Abstract
Background
Autophagy is a highly regulated biological process that mediates the degradation of intracellular components. It is required for tumor cell metabolism and homeostasis. Yin-Yang 1 (YY1) has been reported to be involved in autophagy in several carcinomas. However, its role in autophagy in pancreatic cancer, one of the deadliest human malignancies, is unknown. Here, we investigated the function of YY1 in pancreatic cancer cells autophagy and its mechanisms of action.
Methods
The activity of cells undergoing autophagy was assessed using transmission electron microscopy, immunofluorescence, and Western blotting. A luciferase activity assay, real-time quantitative polymerase chain reaction (RT-qPCR), and chromatin immunoprecipitation (ChIP) were also used to identify putative downstream targets of YY1.
Results
YY1 was confirmed to regulate autophagy in pancreatic cancer cells. It was found to directly regulate the expression of miR-30a, a known modulator of autophagy-associated genes. Furthermore, overexpression of miR-30a attenuated the pro-autophagic effects of YY1.
Conclusions
Cumulatively, our data suggest that miR-30a acts in a feedback loop to modulate the pro-autophagic activities of YY1. Thus, autophagy in pancreatic cancer cells may be regulated, in part, by a tightly coordinated YY1/miR-30a regulatory circuit. These findings provide a potential druggable target for the development of treatments for pancreatic cancer.
Similar content being viewed by others
Background
Yin-Yang 1 (YY1) is a multifunctional zinc-finger transcription factor (also known as DELTA, NF-E1, UCRBP, and INO80S). It is ubiquitously expressed in all tissues and it is highly evolutionarily conserved from Xenopus laevis to humans [1]. The term “Yin-Yang” refers to its two opposing functions as either a transcriptional repressor or transcriptional activator during tumorigenesis. It has fundamental roles in embryogenesis, cell proliferation, and differentiation, which strongly suggests a potentially important function for YY1 during cancer development and progression [2]. YY1 typically binds genomic promoters to transactivate or repress gene expression. However, it can also indirectly activate or repress gene expression by interacting with histone modifiers and chromatin remodeling proteins [3]. In addition to regulating protein-coding genes, YY1 can also modulate the expression of noncoding genes, such as microRNAs (miRNAs) present in human cancer cells. For example, several recent studies have found that YY1 modulates tumorigenesis and the development of tumor cells by repressing the expression of numerous miRNAs, including miR-9, miR-29, miR-146a, and miR-489 [4,5,6,7]. In addition, various types of cancers overexpress YY1 [8]. For instance, our previous study revealed that the expression of YY1 in pancreatic cancers is elevated compared to adjacent non-tumor tissues and normal pancreatic tissues [9].
Autophagy was first reported in 1962 by Ashford and Porter after they observed the accumulation of cytoplasmic components in glucagon-treated hepatic cells [10]. Autophagy has recently gained considerable attention by researchers. It is a highly regulated biological mechanism that facilitates the degradation of intracellular materials within autophagosomes by delivering them to lysosomes for bulk degradation. Autophagy is important for sustaining cell homeostasis and, likewise, is pivotal for tumor cell metabolism. Interestingly, many oncoproteins and oncosuppressors are involved in autophagy. They help cells survive a wide spectrum of stressful conditions [11]. Coincidentally, YY1 has also been reported to be required for autophagy [12]. Many recent studies have focused on the mechanisms of autophagy and its contributions to the development of malignancies, in addition to potential therapies. In pancreatic ductal adenocarcinoma, autophagy plays dual roles. It initially suppresses tumor initiation, but also supports tumor growth during later stages [13]. Furthermore, several studies have reported that autophagy protects pancreatic cancer cells from drugs and environmental stress [14, 15]. On the other hand, some investigators have reported that pancreatic cancer cells respond to various anticancer drugs by undergoing autophagy, which results in enhanced cytotoxicity [16, 17]. Although autophagy is vitally important during the development of pancreatic cancer, the mechanisms that activate autophagy remain largely unknown. In particular, the relationship between YY1 and autophagy in pancreatic cancer cells has not been reported.
In this study, we clearly demonstrate that YY1 modulates autophagy in pancreatic cancer cells by directly targeting miR-30a, an established regulator of the autophagy-associated genes, ATG5 and Beclin 1 [18, 19]. Furthermore, miR-30a regulates the expression of YY1, suggesting a novel YY1/miR-30a regulatory circuit that controls autophagy in pancreatic cancer cells. Cumulatively, this study provides key insights that could aid the development of novel therapeutic strategies for treating pancreatic cancer.
Methods
Cell lines and cell culture
Six human pancreatic cancer cell lines (BxPC-3, CFPAC-1, Colo-357, MiaPaCa-2, PANC-1, and SW1990) were purchased from the Shanghai Cell Bank (Shanghai, China). The normal human pancreatic ductal cell line, HPNE, was purchased from the American Type Culture Collection (ATCC, USA). Cell lines that stably overexpress YY1 (BxPC-3 and PANC-1), cell lines in which YY1 is knocked down, and control cell lines were prepared and cultured as previously described [20]. Human pancreatic cancer cell lines were cultured in DMEM supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL). The HPNE cell line was cultured according to the recommendations of the ATCC.
qRT-PCR
Total RNA was extracted from different cell lines using Trizol reagent and reverse-transcribed into cDNA using the PrimeScript RT Master Mix according to the manufacturer’s instructions. For miRNA quantifications, miRNAs were extracted using the miRNeasy Mini Kit (Qiagen, China). Reverse-transcription was performed using the Mir-XTM miRNA First-Strand Synthesis Kit (Clontech, China) according to the manufacturer’s instructions. The mRNA expression levels were determined by qRT-PCR using the Step One Plus Real-Time PCR System (Applied Biosystems, USA) with the FastStart Universal SYBR Green Master according to the manufacturer’s instructions. The expression levels of the YY1 mRNAs and miR-30a were normalized to GAPDH and U6, respectively, using the 2−ΔΔCT method. Each qRT-PCR experiment was performed in triplicate and independently repeated three times.
Western blotting
Pancreatic cancer cells or xenograft tumor tissues were lysed in ice-cold lysis buffer containing the following reagents: 50 mM Tris–HCl (pH 7.4), 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, and a complete protease inhibitor cocktail (1 tablet per 10 mL, Roche Diagnostics GmbH, Mannheim, Germany). The total protein concentration was determined using a BCA Protein Assay kit (Beyotime Biotechnology, China). Western blotting was performed using standard methods. The following dilutions were used for each antibody: anti-YY1 (ab109228, Abcam, 1:3000), anti-Beclin 1 (#669922, R&D, 1:1000), anti-ATG5 (ab108327, Abcam, 1:3000), anti-P62 (ab109012, Abcam, 1:10,000), anti-LC3A/B (#12741, Cell Signaling, 1:1000), and anti-GAPDH (SAM1003, Sun Shine Bio, 1:5000). Each blot was independently repeated three times.
Immunofluorescence
All reagents for fixation, washing, and blocking were purchased from Beyotime (Beyotime Biotechnology, China). Pancreatic cancer cells were washed with PBS three times for 5 min each and then fixed for 10 min. They were permeabilized with 0.1% Triton X-100 in PBS for 10 min. They were then blocked for 1 h at room temperature, followed by incubation with anti-LC3A/B antibody (#12741, Cell Signaling, 1:1000) at 4 °C overnight. After washing with PBS extensively the following day, cells were incubated with Cy3-Labeled Goat Anti-Rabbit IgG (Beyotime Biotechnology, China) for 3 h at room temperature. Chromatin was stained with DAPI for 10 min, and cells were observed under a fluorescence microscope (Nikon, Eclipse 80i, Japan).
Transmission electron microscopy
Pancreatic cancer cells were washed with PBS twice and fixed in 2.5% glutaraldehyde in PBS at 4 °C overnight. Fixed cells were washed with PBS three times and post-fixed with 1% osmium acid in PBS for 3 h. After washing the cells three times with PBS, the samples were dehydrated in a graded ethanol series (30, 50, 70, 80, 90, 95, and 100%) for about 15–20 min for each incubation. Samples were then transferred to absolute acetone for 20 min. The samples were placed in a 1:1 mixture of absolute acetone, followed by incubation in a Spurr resin mixture for 1 h at room temperature. Samples were incubated in a 1:3 mixture of absolute acetone, followed by the final resin mixture for 3 h and a final Spurr resin mixture overnight. Finally, the specimens were placed in capsules containing embedding medium and heated to 70 °C for about 9 h. Sections 70 nm in thickness were generated using an ultramicrotome (Leica Ultracut R, Germany). Specimen sections were stained with uranyl acetate and lead citrate for 10 min each and observed using a JEM1230 (JEOL, Japan).
miR-30a reagents and transfection
A miR-30a mimic, miR-30a inhibitor, and negative control mimic were purchased from Ribobio (Guangzhou, China). Lentiviruses used to knockdown or overexpress human miR-30a, in addition to negative control lentiviruses, were purchased from Hanbio (Shanghai, China). All transfections were carried out according to the manufacturer’s instructions.
Luciferase assay
The luciferase assay was carried out as previously described [20]. Briefly, dual-luciferase reporter plasmids were constructed by iGeneBio (Guangzhou, China). Transfection of all plasmids was performed using the Lipofectamine 3000 Reagent. Luciferase activity was measured 48 h after transfection using the Dual-Luciferase Reporter Assay Kit (Promega,USA). The assay was repeated at least three times in independent experiments.
ChIP assay
ChIP assays were performed as previously described [20] using the EZ ChIP Kit (Merck Millipore, Germany) according to the manufacturer’s instructions. Briefly, cross-linked chromatin was sonicated into fragments ranging from 100 to 1000 base pairs. The chromatin was then immunoprecipitated using a ChIP-grade anti-YY1 antibody (ab12132, Abcam).
PANC-1 xenograft tumor model
All animal research procedures adhered to the guidelines of The Care and Use of Laboratory Animals published by the National Institutes of Health and were approved by the Laboratory of Animal Care and Use Committee of Nanjing Medical University. Four-week-old female nude mice (BALB/cA-nu) were purchased from the Model Animal Research Center of Nanjing University. Twenty mice were randomly divided into four groups. To establish a xenograft subcutaneous implant tumor model, PANC-1-YY1-knockdown and vector control cells, PANC-1-miR-30a-knockdown and vector control cells (1.5 × 106 cells/100 μL per mouse) were injected subcutaneously. The mice were euthanized after 4 weeks, and tumor xenografts were resected.
Statistical analysis
All data were representative of at least three independent experiments. Statistical analysis was performed using the SPSS software (Version 22.0). Quantitative data are presented as mean ± SD. All statistical tests were two-tailed exact tests with a p < 0.05 considered significant.
Results
YY1 is implicated in autophagy in pancreatic cancer cells
During autophagosome formation, the cytosolic microtubule-associated protein 1 light chain 3 (LC3)-I is converted into LC3-II, it is phosphatidylethanolamine- conjugated form. LC3II then targets autophagic membranes [21]. To determine the state of autophagy in pancreatic cancer cells, Western blotting was performed for LC3 II in six pancreatic cancer cell lines, and results were compared to those of the control cell line, HPNE (Fig. 1a). Autophagy was clearly elevated in pancreatic cancer cells, which was reflected by the increased in LC3II. Immunofluorescence further confirmed it (Fig. 1b). Next, to investigate the potential role of YY1 in autophagy, we either overexpressed or silenced YY1 in BxPC-3 and PANC-1 cells. We found that YY1 overexpression increased LC3II levels, while YY1 knockdown decreased LC3II levels. Furthermore, other autophagy-related genes, ATG5 and Beclin-1 (the mammalian homologue of yeast ATG6), displayed similar expression dynamics as LC3II (Fig. 2a). In addition, Western blotting and immunofluorescence showed that, compared to the YY1-treated group, autophagy was significantly reduced following treatment with a combination of YY1 and the autophagy inhibitor, 3-methyladenine (3-MA), suggesting that YY1-induced autophagy is inhibited by 3-MA (Fig. 2b, c). To better assess autophagy, we observed autophagosome formation and double-membrane autophagic vacuoles by transmission electron microscopy in both BxPC-3 and PANC-1 cells (Fig. 3a, b). From these micrographs, we found that overexpression of YY1 increased the number of autophagosomes, while YY1 knockdown decreased the abundance of autophagosomes. Taken together, YY1 likely plays roles in autophagy in pancreatic cancer cells.
The level of autophagy in pancreatic cancer cells. a The expression of LC3 II were examined by Western blotting in pancreatic HPNE cells and six pancreatic cancer cell lines (BxPC-3, CFPAC-1, Colo357, MiaPaCa-2, PANC-1, and SW1990) cultured under normal growth conditions. Intensity quantified by image J were given in the histogram. b The expression of LC3 II in pancreatic cancer cell lines and HPNE cells was assayed using Immunofluorescence. LC3 subcellular localization is shown in red and nuclear DAPI staining is shown in blue. Results are representative of three independent experiments
YY1 plays roles in autophagy in pancreatic cancer cells. a The expression of the autophagy-associated proteins, LC3, p62, ATG5, and Beclin1, in YY1-overexpressing or YY1 knockdown BXPC-3 and PANC-1 cells assayed by Western blotting. “YY1” indicates YY1-overexpressing cells, “Vector” indicates cells transfected with a control vector, “YY1 shRNA” indicates YY1 knockdown cells, and “Scramble shRNA” indicates cells transfected with a vector expressing Scramble shRNA. b YY1-overexpressing or YY1 knockdown BXPC-3 and PANC-1 cells were treated with 10 mM 3-MA for 8 h. Untreated cells and the vehicle group treated with DMSO were used as controls. Autophagy levels were then assayed using Western blotting. c LC3 levels were assayed using Immunofluorescence. Results are representative of at least three independent experiments
Autophagosomes detected by transmission electron microscopy. a Transmission electron microscopy shows autophagosomes (red circles) in YY1-overexpressing or YY1 knockdown BXPC-3. Images were captured at ×20,000 magnification. The representative autophagosome was standalone enlarged above. The number of autophagosomes per field was acounted in the histogram. ***p < 0.001. b Transmission electron microscopy shows autophagosomes (red circles) in YY1-overexpressing or YY1 knockdown PANC-1. Images were captured at ×20,000 magnification. The representative autophagosomes were standalone enlarged above. The number of autophagosomes per field was accounted in the histogram. ***p < 0.001
YY1 binds upstream of the miR-30a genomic loci and regulates its expression
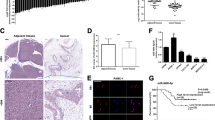
Synergies between YY1 and miRNAs have recently been reported in various cellular processes [5, 12, 22]. Therefore, it is possible that autophagy-related miRNAs might be directly regulated by YY1. Previously, we found that YY1 regulates the expression of ATG5 and Beclin 1 proteins (Fig. 2a). Since ATG5 and Beclin 1 are both known to be direct targets of miR-30a, [19, 23] we hypothesized that YY1 and miR-30a might form a functional regulatory circuit that modulates autophagy in pancreatic cancer cells. Using an RT-PCR assay, miR-30a levels were found to decrease when YY1 was overexpressed and increase when YY1 was knocked down in both BxPC-3 and PANC-1 cells (Fig. 4a, b). This suggested that miR-30a might negatively be regulated by YY1. A bioinformatics prediction algorithm (http://jaspar.binf.ku.dk) revealed three putative YY1 binding sites upstream of the miR-30a genomic loci (Fig. 4c). Using the predicted site sequence that displayed the highest score, we constructed luciferase reporter vectors and performed a dual-luciferase reporter assay. The results showed that overexpression of YY1 significantly decreased luciferase activity for the miR-30a 3′UTR compared to the negative control. In contrast, a mutated version of the miR-30a 3′UTR showed unchanged luciferase activity, demonstrating direct regulatory control of miR-30a (Fig. 4d). To map the YY1 binding sites in the miR-30a promoter under physiological conditions, a ChIP assay was performed. The results revealed that YY1 directly bound the promoter of miR-30a and repressed its expression in BxPC-3 and PANC-1 cells (Fig. 4e). Taken together, these experiments verify miR-30a as a direct target of YY1.
YY1 binds upstream of the miR-30a genomic loci and regulates its expression. a qRT-PCR analysis for miR-30a expression levels in YY1-overexpressing or YY1 knockdown BXPC-3 cells. Results are representative of three independent experiments and are presented as the mean ± SD (bars). *p < 0.05, **p < 0.01, ***p < 0.001. b qRT-PCR analysis for miR-30a expression levels in YY1-overexpressing or YY1 knockdown PANC-1 cells. c Three putative YY1 binding sites upstream of the miR-30a genomic locus predicted using JASPAR bioinformatics software. d A luciferase activity assay was used to determine the effect of YY1 on the expression of a luciferase reporter driven by a miR-30a 3′UTR fragment with or without YY1-specific binding sites in BXPC-3 and PANC-1 cells. e The binding of YY1 to the miR-30a genomic locus was determined by ChIP
Ectopic expression of miR-30a decreases YY1-induced autophagy
Since miR-30a was found to be a direct target of YY1, we next investigated whether miR-30a mediated the autophagy-related effects of YY1. We first manipulated the expression levels of miR-30a in both BxPC-3 and PANC-1 cells using a miR-30a mimic, a miR-30a inhibitor, and a control miRNA. Using Western blotting and immunofluorescence, we found that miR-30a negatively regulated autophagy in pancreatic cancer cells (Fig. 5a, b). Furthermore, we performed a rescue assay in BxPC-3 and PANC-1 cells consisting of a treatment that combined YY1-overexpressing lentiviruses and miR-30a mimics. As expected, overexpression of miR-30a attenuated the pro-autophagic effects of YY1 (Fig. 5c, d), suggesting that miR-30a is indeed a functional target of YY1.
Ectopic expression of miR-30a decreases YY1-induced autophagy. a Western blotting was performed to quantify the expression levels of LC3I/II and p62 in BXPC-3 and PANC-1 cells after transfection with a miR-30a mimic, a miR-30a inhibitor, or a negative control for 48 h. b LC3A/B in BXPC-3 and PANC-1 cells after transfection with a miR-30a mimic, a miR-30a inhibitor, or a negative control for 48 h were detected by Immunofluorescence. c The expression of LC3I/II and p62 in BXPC-3 and PANC-1 cells transfected with YY1-overexpressing lentiviruses or miR-30a mimics were quantified using Western blotting. d The expression of LC3A/B in BXPC-3 and PANC-1 cells transfected with YY1-overexpressing lentiviruses or miR-30a mimics were quantified using Immunofluorescence. Results are representative of at least three independent experiments
miR-30a acts in a feedback loop to inhibit YY1
Since YY1 appears to promote autophagy in pancreatic cancer cells by directly targeting miR-30a, we next explored whether miR-30a modulates YY1 expression, since this type of feedback regulation is commonly observed between transcription factors and miRNAs (such as the Pitx3/miR-133b feedback circuit in midbrain dopamine neurons) [24, 25]. Using various bioinformatics tools, such as Targetscan [26] and RNA22-HAS [27], we found a putative miR-30a binding site in the 3′UTR of YY1 (Fig. 6a). To test whether the predicted YY1 3′UTR binding sites reflect direct regulation by miR-30a, we inserted these sequences downstream of a luciferase reporter gene. We found that a decrease in luciferase activity occurred in the presence of wild type YY1. In contrast, this luciferase inhibition was rescued by mutating the YY1 3′UTR sequences that were predicted to be the miR-30a binding sites (Fig. 6b). We further tested the effect of miR-30a on YY1 mRNA and protein expression using RT-PCR and Western blotting, respectively. This revealed that the expression levels of YY1 were elevated in cells transfected with the miR-30a inhibitor lentivirus and decreased in cells transfected with the miR-30a overexpressing lentivirus (Fig. 6c). Cumulatively, these results suggest that miR-30a acts in a feedback loop to inhibit YY1, thereby regulating autophagy in pancreatic cancer cells.
miR-30a acts in a feedback loop to inhibit YY1. a Predicted binding sites for miR-30a in the YY1 3′UTR. The engineered mutant sequence is also shown. b The luciferase activities of BXPC-3 and PANC-1 cells were measured after co-transfection with the indicated YY1 3′UTR constructs and miR-30a for 24 h. c Western blot and qRT-PCR analysis of YY1 protein or mRNA levels in BXPC-3 and PANC-1 cells transfected with miR-30a-overexpressing or -inhibiting lentivirus. **p < 0.01, ***p < 0.001
YY1/miR30a regulates autophagy in pancreatic xenograft tumors
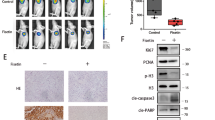
To assess the effects of YY1-induced autophagy in vivo, we implanted xenograft tumors with alternatively varying levels of both YY1 and miR-30a. The mice were euthanized after 4 weeks, and tumor xenografts were resected and photographed (Fig. 7a). The results showed that knockdown of YY1 increased the expression of miR30a (Fig. 7b). We also quantified ATG5 and Beclin-1 expression levels and overall autophagy activity in the xenograft samples (Fig. 7c). Consistent with the in vitro results, knockdown of YY1 inhibited autophagy through miR30a. On the other hand, when nude mice were subcutaneously injected with PANC-1 cells that had been transfected with either a lentiviral vector that inhibited miR30a or a control lentiviral vector, we found that knockdown of miR30a could also increased the expression of YY1 and autophagy activity (Fig. 7d).
YY1/miR30a regulates autophagy in pancreatic xenograft tumors. a The in vivo tumorigenicity of PANC-1 cells with or without YY1/miR30a expression was determined using a nude mouse xenograft assay. Results are representative of three independent experiments. b qRT-PCR analysis of miR30a expression in pancreatic xenograft tumors. c ATG5 and Beclin-1 expression and autophagy activity in the YY1-knockdown xenograft samples assayed by Western blotting. Results are representative of three independent experiments. d ATG5 and Beclin-1 expression and autophagy activity in the miR30a-knockdown xenograft samples assayed by Western blotting. Results are representative of three independent experiments. e A model of the YY1/miR-30a regulatory interaction during autophagy in pancreatic cancer cells
Discussion
This study investigated potential roles for YY1 in autophagy in pancreatic cancer cells. We uncovered the existence of a novel YY1/miR-30a regulatory circuit that modulates autophagy in BxPC-3 and PANC-1 cells. YY1 promotes autophagy in pancreatic cancer cells by suppressing the expression of miR-30a, which modulates the autophagy-associated genes, ATG5 and Beclin 1. Furthermore, YY1 is also targeted by miR-30a via a negative feedback loop (Fig. 7e).
Based on the available literature, YY1 has diverse and complex biological functions. However, research regarding its roles during autophagy is lacking. In pancreatic cancer, autophagy is upregulated during later stages of the disease in a cell-autonomous fashion, and it is required for continued tumor growth in vitro and in vivo [14]. Understanding the role of autophagy in pancreatic cancer pathogenesis is critical. Therefore, we explored the mechanisms of autophagy regulation in pancreatic cancer. In this study, we implicated YY1 in the regulation of autophagy in pancreatic cancer cells. Combined with our previous work, YY1 appears to play critical roles in pancreatic cancer—not only in tumor cell growth, progression, and invasion, but also in autophagy [9]. In our previous study, we showed that YY1 promotes apoptosis and plays a tumor-suppressive role in pancreatic cancer cells [20]. In the present study, we find that YY1 also promotes autophagy and attenuates pancreatic cancer growth. Interestingly, these results seem to contradict a previous study that showed that inhibition of autophagy suppresses proliferation of BxPC-3 and PANC-1 cells [28]. It is possible that these tumor-suppressive effects mainly depend on YY1-induced apoptosis. In addition, many studies have shown that autophagy induces apoptosis, and autophagy-associated proteins (such as ATG5, ATG12, and Beclin1) help initiate apoptosis [29, 30]. Since YY1 has been observed to promote both apoptosis and autophagy in pancreatic cancer, the effect of suppressing tumor growth clearly requires further investigation.
Transcription factors and microRNAs can act in cooperation to regulate target gene expression via feed forward or feedback loops. These regulatory interactions play critical roles in many biological processes and they have been extensively studied over the years [24, 31]. Our findings support the existence of reciprocal regulation between YY1 and a miRNA. Interestingly, the inhibitory feedback interaction between YY1 and miR-30a is similar to the observations of Lu and his team [32], who demonstrated the existence of a novel YY1/miR-1 regulatory circuit during skeletal myogenesis. Feedback loops of this type may be advantageous to cells because it allows the system to remain reversible so that the system will be more stable.
Conclusions
In summary, we have identified a novel signaling circuit consisting of YY1 and miR-30a, which is required for regulating autophagy in pancreatic cancer cell lines. This research sheds new light on mechanisms critical for promote tumorigenesis and tumor progression in pancreatic cancer. Because the pathways downstream of YY1 are highly redundant, other currently unknown mechanisms may be involved in YY1-mediated autophagy. The interaction between YY1 and miR-30a illustrates the importance of feedback control in autophagy, and this circuit may constitute a valuable diagnostic and therapeutic target for treating pancreatic cancer.
Abbreviations
- YY1:
-
Yin-Yang 1
- RT-qPCR:
-
real-time quantitative polymerase chain reaction
- ChIP:
-
chromatin immunoprecipitation
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
fetal calf serum
- NP-40:
-
Nonidet P 40
- EDTA:
-
ethylene diamine tetraacetie acid
- PMSF:
-
phenylmethanesulfonyl fluoride
- BCA:
-
bicinchoninic acid
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DMSO:
-
dimethyl sulphoxide
References
Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–42.
Zhang Q, Stovall DB, Inoue K, Sui G. The oncogenic role of Yin Yang 1. Crit Rev Oncog. 2011;16:163–97.
Inoue K, Fry EA, Frazier DP. Transcription factors that interact with p53 and Mdm2. Int J Cancer. 2016;138:1577–85.
Zhao G, Li Q, Wang A, Jiao J. YY1 regulates melanoma tumorigenesis through a miR-9 ~ RYBP axis. J Exp Clin Cancer Res. 2015;34:F49.
Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, et al. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 2008;14:369–81.
Huang Y, Tao T, Liu C, Guan H, Zhang G, Ling Z, et al. Upregulation of miR-146a by YY1 depletion correlates with delayed progression of prostate cancer. Int J Oncol. 2017;50:421–31.
Yuan P, He X-H, Rong Y-F, Cao J, Li Y, Hu Y-P, et al. KRAS/NF-κB/YY1/miR-489 Signaling axis controls pancreatic cancer metastasis. Can Res. 2017;77:100–11.
Zaravinos A, Spandidos DA. Yin Yang 1 expression in human tumors. Cell Cycle. 2014;9:512–22.
Zhang J-J, Zhu Y, Xie K-L, Peng Y-P, Tao J-Q, Tang J, et al. Yin Yang-1 suppresses invasion and metastasis of pancreatic ductal adenocarcinoma by downregulating MMP10 in a MUC4/ErbB2/p38/MEF2C-dependent mechanism. Mol Cancer. 2014;13:130.
Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962;12:198–202.
Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–80.
Feng L, Ma Y, Sun J, Shen Q, Liu L, Lu H, et al. YY1-MIR372-SQSTM1 regulatory axis in autophagy. Autophagy. 2014;10:1442–53.
Yang S, Kimmelman AC. A critical role for autophagy in pancreatic cancer. Autophagy. 2011;7:912–3.
Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–29.
Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC, et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4:905–13.
Hwang DW, So KS, Kim SC, Park K-M, Lee Y-J, Kim S-W, et al. Autophagy induced by CX-4945, a casein kinase 2 inhibitor, enhances apoptosis in pancreatic cancer cell lines. Pancreas. 2017;46:575–81.
Pardo R, Ré Lo A, Archange C, Ropolo A, Papademetrio DL, Gonzalez CD, et al. Gemcitabine induces the VMP1-mediated autophagy pathway to promote apoptotic death in human pancreatic cancer cells. Pancreatology. 2010;10:19–26.
Yu Y, Cao L, Yang L, Kang R, Lotze M, Tang D. microRNA 30 Apromotes autophagy in response to cancer therapy. Autophagy. 2014;8:853–5.
Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X, et al. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy. 2014;5:816–23.
Zhang J-J, Zhu Y, Yang C, Liu X, Peng Y-P, Jiang K-R, et al. Yin Yang-1 increases apoptosis through Bax activation in pancreatic cancer cells. Oncotarget. 2016;7:28498–509.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8.
Zhao G, Li Q, Wang A, Jiao J. YY1 regulates melanoma tumorigenesis through a miR-9 ~ RYBP axis. J Exp Clin Cancer Res. 2015;34:66.
Hu J, Meng Y, Zhang Z, Yan Q, Jiang X, Lv Z, et al. MARCH5 RNA promotes autophagy, migration, and invasion of ovarian cancer cells. Autophagy. 2017;13:333–44.
Zhang HM, Kuang S, Xiong X, Gao T, Liu C, Guo AY. Transcription factor and microRNA co-regulatory loops: important regulatory motifs in biological processes and diseases. Brief Bioinform. 2015;16:45–58.
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, et al. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–4.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
Miranda KC, Huynh T, Tay Y, Ang Y-S, Tam W-L, Thomson AM, et al. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–17.
Hashimoto D, Bläuer M, Hirota M, Ikonen NH, Sand J, Laukkarinen J. Autophagy is needed for the growth of pancreatic adenocarcinoma and has a cytoprotective effect against anticancer drugs. Eur J Cancer. 2014;50:1382–90.
Rubinstein AD, Eisenstein M, Ber Y, Bialik S, Kimchi A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol Cell. 2011;44:698–709.
Li H, Wang P, Sun Q, Ding WX, Yin XM, Sobol RW, et al. Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of beclin 1. Can Res. 2011;71:3625–34.
Martinez NJ, Walhout AJM. The interplay between transcription factors and microRNAs in genome-scale regulatory networks. BioEssays. 2009;31:435–45.
Lu L, Zhou L, Chen EZ, Sun K, Jiang P, Wang L, et al. A Novel YY1-miR-1 regulatory circuit in skeletal myogenesis revealed by genome-wide prediction of YY1-miRNA network. PLoS ONE. 2012;7:e27596.
Authors’ contributions
CY and JJZ carried out the studies, participated in the experimental design and drafted the manuscript. YPP and YZ helped with Chip, qRT-PCR. LDY and JSW helped with cell culture and Western blotting. WTG and KRJ participated in the in vitro experiments. YM conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (81672449 and 81572337); This work was also supported by the Innovation Capability Development Project of Jiangsu Province (BM2015004), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, JX10231801).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Please contact author for data requests.
Consent for publication
No individual person’s data in any form were involved in this study.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital with Nanjing Medical University.
Funding
The National Natural Science Foundation of China (81672449 and 81572337); The Innovation Capability Development Project of Jiangsu Province (BM2015004); The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, JX10231801).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yang, C., Zhang, JJ., Peng, YP. et al. A Yin-Yang 1/miR-30a regulatory circuit modulates autophagy in pancreatic cancer cells. J Transl Med 15, 211 (2017). https://doi.org/10.1186/s12967-017-1308-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-017-1308-3