Abstract
Background
Established prediction models of Diabetic kidney disease (DKD) are limited to the analysis of clinical research data or general population data and do not consider hospital visits. Construct a 3-year diabetic kidney disease risk prediction model in patients with type 2 diabetes mellitus (T2DM) using machine learning, based on electronic medical records (EMR).
Methods
Data from 816 patients (585 males) with T2DM and 3 years of follow-up at the PLA General Hospital. 46 medical characteristics that are readily available from EMR were used to develop prediction models based on seven machine learning algorithms (light gradient boosting machine [LightGBM], eXtreme gradient boosting, adaptive boosting, artificial neural network, decision tree, support vector machine, logistic regression). Model performance was evaluated using the area under the receiver operating characteristic curve (AUC). Shapley additive explanation (SHAP) was used to interpret the results of the best performing model.
Results
The LightGBM model had the highest AUC (0.815, 95% CI 0.747–0.882). Recursive feature elimination with random forest and SHAP plot based on LightGBM showed that older patients with T2DM with high homocysteine (Hcy), poor glycemic control, low serum albumin (ALB), low estimated glomerular filtration rate (eGFR), and high bicarbonate had an increased risk of developing DKD over the next 3 years.
Conclusions
This study constructed a 3-year DKD risk prediction model in patients with T2DM and normo-albuminuria using machine learning and EMR. The LightGBM model is a tool with potential to facilitate population management strategies for T2DM care in the EMR era.
Similar content being viewed by others
Background
Diabetic kidney disease (DKD) is a leading cause of end-stage renal disease (ESRD), cardiovascular (CV) disease, and all-cause morbidity and mortality in patients with diabetes [1]. Notably, diabetes and chronic kidney disease (CKD) are risk factors for severe COVID-19 infection and poor outcomes [2, 3]. Early identification of patients with diabetes who are at high risk for DKD will inform clinical decision-making. Understanding the risk factors that contribute to DKD and a precise DKD risk prediction model will allow early intervention in DKD and prevent its progression. Accurate prediction of DKD risk will drive the timely use of primary prevention strategies, and facilitate the identification of incident CKD in patients with diabetes before microalbuminuria appears. There remains an unmet clinical need for a precise predictive model of DKD risk that can be used to screen the large population of patients with diabetes and in management decisions.
Predictors of DKD risk include albumin excretion rate (AER), blood pressure, blood glucose, glomerular filtration rate (GFR), diabetic retinopathy, and plasma lipid levels. In the real-world setting, collecting longitudinal data from the large population of patients with diabetes is challenging [4]. In clinical practice, unselective screening for DKD is not cost-effective. The ability to predict DKD risk in individual patients with diabetes may be improved by a comprehensive and integrated evaluation of currently available clinical parameters.
Machine-learning of big medical data derived from electronic medical records (EMR) in the real-world setting is supporting physicians in their clinical diagnoses and management of asthma and life-style related diseases such as diabetes [5, 6]. Models that predict the risk of kidney failure (defined as replacement therapy-treated ESRD) among patients with CKD or the risk of ESRD in patients with DKD have been developed [7, 8]. To the author’s knowledge, there are no predictive models of DKD in patients with diabetes based on EMR constructed using machine learning.
A recent study revealed that the predictive power of a real-world data (RWD)–based model for diabetes-related CKD outperformed published algorithms based on data from clinical trials [9]. The objective of the present study was to construct a 3-year DKD risk prediction model in patients with type 2 diabetes mellitus (T2DM) and normo-albuminuria using machine learning, based on EMR. The model will augment physicians’ empirical judgments with rapid and precise predictions of DKD risk in patients with T2DM and normo-albuminuria and identify predictive risk factors for DKD among this patient population.
Methods
Data source
Data for this study were retrospectively derived from the EMR database at the People’s Liberation Army (PLA) General Hospital, the largest hospital in North China. The EMR database contains patient information and medical records from all hospital departments. The data set was de-identified and spanned from October 2008 to December 2019. This study was approved by the PLA General Hospital ethics committee (S2017-133-01) and conducted according to the guidelines of the Declaration of Helsinki.
Study population
Patients diagnosed with T2DM, according to International Classification of Diseases (ICD)-10 codes, with 3 years of follow-up were eligible for this study. Exclusion criteria were: (1) aged < 18 years; (2) undergoing an invasive procedure; (3) presence of an acute infection; (4) presence of a malignancy; (5) or pregnancy.
At baseline, included patients had no evidence of DKD, defined as urinary albumin/creatinine ratio (UACR) > 30 mg/g, protein excretion rate > 150 mg/24 h, or urine dipstick test ≥ 1 + [10], or eGFR < 60 mL/min/1.73m2, calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI [11]) equation.
At the 3-year follow-up, included patients (n = 2809) were stratified according to the presence (n = 408) or absence (n = 2401) of DKD; under-sampling was used to balance the number of patients with or without DKD to 408 each [12]. Patients were randomly split 8:2 into a training set (n = 652) and a validation set (n = 164) using the Python package (Scikit-learn) [13] (Fig. 1).
Candidate predictor variables
Patients’ demographic and clinical characteristics at baseline and the 3-year follow-up were recorded. Laboratory variables were derived from universally implemented tests. Comorbidities (presence or absence, number and type), including hypertension, cardiovascular disease, peripheral neuropathy, diabetic retinopathy and cerebrovascular disease, were diagnosed according to ICD-10 codes.
The risk prediction model was trained using 46 variables selected from medical reports and published literature, including sex, age, body weight and height, BMI, urine specific gravity (SG), urine red blood cell count (RBC), hemoglobin (Hb), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), white blood cell count (WBC), percent neutrophil granulocytes (N%), percent lymphocytes (L%), neutrophil to lymphocyte ratio (NLR), platelet count (PLT), mean platelet volume (MPV), activated partial thromboplastin time (APTT), plasma fibrinogen (FIB), random blood glucose (RBG), HbA1c, blood urea nitrogen (BUN), serum creatinine (SCR), serum uric acid (SUA), eGFR, total bilirubin (T-BiL), direct bilirubin (D-BiL), ALB, γ-glutamine transferase (GGT), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), LDL, serum potassium (K), serum sodium (Na), calcium (Ca), phosphate (P), bicarbonate, and Hcy. Albuminuria was not used as a predictor, as patients had normal urinary protein excretion at baseline.
Variables with > 25% missing data were excluded. Missing values for included variables were imputed using the random forest (RF) method [14].
Model development and evaluation
Seven machine learning algorithms implemented in the Python package 3.3.8: light gradient boosting machine (LightGBM) [15], eXtreme gradient boosting (XGBoost) [16], adaptive boosting (AdaBoost) [17], artificial neural network [18], decision tree [19], support vector machine (SVM) [20] and logistic regression [21], were used to identify the most informative variables for 3-year DKD risk prediction and develop models that predicted 3-year DKD risk as a binary outcome (presence or absence), according to the baseline values of the selected predictor variables.
LightGBM is a new member of the boosting family of algorithms, which is an accurate and efficient implementation of GBDT, similar to XGBoost. Both LightGBM and XGBoost take the negative gradient of the loss function to fit the residuals and find the optimal solution. Compared with XGBoost, LightGBM has faster training efficiency, lower memory, higher accuracy, and it can handle large-scale data and provide direct support of categories. The AdaBoost algorithm is a boosting method that combines multiple weak classifiers into a single strong classifier. The neural network model represents a (significant) enhancement of the logistic regression method. Decision tree models break down data sets into smaller subsets and develop an associated decision tree. The SVM algorithm is a binary classifier that maps input data into a high-dimensional feature space with a non-linear transformation. The logistic regression algorithm builds linear models with built in attribute selection.
A binary outcome for the prediction model was defined as the presence or absence of DKD. Every subset of data included the baseline values (at patients' first visit) for the predictor variables as well as DKD outcomes at 3 years of follow-up. Data were randomly allocated into separate training and validation data sets for each time window using the Python package (Scikit-learn) [13]. 80% of the data was used for training the model, and the remaining 20% was used to validate the model’s predictive performance.
Statistical analysis
Analyses were conducted using Python version 3.8.3 and SPSS software (version 25.0; SPSS Inc., Chicago, IL, USA). Normally distributed continuous variables were compared using the student's t test. Non-normally distributed continuous variables were compared using the Wilcoxon rank sum test. Categorical variables were compared using the chi-square test. Tests were two-sided. A P value < 0.05 was considered statistically significant.
Performance of the predictive models generated by the seven machine learning algorithms were evaluated using the area under the receiver operating characteristic (ROC) curve (AUC), sensitivity, specificity, accuracy, and the F1 score ( 2* ( (precision*recall)/ (precision + recall)); range from 0 (worst score) to 1 (best score)) [22]. Shapley additive explanation (SHAP) was used to interpret the results of the best performing prediction model by computing the contribution of each variable to the prediction [23, 24]. SHAP values evaluate the importance of the output resulting from the inclusion of feature A for all combinations of features other than A [23].
Results
Patient characteristics
A total of 816 patients were included in this analysis. Of these, patients had a median age of 56 years (IQR, 48–66 years), and 585 (67.7%) patients were male. The incidence of at least one macrovascular or microvascular complication (hypertension, cardiovascular disease, cerebrovascular disease, diabetic retinopathy, diabetic peripheral neuropathy) was 52.6%. Baseline demographic and clinical characteristics of patients with or without DKD (n = 408 each) at the 3-year follow-up are shown in Table 1. At baseline, patients with no DKD at the 3-year follow-up were significantly older and had significantly higher eGFR, ALB, and Hb and significantly lower HbA1c, compared to patients with DKD at the 3-year follow-up.
Feature selection
Recursive feature elimination (RFE) with RF was used to select variables as inputs for the 3-year DKD risk prediction model [25]. Ultimately, the 46 variables were reduced to 8 potential predictors of 3-year DKD risk. Five-fold cross validation combined with RF selected age, Hcy, HbA1c, BMI, Alb, eGFR and bicarbonate as the 7 most relevant variables. LDL was also included as it is a commonly cited risk factor for DKD.
Model building and evaluation
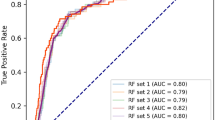
The eight selected variables were used as inputs for the seven machine learning algorithms to predict 3-year DKD risk. Performance evaluation of the models generated by the seven machine learning algorithms is shown in Table 2. The LightGBM model had the highest AUC (0.815, 95% CI 0.747–0.882), sensitivity, positive predictive, and negative predictive values (Fig. 2). The decision tree had the lowest AUC value (0.579, 95% CI 0.503–0.655).
Explanation of risk factor
SHAP was used to interpret the results of the LightGBM model by computing the contribution of each variable to the prediction. The importance matrix plot and SHAP summary plot for the LightGBM model are shown in Fig. 3, and the SHAP dependence plot for the LightGBM model is shown in Fig. 4.
a Importance matrix plot of the LightGBM model, depicting the importance of each variable for predicting 3-year DKD risk in patients with T2DM and normo-albuminuria. b SHAP summary plot of the top 8 clinical features of the LightGBM model. There is one dot per patient per feature colored according to an attribution value, where red represents a higher value and blue represents a lower value. Hcy, homocysteine; BMI, body mass index; ALB, serum albumin; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein
The importance matrix plot ranked the variables contributing to 3-year DKD risk prediction from most to least important as patients’ baseline age, Hcy, HbA1c, BMI, Alb, eGFR, bicarbonate, and LDL (Fig. 3a). The SHAP summary plot (Fig. 3b) and SHAP dependence plot (Fig. 4) identified how each baseline variable influenced the outcome of DKD. On the SHAP summary plot, baseline variables with higher SHAP feature values increased the risk of developing DKD over the next 3 years. On the SHAP dependence plot, each dot represented a patient, such that the plot depicted how the attributed importance of a baseline variable changed with its value. SHAP values exceeding zero represented an increased risk of 3-year DKD. In general, older patients (Fig. 4a) with high Hcy (Fig. 4b), poor glycemic control (Fig. 4c), low Alb (Fig. 4e), low eGFR (Fig. 4f), and high bicarbonate (Fig. 4g) had an increased risk of developing DKD over the next 3 years. High or low BMI (Fig. 4d) and LDL (Fig. 4h) are risk factors for DKD progression.
Applying the prediction model
SHAP force plots illustrate profiles of patients at high or low risk for developing an outcome and show how a predictive model based on EMR can facilitate individualized care planning. SHAP force plots for the LightGBM model are shown in Fig. 5.
SHAP force plot for patients in the dataset at high (a) or low (b) risk of developing DKD; c SHAP values (global interpretation) for the training set. The abscissa represents each patient, and the ordinate represents the SHAP value. More red indicates a higher overall risk. Hcy, homocysteine; BMI, body mass index; eGFR, estimated glomerular filtration rate
In the study population, the risk of developing DKD over the next 3 years was 49.6%. Figure 5a shows a 72-year-old female patient with a predicted 92.4% [26] probability of developing DKD over the next 3 years. Addressing the modifiable risk factors of BMI, HbA1c, Hcy, eGFR, and bicarbonate may reduce this risk. Figure 5b shows a 61-year-old female patient with a lower risk profile; this patient had a predicted 31.7% [26] probability of developing DKD over the next 3 years. Figure 5c shows the risk of developing DKD over the next 3 years in the training set was 49.6%. Predictive values for each patient are listed in supplementary materials [27].
Discussion
This study identified predictive risk factors for DKD and constructed a 3-year DKD risk prediction model in patients with T2DM and normo-albuminuria using machine learning and clinical variables easily extracted from EMR. The performance of predictive models generated by seven machine learning algorithms were compared. Findings showed the LightGBM model had the highest AUC, sensitivity, positive predictive, and negative predictive values. LightGBM is a high-performance gradient boosting framework [28, 29] that has been used for the prediction of undiagnosed T2DM, based on EMR [30]. To the author’s knowledge, this is the first published study to apply the LightGBM algorithm to predict the 3-year risk of DKD in patients with T2DM and normo-albuminuria who attended a hospital.
EMR has increased access to large amounts of patient data. This, combined with machine learning, is facilitating the development of sophisticated prediction models [31, 32]. Previous reports have presented machine learning techniques as black boxes, providing little information on how predictions have been made. This has hampered uptake by clinicians, who are reluctant to make medical diagnoses based on non-transparent decision-making. In this study, to facilitate interpretation of the decision process of the LightGBM algorithm, we used SHAP methodology to explain our predictions [33]. Baseline age, Hcy, HbA1c, BMI, Alb, eGFR, bicarbonate and LDL were selected as variables relevant for predicting 3-year DKD risk in patients with T2DM and normo-albuminuria. Previous studies have identified these as medically, socially, and economically important variables for quantifying the risk of CKD as a microvascular long-term complication of diabetes [34,35,36]. Consistent with this, our SHAP summary and dependence plots showed that baseline age, Hcy, HbA1c, Alb, eGFR and bicarbonate could distinguish patients at high or low 3-year risk of developing DKD. Specifically, older patients with high Hcy, poor glycemic control, low Alb, low eGFR, and high bicarbonate had a high 3-year risk of developing DKD. SHAP visualizations provide clinical insight and inform clinical decision-making, but highlight the complexity of predictive models. In this case, SHAP dependence plots revealed an increased 3-year risk of DKD in patients with T2DM and normo-albuminuria who had high or low eGFR, BMI or LDL.
Machine learning has confirmed that several biomarkers have prognostic use and may help investigators identify novel risk factors and provide insight into disease pathogenesis [37]. Ongoing research has identified multiple risk factors for DKD. In the present study, patients with T2DM aged > 60 years, eGFR < 90 ml/min/1.73m2, poor glycemic control, and high or low BMI had a high 3-year risk of developing DKD. Accordingly, older age was identified as a risk factor for DKD progression, independent of diabetes duration, in patients with T2DM [38]; a prospective observational cohort study of patients with T2DM followed for 10-years reported that albuminuria, older age, hypertension, insulin therapy, and lower baseline eGFR were independent predictors of annual eGFR decline [39]; and poor glycemic control and elevated BMI have been associated with the development and progression of DKD [40]. eGFR, glycemic control, and BMI are modifiable risk factors for DKD, such that the rational use of sodium/glucose cotransporter-2 inhibitors (SGLT2i) and other drugs in patients with T2DM may be beneficial. Interestingly, the present study also showed patients with T2DM, normo-albuminuria and an eGFR120-130 ml/min/1.73m2 (hyperfiltration) had a high 3-year risk of developing DKD. While more research is required, evidence suggests that glomerular or whole kidney hyperfiltration is a major contributing factor to the development of DKD in patients with type 1 or T2DM [41, 42]. Specifically, cohort studies with 3–18 years of follow-up showed that GFR declines more rapidly in patients with hyperfiltration at baseline compared to those with normal GFR [43].
The relationship between lipid profile and DKD is complex. Previous reports suggest dyslipidemia as a potential risk marker for DKD, but it is unclear which lipids or lipoproteins should be targeted for intervention [44]. In this study, LDL was included as a potential predictor of 3-year DKD risk, and it had a small impact on the output of the prediction model. Consistent with this, renal progression was significantly associated with LDL-cholesterol in patients with T1DM and normoalbuminuria followed for 8–9 years [44], substantiating experimental data and clinical studies that show targeted use of statins may represent a successful renoprotective strategy in diabetes [45, 46]. Irrespective of the association with DKD, dyslipidemia has a strong association with overall cardiovascular risk, making the control of dyslipidemia, especially LDL, essential for patients with diabetes. The benefits of pursuing lipid targets in patients without known cardiovascular disease are controversial [47]. In the present study, 23.8% of patients had cardiovascular disease at baseline, and SHAP dependence plots revealed patients with T2DM and high or low or LDL had an increased 3-year risk of DKD.
Homocysteine, Alb, and bicarbonate are not traditionally associated with increased risk for DKD. However, one study in Chinese patients with diabetes indicated a causal relationship between elevated circulating homocysteine levels and risk of DKD [48]; in hospitalized Han patients with T2DM, low serum Alb concentration was independently associated with diabetic retinopathy and DKD [30]; and serum albumin was identified as an important predictor of ESRD in patients with T2DM and DKD from three clinical trials (RENAAL [n = 1513], IDNT [n = 1715]and ALTITUDE [n = 8561]) using a feedforward neural network [40]. Bicarbonate may represent a novel risk factor for DKD [49]. Patients with diabetes with advanced renal failure show a lower prevalence or a less severe degree of metabolic acidosis [50], potentially through feedback control involving systemic acid–base status and hydrogen ion production that inhibits ketoacid anion production [51].
This study has several strengths. First, we used RWD derived from EMR, which is likely more representative of the diverse T2DM patient population than data derived from clinical trials. Second, among the other algorithms, the LightGBM model performed the best. LightGBM is a highly optimized gradient boosting decision tree algorithm that can incorporate multiple clinical variables Third, we identified risk factors that have not been traditionally associated with increased risk for DKD. Fourth, most studies have targeted patients with CKD and an eGFR < 60 ml/min/1.73m2 or ESRD [52]. We included patients with DKD presenting with new-onset micro- and macro- albuminuria. Fifth, our model can be used by clinicians and nurses as a visual approach to predict 3-year risk of DKD in patients with T2DM and normo-albuminuria, appropriately manage patients with T2DM and normo-albuminuria at high-risk for DKD and to target risk factors for DKD, thus informing the allocation of healthcare resources. Last, the model can be used as a screening tool for clinical trials. Enriching trials with patients at high 3-year risk of developing DKD may reduce sample sizes and lead to more efficient drug development programs.
This study was associated with some limitations. It was conducted at a single institution, included a small sample size, and the missing information (e.g., use of hypoglycemic drugs, history of diabetes, blood pressure) in our EMR-derived data represented a potential bias. However, we believe our rigorous methodology generated a robust predictive model of 3-year DKD risk in patients with T2DM and normo-albuminuria. External validation using another data set is required to establish stability in the performance of our prediction model.
Conclusion
In conclusion, we identified baseline demographic and clinical variables as predictive risk factors for DKD and constructed a 3-year DKD risk prediction model in patients with T2DM and normo-albuminuria using machine learning and EMR. We established the LightGBM model as a tool with potential to facilitate population management strategies for T2DM care in the EMR era.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DKD:
-
Diabetic kidney disease
- T2DM:
-
Type 2 diabetes mellitus
- EMR:
-
Electronic medical records
- LightGBM:
-
Light gradient boosting machine
- AUC:
-
Area under the receiver operating characteristic curve
- SHAP:
-
Shapley additive explanation
- Hcy:
-
Homocysteine
- ALB:
-
Albumin
- eGFR:
-
Estimated glomerular filtration rate
- GFR:
-
Glomerular filtration rate
- ESRD:
-
End-stage renal disease
- CV:
-
Cardiovascular
- CKD:
-
Chronic kidney disease
- AER:
-
Albumin excretion rate
- RWD:
-
Real-world data
- ICD:
-
International Classification of Diseases
- UACR:
-
Urinary albumin/creatinine ratio
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- SG:
-
Specific gravity
- RBC:
-
Red blood cell count
- Hb:
-
Hemoglobin
- Hct:
-
Hematocrit
- MCV:
-
Mean corpuscular volume
- MCHC:
-
Mean corpuscular hemoglobin concentration
- WBC:
-
White blood cell count
- N%:
-
Percent neutrophil granulocytes
- L%:
-
Ercent lymphocytes
- NLR:
-
Neutrophil to lymphocyte ratio
- PLT:
-
Platelet count
- MPV:
-
Mean platelet volume
- APTT:
-
Activated partial thromboplastin time
- FIB:
-
Plasma fibrinogen
- RBG:
-
Random blood glucose
- BUN:
-
Blood urea nitrogen
- SCR:
-
Serum creatinine
- SUA:
-
Serum uric acid
- T-BiL:
-
Total bilirubin
- D-BiL:
-
Direct bilirubin
- GGT:
-
γ-Glutamine transferase
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL:
-
High-density lipoprotein
- K:
-
Serum potassium
- N:
-
A serum sodium
- Ca:
-
Calcium
- P:
-
Phosphate
- XGBoost:
-
EXtreme gradient boosting
- AdaBoost:
-
Adaptive boosting
- SVM:
-
Support vector machine
- GBDT:
-
Gradient Boosted Decision Tree
References
Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018.
Sardu C, Gargiulo G, Esposito G, Paolisso G, Marfella R. Impact of diabetes mellitus on clinical outcomes in patients affected by Covid-19. Cardiovasc Diabetol. 2020;19:76.
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:1985.
Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–14.
Makino M, Yoshimoto R, Ono M, Itoko T, Katsuki T, Koseki A, et al. Artificial intelligence predicts the progression of diabetic kidney disease using big data machine learning. Sci Rep. 2019;9:11862.
Weber C, Röschke L, Modersohn L, Lohr C, Kolditz T, Hahn U, et al. Optimized Identification of Advanced Chronic Kidney Disease and Absence of Kidney Disease by Combining Different Electronic Health Data Resources and by Applying Machine Learning Strategies. J Clin Med. 2020;9:89.
Sun L, Shang J, Xiao J, Zhao Z. Development and validation of a predictive model for end-stage renal disease risk in patients with diabetic nephropathy confirmed by renal biopsy. PeerJ. 2020;8:e8499.
Ramspek CL, Evans M, Wanner C. Kidney Failure Prediction Models: A Comprehensive External Validation Study in Patients with Advanced CKD. J Am Soc Nephrol. 2021;32:1174–86.
Ravizza S, Huschto T, Adamov A, Böhm L, Büsser A, Flöther FF, et al. Predicting the early risk of chronic kidney disease in patients with diabetes using real-world data. Nat Med. 2019;25:57–9.
Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Lemaître G, Nogueira F, Aridas CK. Imbalanced-learn: A python toolbox to tackle the curse of imbalanced datasets in machine learning. J Mach Learn Res. 2017;18:559–63.
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: Machine learning in Python. J Mach Learn Res. 2011;12:2825–30.
Tang F, Ishwaran H. Random Forest Missing Data Algorithms. Stat Anal Data Min. 2017;10:363–77.
Ke G, Meng Q, Finley T, Wang T, Chen W, Ma W, et al. Lightgbm: A highly efficient gradient boosting decision tree. Adv Neural Inf Process Syst. 2017;30:3146–54.
Chen T, Guestrin C: Xgboost: A scalable tree boosting system. In: Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining: 2016. p. 785–94.
Freund Y, Schapire R, Abe N. A short introduction to boosting. Journal-Japanese Society For Artificial Intelligence. 1999;14:1612.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–44.
Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. New York: Springer. 2009.
Cortes C, Vapnik V. Support-vector networks Machine learning. 1995;20:273–97.
Fitzmaurice G, Laird N. Multivariate analysis: Discrete variables (logistic regression). 2001.
Handelman G, Kok H, Chandra R, Razavi A, Lee M, Asadi H. eD octor: machine learning and the future of medicine. J Intern Med. 2018;284:603–19.
Tseng PY, Chen YT, Wang CH, Chiu KM, Peng YS, Hsu SP, et al. Prediction of the development of acute kidney injury following cardiac surgery by machine learning. Crit Care. 2020;24:478.
Lundberg SM, Lee S-I. A unified approach to interpreting model predictions. Adv Neural Inf Process Syst. 2017;30:346.
Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn. 2002;46:389–422.
Lundberg SM, Lee S-I. A unified approach to interpreting model predictions. In: Proceedings of the 31st international conference on neural information processing systems: 2017. p. 4768–77.
Dong ZY. Data from: supplementary materials for JCEM. OSF. Deposited October 10, 2021.
Zhao Y, Wang T, Bove R, Cree B, Henry R, Lokhande H, et al. Ensemble learning predicts multiple sclerosis disease course in the SUMMIT study. NPJ Digit Med. 2020;3:135.
Grissa D, Nytoft RD. Alcoholic liver disease: A registry view on comorbidities and disease prediction. PLoS Comput Biol. 2020;16:e1008244.
Zhu Y, Cai X, Liu Y, Hu M, Zhou L, Liu W, et al. Serum Albumin, but not Bilirubin, is Associated with Diabetic Chronic Vascular Complications in a Chinese Type 2 Diabetic Population. Sci Rep. 2019;9:12086.
Damotte V, Lizée A, Tremblay M, Agrawal A, Khankhanian P, Santaniello A, et al. Harnessing electronic medical records to advance research on multiple sclerosis. Mult Scler. 2019;25:408–18.
Cheung M, Cobb AN, Kuo PC. Predicting burn patient mortality with electronic medical records. Surgery. 2018;164:839–47.
Voosen P. The AI detectives. Science. 2017;357:22–7.
Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390:1888–917.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323–34.
Fioretto P, Dodson PM, Ziegler D, Rosenson RS. Residual microvascular risk in diabetes: unmet needs and future directions. Nat Rev Endocrinol. 2010;6:19–25.
Rodriguez-Romero V, Bergstrom RF, Decker BS, Lahu G, Vakilynejad M, Bies RR. Prediction of Nephropathy in Type 2 Diabetes: An Analysis of the ACCORD Trial Applying Machine Learning Techniques. Clin Transl Sci. 2019;12:519–28.
Elley CR, Robinson T, Moyes SA, Kenealy T, Collins J, Robinson E, et al. Derivation and validation of a renal risk score for people with type 2 diabetes. Diabetes Care. 2013;36:3113–20.
Zoppini G, Targher G, Chonchol M, Ortalda V, Negri C, Stoico V, et al. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol. 2012;7:401–8.
Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63:S39-62.
Silveiro SP, Friedman R, de Azevedo MJ, Canani LH, Gross JL. Five-year prospective study of glomerular filtration rate and albumin excretion rate in normofiltering and hyperfiltering normoalbuminuric NIDDM patients. Diabetes Care. 1996;19:171–4.
Thomson HJ, Ekinci EI, Radcliffe NJ, Seah JM, MacIsaac RJ, Jerums G, et al. Elevated baseline glomerular filtration rate (GFR) is independently associated with a more rapid decline in renal function of patients with type 1 diabetes. J Diabetes Complications. 2016;30:256–61.
Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28:1023–39.
Thomas MC, Rosengård-Bärlund M, Mills V, Rönnback M, Thomas S, Forsblom C, et al. Serum lipids and the progression of nephropathy in type 1 diabetes. Diabetes Care. 2006;29:317–22.
Bonnet F, Cooper ME. Potential influence of lipids in diabetic nephropathy: insights from experimental data and clinical studies. Diabetes Metab. 2000;26:254–64.
Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–16.
Alla VM, Agrawal V, DeNazareth A, Mohiuddin S, Ravilla S, Rendell M. A reappraisal of the risks and benefits of treating to target with cholesterol lowering drugs. Drugs. 2013;73:1025–54.
Ma L, Liu Q, Jiang Y, Zhao H, Zhao T, Cao Y, et al. Genetically elevated circulating homocysteine concentrations increase the risk of diabetic kidney disease in Chinese diabetic patients. J Cell Mol Med. 2019;23:2794–800.
Schutte E, Lambers Heerspink HJ, Lutgers HL, Bakker SJ, Vart P, Wolffenbuttel BH, et al. SeruM BICARBONATE AND KIDNEY DISEASE PROGRESSION AND CARDIOVASCULAR OUTCOME IN PATIENTS WITH DIABETIC NEPHROPATHY: A POST HOC ANALysis of the RENAAL (Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan) Study and IDNT (Irbesartan Diabetic Nephropathy Trial). Am J Kidney Dis. 2015;66:450–8.
Caravaca F, Arrobas M, Pizarro JL, Espárrago JF. Metabolic acidosis in advanced renal failure: differences between diabetic and nondiabetic patients. Am J Kidney Dis. 1999;33:892–8.
Hood VL, Danforth E Jr, Horton ES, Tannen RL. Impact of hydrogen ion on fasting ketogenesis: feedback regulation of acid production. Am J Physiol. 1982;242:F238–45.
Gurudas S, Nugawela M, Prevost AT, Sathish T, Mathur R, Rafferty JM, et al. Development and validation of resource-driven risk prediction models for incident chronic kidney disease in type 2 diabetes. Sci Rep. 2021;11:13654.
Acknowledgements
We are grateful to Yue Niu, SM for support with statistical analyses. We thank Medjaden Inc. for its linguistic assistance during the preparation of this manuscript.
Funding
This study was supported by Science & Technology Project of Beijing, China (D171100002817002, D17110700280000, D181100000118002, D181100000118004), Big Data Program from Chinese PLA General Hospital (2019MBD-053, 2019XXMBD-005, 2019XXJSYX01), Up-and-coming Youngster Fund of PLA General Hospital, Fostering Fund of Chinese PLA General Hospital for National Distinguished Young Scholar Science Fund (2019-JQPY-002), National Natural Science Foundation of China (81870491, 82070741), National Key Research and Development Program of China (2018YFC1704203, 2018YFE0126600, 2017YFA0103203, 2019YFC1709903, 2017YFC0908400).
Author information
Authors and Affiliations
Contributions
Each author participated sufficiently in this work to take public responsibility for the content. XC and SS contributed to the design and conduct of the study. ZD and QW wrote the first draft of the manuscript. WZ, QH, CL, XL, JY, YX, YZ and QL contributed to data collection, and analysis. SQ and CY contributed to results’ interpretation. JS, LZ, YW, JW, XS and GC critically appraised and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the PLA General Hospital ethics committee (S2017-133-01) and conducted according to the guidelines of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, Z., Wang, Q., Ke, Y. et al. Prediction of 3-year risk of diabetic kidney disease using machine learning based on electronic medical records. J Transl Med 20, 143 (2022). https://doi.org/10.1186/s12967-022-03339-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03339-1