Abstract
Background
Typically minor ABO incompatible platelet products are transfused without any incident, yet serious hemolytic transfusion reactions occur. To mitigate these events, ABO ‘low titer’ products are used for minor ABO incompatible transfusions. We sought to understand the role of IgM/IgG and complement activation by anti-A on extravascular hemolysis.
Methods
Samples evaluated included (i) Group O plasma from a blood donor whose apheresis platelet product resulted in an extravascular transfusion reaction, (ii) Group O plasma from 12 healthy donors with matching titers that activated complement (N = 6) or not (N = 6), and (iii) Group O sera from 10 patients with anti-A hemolysin activity. A flow cytometric monocyte erythrophagocytosis assay was developed using monocytes isolated by immunomagnetic CD14-positive selection from ACD whole blood of healthy donors. Monocytes were frozen at − 80 °C in 10% dimethyl sulfoxide/FBS and then thawed/reconstituted on the day of use. Monocytes were co-incubated with anti-A-sensitized fluorescently-labeled Group A1 + RBCs with and without fresh Group A serum as a source of complement C3, and erythrophagocytosis was analyzed by flow cytometry. The dependency of IgM/IgG anti-A and complement C3 activation for RBC erythrophagocytosis was studied. Anti-A IgG subclass specificities were examined for specific samples.
Results
The plasma and sera had variable direct agglutinating (IgM) and indirect (IgG) titers. None of 12 selected samples showed monocyte-dependent erythrophagocytosis with or without complement activation. The donor sample causing a hemolytic transfusion reaction and 2 of the 10 patient sera with hemolysin activity showed significant erythrophagocytosis (> 10%) only when complement C3 was activated. The single donor plasma and two sera demonstrating significant erythrophagocytosis had high IgM (≥ 128) and IgG titers (> 1024). The donor plasma anti-A was IgG1, while the patient sera were an IgG3 and an IgG1 plus IgG2.
Conclusion
High anti-A IgM/IgG titers act synergistically to cause significant monocyte erythrophagocytosis by activating complement C3, thus engaging both Fcγ- and CR1-receptors.
Similar content being viewed by others
Background
Hemolytic reactions due to minor ABO incompatible transfusions have been documented [1,2,3,4,5,6,7,8,9] and are most often thought to be associated with high titer anti-A/A,B [6]. Various methods have been used to titer platelet products, and high titer minor ABO incompatible products have been transfused to patients without clinical impact [6, 7].
Antibody-associated extravascular hemolysis is the destruction of red blood cells (RBCs) by resident monocytes/macrophages in spleen and liver [10]. The process is largely governed by IgG and is mainly seen in delayed hemolytic transfusion reactions [11, 12]. Fcγ-receptors present on monocytes/macrophages bind the Fc portion of IgG on sensitized RBCs leading to their phagocytosis [10, 13]. Monocytes/macrophages also possess complement C3b receptors (CR1) on their surface, which can bind and engulf complement coated RBCs. It has been reported that ABO antibody-dependent complement activation alone is insufficient to cause significant erythrophagocytosis [13,14,15].
A strategy to mitigate extravascular hemolysis based on ABO titer alone may unduly limit the use of platelet products that otherwise pose little risk. In vitro monocyte monolayer assay (MMA) is a traditional, laboratory based testing method that is used to predict the outcome of a transfusion in patients with alloantibodies against RBC antigens [16]. To assess erythrophagocytosis, we used a rapid, automated flow cytometry-based monocyte suspension assay (MSA) in which monocytes and antibody-sensitized fluorescent RBCs are co-incubated in suspension rather than using a glass slide adherent monocyte monolayer. We used anti-A as a model to evaluate IgG and IgM-mediated complement C3b activation and the subsequent influence on monocyte/macrophage erythrophagocytosis. The outcome of this study underscores the need to establish ABO IgM/IgG titers to identify specific risk for hemolytic reactions. Although preliminary in nature, our study provides a framework to free up more units for minor ABO incompatible platelet transfusions. Extravascular hemolysis due to anti-A or anti-B IgG is related to patient factors like ABO zygosity [17], and in the present study, we identified other immune characteristics that contribute to monocyte-mediated erythrophagocytosis. The outcomes of the study have implications on the criteria used to qualify apheresis platelets for minor ABO incompatible transfusions.
Results
Anti-A immunoglobulin titers and complement C3b activation
EH-PT had an IgM titer of 256 and an IgG titer of 1024. All 30 donor plasma had IgM titers ≤ 64. Thirteen donor plasma had IgG anti-A titers ≤ 128 while 17 were ≥ 256. Ten out of 30 plasma samples activated complement C3b and were independent of the IgG titer (Table 1) [18]. IgG anti-A titers for the donor plasma causing the minor ABO transfusion reaction and sera H1–H5 are summarized in Table 2.
Erythrophagocytosis
Functional studies were performed with monoclonal BRAD3 anti-D at a concentration of 1:800 and BRAD5 at a concentration of 1:100. BRAD3 sensitization caused significant erythrophagocytosis with all the 3 Rh phenotypes (> 65%) while BRAD5 sensitization caused lower phagocytosis (≤ 6%) (Additional file 1: Figure S1). IVIG inhibited monocyte phagocytosis, with a 50 percent inhibitory concentration (IC50) of 5.3 ± 0.4 µg/mL (Additional file 1: Figure S2). IgG1 (IC50 = 6 µg/mL) and IgG3 (IC50 = 2 µg/mL) inhibited phagocytosis more efficiently than IgG4 (IC50 = 79 µg/mL) and IgG2 (IC50 = 260 µg/mL) (Additional file 1: Figure S3). Effect of diluent on monocyte mediated erythrophagocytosis was measured. Percent erythrophagocytosis of CFDA-SE stained RBCs was observed to remain same (~ 90%) when 1/800 BRAD3 prepared in different diluents 0.2% FBS/PBS, Plasma, and Serum was used for the sensitization of RBCs (Additional file 1: Figure S4).
Anti-A-dependent erythrophagocytosis studies
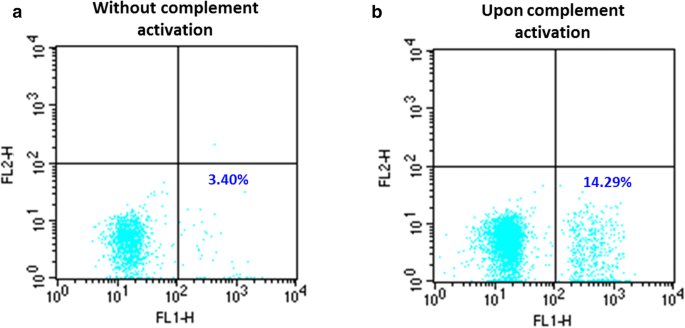
A selection of 12 donor plasma with low or high anti-A IgG titers had no significant erythrophagocytosis whether complement was activated or not. No significant erythrophagocytosis was observed in EH-PT or any of the patient sera in the absence of complement activation. EH-PT and 2of 10 sera (H3 and H4) showed significant phagocytosis upon complement activation; EH-PT = 14.3% (Fig. 1), H3 = 17.0%, H4 = 41.8% (Fig. 2). Controls consisting of anti-D sensitized RBCs (1:10,000 BRAD3 or 1:800 BRAD5) did not show any significant erythrophagocytosis (≤ 2%) in the absence of complement activation. RBCs coated with complement alone using the anti-A IgM clone demonstrated < 5% phagocytosis. Significant erythrophagocytosis was observed with BRAD3 (30% erythrophagocytosis) and BRAD5 (51% erythrophagocytoisis) when the anti-A IgM clone was added along with complement activation (Table 3).
Erythrophagocytosis by plasma from a Group O donor which caused hemolysis in a Group A patient after transfusion of a minor ABO incompatible apheresis platelet unit (EH-PT). a No significant phagocytosis was observed when Group A1 + RBCs were coated with the donor plasma without complement activation. b A significant increase in phagocytosis was observed upon complement C3b activation
High titer IgG anti-A hemolysins mediated monocyte erythrophagocytosis. a–e No significant phagocytosis was observed when Group A1 + RBCs were coated with high titer IgG anti-A hemolysin only. f, j No significant increase in phagocytosis was observed when Group A1 + RBCs were coated with high titer IgG anti-A hemolysin H1 (f), H2 (f), and H5 (j) and fresh Group A serum as a source of complement. A significant increase in phagocytosis was observed upon complement C3b activation for hemolysin H3 (17.01%; h) and H4 (41.8%; i). Fresh Group A sera was used as a source of complement. RBCs: red blood cells
Discussion
Transfusion of ABO incompatible platelets is an accepted clinical practice. However, IgG and IgM anti-A/B present in the plasma could lead to RBCs destruction [19]. To prevent occurrence of such adverse hemolytic events, mitigation strategies like transfusion of platelets containing low titer antibodies has been adopted [20]. Nonetheless, lack of consensus on a standard method for evaluating anti-A/B titer for incompatible plasma has led to a difficulty in adopting an appropriate cut-off titer. There is little information on the characteristics of ABO antibodies causing hemolysis other than the titer. We studied the plasma from a Group O donor that caused an extravascular transfusion reaction in a Group A recipient (EH-PT), and we correlated the extravascular hemolysis using an in vitro monocyte suspension assay (MSA) that demonstrated both Fcγ and CR1 receptor-dependent erythrophagocytosis [21].
The sensitivity of purified monocytes to phagocytose RBCs in suspension was explored. R2R2 RBCs with the highest copy number of RhD antigen had maximum amount of erythrophagocytosis (Additional file 1: Figure S1). Further, consistent with previous published works on IgG subclass, higher phagocytosis by IgG3 anti-D (clone BRAD3) was observed when compared to IgG1 anti-D (BRAD5) [22]. Also, the pattern of Fcγ receptor blockade by IVIG and IgG subclasses was consistent with the previously published literature [16, 23].
We analyzed a subset of anti-A in plasma from random blood donors as a model of ABO antibody-mediated monocyte erythrophagocytic system and found that no monocyte erythrophagocytosis occurred with high titer IgG anti-A even when complement C3b was activated. We then evaluated selected Group O patient sera with high and low IgG anti-A titers that demonstrated hemolysin activity at low titer, with the possible rationale that immune anti-A would contribute to complement-dependent CR1 erythrophagocytosis. Consistent with the random donor plasma, patient sera demonstrating hemolysin activity with low IgM and low IgG titers did not cause significant erythrophagocytosis whether complement was activated or not (H6–H10, data not shown). Further, no monocyte erythrophagocytosis was observed among the sera with high IgM and IgG titers in the absence of complement activation (Fig. 2a–e). In contrast, significant erythrophagocytosis was observed with high IgM and IgG titers (H3 and H4) when complement was activated (Fig. 2f, j). We were able to mimic the need for complement activation using a murine anti-A IgM clone to activate complement and engage CR1 and a monoclonal IgG3 or IgG1 anti-D to engage Fcγ receptors (Table 3).
Landim et al. [24] saw no correlation between ABO isohemagglutinin titer and hemolysin activity. In addition, they found that using both isohemagglutinin titer and hemolysin activity as exclusion criteria increased the percentage of platelet units which were otherwise found unsuitable for minor ABO incompatible transfusions, and that such an approach lacked clinical support as an implementation strategy. Our in vitro erythrophagocytosis data is in agreement with Landim [24]. High titer IgG and the ability to activate complement do not cause significant erythrophagocytosis, and hemolysin activity is not a predictor of complement-dependent erythrophagocytosis. However, we found that both high titer IgM and IgG along with the ability to activate complement are factors that result in significant erythrophagocytosis. These samples contained subclass IgG1/2 or IgG3 anti-A (Table 2). Both IgG3 and IgG1 have the ability to activate complement and IgG2 can play a significant role in monocyte erythrophagocytosis [25, 26]. Taken together, this preliminary study demonstrates that both high titer complement-activating IgM and IgG anti-A are necessary to engage both Fcγ and CR1 receptors and result in significant erythrophagocytosis.
Since the antigen copy number per RBC is similar between A and B, and anti-A, anti-B, and anti-A,B titers can be elevated, the criteria we defined for significant monocyte erythrophagocytosis should be generally consistent regardless of the ABO antigen. However, our observation with anti-A needs to be confirmed with Group B and A,B. Furthermore, the impact of anti-A1 specific hemolysis was not explored but might be an important factor [27]. The limitations of our study are the relatively low number of samples analyzed and the lack of a method to assess the potential for intravascular hemolysis. Also, we have not evaluated IgG titers ≥ 2048; rare donors with extremely high IgG titers, e.g. > 4000, are likely significant. We did not detect blood donors with both high titer IgM and IgA anti-A in our small survey of 30 plasma. Therefore, we do not know frequency of blood donors with both high-titer complement-activating IgM and high IgG anti-A. However, one donor apheresis platelet unit with a similar profile of high-titer complement-activating IgM and IgG caused clinically significant extravascular hemolysis in a minor ABO incompatible transfusion recipient. In our small survey, 30 donors did not have the anti-A characteristics necessary to induce significant monocyte erythrophagocytosis. A more comprehensive analysis is necessary to determine the frequency of donors with such characteristics and confirm our findings.
Conclusions
We have identified serologic conditions necessary for significant monocyte erythrocyte phagocytosis that correlated with extravascular hemolysis in a single transfusion recipient. Our analyses suggest minor ABO incompatible platelet transfusions could be limited to those donors with high-titer complement-activating IgM and IgG titers. Among 30 donor plasma tested, 57% had IgG titers > 256 alone. Limiting half of all donors on the basis of an IgG titer cut-off < 250 places unnecessary constraints on inventory management. Furthermore, IgM titers performed at room temperature do not reflect high levels of IgG. A titer strategy that identifies high-titer complement-activating anti-A has the potential to free up more apheresis units for minor ABO incompatible transfusions. A single method alone that identifies either high-titer IgM or IgG ABO antibodies alone appears insufficient to cause significant extravascular erythrophagocytosis. Larger studies correlating antibody titer and monocytes phagocytosis are warranted to confirm these preliminary findings.
Methods
Plasma containing Group O apheresis platelet product transfused to a Group A patient resulted in an immune transfusion reaction. For the purposes of this study, the effect of anti-A with Group A1 + sensitized RBCs were evaluated from several sources.
Sample characteristics
The following samples were evaluated in this study: (1) plasma from a single Group O donor that caused extravascular hemolysis in a Group A patient of a minor ABO incompatible apheresis platelet transfusion (EH-PT), (2) random Group O plasma (n = 30) from healthy whole blood donors, and (3) selected Group O sera (n = 10) from outpatients being evaluated for possible ABO incompatible kidney transplantation. The plasma from healthy donors had known IgM/IgG anti-A titers, some with the ability to activate complement as previously published [18]. The serum samples had known IgG anti-A titers and demonstrated anti-A hemolysis at low titer when tested with freshly clotted serum. Samples demonstrating hemolysis were selected since ‘hemolysin activity’ has been associated with ‘immune’ anti-A rather than naturally occurring anti-A [28].
IgM and IgG anti-A titers were evaluated using buffered and anti-IgG gel agglutination cards (Ortho Clinical Diagnostics, Pompano Beach, FL, USA), respectively according to the manufacturer’s instructions. To evaluate the effect of direct agglutination by pentameric IgM, the serum samples with high anti-A titers were treated with 1.1 M 2-mercaptoethanol (2-ME) in a 10:1 ratio at 37 °C for 15 min to assess hemagglutinin titers using buffered and anti-IgG gel agglutination cards. Complement activation of anti-A sera was evaluated at a dilution of 1:100 (vol:vol) according to the previously published protocol [18]. Briefly, a 5% Group A1 + RBC suspension was incubated at 37 °C for 30 min with 200 µL 1:100 serum and 200 µL pooled freshly clotted Group A serum from healthy donors as a source of complement. Sensitized RBCs were washed and checked for complement C3 activation using anti-C3b/d (Gamma-clone, Immucor, Norcross, GA, USA). IgG titers ≥ 256 were deemed ‘high titer’ for the purposes of this publication.
RBC fluorescent labeling
Group A1 + RBCs were used in the study. Rh-positive (R1r) RBCs were used in initial studies to establish the MSA. A 5% RBC suspension in 0.2% bovine serum albumin/phosphate buffered saline (pH 7.4) was labeled with 6 mM 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester (CFDA-SE; Sigma Aldrich, St. Louis, MO, USA). The labeling reaction was stopped with heat inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA). The reaction mix was washed and the RBC pellet was resuspended to 5% with 0.2% FBS/PBS (pH 7.4).
RBC sensitization
Based on initial observations from 30 Group O plasma [18], the samples were divided into 4 groups: (1) low IgG titer, non-complement activating, (2) high IgG titer, non-complement activating, (3) low IgG titer, complement activating, and (4) high IgG titer, complement activating. The patient sera with hemolysin activity were divided into 2 clusters (1) low IgG titer and (2) high IgG titer. Representative donor plasma and patient sera from each cluster (n = 12, highlighted in Table 1) were diluted 1:100 and used to sensitize Group A1 + red cells at 37 °C for 30 min with and without fresh Group A serum. All sensitized RBCs were washed and checked for IgG sensitization and complement C3 activation using anti-IgG and anti-C3b/d (Gamma-clone, Immucor, Norcross, GA, USA), respectively. An aliquot of sensitized, washed RBCs resuspended at 1% in monocyte media (CO2 independent media containing 10% FBS and 1% l-Glutamine; Thermo Fisher Scientific, Waltham, MA, USA) was used to evaluate monocyte erythrophagocytosis.
To monitor the performance of MSA, control experiments were performed as described (see results in supplemental files). Controls: Mock controls consisting of IgG and C3b sensitized RBCs were prepared using a 1:10,000 dilution of anti-D IgG3 (clone BRAD3; American Research Products Inc., Waltham, MA, USA) or a 1:800 dilution of anti-D IgG1 (clone BRAD5; American Research Products Inc.) with or without a 1:6000 dilution of murine monoclonal IgM anti-A plus fresh Group A serum as a source of complement C3. Controls were prepared also without fresh Group A serum. When tested alone, BRAD3, BRAD5, plus the murine IgM anti-A clone did not demonstrate monocyte erythrophagocytosis; a fresh source of pooled A serum was needed to activate complement and cause significant erythrophagocytosis. The effect of the diluent used in the MSA was evaluated using BRAD3 diluted in human AB serum and plasma. The results were compared to the MSA for RBCs suspended in 0.2% BFBS/PBS.
Erythrophagocytosis: monocyte suspension assay (MSA)
Peripheral blood mononuclear cells (PBMCs) from a healthy donor were isolated from freshly collected ACD whole blood using density gradient medium (Stemcell Technologies, Vancouver, BC, Canada). Monocytes were purified from PBMCs by immunomagnetic CD14-positive selection (Stemcell Technologies, Vancouver, BC, Canada), frozen in 10% dimethyl sulfoxide/FBS (Thermo Fisher Scientific, Waltham, MA, USA) at − 80 °C and then thawed on the day of use. Before performing MSA, monocytes were thawed rapidly at 37 °C, washed once using saline, and resuspended in monocyte media. The cells were counted and assessed for viability using counting beads (Thermo Fisher Scientific, Waltham, MA, USA) and propidium iodide (Stemcell Technologies, Vancouver, BC, Canada). To measure phagocytosis, 50 µL monocytes (0.5–1 × 105) were incubated with 60 µL 1% sensitized RBCs in 190 µL monocyte media (total reaction volume = 300 µL) in the dark at 37 °C for 30 min. Fluorescent labeled unsensitized RBCs served to evaluate as a negative control for erythrophagocytosis. Phagocytosis was stopped by one wash with saline and non-phagocytosed RBCs were lysed with 4 °C ammonium chloride for 5 min followed by one wash in saline. Flow cytometry was performed on FACSCalibur (BD Biosciences, CA). Data was analyzed using CellQuest Pro software (BD Biosciences, Franklin Lakes, NJ, USA). Increase in fluorescence in the FL1 channel (517 nm) was recorded as a positive signal for phagocytosis (Fig. 3). The phagocytic function of monocytes in suspension was evaluated by showing the effect of (1) Rh D antigen dosage on erythrophagocytosis using BRAD3 and BRAD5, (2) the dose–response inhibition by IVIG, and (3) the variable inhibitory effect of IgG subclasses (Additional file 1). Erythrophagocytosis ≥ 5% was deemed a significant observation based on reproducibility studies and the values obtained for the negative controls.
IgG subclass
EH-PT and sera with high IgG titers (N = 5) were evaluated for IgG subclass after 2-ME treatment. 2-ME treatment was needed as high IgM in the samples was causing direct agglutination thereby obscuring the evaluation of IgG subclass at 1:50 dilution. A 1:50 diluted 2-ME treated sera were used to sensitize A1 + RBCs at 37 °C for 30 min. The reactions were washed and evaluated for the subclass using 1:50 diluted Anti-IgG1, -IgG2, -IgG3, and -IgG4 (Sigma Aldrich, St. Louis, MO, USA).
Availability of data and materials
All data generated and analyzed during this study are included in this published article and its additional files.
Abbreviations
- 2-ME:
-
2-Mercaptoethanol
- CFDA-SE:
-
Carboxyfluorescein diacetate N-succinimidyl ester
- EH-PT:
-
Extravascular hemolysis-platelet transfusion
- FBS/PBS:
-
Fetal bovine serum/phosphate buffered saline
- Fc:
-
Fragment crystallizable
- IgM/G:
-
Immunoglobulin G/M
- MMA:
-
Monocyte monolayer assay
- MSA:
-
Monocyte suspension assay
- RBC:
-
Red blood cell
References
Carr R, Hutton JL, Jenkins JA, Lucas GF, Amphlett NW. Transfusion of ABO-mismatched platelets leads to early platelet refractoriness. Br J Haematol. 1990;75:408–13.
Dunbar NM, Ornstein DL, Dumont LJ. ABO incompatible platelets: risks versus benefit. Curr Opin Hematol. 2012;19:475–9.
Fauzie DS, Shirey R, Thoman S, Bensen-Kennedy D, King KE. The risk of hemolytic transfusion reactions due to passively acquired ABO antibodies: a retrospective study of Non-Group O Adult Recipients of Group O Plateletpheresis. Transfusion. 2004;44:36.
Fung MK, Downes KA, Shulman IA. Transfusion of platelets containing ABO-incompatible plasma: a survey of 3156 North American laboratories. Arch Pathol Lab Med. 2007;131:909–16.
Heal JM, Rowe JM, McMican A, Masel D, Finke C, Blumberg N. The role of ABO matching in platelet transfusion. Eur J Haematol. 1993;50:110–7.
Josephson CD, Mullis NC, Van Demark C, Hillyer CD. Significant numbers of apheresis-derived group O platelet units have “high-titer” anti-A/A, B: implications for transfusion policy. Transfusion. 2004;44:805–8.
Karafin MS, Blagg L, Tobian AA, King KE, Ness PM, Savage WJ. ABO antibody titers are not predictive of hemolytic reactions due to plasma-incompatible platelet transfusions. Transfusion. 2012;52:2087–93.
Lee EJ, Schiffer CA. ABO compatibility can influence the results of platelet transfusion. Results of a randomized trial. Transfusion. 1989;29:384–9.
Mair B, Benson K. Evaluation of changes in hemoglobin levels associated with ABO-incompatible plasma in apheresis platelets. Transfusion. 1998;38:51–5.
Flegel WA. Pathogenesis and mechanisms of antibody-mediated hemolysis. Transfusion. 2015;55(Suppl 2):S47–58.
Josephson CD. Delayed hemolytic transfusion reactions. Transfusion medicine and hemostasis (Second Edition). Amsterdam: Elsevier; 2013. p. 409–12.
Strobel E. Hemolytic transfusion reactions. Transfus Med Hemother. 2008;35:346–53.
Mosser DM, Zhang X. Measuring opsonic phagocytosis via Fcgamma receptors and complement receptors on macrophages. Curr Protoc Immunol. 2011;5(1):14–27.
Kurlander RJ, Rosse WF. Monocyte-mediated destruction in the presence of serum of red cells coated with antibody. Blood. 1979;54:1131–9.
Kurlander RJ, Rosse WF, Logue GL. Quantitative influence of antibody and complement coating of red cells on monocyte-mediated cell lysis. J Clin Invest. 1978;61:1309–19.
Tong TN, Branch DR. Use of a monocyte monolayer assay to evaluate Fcgamma receptor-mediated phagocytosis. J Vis Exp. 2017. https://doi.org/10.3791/55039.
Branch DR, Hellberg A, Bruggeman CW, Storry JR, Sakac D, Blacquiere M, Tong TN, Burke-Murphy E, Binnington B, Parmar N, et al. ABO zygosity, but not secretor or Fc receptor status, is a significant risk factor for IVIG-associated hemolysis. Blood. 2018;131:830–5.
Pandey P, Anani WQ, Gottschall JL, Denomme GA. Potential impact of complement regulator deficiencies on hemolytic reactions due to minor ABO-mismatched transfusions. Blood Adv. 2017;1:1977–82.
Berseus O, Boman K, Nessen SC, Westerberg LA. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion. 2013;53(Suppl 1):114S–23S.
Josephson CD, Castillejo MI, Grima K, Hillyer CD. ABO-mismatched platelet transfusions: strategies to mitigate patient exposure to naturally occurring hemolytic antibodies. Transfus Apher Sci. 2010;42:83–8.
Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623.
Rozsnyay Z, Sarmay G, Walker M, Maslanka K, Valasek Z, Jefferis R, Gergely J. Distinctive role of IgG1 and IgG3 isotypes in Fc gamma R-mediated functions. Immunology. 1989;66:491–8.
Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520.
Landim CS, Gomes FC, Zeza BM, Mendrone-Junior A, Dinardo CL. Prophylactic strategies for acute hemolysis secondary to plasma-incompatible platelet transfusions: correlation between qualitative hemolysin test and isohemagglutinin titration. Rev Bras Hematol Hemoter. 2015;37:217–22.
Michaelsen TE, Garred P, Aase A. Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur J Immunol. 1991;21:11–6.
Yung GP, Seebach JD, Baerenzung N, Pendergrast J, Cserti-Gazdewich C, Branch DR. Eluates from DAT-positive patients with or without hemolysis after high-dose IVIG yield predominantly IgG isoagglutinins of IgG2 subclass. Transfusion. 2019;59:1882–3.
Flegel WA, Henry SM. Can anti-A1 cause hemolysis? Transfusion. 2018;58:3036–7.
Mollison PL. Blood transfusion in clinical medicine. 6th ed. Oxford: Blackwell Scientific; 1979.
Acknowledgements
Not applicable.
Funding
This work was funded by a research grant to GAD from the Commonwealth Transfusion Foundation (formerly the Virginia Blood Foundation). The funding body had no role in the design of the study and collection, analysis, and interpretation of data, nor did it have a role in the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
GAD conceptualized and designed the study and obtained the funding. PP and TMP performed experiments. PP and GAD analyzed the data. WQA and JLG contributed to introduction and discussion. PP and GAD edited the manuscript and approved the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The biospecimens used in this manuscript were provided with waiver of consent as approved by the Medical College of Wisconsin Institutional Review Board, project PRO00031384 and Versiti Inc.
Consent for publication
There are no individual person identifiers in this manuscript. Consent for publication was not sought.
Competing interests
All authors declare they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Monocyte suspension assay supplemental Figures S1–S4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pandey, P., Anani, W.Q., Pugh, T. et al. Complement activating ABO anti-A IgM/IgG act synergistically to cause erythrophagocytosis: implications among minor ABO incompatible transfusions. J Transl Med 18, 216 (2020). https://doi.org/10.1186/s12967-020-02378-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-020-02378-w