Abstract
Background
The onset of the SARS-CoV-2 pandemic has resulted in ever-increasing casualties worldwide, and after 15 months, standard therapeutic regimens are yet to be discovered.
Main body
Due to the regenerative and immunomodulatory function of MSCs, they can serve as a suitable therapeutic option in alleviating major COVID-19 complications like acute respiratory distress syndrome. However, the superior properties of their cognate exosomes as a cell-free product make them preferable in the clinic. Herein, we discuss the current clinical status of these novel therapeutic strategies in COVID-19 treatment. We then delve into the potential of interfering RNAs incorporation as COVID-19 gene therapy and introduce targets involved in SARS-CoV-2 pathogenesis. Further, we present miRNAs and siRNAs candidates with promising results in targeting the mentioned targets.
Conclusion
Finally, we present a therapeutic platform of mesenchymal stem cell-derived exosomes equipped with exogenous iRNAs, that can be employed as a novel therapeutic modality in COVID-19 management aiming to prevent further viral spread within the lung, hinder the virus life cycle and pathogenesis such as immune suppression, and ultimately, enhance the antiviral immune response.
Similar content being viewed by others
Background
As of December 2020, roughly 67 million confirmed cases with severe acute respiratory syndrome coronavirus 2 (SRAS-CoV-2) infection had been reported, with over a million and a half demises [1]. In response to the SARS-CoV-2 outbreak, many therapeutic approaches have been proposed and clinically evaluated to reduce the Coronavirus Disease 2019 (COVID-19) mortality rate. However, there is no unanimously approved product in the global market as COVID-19 therapy for SARS-Cov-2 positive patients [1]. Hence, developing new therapeutics, particularly advanced therapeutic platforms, is still enduring.
Although gene transfer-based approaches have been singularly exploited in vaccine design and multiple candidates are now under clinical evaluation [2], except DeltaRex-G, no gene therapy has been clinically tested for COVID-19 treatment (NCT04378244). Considering the extensive research aimed at developing short interfering RNAs for therapeutic purposes, RNA interference can serve as a genetic treatment approach for SARS-CoV-2 critically ill cases. Due to their natural characteristics, exosomes are considered suitable carriers for interfering RNA (iRNA) delivery.
Exosomes collected from several cell types have shown promise in inducing remission in virally infected patients, especially SARS-CoV-2 positive individuals [3, 4]. Multiple clinical trials are now assessing the administration of mesenchymal stem cell (MSC)-derived exosomes in critically ill COVID-19 patients [4].
This article reviews the current status of exosome-based therapies, particularly those derived from MSCs and their promise as genetic material-delivery vectors. The MSCs’ immune-modulatory and regenerative capabilities in alleviating pulmonary complications, specifically COVID-19, are then elaborated, serving as a rationale for their assignment as the exosome source. Afterward, we delve into the ongoing clinical studies on the administration of exosomes on COVID-19 treatment. Thereafter, the promise of RNA interference (RNAi) -based gene therapy for COVID-19 is explained.
Then, the potential interfering RNA candidates and their cognate targets are introduced in four classes, pro-viral microRNAs (miRNAs), the viral genes themselves, host genes mediating the virus entry and replication, and those of hosts playing roles in the induction of hyper-inflammation.
We culminate by depicting a pipeline for the administration of MSC-derived secretomes carrying a cocktail of the mentioned iRNAs as a novel therapeutic approach for COVID-19 patients in critical status.
Main text
Immunomodulatory and regenerative capacity of MSCs
Upon its entry into the lung through respiration, SARS-CoV-2 primarily invades and destroys pulmonary epithelial cells. The released viral molecular structures are then recognized via pattern recognition receptors (PRRs) on lung-resident innate immune cells, including dendritic cells and macrophages. The local immune response is then triggered, and inflammatory cytokines and chemokines are produced, attracting other immune cells, including T lymphocytes and monocytes. Under normal circumstances, the subsequent anti-viral immune response wipes out the virus with minimal damage before its extensive spread throughout the body. However, the aberrant hyper-inflammatory response in some individuals results in a sudden release of an excessive amount of pro-inflammatory cytokines, a process known as “cytokine storm” [5,6,7]. Cumulative reports correlate the severity of COVID-19 with the excessively-heightened level of pro-inflammatory mediators including interleukin 1 (IL-1), interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), alongside multiple agents, lists of which reported elsewhere [8,9,10,11]. As a result of cytokine storm, blood circulating immune cells, including neutrophils and T lymphocytes, are outrageously recruited into the lung, leading to significant tissue damage, and consequently, lung injury. Lung injury may progress into acute respiratory distress syndrome (ARDS), which is the leading cause of morbidity among COVID-19 patients [12].
MSCs are well known for their profound performance in immunomodulation and tissue repair when encountering a highly-inflamed milieu, particularly in the lung. These cells exert their immunomodulatory and reparative impacts either via direct cell–cell interaction or through the paracrine release of the underlying mediators, including the cell’s migratory elements, immune regulatory agents, antiapoptotic factors, and angiogenic mediators [13,14,15].
They shift the immune system status from inflammation toward regulatory mode by suppressing T lymphocytes’ proliferation and converting the balance between Th1 inflammatory cells and T regulatory cells toward the latter [16, 17]. They also induce the conversion of pro-inflammatory M1 macrophages to anti-inflammatory M2 ones, which in turn results in reduced neutrophil infiltration into the lung [18, 19]. MSCs are also reported to inhibit dendritic cell maturation and activation and also prevent natural killer cell function and proliferation [74], and prevent dendritic cell (DC) maturation and activation [20, 21].
Following modulating the immune response within the lung, they instigate the regeneration of the injured tissues through reversing lung dysfunction and halting pulmonary fibrosis [22]. These dual beneficial therapeutic mechanisms paved MSCs’ way into multiple clinical studies on COVID-19 therapy [23].
Although the safety and efficacy of MSC-based treatment are demonstrated by a handful of studies, the possibility of SARS-Cov-2 infection on MSCs was unknown. Recently a study conducted in china illustrates that the ACE2 and TMPRSS2—the two vital receptors for viral entry—are not expressed on MSC cells, and injection of MSC has no role in inducing infection of other cell types [24]. The result of this study revealed the safety of MSC-based therapy for COVID-19 patients.
Despite its tremendous benefits, utilizing MSCs as immunomodulatory and regenerative agents is not devoid of limitations, particularly when administered through the IV route [25]. For example, these cells might be entrapped at the capillary level and can be almost cleared from the circulation, with a small proportion of them surviving on their way to the target site [26, 27]. Therefore, for efficient trafficking of the cells to the target site, a sufficient cell number needs to be administered and monitored, which can be a resource-consuming process [28]. Moreover, MSCs express tissue factor (TF/CD142) that raises the concern of thromboembolic events [25].
Exosomes as therapeutic agents
After almost three decades since the first report [29], exosomes are now recognized as vital mediators of cell–cell communication [3, 29,30,31]. They are also key players in fundamental cell biology and pathologies, including cancer [32] and cardiovascular diseases [33]. Exosomes are extracellular vesicles of endocytic origin that are secreted by almost every cell type and typically range in 30–100 nm size [30]. They carry macromolecules, including lipids, proteins, and nucleic acids (mainly RNA), and their composition depends on their parent cell [3, 4, 29,30,31].
Secreted exosomes containing biologically active macromolecules can deliver their cargo to the target cell by two distinct mechanisms. First, following the selective binding to cell surface receptors, exosomes are thought to transduce specific intracellular signaling, thereby inducing physiological changes in recipient cells [34]. The second mechanism is the direct transfer of intra-exosomal content such as mRNA and miRNAs into the recipient cells by fusion with the cell membrane [35].
From the pharmaceutical aspect, exosomes demonstrate therapeutic potential when utilized in their native form. For instance, exosomes originated from stimulated platelets have demonstrated superior efficiency in occlusive thrombosis suppression [36]. Furthermore, MCS-derived secretomes, which will be discussed later, display a wide range of capabilities as native extracellular vesicles, including regenerative function in skin, muscle, cardiac and skeletal injuries [37]. However, as illustrated later, exosomes can be manipulated as nanocarriers to deliver various medicinal cargo, including non-coding RNAs (ncRNAs), to the cells of interest as well [38].
Clinical studies on exosome-based COVID-19 therapy
The present pandemic renewed many researchers' interest in the applicability of exosomes as an effective and safe therapeutics for combating COVID-19 associated diseases. As of November 2020, seven clinical trials have been submitted on clinicaltrials.gov to evaluate the safety and/or efficacy of exosome-based therapeutic regimens on SARS-CoV-2 positive patients (Table 1).
MSC-derived exosome therapy in COVID-19
MSC-derived exosomes can be considered as an alternative since they are repeatedly proven to exert similar immune-modulatory and regenerative impacts under distinct circumstances, including hyper-inflammatory situations during pulmonary complications [39,40,41,42,43,44,45]. Despite the potency of MSCs for COVID-19 therapy, MSC-derived exosomes are a better option in the clinic in comparison to their cellular counterparts. While they lead to the same result, MSC-derived exosomes as a cell-free product are more stable, easier to store, and less immunogenic [37], making it a superb substitute as a treatment for several diseases, including lung injury [46]. Furthermore, the cost-effectivity of these natural products makes them a superior therapeutic option for pandemics. Especially in underdeveloped countries, that lack of proper facilities hampers the utilization of any cell-based therapies, whereas the easier delivery of exosomes as freeze-dried powder augment their accessibility in these regions [47, 48]. As another advantage over cell-based therapies, exosomes can also be administrated non-invasively through inhalation [49], which lowers the dosage and prevents the costs and side effects accompanying IV injection.
As the first of its kind, in a pilot phase I study in Ruijin Hospital, China, allogenic adipose MSC-derived exosomes (MSCs-Exo) were administrated to severe patients afflicted with SARS-CoV-2 pneumonia through aerosol inhalation (NCT04276987). Although the study was reportedly completed in July 2020, the results are yet to be published. In another ongoing parallel clinical study on healthy volunteers, Rujin Hospital evaluates the safety and tolerance and determines the clinical dose reference for the aerosol inhalation of the exosomes mentioned above (NCT04313647).
In phase I prospective nonrandomized open-label cohort study during April 2020, Direct Biologics demonstrated the safety and efficacy of its product ExoFlo™. This product is made of allogeneic bone marrow mesenchymal stem cells-derived exosomes and has been tested on 24 severe COVID-19 patients with moderate-to-severe ARDS. A single intravenous injection of ExoFlo displayed no adverse event, and a survival rate of 83% was observed with the restoration of oxygenation, significant improvements in absolute neutrophil count and lymphopenia, and reduction in acute phase reactants including C-reactive protein, ferritin, and d-dimer [4]. Consequently, a phase II multicenter randomized double-blinded placebo-controlled trial study (EXIT COVID-19) is planned to assess ExoFlo’s potential in treating moderate-to-severe ARDS in COVID-19 patients (NCT04493242).
In July 2020, Russia launched a study to assess the efficacy of aerosol inhalation of the exosomes in treating severe patients hospitalized with novel coronavirus pneumonia, joining the race toward having MSC-derived secretome designated against cytokine release syndrome-mediated ARDS (NCT04491240).
However, it is worth noting that there are hurdles that need to be considered in the application of MSC-derived exosome therapy in the COVID-19. These hurdles include developing established methods of isolation, loading, real-time monitoring of trafficking, and potential off-target effects of the exosomes. For example, the targeting efficacy of theses delivery tools can be enhanced by attaching novel ligands specific to the target tissue [50]. Moreover, similar to the MSC administration, the pro-coagulant ingredients of the exosomes are a major aspect that needs to be focused on. COVID-19 patients are at the risk of hypercoagulable state (e.g., disseminated intravascular coagulation (DIC) and thrombo-embolism), and these adverse effects have been reported following the administration of some MSC products [25, 51]. Hence, in the clinical settings, the coagulative state of patients needs to be monitored, and preventive measures have to be adopted. Finally, due to the ongoing pandemic’s complexities, along with growing global demand, it is crucial yet challenging to develop robust logistics to provide sufficient and efficient MSCs and exosomes as their products in a consistent manner.
Non-MSC-derived exosome therapy in COVID-19
Extracellular vesicles derived from other cells have also paved their way to clinical studies for COVID-19 treatment. CSTC-Exo is a product based on the exosomes derived from virus-specific T lymphocytes, which are activated and expanded in vitro by their exposure to viral peptide fragments in the presence of activating and co-stimulatory signals. These T cells-secreted exosomes carry immune mediators, and furthermore, they can serve as off-the-shelf therapeutics in contrast with the virus-specific T cell-based immunotherapy, which is mostly HLA-restricted. In a single-arm open-labeled combined interventional (phase I/II trials) clinical trial in TC Erciyes University, Turkey, CSTC-Exo is being administrated to patients at early stages of SARS-CoV-2-related pulmonary disease. This medication is being delivered via a metered-dose inhaler to assess its potential in halting the disease progression (NCT04389385).
Zofin (Organicell Flow) is another non-MSC-derived exosome-based medication developed by Organicell Regenerative Medicine and is under evaluation for its safety and potential efficacy profile in a phase1/2 clinical trial for the treatment of COVID-related moderate to severe acute respiratory syndrome. Zofin is an acellular, minimally manipulated product derived from human amniotic fluid (HAF) and contains various anti-inflammatory agents such as commonly known miRNAs (NCT04384445).
RNAi as a gene therapy agent
ncRNAs are post-translational gene silencers and guide the mechanism of sequence-specific gene regulation through a process called RNA interference (RNAi). There are two types of RNAi mediators in this process: small interfering RNAs (siRNAs) and miRNAs.
siRNAs are a part of antiviral immunity that target viral genes and silence their expression. siRNA therapeutic potentials were recently (2018) confirmed after the FDA-approval of the first siRNA-based drug (i.e., Patisiran by Alnylam) for the treatment of nerve damage in hereditary transthyretin-mediated amyloidosis (hATTR) in adults [52]. Antiviral siRNA-based therapeutics have also entered clinical trials against various viral infections, including HIV, Ebolavirus, and RSV, illustrating efficacy in inhibiting the replication of various viral pathogens despite distinct mechanisms exploit to evade host immunity [53,54,55].
MicroRNAs, as another class of RNAi, can regulate post-transcriptional-level gene expression in a broader range [56]. In viral infections, the host miRNA expression plays a major role in controlling the replication of the virus by direct binding to the viral genome [57] and mediating T cells and antiviral effector functions [58]. miR-32, the first-ever miRNA targeting viral RNA, binds to the retrovirus PFV-1 transcripts and diminishes the virus replication [58].
Two modalities are mostly recruited concerning miRNA-based therapies, miRNA mimics, and anti-miRNA oligonucleotides. miRNA mimics delivery serves to restore a given miRNA concentration, which had been suppressed as a part of the pathology of the disease. Conversely, anti-miRNA oligonucleotides target perilously overexpressed miRNAs. Both strategies are being vastly assessed in clinical trials for various complications [59, 60].
Since RNAi-based therapeutics have demonstrated promising outcomes in treating various pulmonary diseases [61, 62], including earlier SARS virus [63], RNAi-based drugs for SARS-CoV-2 could emerge as a potential treatment for hospitalized patients.
Choosing the right cocktail of iRNAs for COVID-19 therapy
SARS-CoV-2 entry mechanisms into the lung epithelium have long been established to be mediated by binding of the virus spike (S) protein to the angiotensin-converting enzyme 2 (ACE2) receptor and the subsequent S protein priming via transmembrane serine protease 2 (TMPRSS2) processing [64, 65]. Upon cell entry, SARS-CoV-2 hijacks multiple cellular pathways and machinery to propagate and damp immune response and ultimately debilitate the host’s survival upon the virus infection.
Intervention in the pathways involved in the virus pathophysiology can theoretically block the virus propagation and pathogenesis via targeting either the virus genes or the host genes harnessed by the virus, mostly the ones involved in the viral entry and replication and immune escape and the following hyper-inflammation induction.
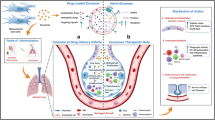
Multiple studies have unveiled RNAi candidates that target the virus transcripts and also the host mRNAs, genes of which take part in the virus pathogenesis. RNAi-dependent gene expression manipulation has also been repeatedly demonstrated to partly mediate the virus pathophysiology. Virus-originating miRNAs and the host cell’s upregulated miRNAs, which contribute to the virus replication cycle, can additionally serve as potential therapeutic targets (Fig. 1) [66].
Potential targets for interfering RNAs in COVID-19. Targeting essential viral genes within the conserved regions of its genome hampers the virus’s cycle of life. As the virus-encoded miRNAs and host pro-viral miRNAs contribute to the virus’s pathogenesis, their hindrance via anti-miRNA oligonucleotides can disrupt the mentioned mechanisms. Human genes responsible for viral entry and the ones hijacked by the virus can also serve as promising iRNAs targets. Targeting various inflammatory genes associated with the SARS-CoV-2 clinical manifestations like ARDS can alleviate the COVID-19 respiratory complications
Viral genes
SARS-CoV-2 genome has 14 open reading frames (ORFs) and encodes 27 proteins, of which four are structural, an envelope protein (E), Nucleocapsid protein (N), matrix protein (M), and spike protein (S). Fifteen non-structural proteins (NSPs) within the ORF1a and ORF1b regions are located at the 5′ end of the genome, and the 3′ end of the genome comprises the sequences pertaining to eight accessory proteins and structural proteins [67]. The regions of interest for iRNAs targeting should be highly conserved in terms of mutational rate and essential for the viral life cycle. N and E proteins-encoding genes and RNA-dependent RNA polymerase (RdRp) gene are highly conserved and encode elements that are indispensable for viral replication and spread; hence they can serve as appropriate iRNA candidates design [68,69,70,71].
Designing siRNAs is a versatile process, and multiple siRNA designing approaches exist and have been reviewed elsewhere [72]. Several groups have been developing siRNAs against conserved regions of the virus genome. Using computational analysis, Lin et al. introduced nine potential siRNAs against RdRp and N and other genes, but their efficacy is yet to be assessed experimentally [73]. In another study, of 78 siRNA candidates, eight were predicted to effectively target N and S genes [74]. Major pharmaceuticals have also initiated the development of siRNA-based therapy for COVID-19. Vir Biotechnology and Alnylam Pharmaceuticals have joined forces to assess 350 siRNAs designed against SARS-coronavirus genomes, including the conserved regions [75]. Throughout separate projects, OilX Pharmaceuticals and Sirnaomics are also exploring the potential of siRNAs targeting the virus’s crucial genes [76, 77].
When designing siRNAs, it is worth considering that following SARS-CoV-2 entry to the cell, the positive-sense, single-stranded genomic RNA is translated into viral polymerase proteins. Subsequently, the complementary negative-sense RNA is synthesized and the regions encoding the structural proteins, and some accessory proteins begin to serve as a template for viral mRNA transcription [78, 79]. Therefore, the RNAs encoding these proteins are present with higher copy numbers than those encoded by the ORF1a and ORF1b.
Accordingly, the virus’s various genome loci are differently present in the host and hence will be targeted disproportionately. Genes within the first 20 kb portion of the virus’s genome are present in two forms, positive and negative sense strands. This portion holds the sequences of ORF1a and ORF1b, and designing a siRNA against the sequence within these loci would theoretically lead to the viral genome double-targeting. With respect to the 10 kb of the genome at the 3′ end, the negative and positive sense strands alongside the viral mRNAs could be triple-targeted via both siRNA strands, yielding a higher viral propagation inhibition [80]. However, recently there has been evidence that targeting sequences within the 10 kb of the genome at 3′ end would decrease siRNA's drug efficacy. The reason could be that high amounts of sub-genomic replicates compete with genomic RNAs for binding to siRNAs and RNA-induced silencing complex (RISC) for subsequent cleavage. Therefore, the ORF1, which is contained solely in genomic RNA, was found to be the most effective target against the SARS-CoV-2 genome. However, this higher efficiency may also be due to better accessibility of ORF1 for RNAi machinery because of the ORF1’s secondary RNA structure or lower abundance of the nucleocapsid proteins [81]. The study also showed that during the RNAi targeting, the negative-sense genomic RNA remains untouched; however, we believe that this strand could also be targeted by changing siRNA strands’ thermodynamic stability at 5′ ends. In this unique situation, the lack of preference between the two siRNA strands gets Argonaute to load both of them into the RISC, which in turn leads to simultaneous targeting of target both negative and positive-sense viral genomic RNAs [82]. Further empirical evidence is needed to identify the best target site in the SARS-CoV-2 genome for RNAi machinery.
Human miRNAome has been exhaustively explored to select miRNAs with prospective potential against the virus genes. Of the human miRNA repertoire, seven miRNAs were predicted to target and inhibit SARS-CoV-2 genes, including N [83]. In a study by Liu et al., human hsa-miR-4661-3p was revealed to target the N gene, serving as a host antiviral response [84]. Adan et al. also identified 479 human miRNAs against various SARS-CoV-2 genes, including N, E, and RdRp [85]. In an attempt to differentiate the epigenetic regulation between various pathogenic coronaviruses, Khademul Islam et al. identified 106 host antiviral miRNAs against SARS-CoV-2, of which three had displayed experimental evidence of having antiviral roles during infections [86]. In an integrated sequence-based analysis of SARS-CoV2 genomes, nine miRNAs were identified to target the SARS-CoV-2 genome, of which six also had targets on human genes, including IFNB as well [87].
Pro-viral miRNAs
Numerous studies have discovered viral miRNAs and pro-viral human miRNAs contribution to virus pathogenesis, some of which shedding light on their ablation via anti-miRNA oligonucleotides. Anti-miRNA oligonucleotides are synthetic oligonucleotides neutralizing miRNAs of interest [88]. Computational analysis of SARS-CoV-2 genome predicted putative viral miRNAs against antiviral response-mediating genes, including human genes involved in pathways like EGF receptor signaling, apoptosis signaling, VEGF signaling, FGF receptor signaling [89]. Using the same approach, Liu and colleagues predicted 45 miRNAs on the virus genome, of which 40 targeted 3′ UTR of 73 human genes, mostly involved in immune response, and 11 targeted 5′ UTR of 13 genes, and many of them are engaged in cytoskeleton organization. This study further demonstrated that viral MR147-3p elevated TMPRSS2 expression in the gut. Several virus-encoded miRNAs were also found to target 5′ UTR of viral genes encoding structural proteins [84]. In a study by Adan et al., viral miRNA-like oligonucleotides were found to target 1367 human genes, resulting in nullifying the immune system’s impact and decreasing the host transcription rate to benefit viral gene expression, a phenomenon named “Host shutoff” [85]. Khademul Islam and colleagues disclosed 170 SARS-CoV-2 mature miRNAs with the potential to target host genes involved in host immune responses, such as autophagy, ErbB signaling, VEGF signaling, Wnt signaling, FGF receptor binding, T-cell-mediated immunity, mTOR signaling, TGF-beta signaling, TNF-alpha signaling, and MAPK signaling [86].
Host genes
The number of identified host genes and pathways involved in viral entry, replication, and pathogenesis is on the rise, and their targeting has been introduced as a therapeutic intervention for COVID-19. Numerous studies are evaluating their perturbation to identify proteins and pathways exhibiting antiviral impacts.
Viral entry is mediated via membrane-bound ACE2 protein binding on lung cells, and its presence on infected cell surface declines due to endocytosis with the viral particle, and this event participates in the disease’s pathogenesis. Although it may seem like an exciting candidate, its knockdown accompanies serious side effects [90, 91]. Nonetheless, inhibition of Type 1 Angiotensin II Receptor (ATR1), which is stimulated during the virus infection, is proven to ameliorate acute lung failure in mice models [92]. Furthermore, TMPRSS2 protein convertase (PC) and cathepsin B/L also contribute to virus entry, and their blockade has been proposed as a promising therapeutic strategy [65, 93, 94].
Furin, namely paired basic amino acid cleaving enzyme (PACE), is another PC and mediates the exposure of S protein binding and fusion domains and is indispensable for the virus entering the cell. Inhibition of furin may have a therapeutic potential via blocking viral entry in SARS-CoV-2 and other viruses that possess the furin cleavage domain. Furin protein inhibitors demonstrated promising outcomes in various pathogens disease models, including influenza A virus, Pseudomonas aeruginosa, and HIV. GM-CSF bi-shRNA furin plasmid (VP) carries two short hairpin RNAs (shRNAs) against furin is now under clinical evaluation for Ewing’s sarcoma and ovarian cancer and is proposed as a repurposing drug for inhibition of viral propagation and immune response promotion [95, 96].
In a genome-wide CRISPR-based screening assay, Wilen et al. identified Cathepsin L, a mediator of viral entry through endocytosis [97], the SWI/SNF chromatin remodeling complex, and SMAD3 protein, a member of the TGF-β signaling pathway, as novel pro-viral agents and their inhibition via small molecules demonstrated therapeutic potential [98]. Construction of the gene network expression revealed genes co-expressed with ACE2 and TMPRSS2 and presented ADK, DPP4, IL13RA2, HDAC8, and CD55 as potential therapeutic targets [99]. Krogan et al. also identified 66 druggable human proteins or host factors by constructing a protein–protein interaction map between the host and SARS-CoV-2 proteins [100].
The possibility of the iRNAs’ efficacy against the mentioned genes as COVID-19 therapy is yet to be assessed, and to our knowledge, only one study has been conducted from this perspective. Seven candidate miRNAs were revealed in a study by Ramakrishnan and colleagues to target host-encoded proteins in signaling pathways involved in receptor activation and host protein hijacking machinery during the pathogenesis of SARS-CoV-2 [101].
Anti-miRNA oligonucleotides can also be designed to target the host miRNAs that assist viral pathogenesis. SARS-CoV-2 infection-induced human miRNAs are found to downregulate multiple pathways in antiviral defense response, including different Toll-Like Receptors (TLRs) [86].
Inflammatory genes
One of the clinical manifestations of COVID-19 is viral-induced inflammation, leading to ARDS. This syndrome is preceded by a significant rise in inflammatory parameters, such as C-reactive protein (CRP) levels, serum ferritin, the erythrocyte sedimentation rate, and d-dimers as a result of pro-inflammatory cytokines increase [102].
Inflammation generally consists of four steps, stimuli recognition by PRRs, inflammatory pathways activation, the release of inflammation mediators, and recruitment of immune cells to the inflammation site. Upon binding damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) to PRRs like TLRs and nod like receptors (NLRs), transcription factors within multiple inflammatory pathways including nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and the Janus kinase signal transducer and activator of transcription (JAK–STAT) pathways translocate into the nucleus and upregulate the expression of various inflammatory cytokines and chemokines. A handful of inflammatory elements such as CRPs, high mobility group box protein 1 (HMGB1), superoxide dismutase (SOD), glutathione peroxidase-1 (GPx), NADPH oxidases (NOX), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (Cox-2) are released from the afflicted cells and promote inflammation through their binding to inflammatory receptors [103]. Myriad of inflammatory signaling cascades involved in pulmonary diseases have been characterized, some of which have proof-of-concept contribution to the SARS-CoV-2 pathogenesis.
Cox-2 synthesizes prostaglandins in response to cytokines and mediates inflammation and tissue damage. Its promoter contains regulatory response elements to NF-kB and IL-6, and SARS-CoV N protein has been previously shown to induce its expression [104, 105]. Although in a recent clinical trial, Cox-2 targeting non-steroidal anti-inflammatory drugs h demonstrated safety in treating COVID-19 patients [106], its natural expression in the kidney poses a major drawback for its systematic blockade, making its localized inhibition an optimal situation [96].
Despite lack of experience in iRNA-mediated Cox-2 silencing in COVID-19, its knockdown and subsequent inflammation modulation have been vastly investigated for other diseases, including cancer and hepatic fibrosis. In a comprehensive review by Espisni et al., lists of studies analyzing various siRNAs and miRNAs are provided [107, 108]. In another study, miR-146a is proven to specifically inhibit Cox-2 in lung epithelial cells [109].
Of the MAPK signaling groups, the p38 MAPK pathway is aberrantly upregulated during SARS-CoV-2 infection, leading to the production of pro-inflammatory cytokines such as IL-6, TNF-α, and interleukin 1 beta (IL-1β). Multiple clinical studies are evaluating p38 inhibitors for a variety of complications [110]. Numerous studies have assessed siRNA candidates for p38 down-regulation in various afflictions, including breast cancer, in an ischemia–reperfusion injury lung transplantation model, and more importantly, lung adenocarcinoma cancer and all have been proven to be efficient suppressors leading to ameliorated inflammation [111,112,113].
Several miRNAs have also demonstrated efficacy in downregulating p38 and can serve as therapeutic candidates. In an attempt to reveal the mechanisms of action of three antiviral miRNAs, miR-124, miR-24, and miR-744, p38 was identified as a ubiquitous antiviral target in multiple viral infections, including influenza and respiratory syncytial virus (RSV) infection [114]. In an early pulmonary fibrosis mouse model caused by ARDS, miR-200b/c overexpression was concomitant with the inhibition of p38 MAPK and TGF-β/smad3 signaling pathways and alleviation of ARDS [115]. In a rat model of chronic inflammation, the miR-16 carrying vector administration palliated the inflammation-induced pain by inhibiting p38 activation [116]. miR-375 is found to prevent myofibroblast trans-differentiation and collagen synthesis by blocking the p38, which is a crucial pathophysiological process in pulmonary fibrosis [117].
NF-kB has repeatedly been demonstrated to orchestrate inflammation and contribute to inflammation-consequent pulmonary complications, including ARDS [118]. NF-kB is an established transcription factor in SARS-CoV pathogenesis. It is activated in response to the virus elements, including N protein [119], and accumulating evidence is attributing the same feature to it in COVID-19 as well. The binding of DAMPs to TLRs and cytokine receptors triggers NF-kB. Its activation upregulates pro-inflammatory agents, including IL-1b, IL-6, and TNF-a, leading to complications such as cytokine release syndrome CRS and pro-inflammatory immune cell recruitment. In a feedback-positive looping manner, these cytokines induce further activation of NF-kB [120, 121].
As a significant regulator of numerous inflammatory cytokines and chemokines, targeting NF-kB transcription factors inhibits multiple pro-inflammatory cascades simultaneously, serving as a superior therapeutic candidate. Cumulating evidence pinpoint the potential of NF-kB suppression in coronavirus-mediated SARS treatment as NF-kB inhibition in SARS-CoV animal models increased its survival and decreased pro-inflammatory agents’ expression [122]. The preliminary results of the RECOVERY clinical trial (NCT04381936) also ratify the rationale of NF-kB inhibition, wherein Dexamethasone, a chemical with NF-kB suppression as its mechanism of action, resulted in a significant reduction in COVID-19 critically ill patients [123, 124].
A manifold of siRNAs is designed and proven to effectively downregulate NF-kB or members of NF-kB signaling pathway and subsequently reduce expression of the NF-kB-regulated genes associated with inflammatory pathways in various pulmonary settings, including sepsis-induced acute lung injury in mice models, lipopolysaccharide-induced acute lung injury in rat models, lung cancer cells [125,126,127,128].
A myriad of miRNAs has been discovered which down-regulate the NF-kB pathway in various organs and modalities. Several papers listed the major miRNAs with altered expression levels in cancer with an impact on this pathway, some of which can serve as therapeutic candidates [129, 130]. Concerning the lung complications, upregulation of miR-140-5p is shown to dampen inflammatory cytokine production in acute lung injury via targeting the TLR4/MyD88/NF-κB signaling pathway [131]. miR-23b cluster and miR-125a-5p are confirmed to silence multiple components of KRAS and NF-kB pathways hence suppressing lung tumorigenesis [132]. By regulating the NF-κB/MMP-9/VEGF pathway, MicroRNA-26b is shown to suppress metastasis in lung cancer [133]. miR-449a also suppresses invasion of lung cancer through blocking HMGB1-Mediated NF-κB Signaling Pathway [134].
RNAi suppressors as a challenge of using RNAi against SARS-CoV-2
It has long been established that interfering RNA-mediated defense mechanism against viruses is mostly confined to fungi, invertebrates, and plants. However, animal viruses are also discovered to be subject to the host RNAi-mediated suppression. Hence, within the evolutionary arms race between host and viruses, viruses have also evolved ways to nullify RNAi-mediated cellular anti-viral defense. Viral proteins underlying these mechanisms are referred to as RNAi suppressors. Aside from their roles in the viral life cycle in various ways, these proteins also manipulate histone and DNA methyltransferases as the components of the host’s transcriptional gene-silencing mechanisms to dampen the cellular antiviral silencing mechanism [135]. Many mammalian viruses, such as HIV and Ebola, were found to encode RNAi-blocking proteins [136]. Such RNAi suppressors were also found in the SARS-CoV; one is derived from ORF7a, and the other is SARS-CoV’s structural nucleocapsid protein [137, 138]. Given the homology of the two viruses, it is most likely that the 7a and N protein act as RNA interference suppressors as well in the SARS-CoV-2 [139]. Karjee et al. also recommended that both the full RdRP and the spike protein may be candidate RNAi suppressors in the SARS-CoV-2 genome, based on the motifs shared in these proteins and a subset of common RNAi suppressors [136]. Such RNAi suppressors might limit the efficiency of using RNAi technology against the SARS-CoV-2. Thus, targeting them could be considered as a strategy against the virus.
Exosomes-based gene therapy for nucleic acid delivery
Utilizing exosomes as a drug delivery system was proposed in 2011 [140], and it gained much attention so far because of the exosomes’ small size, the capability to escape the immune system, deformable cytoskeleton, similarity to cell membranes, and slightly negative zeta potential, which allows them to circulate in the body for a longer period of time [140]. Noncoding RNAs are highly-suitable cargo for exosomes, that can target specific pathways to diminish inflammation in various diseases, including lung injury [141].
Due to the regenerative functions of exosomes secreted from MSC, they are an ideal source of exosomes in a variety of diseases such as cardiac ischemia, liver fibrosis, and cerebrovascular diseases. The therapeutic effects of miRNAs delivered by MSC-derived exosomes have been demonstrated by a handful of studies as well [142, 143].
In COVID-19, similar to other infectious diseases, the immune cells utilize miRNA-carrying exosomes to target the infected cells’ viral RNA. Thus, further delivery of specifically-designed ncRNAs by MSC-derived exosomes can accelerate the combat against SARS-CoV-2 and induce tissue regeneration [144]. To assess the anti-infectivity capacity of iRNAs inside the host cells’ exosomes, Moon et al. have unveiled anti-SARS-CoV-2 miRNA-content of MSC-derived extracellular vesicles [144]. This study sheds light on the mechanism of action of MSC-derived exosome as a carrier for nucleic acid-based therapies in COVID-19 to some extent.
Production of iRNA-carrying exosomes
To combine the aforementioned anti-COVID-19 impact of MSC-derived exosomes with the iRNAs against SARS-CoV-2 pathogenesis, exosomes collected from MSCs should be loaded with the iRNAs of interest. MSCs of multiple sources can be used as exosome donors, including the umbilical cord, bone marrow, and adipose tissue [145]. Mainly, exosomes can be loaded with small RNAs either by direct insertion of the nucleic acids into them or by their collection from genetically-modified MSCs (Fig. 2).
Pipeline of iRNA-carrying exosome production from MSCs. Therapeutic iRNA-carrying exosomes can be produced in two ways. The plasmid encoding the miRNA or/and shRNA of interest may be transferred into the MSCs, and the iRNA-containing exosomes will subsequently be harvested and enriched. Alternatively, synthesized miRNAs mimics or/and siRNAs or/and anti-miRNA oligonucleotides may be chemically inserted into the MSC-derived exosomes, and the resulting loaded exosomes will be then collected and isolated. The consequent exosomes of either way would then be administrated to the COVID-19 critically-ill patients
When synthesized exogenously, siRNAs, miRNA mimics, and anti-miRNA oligonucleotides can be transferred into the exosomes via electroporation, lipofection, sonication, calcium chloride, co-incubation, or Saponin permeabilization [146, 147]. Multiple studies have reported the successful delivery of exogenous iRNAs into the MSC-derived exosomes and observed the expected functionality [148,149,150,151].
Alternatively, it is established that increasing the concentration of iRNAs in the cytosol of the cell is concomitant with their heightened copy number in exosomes [152]. In this regard, MSCs can be manipulated to express shRNA or miRNA of interest via transfection or transduction. The released exosomes can be isolated following their verification regarding the presence of the desired small RNAs. This methodology has demonstrated applicability in a handful of reports [153,154,155,156].
Conclusion
Multiple clinical trials are assessing the efficacy of MSCs and MSC-derived exosomes in alleviating COVID-19 manifestations in critically-ill patients. Enrichment of MSC-derived exosomes carrying exogenous iRNAs for COVID-19 therapy serves as an unprecedented strategy and is yet to be exploited in clinical settings. The right cocktail of iRNAs would not only impede viral propagation, inflammation induction, and immune escape in already-infected cells but also can obstruct the viral particles’ entrance to the un-infected cells and the virus’s further spread within the lung tissue.
Availability of data and materials
Not applicable.
Abbreviations
- MSC:
-
Mesenchymal stem cell
- SRAS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease 2019
- iRNA:
-
Interfering RNA
- miRNAs:
-
MicroRNAs
- siRNAs:
-
Small interfering RNAs
- PRRs:
-
Pattern recognition receptors
- DC:
-
Dendritic cell
- ncRNAs:
-
Including non-coding RNAs
- HAF:
-
Human amniotic fluid
- RNAi:
-
RNA interference
- hATTR:
-
Hereditary transthyretin-mediated amyloidosis
- S:
-
Spike protein
- ACE-2:
-
Angiotensin-converting enzyme 2
- TMPRSS2:
-
Transmembrane serine protease 2
- ORF:
-
Open reading frame
- E:
-
Envelop protein
- N:
-
Nucleocapsid protein
- M:
-
Matrix protein
- NSP:
-
Non-structural protein
- RdRp:
-
RNA-dependent RNA polymerase
- ATR1:
-
Type 1 Angiotensin II Receptor
- PC:
-
Protein convertase
- PACE:
-
Paired basic amino acid cleaving enzyme
- shRNAs:
-
Short hairpin RNAs
- TLRs:
-
Toll-Like Receptors
- CRP:
-
C-reactive protein
- Cox-2:
-
Cyclooxygenase-2
- DAMP:
-
Damage-associated molecular pattern
- PAMP:
-
Pathogen-associated molecular patterns
- NLRs:
-
Nod like receptors
- NF-κB:
-
Nuclear factor kappa B
- MAPK:
-
Mitogen-activated protein kinase
- JAK–STAT:
-
Janus kinase–signal transducer and activator of transcription
- HMGB1:
-
High mobility group box protein 1
- GPx:
-
Glutathione peroxidase-1
- NOX:
-
NADPH oxidases
- iNOS:
-
Inducible nitric oxide synthase
- IL-1:
-
Interleukin 1
- TNF-α:
-
Tumor necrosis factor alpha
- ARDS:
-
Acute respiratory distress syndrome
- IL-1β:
-
Interleukin 1 beta
- RSV:
-
Respiratory syncytial virus
- CRS:
-
Cytokine release syndrome
- SOD:
-
Superoxide dismutase
- RISC:
-
RNA-induced silencing complex
References
Organization WH. WHO Coronavirus Disease (COVID-19) Dashboard 2020. https://covid19.who.int/.
D’Ascanio L, Pandolfini M, Cingolani C, et al. Olfactory dysfunction in COVID-19 patients: prevalence and prognosis for recovering sense of smell. Otolaryngol Head Neck Surg. 2021;164(1):82–6. https://doi.org/10.1177/0194599820943530.
Crenshaw BJ, Gu L, Sims B, Matthews QL. Exosome biogenesis and biological function in response to viral infections. Open Virol J. 2018;12:134–48.
Salepci E, Turk B, Ozcan SN, et al. Symptomatology of COVID-19 from the otorhinolaryngology perspective: a survey of 223 SARS-CoV-2 RNA-positive patients. Eur Arch Otorhinolaryngol. 2021;278:525–535. https://doi.org/10.1007/s00405-020-06284-1
Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–3.
Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–70.
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–74.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Metcalfe SM. Mesenchymal stem cells and management of COVID-19 pneumonia. Med Drug Discov. 2020;5:100019.
Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452.
Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–31.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43.
Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402.
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–8.
Antebi B, Mohammadipoor A, Batchinsky AI, Cancio LC. The promise of mesenchymal stem cell therapy for acute respiratory distress syndrome. J Trauma Acute Care Surg. 2018;84(1):183–91.
Fan X-L, Zeng Q-X, Li X, Li C-L, Xu Z-B, Deng X-Q, et al. Induced pluripotent stem cell-derived mesenchymal stem cells activate quiescent T cells and elevate regulatory T cell response via NF-κB in allergic rhinitis patients. Stem Cell Res Ther. 2018;9(1):170.
Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–22.
Geng Y, Zhang L, Fu B, Zhang J, Hong Q, Hu J, et al. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res Ther. 2014;5(3):80.
Li S, Zheng X, Li H, Zheng J, Chen X, Liu W, et al. Mesenchymal stem cells ameliorate hepatic ischemia/reperfusion injury via inhibition of neutrophil recruitment. J Immunol Res. 2018;2018:7283703.
Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–33.
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–6.
Akkoc T. COVID-19 and mesenchymal stem cell treatment; mystery or not. In: Turksen K, editor. Cell biology and translational medicine, volume 10: stem cells in tissue regeneration. Cham: Springer International Publishing; 2020. p. 167–76.
Ellison-Hughes GM, Colley L, O'Brien KA, Roberts KA, Agbaedeng TA, Ross MD. The role of MSC therapy in attenuating the damaging effects of the cytokine storm induced by COVID-19 on the heart and cardiovascular system. Front Cardiovasc Med. 2020;7(327).
Cao Y, Wu H, Zhai W, Wang Y, Li M, Li M, et al. A safety consideration of mesenchymal stem cell therapy on COVID-19. Stem Cell Res. 2020;49:102066.
Al-Khawaga S, Abdelalim EM. Potential application of mesenchymal stem cells and their exosomes in lung injury: an emerging therapeutic option for COVID-19 patients. Stem Cell Res Ther. 2020;11(1):437.
Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112(10):1451–61.
Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res. 2009;104(3):398–402.
McBride C, Gaupp D, Phinney DG. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by real-time PCR. Cytotherapy. 2003;5(1):7–18.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20.
Chahar HS, Bao X, Casola A. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses. 2015;7(6):3204–25.
Meckes DG Jr, Raab-Traub N. Microvesicles and viral infection. J Virol. 2011;85(24):12844–54.
Wang J, Zheng Y, Zhao M. Exosome-based cancer therapy: implication for targeting cancer stem cells. Front. Pharmacol. 2017;7:533. https://doi.org/10.3389/fphar.2016.00533
Vacchiano V, Riguzzi P, Volpi L, Tappatà M, Avoni P, Rizzo G, et al. Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci. 2020;41(8):2029–31.
Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1(7):1074–83.
Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:1. https://doi.org/10.3402/jev.v3.24641.
Srikanthan S, Li W, Silverstein RL, McIntyre TM. Exosome poly-ubiquitin inhibits platelet activation, downregulates CD36 and inhibits pro-atherothombotic cellular functions. J Thromb Haemost. 2014;12(11):1906–17.
Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9(1):17.
Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner DE, Coffey A, et al. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl Med. 2015;4(11):1302–16.
Srour N, Thébaud B. Mesenchymal stromal cells in animal bleomycin pulmonary fibrosis models: a systematic review. Stem Cells Transl Med. 2015;4(12):1500–10.
Ahn SY, Park WS, Kim YE, Sung DK, Sung SI, Ahn JY, et al. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med. 2018;50(4):26.
Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K. Clinical application of mesenchymal stem cell-derived extracellular vesicle-based therapeutics for inflammatory lung diseases. J Clin Med. 2018;7(10):355.
Mohammadipoor A, Antebi B, Batchinsky AI, Cancio LC. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir Res. 2018;19(1):218.
Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197(1):104–16.
Monsel A, Zhu YG, Gudapati V, Lim H, Lee JW. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther. 2016;16(7):859–71.
Bari E, Perteghella S, Di Silvestre D, Sorlini M, Catenacci L, Sorrenti M, et al. Pilot production of mesenchymal stem/stromal freeze-dried secretome for cell-free regenerative nanomedicine: a validated GMP-compliant process. Cells. 2018;7(11):190.
Bari E, Perteghella S, Catenacci L, Sorlini M, Croce S, Mantelli M, et al. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine (Lond). 2019;14(6):753–65.
Bari E, Ferrarotti I, Torre ML, Corsico AG, Perteghella S. Mesenchymal stem/stromal cell secretome for lung regeneration: the long way through “pharmaceuticalization” for the best formulation. J Control Release. 2019;309:11–24.
Das CK, Jena BC, Banerjee I, Das S, Parekh A, Bhutia SK, et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharm. 2019;16(1):24–40.
Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringdén O, Volk HD, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149–63.
Solomon SD, Adams D, Kristen A, Grogan M, González-Duarte A, Maurer MS, et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation. 2019;139(4):431–43.
DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci USA. 2010;107(19):8800–5.
Bobbin ML, Burnett JC, Rossi JJ. RNA interference approaches for treatment of HIV-1 infection. Genome Med. 2015;7(1):50.
Cross R, Mire C, Feldmann H, et al. Post-exposure treatments for Ebola and Marburg virus infections. Nat Rev Drug Discov 2018;17:413–434. https://doi.org/10.1038/nrd.2017.251
Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–6.
Trobaugh DW, Klimstra WB. MicroRNA regulation of RNA virus replication and pathogenesis. Trends Mol Med. 2017;23(1):80–93.
Dickey LL, Worne CL, Glover JL, Lane TE, O’Connell RM. MicroRNA-155 enhances T cell trafficking and antiviral effector function in a model of coronavirus-induced neurologic disease. J Neuroinflamm. 2016;13(1):240.
Bonneau E, Neveu B, Kostantin E, Tsongalis GJ, De Guire V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. Ejifcc. 2019;30(2):114–27.
Hanna J, Hossain GS, Kocerha J. The potential for microRNA therapeutics and clinical research. Front Genet. 2019;10:478.
Fujita Y, Takeshita F, Kuwano K, Ochiya T. RNAi therapeutic platforms for lung diseases. Pharmaceuticals (Basel). 2013;6(2):223–50.
Thanki K, Blum KG, Thakur A, Rose F, Foged C. Formulation of RNA interference-based drugs for pulmonary delivery: challenges and opportunities. Ther Deliv. 2018;9(10):731–49.
Wang Z, Ren L, Zhao X, Hung T, Meng A, Wang J, et al. Inhibition of severe acute respiratory syndrome virus replication by small interfering RNAs in mammalian cells. J Virol. 2004;78(14):7523–7.
Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020;117(21):11727–34.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-80.e8.
Uludağ H, Parent K, Aliabadi HM, Haddadi A. Prospects for RNAi therapy of COVID-19. Front Bioeng Biotechnol. 2020;8:916.
Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–8.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81.
Kim JS, Jang JH, Kim JM, Chung YS, Yoo CK, Han MG. Genome-wide identification and characterization of point mutations in the SARS-CoV-2 Genome. Osong Public Health Res Perspect. 2020;11(3):101–11.
Kasibhatla SM, Kinikar M, Limaye S, Kale MM, Kulkarni‐Kale U. Understanding evolution of SARS‐CoV‐2: a perspective from analysis of genetic diversity of RdRp gene. J Med Virol. 2020;92:1932–1937. https://doi.org/10.1002/jmv.25909
Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81:104260.
Naito Y, Ui-Tei K. siRNA design software for a target gene-specific RNA interference. Front Genet. 2012;3:102.
Chen W, Feng P, Liu K, Wu M, Lin H. Computational identification of small interfering RNA targets in SARS-CoV-2. Virol Sin. 2020;35(3):359–61.
Chowdhury UF, Sharif Shohan MU, Hoque KI, Beg MA, Moni MA, Sharif Siam MK. A computational approach to design potential siRNA molecules as a prospective tool for silencing nucleocapsid phosphoprotein and surface glycoprotein gene of SARS-CoV-2. bioRxiv. 2020:2020.04.10.036335.
Pharmaceuticals A. Vir and Alnylam Expand Collaboration to advance RNAi therapeutics for the treatment of coronavirus infection, including Covid-19. 2020. https://investors.alnylam.com/press-release?id=24656.
Healthcare N. Sirnaomics to develop RNAi-based therapeutics for 2019-nCoV infections. 2020. https://www.ns-healthcare.com/news/sirnaomics-rnai-2019-ncov.
Pharmaceuticals O. Olix pharmaceuticals advances RNAi approaches to target highly conserved regions of coronavirus RNAS 2020. https://www.globenewswire.com/news-release/2020/03/20/2004106/0/en/OliX-Pharmaceuticals-Advances-RNAi-Approaches-to-Target-Highly-Conserved-Regions-of-Coronavirus-RNAs.html.
V’Kovski P, Kratzel A, Steiner S, et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 2021;19:155–170. https://doi.org/10.1038/s41579-020-00468-6
Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41(5):355–9.
Alexandersen S, Chamings A, Bhatta TR. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat Commun. 2020;11(1):6059.
Ambike S, Cheng CC, Afridi S, et al. Systematic analysis of RNAi-accessible SARS-CoV-2 replication steps identifies ORF1 as promising target. 2020.
Lisowiec-Wąchnicka J, Bartyś N, Pasternak A. A systematic study on the influence of thermodynamic asymmetry of 5’-ends of siRNA duplexes in relation to their silencing potency. Sci Rep. 2019;9(1):2477.
Rakhmetullina A, Ivashchenko A, Akimniyazova A, Aisina D, Pyrkova A. The miRNA complexes against coronaviruses COVID-19, SARS-CoV, and MERS-CoV. Research Square. 2020.
Liu Z, Wang J, Xu Y, Guo M, Mi K, Xu R, Pei Y, Zhang Q, Luan X, Hu Z. Implications of the virus-encoded miRNA and host miRNA in the pathogenicity of SARS-CoV-2. 2020.
Saçar Demirci MD, Adan A. Computational analysis of microRNA-mediated interactions in SARS-CoV-2 infection. PeerJ. 2020;8:e9369.
Khan MA-A-K, Sany MRU, Islam MS, Islam ABMMK. Epigenetic regulator miRNA pattern differences among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 world-wide isolates delineated the mystery behind the epic pathogenicity and distinct clinical characteristics of pandemic COVID-19. Front Genet. 2020;11:765.
Sardar R, Satish D, Birla S, Gupta D. Comparative analyses of SAR-CoV2 genomes from different geographical locations and other coronavirus family genomes reveals unique features potentially consequential to host-virus interaction and pathogenesis. bioRxiv. 2020:2020.03.21.001586.
Lima JF, Cerqueira L, Figueiredo C, Oliveira C, Azevedo NF. Anti-miRNA oligonucleotides: a comprehensive guide for design. RNA Biol. 2018;15(3):338–52.
Saini S, Saini A, Jyoti Thakur C, Kumar V, Gupta RD, Sharma J. Genome-wide computational prediction of miRNAs in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) revealed target genes involved in pulmonary vasculature and antiviral innate immunity. Mol Biol Res Commun. 2020;9(2):83–91.
Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84(2):1198–205.
Ciulla MM. SARS-CoV-2 downregulation of ACE2 and pleiotropic effects of ACEIs/ARBs. Hypertens Res 2020;43:985–986. https://doi.org/10.1038/s41440-020-0488-z
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875–9.
Liu T, Luo S, Libby P, Shi GP. Cathepsin L-selective inhibitors: a potentially promising treatment for COVID-19 patients. Pharmacol Ther. 2020;213:107587.
Wang X, Dhindsa R, Povysil G, Zoghbi A, Motelow J, Hostyk J, Goldstein D. Transcriptional inhibition of host viral entry proteins as a therapeutic strategy for SARS-CoV-2. 2020.
Nemunaitis J, Stanbery L, Senzer N. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection: let the virus be its own demise. Future Virol. 2020;10(10):2020–68. https://doi.org/10.2217/fvl-2020-0068.
Abassi ZA, Skorecki K, Heyman SN, Kinaneh S, Armaly Z. Covid-19 infection and mortality: a physiologist’s perspective enlightening clinical features and plausible interventional strategies. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L1020–2.
Simmons G, Zmora P, Gierer S, Heurich A, Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antivir Res. 2013;100(3):605–14.
Wei J, Alfajaro MM, Hanna RE, DeWeirdt PC, Strine MS, Lu-Culligan WJ, et al. Genome-wide CRISPR screen reveals host genes that regulate SARS-CoV-2 infection. bioRxiv. 2020:2020.06.16.155101.
Hernández Cordero AI, Li X, Yang CX, Milne S, Bossé Y, Joubert P, et al. Gene expression network analysis provides potential targets against SARS-CoV-2. bioRxiv. 2020:2020.07.06.182634.
Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–68.
Ahmed S, Paramasivam P, Raj K, Kumar V, Murugesan R, Ramakrishnan V. Regulatory cross talk between SARS-CoV-2 receptor binding and replication machinery in the human host. Front Physiol. 2020;11:802.
Prete M, Favoino E, Catacchio G, Racanelli V, Perosa F. SARS-CoV-2 inflammatory syndrome. Clinical features and rationale for immunological treatment. Int J Mol Sci. 2020;21(9):3377.
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–18.
Liu M, Yang Y, Gu C, Yue Y, Wu KK, Wu J, et al. Spike protein of SARS-CoV stimulates cyclooxygenase-2 expression via both calcium-dependent and calcium-independent protein kinase C pathways. FASEB J. 2007;21(7):1586–96.
Lee IT, Yang C-M. Inflammatory signalings involved in airway and pulmonary diseases. Mediat Inflamm. 2013;2013:791231.
Zhou J, Li C, Liu X, Chiu MC, Zhao X, Wang D, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077–83.
Strillacci A, Griffoni C, Valerii MC, Lazzarini G, Tomasi V, Spisni E. RNAi-based strategies for cyclooxygenase-2 inhibition in cancer. J Biomed Biotechnol. 2010;2010:828045.
Xie N, Liao HW, Ou WS, Zhou X, Hu Y, Fu N, et al. Construction of COX-2 short hairpin RNA expression vector and its inhibitory effect on hepatic fibrosis. Biotechnol Biotechnol Equip. 2018;32(3):653–62.
Cornett AL, Lutz CS. Regulation of COX-2 expression by miR-146a in lung cancer cells. RNA. 2014;20(9):1419–30.
Grimes JM, Grimes KV. p38 MAPK inhibition: a promising therapeutic approach for COVID-19. J Mol Cell Cardiol. 2020;144:63–5.
Wang J, Tan J, Liu Y, Song L, Li D, Cui X. Amelioration of lung ischemia-reperfusion injury by JNK and p38 small interfering RNAs in rat pulmonary microvascular endothelial cells in an ischemia–reperfusion injury lung transplantation model. Mol Med Rep. 2018;17(1):1228–34.
Doğaner F, Turgut Coşan D, Güneş HV, Değirmenci I, Bal C. The effects of p38 gene silencing on breast cancer cells. Mol Biol Rep. 2014;41(5):2923–7.
Zarredar H, Farajnia S, Ansarin K, Baradaran B, Aria M, Asadi M. Synergistic effect of novel EGFR inhibitor AZD8931 and p38α siRNA in lung adenocarcinoma cancer cells. Anticancer Agents Med Chem. 2019;19(5):638–44.
McCaskill JL, Ressel S, Alber A, Redford J, Power UF, Schwarze J, et al. Broad-spectrum inhibition of respiratory virus infection by microRNA mimics targeting p38 MAPK signaling. Mol Ther Nucleic Acids. 2017;7:256–66.
Cao Y, Liu Y, Ping F, Yi L, Zeng Z, Li Y. miR-200b/c attenuates lipopolysaccharide-induced early pulmonary fibrosis by targeting ZEB1/2 via p38 MAPK and TGF-β/smad3 signaling pathways. Lab Invest. 2018;98(3):339–59.
Chen W, Guo S, Wang S. MicroRNA-16 alleviates inflammatory pain by targeting Ras-related protein 23 (RAB23) and inhibiting p38 MAPK activation. Med Sci Monit. 2016;22:3894–901.
Zhang X, Chen Q, Song H, Jiang W, Xie S, Huang J, et al. MicroRNA-375 prevents TGF-β-dependent transdifferentiation of lung fibroblasts via the MAP2K6/P38 pathway. Mol Med Rep. 2020;22(3):1803–10.
Moine P, McIntyre R, Schwartz MD, Kaneko D, Shenkar R, Le Tulzo Y, et al. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock. 2000;13(2):85–91.
Zhang X, Wu K, Wang D, Yue X, Song D, Zhu Y, et al. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology. 2007;365(2):324–35.
Battagello Daniella S, Dragunas G, Klein Marianne O, Ayub ALP, Velloso Fernando J, Correa RG. Unpuzzling COVID-19: tissue-related signaling pathways associated with SARS-CoV-2 infection and transmission. Clin Sci. 2020;134(16):2137–60.
Neufeldt CJ, Cerikan B, Cortese M, Frankish J, Lee J-Y, Plociennikowska A, et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-κB. bioRxiv. 2020:2020.07.21.212639.
DeDiego ML, Nieto-Torres JL, Regla-Nava JA, Jimenez-Guardeño JM, Fernandez-Delgado R, Fett C, et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol. 2014;88(2):913–24.
Kircheis R, Haasbach E, Lueftenegger D, Heyken WT, Ocker M, Planz O. NF-κB Pathway as a Potential Target for Treatment of Critical Stage COVID-19 Patients. Front Immunol. 2020;11:598444. https://doi.org/10.3389/fimmu.2020.598444.
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. https://doi.org/10.1056/NEJMoa2021436.
Li N, Song Y, Zhao W, Han T, Lin S, Ramirez O, et al. Small interfering RNA targeting NF-κB attenuates lipopolysaccharide-induced acute lung injury in rats. BMC Physiol. 2016;16(1):7.
Wu C, Zhao J, Zhu G, Huang Y, Jin L. SiRNA directed against NF-κB inhibits mononuclear macrophage cells releasing proinflammatory cytokines in vitro. Mol Med Rep. 2017;16(6):9060–6.
Chen W, Wang X, Bai L, Liang X, Zhuang J, Lin Y. Blockage of NF-kappaB by IKKbeta- or RelA-siRNA rather than the NF-kappaB super-suppressor IkappaBalpha mutant potentiates adriamycin-induced cytotoxicity in lung cancer cells. J Cell Biochem. 2008;105(2):554–61.
Jin LY, Li CF, Zhu GF, Wu CT, Wang J, Yan SF. Effect of siRNA against NF-κB on sepsis-induced acute lung injury in a mouse model. Mol Med Rep. 2014;10(2):631–7.
Wu J, Ding J, Yang J, Guo X, Zheng Y. MicroRNA roles in the nuclear factor kappa B signaling pathway in cancer. Front Immunol. 2018;9:546.
Tong L, Yuan Y, Wu S. Therapeutic microRNAs targeting the NF-kappa B signaling circuits of cancers. Adv Drug Deliv Rev. 2015;81:1–15.
Yang Y, Liu D, Xi Y, Li J, Liu B, Li J. Upregulation of miRNA-140-5p inhibits inflammatory cytokines in acute lung injury through the MyD88/NF-κB signaling pathway by targeting TLR4. Exp Ther Med. 2018;16(5):3913–20.
Naidu S, Shi L, Magee P, Middleton JD, Laganá A, Sahoo S, et al. PDGFR-modulated miR-23b cluster and miR-125a-5p suppress lung tumorigenesis by targeting multiple components of KRAS and NF-kB pathways. Sci Rep. 2017;7(1):15441.
Li D, Wei Y, Wang D, Gao H, Liu K. MicroRNA-26b suppresses the metastasis of non-small cell lung cancer by targeting MIEN1 via NF-κB/MMP-9/VEGF pathways. Biochem Biophys Res Commun. 2016;472(3):465–70.
Wu D, Liu J, Chen J, He H, Ma H, Lv X. miR-449a suppresses tumor growth, migration, and invasion in non-small cell lung cancer by targeting a HMGB1-mediated NF-κB signaling pathway. Oncol Res. 2019;27(2):227–35.
Sanan-Mishra N, Chakraborty S, Gupta D, Mukherjee SK. RNAi suppressors: biology and mechanisms. 2017.
Karjee S, Mukherjee SK. RNAi suppressor: the hidden weapon of SARS-CoV. J Biosci. 2020. https://doi.org/10.1007/s12038-020-00071-0.
Karjee S, Minhas A, Sood V, Ponia SS, Banerjea AC, Chow VTK, Mukherjee SK, Lal SK. J Virol. 2010;84(19):10395–10401. https://doi.org/10.1128/JVI.00748-10
Cui L, Wang H, Ji Y, Yang J, Xu S, Huang X, et al. The nucleocapsid protein of coronaviruses acts as a viral suppressor of RNA silencing in mammalian cells. J Virol. 2015;89(17):9029–43.
Henzinger H, Barth DA, Klec C, Pichler M. Non-coding RNAs and SARS-related coronaviruses. Viruses. 2020;12(12):1374.
Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays. 2011;33(10):737–41.
Wu X, Liu Z, Hu L, Gu W, Zhu L. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126. Exp Cell Res. 2018;370(1):13–23.
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–22.
Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103(4):530–41.
Park JH, Choi Y, Lim C-W, Park J-M, Yu S-H, Kim Y, et al. Antiviral effects of miRNAs in extracellular vesicles against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and mutations in SARS-CoV-2 RNA virus. bioRxiv. 2020:2020.07.27.190561.
Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells. 2019;37(7):855–64.
Orefice NS. Development of new strategies using extracellular vesicles loaded with exogenous nucleic acid. Pharmaceutics. 2020;12(8):705.
Li S-P, Lin Z-X, Jiang X-Y, Yu X-Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol Sin. 2018;39(4):542–51.
Umezu T, Imanishi S, Azuma K, Kobayashi C, Yoshizawa S, Ohyashiki K, et al. Replenishing exosomes from older bone marrow stromal cells with miR-340 inhibits myeloma-related angiogenesis. Blood Adv. 2017;1(13):812–23.
Naseri Z, Oskuee RK, Jaafari MR, Forouzandeh MM. Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int J Nanomed. 2018;13:7727–47.
Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm. 2020;17(5):1723–33.
Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L, et al. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int. 2018;2018:3290372.
Munir J, Yoon JK, Ryu S. Therapeutic miRNA-enriched extracellular vesicles: current approaches and future prospects. Cells. 2020;9(10):2271.
Yu T, Zhao C, Hou S, Zhou W, Wang B, Chen Y. Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz J Med Biol Res. 2019;52(12):e8735.
Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–4.
Tapparo M, Bruno S, Collino F, Togliatto G, Deregibus MC, Provero P, et al. Renal regenerative potential of extracellular vesicles derived from miRNA-engineered mesenchymal stromal cells. Int J Mol Sci. 2019;20(10):2381.
Che Y, Shi X, Shi Y, Jiang X, Ai Q, Shi Y, et al. Exosomes derived from miR-143-overexpressing MSCs inhibit cell migration and invasion in human prostate cancer by downregulating TFF3. Mol Ther Nucleic Acids. 2019;18:232–44.
Acknowledgements
Part of the figures in this article was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; http://smart.servier.com.
Funding
The research was conducted in the absence of any commercial or financial relationships.
Author information
Authors and Affiliations
Contributions
MJ, YA, JK, and MA-K performed the literature search and data analysis, drafted and revised the work. MS, EA and JK critically revised the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jamalkhah, M., Asaadi, Y., Azangou-Khyavy, M. et al. MSC-derived exosomes carrying a cocktail of exogenous interfering RNAs an unprecedented therapy in era of COVID-19 outbreak. J Transl Med 19, 164 (2021). https://doi.org/10.1186/s12967-021-02840-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-021-02840-3