Abstract
Background
Determining the risk of Cardiovascular Disease (CVD) is a necessity for timely preventive interventions in high-risk groups. However, laboratory testing may be impractical in countries with limited resources. This study aimed at comparison and assessment of the agreement between laboratory-based and non-laboratory-based WHO risk charts models.
Methods
This study was performed using the baseline data of 8138 participants in the pars cohort study who had no history of CVD and stroke. The updated 2019 WHO model was used to determine the 10-year fatal and non-fatal CVD risks. In general, there are two types of new WHO risk prediction models for CVD. The scores were determined based on age, sex, smoking status, diabetes, Systolic Blood Pressure (SBP), and total cholesterol for the laboratory-based model and age, sex, smoking status, SBP, and Body Mass Index (BMI) for the non-laboratory-based model. The agreement of these two models was determined via kappa statistics for the classified risk (low: < 10%, moderate: 10–< 20%, high: ≥ 20%). Correlation coefficients (r) and scatter plots was used for correlation between scores.
Results
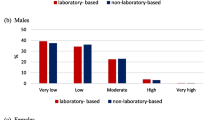
The results revealed very strong correlation coefficients for all sex and age groups (r = 0.84 for males < 60 years old, 0.93 for males ≥ 60 years old, 0.85 for females < 60 years old, and 0.88 for females ≥ 60 years old). In the laboratory-based model, low, moderate, and high risks were 76.10%, 18.17%, and 5.73%, respectively. These measures were respectively obtained as 77.00%, 18.08%, and 4.92% in the non-laboratory-based model. Based on risk classification, the agreement was substantial for males < 60 years old and for both males and females aged ≥ 60 years (kappa values: 0.79 for males < 60 years old, 0.65 for males ≥ 60 years old, and 0.66 for females ≥ 60 years old) and moderate for females < 60 years old (kappa = 0.46).
Conclusions
The non-laboratory-based risk prediction model, which is simple, inexpensive, and non-invasive, classifies individuals almost identically to the laboratory-based model. Therefore, in countries with limited resources, these two models can be used interchangeably.
Similar content being viewed by others
Background
Cardiovascular Diseases (CVDs) are the most important public health problems worldwide, with higher disproportionate consequences in developing countries. In 2015, 422.7 million cases with CVDs and 17.92 million CVD-related deaths occurred worldwide [1]. In Iran, nearly 50% of premature deaths have been attributed to CVDs [2]. Classification of people at risk of CVDs and its impact on the choice of preventive interventions is necessary for high-risk groups. Calculating the overall risk of CVDs to identify high-risk individuals can be cost-effective in Low- and Middle-Income Countries (LMICs) that have limited health resources [3, 4].
Up to now, several risk prediction models have been developed around the world to determine the overall risk of CVDs. These models aim to estimate the probability of a particular disease now or in the future. Determining the risk of CVDs has become essential in the prevention of these diseases and clinical trials [5]. One of the risk prediction models for CVDs is WHO risk charts. The first WHO risk charts were introduced in 2007. These charts estimate the 10-year risk of fatal and non-fatal CVDs for people without CVDs. They also classify each individual into different risk groups, so that they can manage their situations by modifying the lifestyle or medications, if needed [6]. In 2019, WHO updated the risk charts based on validated risk prediction models to estimate the CVDs risk in 21 Institute for Health Metrics and Evaluation (IHME) Global Burden of Disease (GBD) areas [7]. The new risk prediction models were calibrated using the data obtained from the GBD study including estimates for LMIC. The external validity of this model has been confirmed in several cohorts [8]. The previous risk charts published by the WHO provided CVDs risk for 14 global regions. There are two new WHO risk prediction models for CVDs: (1) a laboratory-based model that includes age, sex, smoking status, SBP), history of diabetes, and total cholesterol and (2) a non-laboratory-based model including age, sex, smoking status, SBP, and BMI [7]. Choosing the non-laboratory-based model depends on the setting and goals of CVDs risk assessment, which may be the best model for deciding on primary and secondary preventive interventions in the general community. In many LMICs, laboratory measurements may not be available at primary healthcare centers or people may not afford the tests due to their high costs. Therefore, the non-laboratory-based model can be used [9].
In Iran, some studies have used different risk prediction models. In Iran, the American College of Cardiology/American Heart Association (ACC/AHA) and World Health Organization/International Society of Hypertension (WHO/ISH) risk models have been used for risk prediction [10, 11]. In another study, the agreement between laboratory-based and non-laboratory-based models was calculated using the Framingham risk score for the pars cohort population [12]. However, no study has compered the laboratory-based and non-laboratory-based WHO CVD risk prediction charts.
It is important to find out whether the laboratory- and non-laboratory-based risk models provide similar estimates of the 10-year CVDs risk in an individual. Thus, the present study aims at comparison and evaluation of the agreement between the updated 2019 WHO CVD risk based on laboratory-based and non-laboratory-based risk models in a large population.
Methods
Study population
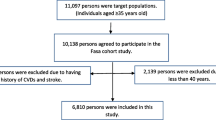
This cross-sectional study was conducted using the baseline data of the pars cohort study, which was proceeded in Valashahr and its neighboring villages in southern Iran in fall 2012. Valashahr includes about 40.000 residents. Details of the pars cohort study have already been published [13]. Briefly, it was conducted on 9264 individuals aged 40–75 years from 2012 to 2014. The participants’ demographic characteristics including age, sex, lifestyle variables including smoking, and disease history including heart disease, stroke, hypertension, diabetes were collected using structured questionnaires by trained interviewers. The physical exam was done by trained physicians and nurses who were employed at the Pars Cohort Center. The physical examination included anthropometric indexes (height and weight) and the determination of systolic and diastolic blood pressure, as well. Staffs were native residents. They were familiar with residents. So, people were able to communicate with them confidently and effectively for more information about any issue. Totally, 8138 cases without a history of CVDs or stroke were recruited.
CVDs risk
In the present study, the 10-year risk of CVDs was calculated using WHO laboratory-based and non-laboratory-based models. The laboratory-based model included age, sex (male/female), SBP (mmHg), smoking status (current/other), history of diabetes (yes/no), and cholesterol (mmol/l), while the non-laboratory-based model included age, sex, SBP (mmHg), smoking status (current/other), and BMI.
Smoking status was determined through the interviews. In addition, the history of diabetes was determined by the previous history of the disease or Fasting Blood Sugar (FBS) ≥ 126 mg/dL. BP was measured for each participant using a mercury sphygmomanometer after a 5-min rest. BP was measured twice, with a 10-min interval, from each arm and the mean BP was recorded. Cholesterol was tested in the laboratory. Finally, BMI was computed by dividing weight by height squared (kg/m2).
Statistical analysis
Percentage was reported for the categorical variables and mean and Standard Deviation (SD) for quantitative ones. Chi-square and t-test were used for categorical and continuous variables, respectively.
The agreement between the laboratory-based and non-laboratory-based models was determined using two models based on the type of risk score, which could be continuous or categorical. Correlation coefficients and scatter plots of the predicted individual-level risk of fatal and non-fatal CVDs were used to present the correlation between the laboratory-based and non-laboratory-based CVDs risk scores. Accordingly, the correlation coefficients 0.00–0.19, 0.20–0.39, 0.40–0.59, 0.60–0.79, and 0.80–1.0 indicated a very weak correlation between the two variables, weak correlation, moderate correlation, strong correlation, and very strong correlation, respectively [14]. The correlation coefficients and scatter plots were presented based on gender and age groups (< 60 and ≥ 60 years).
In the WHO risk model, the predicted risk is classified into five groups; i.e., very low (< 5%), low (5% to < 10%), moderate (10% to < 20%), high (20– < 30%), and very high (≥ 30%). In this study, it was classified into three groups: low (< 10%), moderate (10% to < 20%), and high (≥ 20%). The agreement between the classified risk of laboratory-based and non-laboratory-based models was evaluated using kappa statistics. The agreement less than odds was indicated by kappa values < 0, slight agreement by kappa values between 0.01 and 0.20, fair agreement by kappa values between 0.21 and 0.40, moderate agreement by kappa values 0.41–0.60, substantial agreement by kappa values 0.61–0.80, and almost complete agreement by kappa values 0.81–0.99 [15]. Statistical analyses were performed with Statistical Package for Social Science (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp) and Stata Statistical Software (Stata 14 for windows, Stata Corp., College Station, TX, USA). P-values less than 0.05 were considered as statistically significant.
Results
In this study, 4349 participants (53.44%) were female and the mean age of the participants was 51.65 ± 9.06 years. In addition, the prevalence of smoking was higher among males compared to females. The prevalence of hypertension and diabetes were higher in females than in males. The mean diastolic blood pressure was slightly higher in males than in females. However, the means of SBP, High Density Lipoprotein (HDL), Low Density lipoprotein (LDL), cholesterol, and BMI were higher in females compared to males.
The mean 10-year CVDs risk in the total population was slightly higher in the laboratory-based model than in the non-laboratory-based model (7.60 ± 6.41 vs. 7.49 ± 5.98). In both models, the mean 10-year CVDs risk was higher in males compared to females (Table 1). The risk classification of laboratory-based and non-laboratory-based models was very similar (Fig. 1). Based on the results, 5.73% and 4.92% were high risk in the laboratory-based and non-laboratory-based models, respectively.
Correlation coefficient
The correlation coefficients between the laboratory-based and non-laboratory-based WHO CVDs risks have been presented in Table 2. The results revealed a very strong positive correlation between the laboratory-based and non-laboratory-based models amongst males (r = 0.94, p < 0.001) and females (r = 0.94, p < 0.001). A very strong positive correlation coefficient was also observed among both males and females in the two age groups. Accordingly, a very strong positive correlations were found in males < 60 years old (r = 0.84, p < 0.001), males ≥ 60 years old (r = 0.93, p < 0.001), females < 60 years old (r = 0.85, p < 0.001), and females ≥ 60 years old (r = 0.88, p < 0.001).
Scatter plots of the predicted individual-level risk of fatal and non-fatal CVDs using the laboratory-based and non-laboratory-based scores for males and females in the two age groups have been shown in Fig. 2. Accordingly, a very strong positive correlation was found amongst males and females in the two age groups.
Categorical agreement
In the total population, the agreement between the two risk scores according to the risk score categories was 91.37% (kappa = 0.77, Standard Error (SE) = 0.00). Categorical agreements between the two risk scores have been presented in Table 3 (males) and Table 4 (females). For males, the agreement between the risk categories was 91.39% (kappa = 0.79, SE = 0.01). In addition, the number of males in the high-risk group was higher in the laboratory-based model than in the non-laboratory-based model (272 vs. 245). Moreover, the agreement was 92.95% for the males aged < 60 years (kappa = 0.65, SE = 0.02) and 85.25% for those aged ≥ 60 years (kappa = 0.75, SE = 0.02). The agreement was substantial for the two age groups. Considering females, the agreement between the risk categories was 91.35% (kappa = 0.75, SE = 0.01). Besides, the number of females in the high-risk group was higher in the laboratory-based model compared to the non-laboratory-based model (194 vs. 155). Furthermore, the agreement was 94.20% for the females < 60 years old (kappa = 0.46, SE = 0.03) and 80.65% for those ≥ 60 years old (kappa = 0.66, SE = 0.02). The agreement was moderate and substantial for the females aged < 60 and ≥ 60 years, respectively.
Discussion
In this study, the new WHO model updated in 2109 was used to predict the 10-year risk of CVDs. These models help CVDs risk prediction in primary health centers and can result in public health interventions. In many LMICs, laboratory testing is not always available in primary care centers due to the cost and lack of resources. Hence, a non-laboratory model is used to determine the risk of CVDs.
In the present study, the correlation between the laboratory-based and non-laboratory-based models was measured using correlation coefficients. In addition, kappa statistics were employed to determine whether the non-laboratory-based model could replace the laboratory-based one. Several studies have recommended the use of WHO risk charts in low-income countries [3, 16]. In some studies, the agreement between different CVDs risk prediction models has been investigated [17, 18]. Moreover, some other studies have evaluated the agreement or correlation between laboratory-based and non-laboratory-based Framingham risk scores [12, 19, 20].
According to the correlation coefficients and scatter plots in the present study, there was a strong correlation between the two risk scores in both males and females. The correlation coefficients were also strong for all sex and age groups (r = 0.84 for the males < 60 years old, 0.93 for the males ≥ 60 years old, 0.85 for the females < 60 years of age, and 0.88 for the females ≥ 60 years old). However, the correlation coefficients were higher in elderly people. In the same line, another study indicated a high correlation between the mean scores of laboratory-based and non-laboratory-based models [20]. Another study also revealed a strong positive correlation between lipid-based and BMI-based-models in predicting the Framingham risk score [21].
Due to the fact that the risk was classified into three groups of low (< 10%), moderate (10% to < 20%), and high (≥ 20%) in the present study, the agreement between the classified risks of laboratory-based and non-laboratory-based models was evaluated using kappa statistics. According to the results, the agreement was better in males than in females. Among males, the agreement was substantial in both age groups. On the other hand, the agreement was moderate in the females < 60 years old and substantial in those ≥ 60 years of age. In another study, the agreement between the two risk scores of Framingham laboratory-based and non-laboratory-based models was 74.8% and the kappa statistic was 0.63, which was slightly lower than the overall agreement in the current research [22]. Another study performed in Sri Lanka revealed a good agreement between the cholesterol-based and BMI-based WHO/ISH models (kappa = 0.804) [23].
The comparability of the laboratory-based and non-laboratory based CVDs risk estimation has been shown in settings where resources are limited, which can further optimize cost-effective strategies [20]. For instance, the Heart Wellness Study indicated that the BMI-based risk score could be used to distinguish low-risk people and potentially decrease additional laboratory testing [24]. Other studies also emphasized that the non-laboratory-based model could accurately predict the consequences of CVDs [25, 26].
The results of the present study showed that there were a larger number of people in the high-risk group in the laboratory-based model than in the non-laboratory-based model (5.73% vs. 4.92%). In contrast, another study found that there were a larger number of people in the high-risk group in the Framingham BMI-based risk model than in the cholesterol-based model. In that study, the agreement was good for all sex and age groups, except for older men that was fair [19]. Another study reported that the BMI-based model overestimated the risk of CVDs [27]. In the present study, however, a large proportion of the high-risk participants in the laboratory-based model were also identified in the non-laboratory-based model. In another study, laboratory-based and non-laboratory-based models indicated that 9.4% and 12.7% of the participants were high-risk. In addition, a larger number of people were in the high-risk group in the non-laboratory-based model compared to the laboratory-based model [20]. On the contrary, the findings of the research carried out in Sri Lanka demonstrated that more participants belonged to the high-risk group in the non-laboratory-based model compared to the laboratory-based model (10.7% vs. 9.5%) [23]. The techniques used for measurement of BMI, BP, and cholesterol can result in the underestimation or overestimation of the CVDs risk, eventually increasing or decreasing the risk scores of these models [19]. However, cohort studies in Iran are conducted by skilled staff and accurate tools that can be reliable. Furthermore, ethnicity, socioeconomic status, and genetics in different geographical areas can increase or decrease the risk of CVDs. Another reason for the discrepancy among the results is that most studies comparing the agreement between laboratory-based and non-laboratory-based models have used the Framingham risk prediction model, while the WHO model was utilized in the current study. The Framingham risk model is based on a single Caucasian cohort.
The present study findings demonstrated that the non-laboratory-based model could be used instead of the laboratory-based model. Thus, CVDs risk assessment can be done using the non-laboratory-based model in settings with low resources [28]. Kariuki et al. also disclosed that the non-lab based model decreased the costs by 11% compared to the laboratory-based model [29]. Therefore, the non-laboratory-based model is recommended, especially in settings with limited resources where extensive laboratories are not available and it is not economically feasible to perform laboratory tests.
Study strengths and limitations
The main strength of the present study was the large sample size and the use of carefully collected data from a population-based study. Therefore, the findings can be generalized. To the best of our knowledge, this was the first study comparing two laboratory-based and non-laboratory-based models using the updated 2019 WHO risk chart in a large population. However, this cross-sectional study is based on the baseline data of a cohort study. Thus, a longitudinal study with a 10-year follow-up period is suggested to be conducted to validate laboratory-based and non-laboratory-based risk models. Another limitation is the lack of HbA1c test for diabetes. Because, in low-income countries, the HbA1c test is expensive, especially for studies that are performed on a large population.
Conclusions
The present study results revealed that the correlation coefficients were very strong for all sex and age groups. A substantial agreement was also observed between the scores when classified as low, moderate, and high. Thus, the non-laboratory-based risk prediction model classified individuals almost similarly to the laboratory-based model. This model, which is measured without a blood test, can lead to the beginning of the treatment without the need for additional costs or the inconvenience of laboratory testing. Additionally, the healthcare providers who have limited resources and time in primary health centers can use this model to evaluate the risk inexpensively and to make treatment decisions in a timely manner. Therefore, in countries with limited resources where individuals cannot afford laboratory tests, these two models can be used interchangeably. Yet, future longitudinal cohort studies with 10-year follow-up periods are required to validate laboratory-based and non-laboratory-based risk models for the pars cohort population.
Availability of data and materials
The data used in this study are available from the corresponding author on reasonable request.
Abbreviations
- CVD:
-
Cardiovascular disease
- WHO:
-
World health organization
- LMICs:
-
Low- and Middle-Income Countries
- IHME:
-
Institute for health metrics and evaluation
- GBD:
-
Global burden of disease
- ACC/AHA:
-
American College of Cardiology/American Heart Association;
- ISH:
-
International Society of Hypertension
- BP:
-
Blood pressure
- DBP:
-
Diastolic blood pressure
- SBP:
-
Systolic blood pressure
- FBS:
-
Fasting blood sugar
- HDL:
-
High density lipoprotein
- Chol:
-
Cholesterol
- TG:
-
Triglyceride
- BMI:
-
Body mass index
References
Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25.
Eslami A, Irvani SSN, Ramezankhani A, Fekri N, Asadi K, Azizi F, et al. Incidence and associated risk factors for premature death in the Tehran lipid and glucose study cohort. Iran BMC Public Health. 2019;19(1):1–7.
Mendis S, Lindholm LH, Anderson SG, Alwan A, Koju R, Onwubere BJ, et al. Total cardiovascular risk approach to improve efficiency of cardiovascular prevention in resource constrain settings. J Clin Epidemiol. 2011;64(12):1451–62.
Ndindjock R, Gedeon J, Mendis S, Paccaud F, Bovet P. Potential impact of single-risk-factor versus total risk management for the prevention of cardiovascular events in Seychelles. Bull World Health Organ. 2011;89:286–95.
Damen JA, Bots ML. Prediction of cardiovascular risk: it is not only in the details. Eur J Prev Cardiol. 2019;26(17):1886–7.
WHO. Geneva: World Health Organization; 2007. Prevention of cardiovascular disease—guidelines for assessment and management of cardiovascular risk.
Kaptoge S, Pennells L, De Bacquer D, Cooney MT, Kavousi M, Stevens G, et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332–45.
WHO. Hearts: technical package for cardiovascular disease management in primary health care. 2020.
D’agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care. Circulation. 2008;117(6):743–53.
Hajian-Tilaki K, Heidari B. Comparison of abdominal obesity measures in predicting of 10-year cardiovascular risk in an Iranian adult population using ACC/AHA risk model: a population based cross sectional study. Diabetes Metab Syndr. 2018;12(6):991–7.
Momeni M, Danaei M, Ebrahimi S. Estimating the Frequency of Risk Factors and the 10-Year Risk of Developing Cardiovascular Diseases in Middle-aged Population in Kerman, Iran. Shiraz E Medical J. 2020;21(1).
Rezaei F, Seif M, Gandomkar A, Fattahi MR, Hasanzadeh J. Agreement between laboratory-based and non-laboratory-based Framingham risk score in Southern Iran. Sci Rep. 2021;11(1):1–8.
Gandomkar A, Poustchi H, Moini M, Moghadami M, Imanieh H, Fattahi MR, et al. Pars cohort study of non-communicable diseases in Iran: protocol and preliminary results. Int J Public Health. 2017;62(3):397–406.
Evans JD. Straightforward statistics for the behavioral sciences. Thomson Brooks/Cole Publishing Co; 1996.
Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. J Fam med. 2005;37(5):360–3.
Ofori SN, Odia OJ. Risk assessment in the prevention of cardiovascular disease in low-resource settings. Indian Heart J. 2016;68(3):391–8.
Wekesah FM, Mutua MK, Boateng D, Grobbee DE, Asiki G, Kyobutungi CK, et al. Comparative performance of pooled cohort equations and Framingham risk scores in cardiovascular disease risk classification in a slum setting in Nairobi Kenya. IJC Heart Vasc. 2020;28:100521.
Bazo-Alvarez JC, Quispe R, Peralta F, Poterico JA, Valle GA, Burroughs M, et al. Agreement between cardiovascular disease risk scores in resource-limited settings: evidence from 5 Peruvian sites. Crit Pathw Cardiol. 2015;14(2):74.
Jones CA, Ross L, Surani N, Dharamshi N, Karmali K. Framingham ten-year general cardiovascular disease risk: agreement between BMI-based and cholesterol-based estimates in a South Asian convenience sample. PLoS ONE. 2015;10(3):e0119183.
Peer N, Lombard C, Steyn K, Gaziano T, Levitt N. Comparability of total cardiovascular disease risk estimates using laboratory and non-laboratory based assessments in urban-dwelling South Africans: the CRIBSA study. S Afr Med J. 2014;104(10):691–6.
Niyibizi JB, Joseph OK, Naomi L, Rulisa S, Ntawuyirushintege S, Tumusiime D, et al. The Comparability of Lipid-based and Body Mass Index-based Cardiovascular Disease Risk Scores: using the Rwanda 2012–2013 Non-communicable Diseases Risk Factors Survey Data. Rwanda J Med Health Sci. 2021;4(1):166–84.
Boateng D, Agyemang C, Beune E, Meeks K, Smeeth L, Schulze MB, et al. Cardiovascular disease risk prediction in sub-Saharan African populations—comparative analysis of risk algorithms in the RODAM study. Int J Cardiol. 2018;254:310–5.
Mettananda KCD, Gunasekara N, Thampoe R, Madurangi S, Pathmeswaran A. Place of cardiovascular risk prediction models in South Asians; agreement between Framingham risk score and WHO/ISH risk charts. Int J Clin Pract. 2021;75:e14190.
Green BB, Anderson ML, Cook AJ, Catz S, Fishman PA, McClure JB, et al. Using body mass index data in the electronic health record to calculate cardiovascular risk. Am J Prev Med. 2012;42(4):342–7.
Joseph P, Yusuf S, Lee SF, Ibrahim Q, Teo K, Rangarajan S, et al. Prognostic validation of a non-laboratory and a laboratory based cardiovascular disease risk score in multiple regions of the world. Heart. 2018;104(7):581–7.
Cui J, Forbes A, Kirby A, Simes J, Tonkin A. Laboratory and non-laboratory-based risk prediction models for secondary prevention of cardiovascular disease: the LIPID study. Eur J Prev Cardiol. 2009;16(6):660–8.
Borhanuddin B, Mohd Nawi A, Shah SA, Abdullah N, Syed Zakaria SZ, Kamaruddin MA, et al. 10-year cardiovascular disease risk estimation based on lipid profile-based and BMI-based Framingham risk scores across multiple sociodemographic characteristics: the Malaysian cohort project. Sci World J. 2018;2018:2979206.
Hanif AAM, Hasan M, Khan MSA, Hossain MM, Shamim AA, Hossaine M, et al. Ten-years cardiovascular risk among Bangladeshi population using non-laboratory-based risk chart of the World Health Organization: findings from a nationally representative survey. PLoS ONE. 2021;16(5):e0251967.
Kariuki JK, Gona P, Leveille SG, Stuart-Shor EM, Hayman LL, Cromwell J. Cost-effectiveness of the non-laboratory based Framingham algorithm in primary prevention of cardiovascular disease: a simulated analysis of a cohort of African American adults. Prev Med. 2018;111:415–22.
Acknowledgements
This manuscript was approved by Shiraz University of Medical Sciences (IR.SUMS.REC.1398.860). The authors are grateful for Shiraz University of Medical Sciences and Digestive Diseases Research Institute affiliated to Tehran University of Medical Sciences for providing facilities for the study. They would also like to appreciate Ms. A. Keivanshekouh at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for improving the use of English in the manuscript. The Iranian Ministry of Health and Medical Education has contributed to the funding used in the Pars Cohort through Grant No. 700/107.
Funding
There is no funding to declare.
Author information
Authors and Affiliations
Contributions
FR and JH designed the project. FR was the main investigator. FM, MS, and AG cleaned the data. FR, SGS, and MS did the statistical analysis. FR, FM, and AG wrote the first draft of the manuscript. JH, SGS, and MRF were the research supervisors and critically and substantially revised the final article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1398.860). The data were collected anonymously and informed consent forms were obtained from the participants.
Consent for publication
All authors have given their consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rezaei, F., Seif, M., Gandomkar, A. et al. Comparison of laboratory-based and non-laboratory-based WHO cardiovascular disease risk charts: a population-based study. J Transl Med 20, 133 (2022). https://doi.org/10.1186/s12967-022-03336-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03336-4