSCLC patients who developed RET fusions following TKI resistance, especially those on 3rd-G EGFR-TKI treatments.
Availability of data and materials
As the study involved human participants, the data cannot be made freely available in the manuscript nor a public repository because of ethical restrictions. However, the datasets generated and/or analyzed during this current study are available from the corresponding author on reasonable request.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- RET :
-
Rearranged during transfection
- RET+:
-
RET fusion-positive
- EGFR :
-
epidermal growth factor
- EGFR-TKIs:
-
EGFR-tyrosine kinase inhibitors
- FDA:
-
Food and Drug Administration
- TRAE:
-
Treatment-related adverse effects
- G:
-
Generation
- NGS:
-
Next-generation sequencing
- PFS:
-
Progression-free survival
- AR:
-
Acquired resistance
- CLIA:
-
Clinical Laboratory Improvement Amendments
- CAP:
-
College of American Pathologists
- ADC:
-
Adenocarcinoma
- ASC:
-
Adenosquamous cell carcinoma
- SCC:
-
Squamous cell carcinoma
- LCC:
-
Large cell carcinoma
- NOS:
-
Not otherwise specified
- FFPE:
-
Formalin-fixed paraffin-embedded
- SNVs:
-
Single-nucleotide variations
- VAF:
-
Variant allele frequency
- IGR:
-
Intergenic region
- TMB:
-
Tumor mutational burden
- CIS:
-
Chromosome instability score
- 19-Del:
-
EGFR Exon 19 deletions
- IGV:
-
Integrative genomics viewer
- 1L:
-
First-line
- 2L:
-
Second-line
- CNVs:
-
Copy-number variations
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, Sakamoto H, Tsuta K, Furuta K, Shimada Y, Iwakawa R, Ogiwara H, Oike T, Enari M, Schetter AJ, Okayama H, Haugen A, Skaug V, Chiku S, Yamanaka I, Arai Y, Watanabe S, Sekine I, Ogawa S, Harris CC, Tsuda H, Yoshida T, Yokota J, Shibata T. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18(3):375–7.
Lipson D, Capelletti M, Yelensky R, Otto G, Parker A, Jarosz M, Curran JA, Balasubramanian S, Bloom T, Brennan KW, Donahue A, Downing SR, Frampton GM, Garcia L, Juhn F, Mitchell KC, White E, White J, Zwirko Z, Peretz T, Nechushtan H, Soussan-Gutman L, Kim J, Sasaki H, Kim HR, Park SI, Ercan D, Sheehan CE, Ross JS, Cronin MT, Janne PA, Stephens PJ. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18(3):382–4.
Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, Asaka R, Hamanaka W, Ninomiya H, Uehara H, LimChoi Y, Satoh Y, Okumura S, Nakagawa K, Mano H, Ishikawa Y. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18(3):378–81.
Wang R, Hu H, Pan Y, Li Y, Ye T, Li C, Luo X, Wang L, Li H, Zhang Y, Li F, Lu Y, Lu Q, Xu J, Garfield D, Shen L, Ji H, Pao W, Sun Y, Chen H. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30(35):4352–9.
Thein KZ, Velcheti V, Mooers BHM, Wu J, Subbiah V. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer. 2021;7(12):1074–88.
Hess LM, Han Y, Zhu YE, Bhandari NR, Sireci A. Characteristics and outcomes of patients with RET-fusion positive non-small lung cancer in real-world practice in the United States. BMC Cancer. 2021;21(1):28.
Ferrara R, Auger N, Auclin E, Besse B. Clinical and translational implications of RET rearrangements in non-small cell lung cancer. J Thorac Oncol. 2018;13(1):27–45.
Michels S, Scheel AH, Scheffler M, Schultheis AM, Gautschi O, Aebersold F, et al. Clinicopathological characteristics of RET rearranged lung cancer in European patients. J Thorac Oncol. 2016;11(1):122–7.
Gautschi O, Milia J, Filleron T, Wolf J, Carbone DP, Owen D, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET Registry. J Clin Oncol. 2017;35(13):1403–10.
Ou SI, Zhu VW. Catalog of 5′ fusion partners in RET+ NSCLC Circa 2020. JTO Clin Res Rep. 2020;1(2): 100037.
Tan AC, Seet AOL, Lai GGY, Lim TH, Lim AST, Tan GS, Takano A, Tai DWM, Tan TJY, Lam JYC, Ng MCH, Tan WL, Ang MK, Kanesvaran R, Ng QS, Jain A, Rajasekaran T, Lim WT, Tan EH, Lim TKH, Tan DSW. Molecular characterization and clinical outcomes in RET-rearranged NSCLC. J Thorac Oncol. 2020;15(12):1928–34.
Drilon A, Wang L, Hasanovic A, Suehara Y, Lipson D, Stephens P, Ross J, Miller V, Ginsberg M, Zakowski MF, Kris MG, Ladanyi M, Rizvi N. Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov. 2013;3(6):630–5.
Drilon A, Rekhtman N, Arcila M, Wang L, Ni A, Albano M, Van Voorthuysen M, Somwar R, Smith RS, Montecalvo J, Plodkowski A, Ginsberg MS, Riely GJ, Rudin CM, Ladanyi M, Kris MG. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17(12):1653–60.
Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Matsumoto S, Kohno T, Tsuta K, Tsuchihara K, Ishii G, Nomura S, Sato A, Ohtsu A, Ohe Y, Goto K. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med. 2017;5(1):42–50.
Zhu VW, Klempner SJ, Ou SI. Receptor tyrosine kinase fusions as an actionable resistance mechanism to EGFR TKIs in EGFR-mutant non-small-cell lung cancer. Trends Cancer. 2019;5(11):677–92.
Clifton K, Rich TA, Parseghian C, Raymond VM, Dasari A, Pereira AAL, Willis J, Loree JM, Bauer TM, Chae YK, Sherrill G, Fanta P, Grothey A, Hendifar A, Henry D, Mahadevan D, Nezami MA, Tan B, Wainberg ZA, Lanman R, Kopetz S, Morris V. Identification of actionable fusions as an anti-EGFR resistance mechanism using a circulating tumor DNA assay. JCO Precis Oncol. 2019. https://doi.org/10.1200/PO.19.00141.
Piotrowska Z, Isozaki H, Lennerz JK, Gainor JF, Lennes IT, Zhu VW, Marcoux N, Banwait MK, Digumarthy SR, Su W, Yoda S, Riley AK, Nangia V, Lin JJ, Nagy RJ, Lanman RB, Dias-Santagata D, Mino-Kenudson M, Iafrate AJ, Heist RS, Shaw AT, Evans EK, Clifford C, Ou SI, Wolf B, Hata AN, Sequist LV. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov. 2018;8(12):1529–39.
Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, Wirth LJ, Stock S, Smith S, Lauriault V, Corsi-Travali S, Henry D, Burkard M, Hamor R, Bouhana K, Winski S, Wallace RD, Hartley D, Rhodes S, Reddy M, Brandhuber BJ, Andrews S, Rothenberg SM, Drilon A. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29(8):1869–76.
Subbiah V, Shen T, Terzyan SS, Liu X, Hu X, Patel KP, Hu M, Cabanillas M, Behrang A, Meric-Bernstam F, Vo PTT, Mooers BHM, Wu J. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann Oncol. 2021;32(2):261–8.
Ali F, Neha K, Chauhan G. Pralsetinib: chemical and therapeutic development with FDA authorization for the management of RET fusion-positive non-small-cell lung cancers. Arch Pharm Res. 2022;45(5):309–27.
Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med. 2020;383(9):813–24.
Gainor JF, Curigliano G, Kim DW, Lee DH, Besse B, Baik CS, Doebele RC, Cassier PA, Lopes G, Tan DSW, Garralda E, Paz-Ares LG, Cho BC, Gadgeel SM, Thomas M, Liu SV, Taylor MH, Mansfield AS, Zhu VW, Clifford C, Zhang H, Palmer M, Green J, Turner CD, Subbiah V. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22(7):959–69.
Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, Brose MS, Zhu VW, Leboulleux S, Bowles DW, Baik CS, Adkins D, Keam B, Matos I, Garralda E, Gainor JF, Lopes G, Lin CC, Godbert Y, Sarker D, Miller SG, Clifford C, Zhang H, Turner CD, Taylor MH. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021;9(8):491–501.
Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825–35.
Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu X, Bao H, Tong X, Wang X, Shao YW, Liu Y, Wang Y, Zhou C. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer patients. Clin Cancer Res. 2018;24(13):3097–107.
Shu Y, Wu X, Tong X, Wang X, Chang Z, Mao Y, Chen X, Sun J, Wang Z, Hong Z, Zhu L, Zhu C, Chen J, Liang Y, Shao H, Shao YW. Circulating tumor DNA mutation profiling by targeted next generation sequencing provides guidance for personalized treatments in multiple cancer types. Sci Rep. 2017;7(1):583.
Fang W, Ma Y, Yin JC, Hong S, Zhou H, Wang A, Wang F, Bao H, Wu X, Yang Y, Huang Y, Zhao H, Shao YW, Zhang L. Comprehensive genomic profiling identifies novel genetic predictors of response to anti-PD-(L)1 therapies in non-small cell lung cancer. Clin Cancer Res. 2019;25(16):5015–26.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Jin Y, Bao H, Lin X, Fan X, Tang M, Shi X, Zhao J, Yan J, Xu Y, Quek K, Elamin YY, Zhang J, Futreal PA, Wistuba I, Heymach JV, Lou G, Shao L, Hel Q, Lin C, Wu X, Shao YW, Wang X, He J, Chen Y, Stebbing J, Chen M, Zhang J, Yu X. Distinct co-acquired alterations and genomic evolution during TKI treatment in non-small-cell lung cancer patients with or without acquired T790M mutation. Oncogene. 2020;39(9):1846–59.
Li B, Qu H, Zhang J, Pan W, Liu M, Yan X, Huang X, He X, Lin D, Liu S, Guan R, Wu Y, Ou Q, Bao H, Xu Y, Wu X, Shao Y, Lin N. Genomic characterization and outcome evaluation of kinome fusions in lung cancer revealed novel druggable fusions. NPJ Precis Oncol. 2021;5(1):81.
Newman AM, Bratman SV, Stehr H, Lee LJ, Liu CL, Diehn M, Alizadeh AA. FACTERA: a practical method for the discovery of genomic rearrangements at breakpoint resolution. Bioinformatics. 2014;30(23):3390–3.
Turajlic S, Xu H, Litchfield K, Rowan A, Chambers T, Lopez JI, et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell. 2018;173(3):581-594 e12.
Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173(2):321-337 e10.
Drilon A, Hu ZI, Lai GGY, Tan DSW. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15(3):151–67.
Rich TA, Reckamp KL, Chae YK, Doebele RC, Iams WT, Oh M, Raymond VM, Lanman RB, Riess JW, Stinchcombe TE, Subbiah V, Trevarthen DR, Fairclough S, Yen J, Gautschi O. Analysis of cell-free DNA from 32,989 advanced cancers reveals novel co-occurring activating RET alterations and oncogenic signaling pathway aberrations. Clin Cancer Res. 2019;25(19):5832–42.
Papadimitrakopoulou VA, Wu YL, Han JY, Ahn MJ, Ramalingam SS, John T, Okamoto I, Yang JCH, Bulusu KC, Laus G, Collins B, Barrett JC, Chmielecki J, Mok TSK. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol. 2018;29:viii741.
Oxnard GR, Hu Y, Mileham KF, Husain H, Costa DB, Tracy P, Feeney N, Sholl LM, Dahlberg SE, Redig AJ, Kwiatkowski DJ, Rabin MS, Paweletz CP, Thress KS, Janne PA. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4(11):1527–34.
Le X, Puri S, Negrao MV, Nilsson MB, Robichaux J, Boyle T, Hicks JK, Lovinger KL, Roarty E, Rinsurongkawong W, Tang M, Sun H, Elamin Y, Lacerda LC, Lewis J, Roth JA, Swisher SG, Lee JJ, William WN Jr, Glisson BS, Zhang J, Papadimitrakopoulou VA, Gray JE, Heymach JV. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res. 2018;24(24):6195–203.
Puri S, Hicks J, Knepper T, Smith M, Boyle T, Gray J. MA 1205 genomic profiling of EGFR T790M mutated non-small cell lung cancer to evaluate the mechanisms of resistance to osimertinib. J Thorac Oncol. 2017;12(11):S1848–9.
Zhang K, Chen H, Wang Y, Yang L, Zhou C, Yin W, Wang G, Mao X, Xiang J, Li B, Zhang T, Fei S. Clinical characteristics and molecular patterns of RET-rearranged lung cancer in chinese patients. Oncol Res. 2019;27(5):575–82.
Tsuta K, Kohno T, Yoshida A, Shimada Y, Asamura H, Furuta K, Kushima R. RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer. 2014;110(6):1571–8.
Offin M, Chan JM, Tenet M, Rizvi HA, Shen R, Riely GJ, Rekhtman N, Daneshbod Y, Quintanal-Villalonga A, Penson A, Hellmann MD, Arcila ME, Ladanyi M, Pe’er D, Kris MG, Rudin CM, Yu HA. Concurrent RB1 and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J Thorac Oncol. 2019;14(10):1784–93.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA, Investigators A. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–40.
Acknowledgements
The authors sincerely thank the patients, their families, investigators, and research staff involved.
Funding
This work was supported by the Science and Technology Project of Nantong City (JC2020071). The funder had no role in the study design, data collection or analysis, preparation of the manuscript, or the decision to publish.
Author information
Authors and Affiliations
Contributions
CYW and ZLZ designed the study. YLS, SW, MMW, QXO and YX performed data acquisition. CYW, ZLZ, YLS, SW, MMW, QXO, and YX analyzed the data. CYW, ZLZ, SW, QXO and YX prepared the manuscript. SW, QXO and YX edited the manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the institution's ethical standards. Written consent was obtained from each patient.
Consent for publication
The informed consent form was obtained from each patient.
Competing interests
SW, MMW, QXO, YX and YS are employees of Nanjing Geneseeq Technology Inc. The remaining authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Novel RET partner genes in NSCLC identified in the study cohort. Table S2. Univariate and multivariate analysis using the refined cohort.
Additional file 2: Figure S1.
Correlation between patient’s clinical characteristics and RET fusion types. a-c Stacked bar plots demonstrate whether gender (a), age (b), and cancer stage at diagnosis (c) are associated with RET fusion subtypes in baseline RET + patients. Asterisks represent the significance level between two categorical variants based on Fisher’s exact test. **P < 0.01. d-e In acquired RET + patients, no significant correlation was observed between the patient’s gender (d) or age (e) and RET fusion types.
Additional file 3: Figure S2.
Baseline RET fusions co-occurred with other oncogenic drivers. a The bar plot illustrates the percentage of baseline patients with (dark blue, 21/380) or without (yellow, 360/380) non-RET oncogenic driver mutations. The pie chart demonstrates the distribution of concurrent driver mutations in these patients. b Clinical characteristics of acquired RET + patients who harbored non-RET driver mutations.
Additional file 4: Figure S3.
RET fusion type has no significant impact on TMB, CIS, or arm-level changes. No significant difference in TMB (a), chromosomal instability score (b) or arm-level changes (c-d) was observed among baseline patients with different RET fusions.
Additional file 5: Figure S4.
The refinement of group 2 patients. a Kaplan–Meier estimates of PFS in patients treated with different EGFR-TKI regimens. b The flowchart demonstrates selecting qualified patients within group 2 for the following survival analyses. Patients with non-classic EGFR mutations or non-ADC histology were excluded.
Additional file 6: Figure S5.
Survival analyses of patients with plasma ctDNA. a Patient stratification. Four patients with only FFPE samples were excluded from the refined cohort. b-d Kaplan–Meier estimates of PFS in patients with bypass pathway genetic alterations (b), RB1 and TP53 double-mutations (c), and ERBB2 copy-number gain (c) versus corresponding wild-type patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, C., Zhang, Z., Sun, Y. et al. RET fusions as primary oncogenic drivers and secondary acquired resistance to EGFR tyrosine kinase inhibitors in patients with non-small-cell lung cancer. J Transl Med 20, 390 (2022). https://doi.org/10.1186/s12967-022-03593-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03593-3