Abstract
Background
To identify the novel epitopes from the human papillomavirus type 18 E7 which can sensitize PBMCs of four different major HLA class I A allele.
Methods
Twenty-four synthetic overlapping 15-amino acid peptides were screened by measuring the frequency of CD8+ cytotoxic T lymphocytes (CTLs)-producing interferon-γ (IFN-γ) by using flow cytometry and ELISpot assays and selected peptides were validated for cytolytic activity by using the 51Cr release assay. Truncated peptides in the selected epitopes were tested to determine the important residues using ELISpot and 51Cr release assay.
Results
Among 24 peptides, E781-95DDLRAFQQLFLNTLS (#21) and E789-103LFLNTLSFVCPWCAS (#23) induced significantly higher Th 1 response including IFN-γ production and in vitro cytotoxicity of PBMCs of four different HLA-A alleles against cervical cancer cells than that of other peptides and the negative control (no peptide sensitization). In E781–95 (#21), amino acid position 81, 82 (N-terminus) and 92, 94, 95 (C-terminus) for HLA-A*02:02 and 24:02, and 81, 82 (N-terminus) and 92, 95 (C-terminus) for HLA-A*11:01 and 33:03 were important to elicit Th1 response of PBMCS. In E789–103 (#23), residue 100 and103 (C-terminus) were important to elicit the CD8+ CTL response in HLA-A*02:01, 11:01 and 33:03 and 100, 101, and 103 (C-terminus) were important to elicit the CD8+ CTL response in HLA-A*24:02.
Conclusions
E781–95 (#21) and E789–103 (#23) were identified as novel epitopes from HPV18 E7 which could sensitized PBMCs of four different HLA class I (HLA-A*02:01, 24:02, 11:01 and 33:03). These epitopes could be useful for immune monitoring and immunotherapy for HPV 18+ cervical cancer.
Similar content being viewed by others
Introduction
A number of previous studies have reported that various types of cancer are caused by Infectious pathogens such as human papilloma virus (HPV), Helicobacter pylori, and hepatitis viruses [1],[2]. HPV has been proven as a major causative pathogen of cervical cancer, anal cancer and nasopharyngeal cancer. Cervical cancer is the third most commonly diagnosed cancer and second leading cause of cancer-related death worldwide in women [3],[4] and the incidence of nasopharyngeal cancer have been markedly increased during last decade in both men and women [5]–[8]. Therefore, the control of HPV infection is critical for the prevention or treatment of not only in precancerous states, but also in HPV-induced cancers. Among over 100 subtypes of HPV, HPV 16 and 18 are most important subtypes and about 70% of cervical cancers are associated with these two types of HPV [9].
Although currently available two prophylactic HPV vaccines have been shown to effectively prevent HPV-associated anogenital disease in young women and men [8],[9], only 32% of teenagers who qualify for immunization have received all three recommended doses of the vaccine in the USA [10]. These vaccines did not show a therapeutic effect on pre-existing cervical infection or cervical lesions [11],[12]. Moreover, conventional primary treatments for early stage cervical cancer showed a recurrence rate of about 15% [13],[14]. In the case of late stage or recurrent disease, treatment results are relatively poor. Thus, more effective ways which can control or treat HPV infection and HPV-related cancer should be needed.
Spontaneous regression of HPV-infected precancerous lesions has been reported in patients who had strong HPV-specific Th1-biased T-cell responses [15]–[18]. In particularly, a strong CD8+ cytotoxic T lymphocyte (CTL) response could play an important role not only for control of HPV infection, but also for treatment of HPV-induced cervical cancer. The peptides from the HPV protein could successfully sensitize and expand HPV-specific CTLs and these peptides can be used for immune monitoring as well as for vaccine or immune therapy against HPV-induced disease [19],[20]. However, one of the major drawback limiting the use of peptide-based immune monitoring or immunotherapy is that identification of at least one immune dominant epitopes from HPV molecules for each HLA class I alleles are needed for wide clinical application because CD8+ CTLs from patients or donors who have different HLA class I genetic background recognized different epitopes of HPV molecules. For overcome this drawback of peptide-based immune monitoring or immunotherapy, the identification of single peptides that can bind to multiple HLA types have been investigated and theses could lead to effective coverage of the human population by a peptide-based immune monitoring or immunotherapy. However, very few studies have addressed PBMC recognition of single peptides in a genetically heterogeneous group previously exposed to an infectious agent or cancer [21]–[24].
In this study, we report that novel single peptides that can bind to 4 different HLA class I which are A*02:01, A*24:02, A*11:01 and A*33:03, most frequent allele in human race. Theses single peptides were successfully elicit HPV 18-speciifc Th 1 responses of PBMCs.
Methods
Donors and peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were collected from four HLA class I (HLA-A*02:01, A*24:02, A*11:01, A*33:03) healthy donors. Human leukocyte antigen (HLA) class I genotypes were determined by the HLA laboratory at the Seoul Medical Science Institute (Seoul, South Korea) via sequence-specific polymerase chain reaction using genomic DNA. PBMCs were isolated via density gradient centrifugation using Ficoll-Hypaque 1.077 (Pharmacia Biotech, Wilkstrom, Sweden). The mononuclear cells were cryopreserved at −160°C in human AB + serum containing 10% dimethylsulfoxide (DMSO; Sigma, St Louis, MO, USA). This research was approved by the institutional review board of Yonsei University Health System, and all participants provided written informed consent.
Synthesis of HPV16 E7 peptides
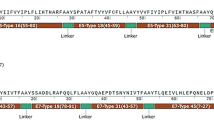
A total of twenty-four, 15-amino acid peptides spanning the HPV18 E7 protein that overlapped by 11 residues, were synthesized commercially (purity of >95%; A & Pep, Yeongi-gun, South Korea; Table 1). After selection of the immune dominant candidate 15-amino acid peptides which were restricted to each HLA class I from the peptide library by screening and confirmation tests, truncated peptides spanning the candidate 15-amino acid peptides were synthesized (Table 2). The peptides were diluted to working solution concentrations (1 μg/μL) in diethylpyrocarbonate-treated water (Invitrogen, Carlsbad, CA, USA) containing 1% DMSO and stored at −80°C before testing.
Generation of autologous dendritic cells and peptide-specific CTLs
Autologous dendritic cells (DCs) were generated as previously described, with minor modifications [25],[26]. PBMCs were incubated for 2 h at 37°C using complete RPMI medium containing 10% fetal bovine serum (FBS). Adherent monocytes were resuspended at a concentration of 5 × 106 cells/mL in complete RPMI medium with granulocyte-macrophage colony-stimulating factor (1500 IU/mL; PeproTech, Rocky Hill, NJ, USA) and interleukin (IL)-4 (1200 IU/mL; PeproTech). On days 2, 4, and 6 of culture, fresh cytokines were added. On day 5 of culture, 10 ng/mL of tumor necrosis factor-α (R&D Systems, Minneapolis, MN, USA) was added for DC maturation. After maturation, autologous DCs were pulsed with peptides for at least 6 h. PBMCs were plated at a concentration of 2 × 106 cells per well in a 24-well culture plate (Nunc, Rochester, NY, USA) with 2 mL of complete RPMI medium. PBMCs were sensitized with synthetic HPV18 E7 peptides (10 μg/mL/well), and 1000 IU/mL/well of recombinant human IL-2 (rhIL-2; PeproTech) was added. Additionally, rhIL-2 (1000 IU/mL/well) was added to the culture every other day. For 1-week expansion, peptide-pulsed autologous DCs (4–10 × 106/well) were added to the PBMCs on day 7, incubated for 6 h, and analyzed with flow cytometry. DC-treated PBMCs were cultured for another 7 days, and cytotoxicity assays were performed.
Intracellular flow cytometric analysis
Phycoerythrin (PE)-conjugated anti–interferon-gamma (anti-IFN-γ), APC-Cy7-conjugated anti-CD44, FITC-conjugated anti-CD3, PerCp-Cy5.5-conjugated anti-CD4, and APC-conjugated anti-CD8 were purchased from BD Biosciences (San Jose, CA, USA). For each sample, 1 × 105 events were gated on a LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). For data analysis (Flowjo software; Tree Star, Ashland, OR, USA), positive cells were expressed as a percentage of the respective reference population. The assessment of responses was previously described in more detail [24]. Briefly, peptide-sensitized PBMCs (1 × 106 cells/mL) stimulated with phytohemagglutinin (PHA, Sigma) and PBMCs pulsed with autologous DCs that were not loaded with any peptide were used as positive and negative controls, respectively. One hour after stimulation, 10 μg of Brefeldin A (Sigma) was added to each well. After 5 additional hours of incubation, PBMCs were washed once with phosphate-buffered saline (PBS) and then incubated in PBS containing 1 mM ethylene-diamine-tetraacetic acid for 10 min. After 2 additional washes with PBS containing 5% FBS, the cells were incubated with fluorescently-labeled monoclonal anti-CD3, anti-CD8, anti-CD4, anti-CD44, and anti–INF-γ antibodies for 15 min on ice in the dark prior to analysis.
IFN-γ enzyme-linked immunosorbent spot assay (ELISpot)
IFN-γ production was determined in PBMCs stimulated with HPV18 E7 peptides. Cryopreserved PBMCs were thawed into complete RPMI (RPMI supplemented with L-Glutamine, penicillin, streptomycin, and 10% heat inactivated fetal calf serum; Invitrogen). Then, 5 × 105 cells/well were plated in triplicate for each treatment in the ELISpot plates (Millipore, Billerica, MA, USA). Plates were previously coated with anti-IFN-γ antibody (Clone AN-18, Ebioscience, San Diego, CA, USA) at 4°C, overnight. PBMCs were incubated with HPV18 E7 peptides for 48 h at 37°C and 5% CO2. Phytohemagglutinin (PHA) was added at 2.5 μg/mL as a positive control and PBMCs sensitized with no peptide were used as a negative control. Biotinylated IFN-γ detection antibody (100 μL; 1 μg/mL in PBS) was added to each well (clone R4-6A2, Ebioscience, San Diego, CA, USA) and incubated at RT for 1.5 hours. After washing the plate 5 times with PBS, 100 μl of streptavidin-alkaline phosphatase (Invitrogen) diluted 1:1000 in PBS was added. The plate was incubated at RT for 1 h in the dark and developed with color solution by mixing 10 mL of Tris-MgCl2 buffer with 100 μL of NBT solution (Bio-Rad, Berkeley, CA, USA) and 100 μL of BCIP solution (Bio-Rad). The plate was dried before reading. The spots were quantified with an ELISpot plate reader and software version 3.5 (AID ELISpot Reader System, Strassberg, Germany). Data were obtained by calculating the means of triplicate wells. ELISpot data were expressed as the total IFN-γ spot-forming units (SFU)/106 PBMCs.
Cell culture
The human HPV 18+, cervical cancer cell lines (HTB-34 for HLA-A*02:01, SNU-1160-A*2402) were purchased from by the Korean Cell Line Bank (Seoul, Korea), and it has been tested and authenticated. Cancer cell line was expanded and frozen in aliquots within 4 weeks of purchase. Cancer cells were thawed and cultured at 37°C and 5% CO2 in Dulbecco’s modified Eagle medium (Gibco, Grand Island, NY, USA) containing 10% FBS and 1% antibiotics for no more than 8 passages.
Immortalized Epstein-Barr virus-B lymphoblastoid cell lines (EBV-BLC) which were restricted each HLA class I (kindly gifted from Dr. David Stroncek, in NIH) were cultured at 37°C and 5% CO2 in RPMI-1640 with 10% FBS. Cells were routinely tested for the absence of mycoplasma.
In vitro cytotoxicity assay
Cytotoxicity assays were performed using the 51Cr release assay. Briefly, cervical cancer cells labeled for 45 min with 51Cr (100 mCi/106 cells; Perkin Elmer, Waltham, MA, USA), washed in PBS, and dispensed in triplicate into 96-well U-bottom plates (Nunc, Rochester, NY, USA) at 4 × 103 cells/well. Peptide-sensitized PBMCs were added at an effector: target ratio of either 10:1, 30:1, 50:1, or 100:1. The cells were pelleted and incubated for 6 h, and the supernatant was analyzed using a WIZARD2 Automatic Gamma Counter (Perkin Elmer). Spontaneous and total release for each target were used to calculate the percentage of specific release according to the following formula: % specific release = (experimental counts per minute – spontaneous counts per minute)/(total counts per minute – spontaneous counts per minute) × 100.
Statistical analysis
Data presented as mean ± standard error are the representative of at least 3 different experiments. To compare between control group and each tested group, a student t- test (two-tailed) was used. P-values less than 0.05 was considered statistically significant.
Results
Screening of 15-amino acid peptides from HPV 18 E7 for elicit of Th1 response
IFN-γ+ spot forming unit (SFU) were counted using ELISpot assay after in vitro sensitization of PBMCs with each candidate peptide to determine which 15-amino acid peptides, from the 24 candidate peptides, were able to elicit CTL-specific immune responses. In HLA-A*02:01, A*11:01 and A*33:03, HPV 18 E789-103LFLNTLSFVCPWCAS (#23) and HPV 18 E781-95DDLRAFQQLFLNTLS (#21) consistently induced the highest and 2nd highest production of IFN-γ+ spots from PBMCs among 24 candidate peptides, respectively (Figure 1A, C, D). In HLA-A*2402, E781–95 (#21) induced the highest production of IFN-γ + spots and E789–103 (#23) was 2nd highest production of IFN-γ + spots from PBMCs among 24 candidate peptides (Figure 1B). E789–103 (#23) and E781–95 (#21) induced at least 3 fold higher numbers of IFN-γ + spot forming units (SFU) from PBMCs than those of negative control (PBMCs sensitized with no peptide) in all four HLA class I (P < 0.05, P < 0.05, respectively). These results indicated that HPV18 E781–95 (#21) and E789–103 (#23) could induce strong Th1 response from donor PBMCs of HLA-A*02:01, A*24:02, A*11:01, A*33:03 simultaneously. Because Th1 response was mainly induce by CD8+ and CD4+ T cells as well as NK cells and the CD8+ CTLs play a major role in anti-viral and anti-tumor responses, we further investigated to determine whether these two candidate peptides induce CD8+ CTL response using flow cytometry analysis.
Screening of immunogenic epitopes of HPV18 E7 which could sensitize PBMCs of four major HLA class I using IFN-γ ELISpot assay. ELISpot assays were performed to measure IFN-γ production from donor’s PBMCs of four major HLA class I that were sensitized with 24 candidate peptides. HPV18 E781–95 (#21) and E789–103 (#23) induced greater number of IFN-γ+ spots from PBMCs of HLA-A*02:01 (A), HLA-A*24:02 (B), HLA-A*11:01 (C), HLA-A*33:03 (D) than other peptides. Data are representative of at least three independent experiments using PBMCs from HLA-A*02:01, HLA-A*24:02, HLA-A*11:01, HLA-A*33:03 subjects. PBMCs sensitized with PHA were used as a positive control (PHA), and PBMCs with sensitized with no peptide (No peptide) were used as the negative control (N.C). Data are presented as mean ± standard error. Statistically significant differences between the tested group and negative control group were determined using a student t-test (two-tailed). **P < .001; *P < .05.
Measuring CD8+ CTL response after sensitization with 15-amino acid peptides
We measured the intracellular IFN-γ production from four HLA class I (HLA-A*02:01, A*24:02, A*11:01, A*33:03) donor’s PBMCs to determine if the CD8+ CTL response could be induced by sensitization of donor PBMCs with candidate peptides. After a week of in vitro sensitization, PBMCs were restimulated with dendritic cells derived from autologous monocytes that were loaded with each candidate peptide. After a 6-hour resensitization, intracellular IFN-γ production from donor’s CD8+ T cells (CD3+CD8+IFN-γ+) was measured by flow cytometry. The fold increases of the percentage of CD8+ T cells that produced intracellular IFN-γ (CD3+CD8+IFN-γ+) after resensitization of candidate peptides among the total CD3+CD8+ T cell population were calculated and compared to that of the negative control (CD3+CD8+IFN-γ + among PBMCs sensitized with no peptide) (Figure 2). HPV18 E781–95 (#21) and E789–103 (#23) consistently induced higher percentage of CD3+CD8+IFN-γ+ than that of other candidate peptides in HLA-A*02:01, A*24:02, A*11:01, A*33:03. E781–95 (#21) and E789–103 (#23) showed at least 2-fold and 2.5-fold higher induction of CD8+IFN-γ+ T cells compared to the negative control in HLA-A*02:01, A*24:02, A*11:01, A*33:03, respectively.
Fold increase of the percentage of CD8 + IFN-γ + within donor’s PBMCs of four major HLA class I after sensitization of candidate peptides. The percentage of CD8+IFN-γ+ from donor’s PBMCs of four major HLA class I were measured to determine whether the CD8+ CTL response were induced by sensitization of donor PBMCs with candidate peptides and the fold increase of the percentage of CD8+IFN-γ+ within the total CD8+ T cell population in donor PBMCs after re-sensitization of each candidate peptide were compared to that of negative control (PBMCs sensitized with no peptide). HPV18 E781-95DDLRAFQQLFLNTLS (#21) and E789-103LFLNTLSFVCPWCAS (#23) consistently induced higher the percentage of CD8+IFN-γ+ T cells than that of other candidate peptides in HLA-A*02:01 (A), HLA-A*24:02 (B), HLA-A*11:01 (C), HLA-A*33:03 (D). PBMCs sensitized with PHA were used as a positive control (PHA), and PBMCs sensitized with no peptide (No peptide) were used as the negative control (N.C). Data are presented as mean ± standard error.
These results suggested that E781–95 (#21) and E789–103 (#23) could successfully induce CD8+ CTL response from PBMC and these two peptides were selected for further study.
Analysis of the peptide-specific cytotoxicity of E781–95 (#21) and E789–103 (#23) peptides
To confirm that HPV18 E781–95 (#21) and E789–103 (#23) are immune-dominant peptides for each HLA class I subjects, PBMCs from four HLA class I donors (HLA-A*02:01, A*24:02, A*11:01, A*33:03) were sensitized in vitro for two weeks with the candidate peptides and were tested for cytotoxic effects using the HLA-matched EBV-BLCs as the target cells because EBV-BLCs could present each peptide successfully in the HLA-peptide complex on their cell surface. HPV18 E781–95 (#21)- and E789–103 (#23)-sensitized CTLs lysed more EBV-BLCs loaded with E781–95 (#21) and E789–103 (#23, respectively, than negative control (EBV-BLCs loaded with no peptide) in all HLA-A*02:01, A*24:02, A*11:01, A*33:03 (Figure 3A-D). PBMCs sensitized for 2 weeks with E789–103 (#23) were slightly higher cytotoxicity to HLA-matched EBV-BLCs loaded with E789–103 (#23) than that of E781–95 (#21) in all four HLA class I. These results confirmed that both HPV-18 E781–95 (#21) and E789–103 (#23) were successfully recognized by CD8+ CTLs from 4 different HLA-A and induced CD8+ cytotoxicity response in all four HLA class I (HLA-A*02:01, A*24:02, A*11:01, A*33:03).
EBV-BCLs cytotoxicity of PBMCs sensitized with HPV 18 E7 81-95 (#21) and E7 89-103 (#23) peptides. HPV18 E781-95 (#21)- and E789-103 (#23)-sensitized CTLs lysed more EBV-BLCs with E781-95 (#21) and E789-103 (#23), respectively, than negative control (EBV-BLCs with no peptide) in all HLA-A*02:01 (A), A*24:02 (B), A*11:01(C), A*33:03 (D). PBMCs sensitized with E781-95 or E789-105 with EBV-BCLs loaded with no peptide or PBMCs sensitized with no peptide and E789-105-loaded EBV-BCLs were used as negative controls. Data are representative of at least three independent experiments using PBMCs from HLA-A*02:01, HLA-A*24:02, HLA-A*11:01, HLA-A*33:03 subjects. Data are presented as mean (point) ± standard error (bar).
Cervical cancer cell cytotoxicity of PBMCs sensitized with E781–95 (#21) and E789–103 (#23) peptides
To confirm the cervical cancer cell cytotoxicity of PBMCs sensitized E781–95 (#21) and E789–103 (#23) peptides, PBMCs from four HLA-A*02:01, A*24:02 donors were sensitized with E781–95 (#21) and E789–103 (#23) peptides in vitro for two weeks and were tested for cytotoxic effects on the HLA-matched cervical cancer cell line (HTB-34 restricted to HLA-A*02:01 and SNU-1160 restricted to HLA-A*24:02). The cytotoxicity assay was carried out by measuring 51Cr release from each HLA-restricted cancer cells. HPV18 E781–95 (#21)- and E789–103 (#23)-sensitized PBMCs lysed more cervical cancer cells than the unsensitized cells. PBMCs from HLA-A*02:01 donors that were in vitro- sensitized for 2 weeks with HPV18 E789–103 (#23) and E781–95 (#21) were highly cytotoxic to HLA-A*02:01-restricted HTB-34 cell lines (Figure 4A). PBMCs from HLA-A*24:02 donors that were in vitro- sensitized for 2 weeks with HPV18 E789–103 (#23) and E781–95 (#21) were also highly cytotoxic to SNU-1160 cells (Figure 3B). These results confirmed that both HPV-18 E781–95 (#21) and E789–103 (#23) epitopes were naturally processed in both HTB-34 and SNU-1160 cervical cancer cell lines and were to be useful as the HLA-A*02:01 and HLA-A*24:02 immunogenic epitopes within the HPV18 E7 protein.
Cervical cancer cell cytotoxicity of PBMCs sensitized with HPV 18 E7 81-95 (#21) and E7 89-103 (#23) peptides. Cytotoxicity assays with HLA-matched cervix cancer cell lines were tested using the 51Cr-release assay to confirmed HPV18 E781-95 (#21)- and E789-103 (#23)-sensitized PBMCs could successfully recognized same peptides that naturally presented on the surface of cancer cells. E781-95 (#21)- and E789-103 (#23)-sensitized PBMCs of HLA-A*02:01 and HLA-A*24:02 lysed greater quantities of HTB-34 cells (HPV 18+, HLA-A*02:01) (A) and SNU-1160 (HPV 18+, HLA-A*24:02) (B) than that of negative control (PBMCs sensitized with no peptide), respectively. Data are representative of at least three independent experiments using PBMCs from HLA-A*02:01, HLA-A*24:02, HLA-A*11:01, HLA-A*33:03 subjects. Data are presented as mean (point) ± standard error (bar).
Determination of the specific residues within E781–95 (#21) and E789–103 (#23) epitopes recognized by CD8+ CTLs
To determine the specific residues within E781–95 (#21) and E789–103 (#23) epitopes recognized by CD8+ CTLs, a panel of N- and C-terminal truncations of HPV18 E781–95 (#21) and E789–103 (#23) was synthesized (Table 2) and tested using ELISpot assay (Figure 5). In all four HLA types, the stepwise removal of amino acids from the N-terminal end of the HPV-18 E781–95 (#21) revealed that the Th1 response was diminished when residue 82 was deleted. This result suggested that residue 81 and 82 of the N-terminus were important to elicit Th1 response of PBMCs in four different HLA class I (Figure 5A-D). In case of HLA-A*02:01 and HLA-24:02, truncations from the C-terminal side of HPV-18 E781–95 (#21) revealed that residue 92, 94, and 95 were important for the CD8+ CTL response. These results indicate that residues 92, 94 and 95 would mark the C-terminal edge of the CD8+ CTL-stimulating epitope (Figure 5A and B). However, in case of HLA-A*11:01 and HLA-33:03, residue 94 and 95 were important for the PBMCs response, and these residues would mark the C-terminal edge of the CD8+ CTL-stimulating epitope (5C, 5D).
Determination the fine specific residues within the 15-aa epitopes recognized by PBMCs using IFN-γ ELISpot assay. IFN-γ ELISpot assays were performed to determination the fine specific residues within HPV18 E781–95(#21) and E789–103 (#23) which were recognized by PBMCs. In HLA-A*02:01 (A), residue 82 of the N-terminus and 92, 94 of C-terminus within HPV18 E781–95 (#21) were important to elicit Th1 response of PBMCS. Residue 100, 103 of C-terminus within E789–103 (#23) were important to elicit the cytotoxic response of PBMCs. In HLA-24:02 (B), residue 82 of the N-terminus and 92, 94 of C-terminus within HPV18 E781-95 (#21) were important to elicit Th1 response of PBMCS. Residue 100, 101, 103 of C-terminus within E789-103 (#23) were important to elicit the cytotoxic response of PBMCs. In HLA-A*11:01 (C) and 33:03 (D), residue 82 of the N-terminus and 94 of C-terminus within HPV18 E781–95 (#21) were important to elicit Th1 response of PBMCS. Residue 100, 103 of C-terminus within E789–103 (#23) were important to elicit the cytotoxic response of PBMCs. Data are representative of at least three independent experiments using PBMCs from HLA-A*02:01, HLA-A*24:02, HLA-A*11:01, HLA-A*33:03 subjects. Data are presented as mean ± standard error. Statistically significant differences between the tested group and negative control group were determined using a student t-test (two-tailed). **P < .001; *P < .05.
In HLA-A*02:01, A*11:01 and A*33:03, truncations from the C-terminal end of HPV18 E789–103 (#23) revealed that residue 100 and 103 were important to elicit the CD8+ CTL response. This suggested that residues 100 and 103 comprise the C-terminal end of the CD8+ CTL-stimulating epitope (5A, 5C, 5D). However, in case of HLA-A*24:02, residue 100, 101 and 103 were important to elicit the CD8+ CTL response and residues 100, 101 and 103 comprise the C-terminal end of the CD8+ CTL-stimulating epitope (5B).
Truncated peptides of HPV-18 E781–95 (#21) and E784–99 (#23) induced the peptide-specific cytotoxicity
To provide further evidence that truncated peptides of HPV18 E781–95 (#21) and E784–99 (#23) induced epitope-specific and HLA class I-restricted cell cytotoxicity, PBMCs were sensitized with the truncated peptides and tested for cytotoxicity, using the 51Cr release assay with HLA-matched EBV-BLCs loaded with same peptides. In case of HLA-A*02:01 and A*24:02, HPV18 E782-95DLRAFQQLFLNTLS (#21-1), E781-94DDLRAFQQLFLNTL (#21-8), and E781–92 DDLRAFQQLFLN (#21-10) from HPV18 E781–95 (#21)-sensitized CTLs lysed greater quantities of EBV-BLCs than that of negative control cells (EBV-BLCs loaded with no peptide or PBMCs sensitized with no peptide) (Figure 6A and C). In HLA-A*11:01 and A*33:03, HPV18 E782-95DLRAFQQLFLNTLS (21–1) and E781–92 DDLRAFQQLFLN (21–10) from HPV18 E781–95 (#21)-sensitized CTLs lysed greater quantities of EBV-BLCs than that of negative control cells (EBV-BLCs pulsed with no peptide (Figure 6E and G). In case of HLA-A*02:01, A*11:01 and A*33:03, CTLs sensitized with E784–97 LFLNTLSFVCPW (#23-10) from HPV18 E789–103 (#23) lysed greater quantities of EBV-BLCs loaded with same peptide than the negative controls (EBV-BLCs loaded with no peptide or PBMCs sensitized with no peptide)) (Figure 6B, F, H). In HLA-A*24:02, CTLs sensitized with E784–98 LFLNTLSFVCPWC (23–9) and E784–97 LFLNTLSFVCPW (23–10) from HPV18 E789–103 (#23) lysed greater quantities of EBV-BLCs pulsed with same peptide than the negative controls (EBV-BLCs loaded with no peptide) (Figure 6D). These results were comparable to the results of ELISpot assays in Figure 5.
Truncated peptides of HPV-18 E7 81–95 (#21) and E7 84–99 (#23) induced the peptide-specific cytotoxicity. PBMCs were sensitized with truncated peptides and tested for cytotoxicity using the 51Cr release assay against HLA-matched, truncated peptide-loaded EBV-BLCs. In HLA-A*02:01, truncated peptides #21-1, #21-8, #21-10 and #23-10-sensitized CTLs lysed more EBV-BLCs loaded with same peptide, respectively, than negative control (A,B), In HLA-A*24:02, #21-1, #21-8, #21-10 and #23-9, #23-10-sensitized CTLs lysed more EBV-BLCs loaded with same peptide, respectively, than negative control (C,D). In HLA-A*11:01, #21-1, #21-10 and #23-10-sensitized CTLs lysed more EBV-BLCs loaded with same peptide, respectively, than negative control (E,F). In HLA-A*33:03, #21-1, #21-10 and #23-10-sensitized CTLs lysed more EBV-BLCs loaded with same peptide, respectively, than negative control (G,H). PBMCs sensitized with truncated-peptide and EBV-BCLs loaded with no peptide were used as the negative control. Data are representative of at least three independent experiments using PBMCs from HLA-A*02:01, HLA-A*24:02, HLA-A*11:01, HLA-A*33:03 subjects. Data are presented as mean (point) ± standard error (bar).
Discussion
A strong Th1-biased T-cell response is important for control or elimination of not only infection of HPV 16 and 18 in precancerous disease, but also HPV-induced cervical cancer. E6 and E7 protein of HPV have been suggested as good targets for immune monitoring or immunotherapy against HPV-associated disease because E6 and E7 are constitutively expressed in both HPV-infected cells and cervical cancer cells and are not express in normal cervical epithelia. Therefore, the identification of CTL specific epitopes from E6 and E7 proteins of HPV 16 and 18 is essential for the development of peptide-based immune monitoring of HPV-specific CTL response and immunotherapy in patients. However, a major drawback of the development of clinically effective peptide-base immune monitoring or immunotherapy is the fact that different epitopes are recognized by T cells from individuals displaying distinct major HLA molecules. Moreover, although HPV 18 is second most common subtype after HPV 16 which is associated with cervical cancer, only few CTL-specific epitopes of HPV18 have been identified.
Until very recently, the studies searching for immune dominant peptides were performed testing of substantial numbers of overlapping peptides or peptide libraries. The identification of major HLA-binding motifs allowed the prediction of potential T cell epitopes (21,22), and supertypes or single peptide which can sensitize multiple HLA molecules simultaneously were found (23). However, very few studies have addressed CTL recognition of single peptide in a genetically heterogeneous group previously exposed to HPV infection or cervical cancer.
In this study, we found that two overlapping 15-amino acid peptides from HPV 18 E7 protein, E781-95DDLRAFQQLFLNTLS (#21) and E789-103LFLNTLSFVCPWCAS (#23), which could sensitize PBMCs of four major HLA class I A molecules including HLA-A*02:01, A*24:02, A*1101, and A*33:03, simultaneously. These four major HLA class I alleles are the most frequent HLA class I allele in human race. These 15-amino acid peptides were also successfully recognized by CD8+ CTLs of four different HLA class I alleles.
In the screening test, E781–95 (#21) and E789–103 (#23) were selected because these peptides induced most strong IFN-γ responses from PBMCs of four different HLA class I than other peptides (Figure 1A-D). However, IFN-γ could be released from many kinds of immune cells, including CD8+ and CD4+ T cells and NK cells. Thus, to determine if IFN-γ was produced from CD8+ T lymphocytes, CD3+CD8+IFN-γ+ T cells were counted using flow cytometry, and HPV-18 E781–95 (#21) and E789–103 (#23) induced greater numbers of CD3+CD8+IFN-γ+ T cells than other peptides and negative control (PBMCs sensitized with no peptide) in all four different HLA types (Figure 2A-D). HPV18 E781–95 (#21)- and E789–103 (#23)-sensitized CTLs lysed more EBV-BLCs loaded with E781–95 (#21) and E789–103 (#23), respectively, than negative control (EBV-BLCs with no peptide) in all four HLA types (Figure 3A-D). It suggested that these epitopes successfully bound to HLA-A*02:01, 24:02, 11:01 and 33:03 molecules on the surface of EBV-BLCs and also were successfully recognized by CD8+ CTLs within the PBMCs that were in vitro sensitized with same peptides. HPV18 E781–95 (#21)- and E789–103 (#23)-sensitized HLA-A*02:01 and 24:02 PBMCs also lysed more HLA-matched cervical cancer cell lines (HTB-34 and SNU-1160, respectively) than negative control (PBMCs sensitized with no peptide) (Figure 5A, B). These results confirmed that both HPV-18 E781–95 (#21) and E789–103 (#23) epitopes were naturally processed in both HTB-34 cell lines and SNU-1160 cell lines and were to be useful HLA-A*02:01 and HLA-A*24:02 immunogenic epitopes within the HPV18 E7 protein. We only used HTB-34 and SNU-1160 for cytotoxicity assay against cervical cancer cell in this study because these two cervical cancer cell lines were only available HPV 18+ E7 expressed cell lines in ATCC and Korean Cell Line Bank.
Virally infected human cells or human cancer cells can be recognized by CD8+ CTLs through immunogenic epitopes of 8 to 12 amino acids that are presented on the cell surface with HLA class I molecules. Thus, we used truncated peptides to determine the critical residues that induce CD8+ CTL responses within the 15-amino acid peptides and to determine the position of MHC anchor residues. Residue 81,82 of the N-terminus of E781–95 (#21) were important to elicit Th1 response of PBMCS in all four HLA class I (Figure 5A-D). Residue 92, 94, 95 would mark the C-terminal edge of the CD8+ CTL-stimulating epitope in HLA-A*02:01 and 24:02 (Figure 5A, B). In HLA-A*11:01 and 33:03, residue 94, 95 would mark the C-terminal edge of the CD8+ CTL-stimulating epitope within E781–95 (#21) (5C, 5D). In HLA-A*02:01, A*11:01 and A*33:03, residue 100 and 103 comprise the C-terminal end of the CD8+ CTL-stimulating epitope in peptide #23 (5A, 5C, 5D). However, in case of HLA-A*24:02, residue 100, 101 and 103 comprise the C-terminal end of the CD8+ CTL-stimulating epitope within E789–103 (#23) (5B). These results were comparable to the results of cytotoxicity assays using PBMCs sensitized with truncated peptides within E781–95 (#21) and E789–103 (#23), and HLA-matched EBV-BLCs loaded with same truncated peptides in Figure 6.
In conclusion, we identified E781-95DDLRAFQQLFLNTLS (#21) and E789-103LFLNTLSFVCPWCAS (#23), which could sensitize PBMCs of four HLA class I A molecules including HLA-A*02:01, A*24:02, A*11:01, and A*33:03, simultaneously, and demonstrated that PBMCs sensitized with these peptides showed cytotoxicity against cervical cancer cells. These epitopes could be useful for immune monitoring or immunotherapy against for HPV18-related diseases including cervical cancer, anal cancer, and oropharyngeal cancer.
References
De Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M: Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012, 13: 607-615. 10.1016/S1470-2045(12)70137-7.
Morrow MP, Yan J, Sardesai NY: Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev Vaccines. 2013, 12: 271-283. 10.1586/erv.13.23.
Pisani P, Bray F, Parkin DM: Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002, 97: 72-81. 10.1002/ijc.1571.
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N: Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999, 189: 12-19. 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F.
Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML: Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011, 29: 4294-4430. 10.1200/JCO.2011.36.4596.
Shiels MS, Pfeiffer RM, Chaturvedi AK, Kreimer AR, Engels EA: Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst. 2012, 104: 1591-1598. 10.1093/jnci/djs371.
Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA: Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007, 356: 1928-1943. 10.1056/NEJMoa061760.
Kim JJ: Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010, 10: 845-852. 10.1016/S1473-3099(10)70219-X.
Muñoz N, Bosch FX, De Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ: International agency for research on cancer multicenter cervical cancer study group; epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003, 348: 518-527. 10.1056/NEJMoa021641.
CDC press release: National survey shows HPV vaccine rates trail other teen vaccines.., [www.cdc.gov/media/releases/2011/p0825_hpv_vaccine.html]
Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR: Costa Rican HPV vaccine trial group. Effect of human papillomavirus 16/18 L1 virus like particle vaccine among young women with preexisting infection. JAMA. 2007, 298: 743-753. 10.1001/jama.298.7.743.
EMA: Annex I Summary of Product Characteristics for the HPV Vaccine ‘Gardasil’ 2008. Updated 2012. ., [www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000703/WC500021142.pdf]
Schorge JO, Molpus KL, Koelliker D, Nikui N, Goodman A, Fuller AF: Stage IB and IIA cervical cancer with negative lymph nodes: the role of adjuvant radiotherapy after radical hysterectomy. Gynecol Oncol. 1997, 66: 31-35. 10.1006/gyno.1997.4691.
Cannistra SA, Niloff JM: Cancer of the uterine cervix. N Engl J Med. 1996, 334: 1030-1038. 10.1056/NEJM199604183341606.
Ma B, Xu Y, Hung CF, Wu TC: HPV and therapeutic vaccines: where are we in 2010. Curr Can Ther Rev. 2010, 6: 81-103. 10.2174/157339410791202583.
van der Burg SH, Melief MC: Therapeutic vaccination against human papilloma virus induced malignancies. Curr Opin Immuol. 2011, 23: 252-257. 10.1016/j.coi.2010.12.010.
Su JH, Wu A, Scotney E, Ma B, Monie A, Hung CF, Wu TC: Immunotherapy for cervical cancer research status and clinical potential. BioDrugs. 2010, 24: 109-129. 10.2165/11532810-000000000-00000.
Lin K, Roosinovich E, Ma B, Hung CF, Wu TC: Therapeutic HPV DNA vaccines. Immunol Res. 2010, 47: 86-112. 10.1007/s12026-009-8141-6.
Jang S, Kim YT, Chung HW, Lee KR, Lim JB, Lee K: Identification of novel immunogenic human leukocyte antigen-A 2402-binding epitopes of human papillomavirus type 16 E7 for immunotherapy against human cervical cancer. Cancer. 2012, 118: 2173-2183. 10.1002/cncr.26468.
Yan J, Harris K, Khan AS, Draghia-Akli R, Sewell D, Weiner DB: Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine. 2008, 26: 5210-5215. 10.1016/j.vaccine.2008.03.069.
Cunha-Neto E: MHC-restricted antigen presentation and recognition: constraints on gene, recombinant and peptide vaccines in humans. Braz J Med Biol Res. 1999, 32: 199-205.
Rammensee HG: Chemistry of peptides associated with MHC class I and class II molecules. Curr Opin Immunol. 1995, 7: 85-96. 10.1016/0952-7915(95)80033-6.
Rammensee HG, Friede T, Stevanoviic S: MHC ligands and peptide motifs: first listing. Immunogenetics. 1995, 41: 178-228. 10.1007/BF00172063.
Meister GE, Roberts CG, Berzofsky JA, De Groot AS: Two novel T-cell epitope prediction algorithms based on MHC-binding motifs; comparison of predicted and published epitopes from Mycobacterium tuberculosis and HIV protein sequences. Vaccine. 1995, 13: 581-591. 10.1016/0264-410X(94)00014-E.
Lim JB, Kim HO, Jeong SH, Ha JE, Jang S, Lee SG, Lee K, Stroncek D: Identification of HLA-A* 2402-restricted HCMV immediate early-1 (IE-1) epitopes as targets for CD8+ HCMV-specific cytotoxic T lymphocytes. J Transl Med. 2009, 7: 72-82. 10.1186/1479-5876-7-72.
Friedl F, Kimura I, Osato T, Ito Y: Studies on a new human cell line (SiHa) derived from carcinoma of uterus. I. Its establishment and morphology. Proc Soc Exp Biol Med. 1970, 135: 543-545. 10.3181/00379727-135-35091a.
Acknowledgement
Authors thank to Dr. Sunphil Jang for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KSH, CHY, LKR, and LJB designed the research. KSH, CHY, and LJB performed the research and analyzed the data. KSH, CHY, LKR, and LJB wrote the paper. All authors read and approved the final manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kim, S., Chung, H.W., Lee, KR. et al. Identification of novel epitopes from human papillomavirus type 18 E7 that can sensitize PBMCs of multiple HLA class I against human cervical cancer. J Transl Med 12, 229 (2014). https://doi.org/10.1186/s12967-014-0229-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-014-0229-7









