Abstract
Background
The associations of the proportion of vigorous physical activity (VPA) to moderate to vigorous physical activity (MVPA) with incident cardiovascular disease (CVD) and all-cause mortality are unclear.
Methods
The present study included 366,566 participants (aged 40–69 years) without baseline CVD from the UK biobank during 2006 to 2010. Cox regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for risks of outcomes.
Results
During a median 11.8 years of follow-up, among 366,566 participants (mean age [SD]: 56.0 [8.1]), 31,894 incident CVD and 19,823 total deaths were documented. Compared with no VPA, 0%-30% of VPA to MVPA was associated with 12% and 19% lower risks of incident CVD (HR, 0.88 [95% CI, 0.86–0.91]) and all-cause mortality (HR, 0.81 [95% CI, 0.78–0.84]), respectively. Furthermore, we found that the maximum reduction of risks of incident CVD and all-cause mortality occurred at performing approximately 30% of VPA to MVPA (P < 0.001). Compared with participants reporting the lowest levels of MVPA (moderate physical activity [MPA], 0–150 min/week; VPA, 0–75 min/week), those performing 150–300 min/week of MPA and ≥ 150 min/week of VPA experienced the lowest risk of incident CVD (HR, 0.87 [95% CI, 0.79–0.95]) and all-cause mortality (HR, 0.71 [95% CI, 0.63–0.80]). Interestingly, we found that smokers yielded more cardiovascular benefits than non-smokers by performing a higher volume of VPA.
Conclusions
Comparing with UK adults reporting no VPA, engaging in 30% of VPA was associated with the lowest risk of incident CVD and all-cause mortality.
Similar content being viewed by others
Background
Physical inactivity is a leading risk factor of cardiovascular disease (CVD) and premature death worldwide [1,2,3]. Current guidelines on physical activity are developed based on the assumption that the same duration of vigorous physical activity (VPA, ≥ 6 metabolic equivalent tasks [METs], such as running) could convey twice the health benefits of moderate physical activity (MPA, 3–5.9 METs, such as brisk walking); the World Health Organization recommended 150–300 min/week of MPA or 75–150 min/week of VPA, or equal combinations [4]. Recent studies have reported that VPA might confer greater benefits in lowering mortality risks than MPA when were conducted at an equivalent amount [5, 6]. A prospective cohort study of 403,681 US adults suggested that VPA was associated with 15% lower all-cause mortality compared with MPA [5]. Another cohort study of 64,913 UK adults also found VPA was associated with 16% lower risk of all-cause mortality versus MPA. However, there were two other studies reported no significant difference in mortality risk were observed among participants performed MPA and VPA [7, 8]. Therefore, more studies are still needed to examine the independent association of VPA in comparison with MPA with mortality risks.
Furthermore, current studies [5,6,7, 9] drew inconsistent conclusions on the association of VPA versus MPA with CVD, the leading cause of mortality worldwide which accounted for 32% of global deaths in 2019 [10]. The US study found that performing more than 50% to 100% of VPA to total MVPA was associated with a 17% lower risk of CVD mortality [5]. However, the UK study did not observe a significant association of higher VPA with CVD mortality [6]. Despite the mixed findings, these studies only focused on CVD mortality, and to date, no study has examined the association of the proportion of VPA to MVPA with incident CVD, as well as its major subtypes (coronary heart disease [CHD], heart failure [HF] and stroke). In addition, previous studies concerning the association between physical activity and CVD usually focused on total amount of MVPA [11, 12], the independent and joint association of MPA and VPA with health outcomes remained largely unclear. Finding the optimal combination of MPA and VPA would benefit in refining the future guideline on physical activity.
Using data from a prospective cohort of 366,566 UK adults, the current investigation aimed to examine the association of the proportion of VPA to MVPA with risks of CVD (overall and major subtypes), and all-cause mortality, to explore the joint effect of MPA and VPA on these risks, with the hypothesis that higher proportion of VPA to MVPA were associated lower risks of incident CVD and all-cause mortality.
Methods
Study population
Our analysis was based on data from the UK Biobank study [13]. Briefly, UK Biobank is a large population-based, prospective cohort study that recruited 502,505 participants aged 40–69 years in the UK from 2006 to 2010 (protocol available at https://www.UKbiobank.ac.UK/key-documents/). All participants provided questionnaire information, physical measurements, and biological samples at the baseline. UK Biobank was approved by the North West Multi-Center Research Ethical Committee (REF: 11/NW/03820). All participants gave written informed consent before enrolment in the study, which was conducted in accordance with the principles of the Declaration of Helsinki.
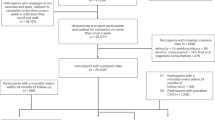
Among the 502,505 participants recruited at baseline, the present study excluded participants who have withdrawn from the UK Biobank (n = 46), pregnant women (n = 371), those with baseline CVD (n = 36,449), and those with missing information on physical activity (n = 91,624) or reported no physical activity (n = 7,449), leaving 366,566 participants for the final analytic sample (see Additional file 1). Baseline CVD was ascertained by self-reported information and hospital records [14]. The distribution of baseline characteristics was comparable among participants before and after exclusion (see Additional file 2).
Physical activity assessment
Data on physical activity was obtained through questionnaires adapted from the IPAQ short version (https://biobank.ndph.ox.ac.uk/showcase/browse.cgi?id=1008&cd=browse), which was reported to have acceptable test–retest reliability and criterion validity in the UK population [15]. Accordingly, we multiplied the energy expended for a specific category of activity (MET: 3.3 for walking, 4.0 for moderate activity and 8.0 for vigorous activity) [15, 16] by the corresponding frequency (times/week) and duration (minutes/time) and summed the corresponding amount to estimate MET-minutes/week of total MVPA. In the present study, we further defined MPA as physical activity of 3.0–5.9 METs therefore MET-minutes/week of walking and moderate activity were summed as the total amount of MPA, and VPA as physical activity of 6.0 METs or more such as running [15, 16]. Among participants with any MVPA, the proportion of VPA to MVPA was calculated by dividing the amount of VPA by the amount of MVPA. The proportion of VPA to MVPA was categorized into one of the three categories: 0% (no VPA), > 0% to ≤ 30%, and > 30%. The 30% cut-off was chosen according to a previous study, which showed a maximum reduction in risk of all-cause mortality appeared at 30% of VPA to MVPA [17].
Covariates
The present study included demographic characteristics (age, sex [male or female]), sociodemographic characteristics (education [college/university or below college], income [< 18,000 or 18,000–52,000 or ≥ 52,000 £/year] [18], race [white or others], Townsend index), lifestyle factors (smoking status [never or current or former], alcohol consumption [0 or 0.1–30 or ≥ 30 g/day] [19, 20], diet quality score [see Additional file 3], sedentary behavior [hours/day] and MVPA [MET-minutes/week]), body mass index (BMI, kg/m2) and family history of CVD (yes or no) as covariates according to previous studies on similar topic [5, 7, 17]. Missing indicator approach was used in the regression model by coding missingness as a dummy variable.
Ascertainment of outcome
UK Biobank data are linked to Hospital Episode Statistics (HES; hospital diagnoses from the National Health Service) and the UK Biobank Cause of Death Registry [21]. Incident CVD and mortality were determined based on the International Classification of Diseases edition 10 (International Classification of Diseases, ICD-10) [22]. HES and mortality data were updated up until December 31, 2020. The primary outcome was incident CVD (ICD-10: I00-I99), its main subtypes including CHD (I20-25), heart failure (HF; I50, I500, I501, I509), and stroke (I60, I61, I63, I64). The secondary outcome was all-cause mortality, its main components including CVD mortality.
Statistical analysis
Standardized difference was calculated to compare the baseline characteristics across three groups of VPA to MVPA (0%, > 0% to ≤ 30% and > 30%) [23]. A standardized difference less than 0.20 indicates a small difference of characteristic across different groups of VPA to MVPA (0%, > 0% to ≤ 30% and > 30%) [24]. Cox proportional hazard models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for incident CVD and all-cause mortality according to three groups of VPA to MVPA. Models were adjusted for age, sex, education, income, race, Townsend index, smoking status, alcohol consumption, sedentary behavior (hours/day), MVPA (MET-minutes/week), BMI (kg/m2), diet quality score, and family history of CVD. To test for P trend, participants were assigned to the median value of each group of VPA to MVPA (0%, > 0% to ≤ 30% and > 30%), and then this continuous variable was entered into the Cox model [25]. Comparison between adjusted HRs for the first half of follow-up years and for the subsequent years revealed no evidence of departure from the proportional hazards assumption for main analyses.
In order to explore the potential interaction, stratified analyses were performed by socio-demographic characteristics (age, sex, education, Townsend index), lifestyle risk factors (smoking status, alcohol consumption, total MVPA, sedentary behavior), BMI and chronic diseases (baseline hypertension and diabetes) [26], with adjustment for covariates the same as in the main model except for the stratified variable itself. The interaction effect of VPA to MVPA with stratified variable was assessed by introducing a multiplicative interaction term into the Cox models and the Wald test was used to calculate the P value for the interaction term [27].
We used the restricted cubic spline function to delineate the continuous exposure–response association between the proportion of VPA to MVPA and incident CVD and all-cause mortality with the %LGTPHCUTV9 macro, which fits restricted cubic splines to proportional hazards regression models to examine non-parametrically the relation between an exposure and the incidence rate ratio of the outcome of interest [28]. The output includes the set of P-values from the likelihood ratio tests for non-linearity, a linear association, and any association. We set three knots at the 50th, 75th and 95th percentiles of the proportion of VPA to MVPA.
To find the optimal combination of MPA and VPA, we also conducted an analysis to examine the joint association of MPA (0 to < 150, 150 to < 300, ≥ 300 min/week) and VPA (0 to < 75, 75 to < 150, ≥ 150 min/week) [4] with different outcomes by creating a combined variable with 3×3 mutually exclusive groups, taking the lowest MPA and VPA as reference.
Finally, to test the robustness of our main findings, we conducted sensitivity analyses by extended adjustment for hypertension, diabetes status, medical center, lipid-lowering treatment, antihypertensive medications, and diabetes medication, by extended adjustment for employment information, by conducting competing risks analyses to compare end point-specific survival between the proportion of VPA and CVD events using the Fine and Gray competing risk model [29], by excluding participants who developed CVD or died within the first two years of follow-up, and by excluding participants with missing covariates.
All statistical analyses were performed using the SAS 9.4 (SAS Institute, Cary, North Carolina, USA) and R (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a two-sided P < 0.05 except for the subgroup analyses, in which statistical significance was set at P < 0.0045 (0.05 / 11 subgroups) to account for the possible type I error generating from multi-comparisons [30].
Results
Characteristics at baseline
A total of 366,566 participants (46.1% were men, mean [SD] age: 56.0 [8.1] years) were included in the present study. Baseline characteristics by the proportion of VPA to MVPA were presented in Table 1. Of these, 142,476 (38.9%) reported 0% of VPA, 108,068 (29.5%) reported 0–30% of VPA, and 116,022 (31.6%) reported > 30% of total MVPA being VPA. Except for the exposure variable (MVPA, VPA and MPA) used for categorizing participants, standardized differences of baseline characteristics ranged from 0.024 to 0.184, indicating a small difference among groups (Table 1).
Association of proportion of VPA to MVPA with incident CVD and all-cause mortality
During a median 11.8 years of follow-up, we documented 31,894 incident CVD cases (including 23,657 CHD cases, 6,905 HF cases and 6,293 stroke cases) and 19,823 total deaths (including 3,359 CVD deaths). In the fully adjusted models, compared with participants with 0% of VPA to MVPA, the fully-adjusted HRs for participants with 0% to 30% of VPA to MVPA were 0.88 (95% CI, 0.86–0.91) and 0.81 (95% CI, 0.78–0.84), respectively for incident CVD and all-cause mortality. The corresponding HRs were 0.89 (95% CI, 0.86–0.92) and 0.82 (95% CI, 0.79–0.85) for those reporting more than 30% of VPA to MVPA (P trend < 0.001, Table 2). Similar associations were observed across different subtypes of CVD and mortality, the corresponding HRs (95% CIs) were 0.89 (0.86–0.93), 0.78 (0.72–0.84) and 0.86 (0.77–0.96) for incident CHD, HF and CVD mortality, respectively, while non-significant association was seen for incident stroke (HR [95% CI]: 0.93 [0.86–1.00]; Table 2). The results did not change materially by further adjustments for conventional CVD risk factors or employment, excluding subjects developed CVD or died within 2 years, using the competing risk model, or excluding participants with missing covariates. The corresponding HRs for incident CVD were 0.89 (0.87–0.92), 0.88 (0.85–0.91), 0.89 (0.86–0.92), 0.88 (0.86–0.91), 0.90 (0.86–0.93), respectively (see Additional file 4).
In the subgroup analysis, we found a significant interaction effect of VPA to MVPA and age on the incident CVD and all-cause mortality (all P interaction < 0.005), the associations of VPA with incident CVD and all-cause mortality tended to be more obvious among participants aged less than 65 years old, the HRs (95% CIs) of > 30% of VPA to MVPA were 0.79 (0.76–0.82) and 0.72 (0.69–0.76), respectively, for incident CVD and all-cause mortality. Similarly, we observed significant interaction of VPA to MVPA and smoking on the incident CVD and all-cause mortality (all P interaction < 0.001), stronger association were seen among current smokers than non-smokers, the corresponding HRs (95% CIs) were 0.81 (0.78–0.85) and 0.75 (0.71–0.78), respectively. In addition, the health benefits were consistent across sex, education, Townsend index, alcohol consumption, BMI, hypertension, diabetes, total MVPA and daily sedentary hours. (P interaction > 0.005; see Additional file 5 and 6).
Exposure–response association of the proportion of VPA to MVPA with incident CVD and all-cause mortality
The cubic spline showed an L-shape in both incident CVD and all-cause mortality (P association < 0.001). Cubic spline showed a decrease in risks of incident CVD with increasing proportion of VPA to MVPA before 30% of VPA to MVPA, and plateaued thereafter without upward trend (Fig. 1A). For all-cause mortality, similar pattern of association was observed, but the mortality risk reached a nadir at about 30% of VPA to MVPA, and then rose slowly but never reached the level of no VPA (Fig. 1B). The maximum risk reduction occurred at approximately 30% of VPA to MVPA, with the HRs (95% CIs) of 0.89 (0.87–0.92) and 0.81 (0.78–0.84), respectively, for incident CVD and all-cause mortality (Fig. 1). Consistent associations were seen for CVD subtypes and CVD mortality (P association < 0.05). The corresponding HRs (95% CIs) at 30% of VPA to MVPA were 0.87 (0.85–0.90), 0.81 (0.77–0.87), 0.92 (0.86–0.98), and 0.79 (0.72–0.86), respectively, for incident CHD, HF, stroke, and CVD mortality (see Additional file 7 and 8).
Associations of VPA proportion with incident CVD and all-cause mortality. Abbreviations: CVD, cardiovascular disease; MVPA, moderate-to-vigorous intensity physical activity; VPA, vigorous physical activity. Models were adjusted for age, sex, education, income, race, Townsend index, smoking status, alcohol consumption, BMI, sedentary behavior, MVPA, diet quality score and family history of CVD
Joint associations of MPA and VPA with incident CVD and all-cause mortality
Compared with those who reported the least MPA and VPA (0 to < 150 min of MPA and 0 to < 75 min of VPA), those performing 150 to 300 min of MPA and ≥ 150 min of VPA per week had the lowest risk of incident CVD (HR [95% CI], 0.87 [0.79–0.95]) and all-cause mortality (HR [95% CI], 0.71 [0.63–0.80]; Table 3). Similar results were observed for incident CHD (HR [95% CI], 0.86 [0.77–0.95]). For incident HF and CVD mortality, the optimum combination of MPA and VPA was 150 to 300 min/week of MPA and 75–150 min/week of VPA, the corresponding HRs (95% CIs) were 0.77 (0.65–0.92) and 0.72 (0.57–0.93), respectively (see Additional files 9 and 10).
Discussion
In this large prospective cohort study of UK adults, performing VPA was associated with lower risks of incident CVD and all-cause mortality. Participants reporting 30% of VPA appeared to experience the lowest risks of incident CVD and all-cause mortality. Moreover, we found that participants performing 150–300 min of MPA and ≥ 150 min of VPA per week had the lowest risks of CVD and all-cause mortality. To our best knowledge, this is the first prospective study investigating the association of the proportion of VPA to MVPA as well as the joint association of MPA and VPA with incident CVD.
Comparison with other studies
In the present study, we found participants who performed VPA had significantly lower risks of all-cause mortality than no VPA, with the lowest risk occurring at about 30% VPA to MVPA, which was generally in line with previous studies [6, 17]. The study of 11 cohorts reported similar magnitude of HR (0.84) in association between VPA and all-cause mortality among those performed 0%-30% and 30% or more of VPA [6]. Another cohort study of 204,542 middle-aged and older Australians reported that 0%-30% of VPA was associated with a 9% reduction of all-cause mortality (HR, 0.91 [95% CI, 0.84–0.98]), while no additional benefits in lowering mortality risk were seen among those with 30% or more of VPA [17], which was aligned with our study. The reason might be that high levels of VPA was positively associated with oxidative stress [31, 32] which might counteract the beneficial effects from physical activity.
Several prospective cohort studies have investigated the associations of VPA versus MPA with CVD mortality [5, 6, 33]. A prospective study of 403,681 US adults (mean age, 42.8 years) [5] identified 17% lower risks of CVD mortality among those performing 50%-75% and 75%-100% of VPA to MVPA. Another study of 7,979 men and 38,671 women from the Harvard Alumni Health Study only identified a 26% lower risk of CVD mortality (HR, 0.74 [95% CI, 0.58–0.93]) among men who performed 25%-50% of VPA to MVPA [33]. However, a study of 64,913 adults from 11 population cohorts and observed non-significant associations of VPA with CVD mortality (HR [95% CI] of 0%-30% VPA and ≥ 30% VPA, 0.83 [0.57–1.18] and 0.84 [0.68–1.04]) [6].The present study found new evidence that a higher proportion of VPA is associated with lower risks of CVD mortality compared with MPA, while the US study [5] only observed significant associations among participants with 50%-99% of VPA for CVD mortality, which might partly be due to the small number of deaths collected in the 0%-50% group (306 CVD deaths). Notably, the present study identified for the first time that 0%-30% and ≥ 30% VPA to MVPA were associated with 12% and 11% lower risk of incident CVD, which were not examined in previous studies. The possible reason that VPA was associated with larger cardiovascular benefits might be that VPA might increase peak oxygen uptake thus improving cardiorespiratory fitness [34].
The inverse association was less obvious among older adults than their younger counterparts. This might be because older adults are more likely to suffer from multiple comorbidities, and their cardiac condition may also limit their capacity in performing VPA [35, 36]. Another interesting finding of our study was that current smokers experienced more health benefits than non-smokers by performing a higher volume of VPA. A previous study of 17,944 British men also found that smoked men who engaged in VPA experienced approximately half of the CHD incidence than smokers with no VPA (CHD incidence: 4.9 VS 9.7) [37]. The current study provides evidence that guideline on physical activity should be specified for target pollution such as middle-aged adults and smokers, and highlights the need for more epidemiological studies to validate these findings in different population [38].
Strengths and limitations
The major strengths include the large sample size, approximately 12 years of follow-up, accounting for a total amount of MVPA, examining subtypes of CVD in addition to overall CVD, and exploring the joint association of MPA and VPA for the first time to our knowledge. This study has several limitations. First, information on physical activity was obtained once by self-report and might be inaccurate in differentiating different intensities of physical activity. Second, the UK Biobank sample was not representative of the UK population and more than 90% of the participants included were white, therefore the generalization of our results to other population should be cautious. Finally, the possibility of reverse causation still existed because participants with underlying diseases were less likely to perform VPA and more likely to have a CVD event or die; however, we adjusted for pre-existing diseases and medications at baseline, excluded events or deaths in the first 2 years of follow-up, and the results were largely unchanged.
Conclusions
The current study indicated lower risks of incident CVD and all-cause mortality at 0% ~ 30% of VPA to MVPA, no additional benefit at more than 30% of VPA to MVPA. To maximum population health, we recommended a combination of 150–300 min/week of MPA and ≥ 150 min/week of VPA for middle-aged and older adults.
Availability of data and material
All the data were extracted from the UK Biobank, which are publicly available to qualified researchers on application to the UK Biobank (www.ukbiobank.ac.uk). The code is available from the corresponding author upon reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CHD:
-
Coronary heart disease
- CIs:
-
Confidence intervals
- CVD:
-
Cardiovascular disease
- HES:
-
Hospital diagnoses from the National Health Service
- HF:
-
Heart failure
- HR:
-
Hazard ratios
- MET:
-
Metabolic equivalent task
- MPA:
-
Moderate physical activity
- PA:
-
Physical activity
- VPA:
-
Vigorous physical activity
References
Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124(5):799–815. https://doi.org/10.1161/circresaha.118.312669.
Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev. 2017;97(4):1351–402. https://doi.org/10.1152/physrev.00019.2016.
Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(14):1622–39. https://doi.org/10.1016/j.jacc.2018.08.2141.
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. https://doi.org/10.1136/bjsports-2020-102955.
Wang Y, Nie J, Ferrari G, Rey-Lopez JP, Rezende LFM. Association of physical activity intensity with mortality: a national cohort study of 403,681 US adults. JAMA Intern Med. 2021;181(2):203–11. https://doi.org/10.1001/jamainternmed.2020.6331.
Rey Lopez JP, Gebel K, Chia D, Stamatakis E. Associations of vigorous physical activity with all-cause, cardiovascular and cancer mortality among 64 913 adults. BMJ Open. 2019;5(1):e000596. https://doi.org/10.1136/bmjsem-2019-000596 (https://bmjopensem.bmj.com/content/5/1/e000596.abstract).
Lee DH, Rezende LF, Joh H-K, Keum NN, Ferrari G, Rey-Lopez JP, et al. Long-term leisure-time physical activity intensity and all-cause and cause-specific mortality: a prospective cohort of US adults. Circulation. 2022;146(7):523–34. https://doi.org/10.1161/CIRCULATIONAHA.121.058162.
Kikuchi H, Inoue S, Lee IM, Odagiri Y, Sawada N, Inoue M, et al. Impact of moderate-intensity and vigorous-intensity physical activity on mortality. Med Sci Sports Exerc. 2018;50(4):715–21. https://doi.org/10.1249/mss.0000000000001463.
Zhao M, Veeranki SP, Li S, Steffen LM, Xi B. Beneficial associations of low and large doses of leisure time physical activity with all-cause, cardiovascular disease and cancer mortality: a national cohort study of 88,140 US adults. Br J Sports Med. 2019;53(22):1405–11. https://doi.org/10.1136/bjsports-2018-099254.
World Health Organization. Cardiovascular diseases (CVDs). n.d. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 2 Nov 2022.
Kraus WE, Powell KE, Haskell WL, Janz KF, Campbell WW, Jakicic JM, et al. Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med Sci Sports Exerc. 2019;51(6):1270–81. https://doi.org/10.1249/mss.0000000000001939.
Hu G, Jousilahti P, Borodulin K, Barengo NC, Lakka TA, Nissinen A, et al. Occupational, commuting and leisure-time physical activity in relation to coronary heart disease among middle-aged Finnish men and women. Atherosclerosis. 2007;194(2):490–7. https://doi.org/10.1016/j.atherosclerosis.2006.08.051.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Daneshet J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. https://doi.org/10.1371/journal.pmed.1001779.
Shahid H. Association between neuroticism and risk of incident cardiovascular disease in UK Biobank cohort. 2020;26. https://xs.lsqwl.org/scholar?q=Association+between+neuroticism+and+risk+of+incident+cardiovascular+disease+in+UK+Biobank+cohort. Accessed 13 Sep 2022.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. https://doi.org/10.1249/01.Mss.0000078924.61453.Fb.
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504. https://doi.org/10.1097/00005768-200009001-00009.
Gebel K, Ding D, Chey T, Stamatakis E, Brown WJ, Bauman AE. Effect of moderate to vigorous physical activity on all-cause mortality in middle-aged and older Australians. JAMA Intern Med. 2015;175(6):970–7. https://doi.org/10.1001/jamainternmed.2015.0541.
Zhu J, Ge F, Zheng Y, Qu Y, Chen W, Yang H, et al. Physical and mental activity, disease susceptibility, and risk of dementia: a prospective cohort study based on UK biobank. Neurology. 2022;99(8):e799–813. https://doi.org/10.1212/wnl.0000000000200701.
Dai L, Liu M, Chen L. Association of serum 25-hydroxyvitamin D concentrations with all-cause and cause-specific mortality among adult patients with existing cardiovascular disease. Front Nutr. 2021; 693. https://doi.org/10.3389/fnut.2021.740855.
Lee CH, Lee JM, Wu DC, Goan YG, Chou SH, Wu IC, et al. Carcinogenetic impact of ADH1B and ALDH2 genes on squamous cell carcinoma risk of the esophagus with regard to the consumption of alcohol, tobacco and betel quid. Int J Cancer. 2008;122(6):1347–56. https://doi.org/10.1002/ijc.23264.
Caleyachetty R, Littlejohns T, Lacey B, Bešević J, Conroy M, Collins R, et al. United Kingdom Biobank (UK Biobank): JACC Focus Seminar 6/8. J Am Coll Cardiol. 2021;78(1):56–65. https://doi.org/10.1016/j.jacc.2021.03.342.
World Health Organization. International statistical classification of diseases and related health problems. 10th revision. 2010. https://www.who.int/news-room/spotlight/international-classification-of-diseases#:~:text=The%20International%20Statistical%20Classification%20of%20Diseases%20and%20Related,anything%20we%20might%20die%20of%20%E2%88%92%20is%20coded. Accessed 21 Dec 2021.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786 (https://www.tandfonline.com/doi/full/10.1080/00273171.2011.568786).
Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale: Erlbaum; 1988.
Keum N, Bao Y, Smith-Warner SA, Orav J, Wu K, Fuchs CS, et al. Association of physical activity by type and intensity with digestive system cancer risk. JAMA Oncol. 2016;2(9):1146–53. https://doi.org/10.1001/jamaoncol.2016.0740.
Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175(6):959–67. https://doi.org/10.1001/jamainternmed.2015.0533.
VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3(1):33–72 (https://www.degruyter.com/document/doi/10.1515/em-2013-0005/html).
Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. https://doi.org/10.1002/sim.4780080504.
Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–9. https://doi.org/10.1161/circulationaha.115.017719.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300 (https://rss.onlinelibrary.wiley.com/doi/abs/10.1111/j.2517-6161.1995.tb02031.x).
Kruk J, Aboul-Enein HY, Kładna A, Bowser JE. Oxidative stress in biological systems and its relation with pathophysiological functions: the effect of physical activity on cellular redox homeostasis. Free Radic Res. 2019;53(5):497–521. https://doi.org/10.1080/10715762.2019.1612059.
Boccatonda A, Tripaldi R, Davì G, Santilli F. Oxidative stress modulation through habitual physical activity. Curr Pharm Des. 2016;22(24):3648–80. https://doi.org/10.2174/1381612822666160413123806.
Shiroma EJ, Sesso HD, Moorthy MV, Buring JE, Lee IM. Do moderate-intensity and vigorous-intensity physical activities reduce mortality rates to the same extent? J Am Heart Assoc. 2014;3(5):e000802. https://doi.org/10.1161/jaha.114.000802.
Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97(1):141–7. https://doi.org/10.1016/j.amjcard.2005.07.130.
Chen L, Nelson DR, Zhao Y, Cui Z, Johnston JA. Relationship between muscle mass and muscle strength, and the impact of comorbidities: a population-based, cross-sectional study of older adults in the United States. BMC Geriatr. 2013;13:74. https://doi.org/10.1186/1471-2318-13-74.
Plooij B, Scherder EJ, Eggermont LH. Physical inactivity in aging and dementia: a review of its relationship to pain. J Clin Nurs. 2012;21(21–22):3002–8. https://doi.org/10.1111/j.1365-2702.2011.03856.x.
Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet. 1980;2(8206):1207–10. https://doi.org/10.1016/s0140-6736(80)92476-9.
Persoskie A, Kaufman AR, Leyva B. Receiving and adhering to lifestyle modification counseling for hypertension: disparities between smokers and nonsmokers. J Clin Hypertens (Greenwich). 2014;16(6):429–36. https://doi.org/10.1111/jch.12314.
Acknowledgements
We wish to acknowledge the UK Biobank participants who provided the questionnaire information that made the data available.
Funding
This work was supported by the National Natural Science Foundation of China (82003422), the Fundamental Research Funds for the Central Universities (2019kfyXJJS035), and National Key Research and Development Program of China (2020YFC2006300). The founders had no role in the design of the study and collection, analysis, and interpretation of data and in writing of this manuscript.
Author information
Authors and Affiliations
Contributions
KY and LC planned the study. XM carried out the statistical analyses, interpreted the data, and drafted the manuscript. SL, MF, ML and DD provided substantial scientific input in interpreting the results and revised drafts critically. All authors approved the final version for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
UK Biobank was approved by the North West Multi-Center Research Ethical Committee (REF: 11/NW/03820). All participants gave written informed consent before enrolment in the study.
Consent for publication
All participants were consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mu, X., Liu, S., Fu, M. et al. Associations of physical activity intensity with incident cardiovascular diseases and mortality among 366,566 UK adults. Int J Behav Nutr Phys Act 19, 151 (2022). https://doi.org/10.1186/s12966-022-01393-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12966-022-01393-y