Abstract
Background
In the current Physical Activity Guidelines (PAG) for moderate- and vigorous-intensity physical activity (MVPA), abrupt transition from ≥ 60 min/day [youth PAG] to ≥ 150 min/week (≥ 22 min/day on average) [adult PAG] during emerging adulthood is poorly justified. The aim of this study was to examine body fat mass changes according to whether meeting the youth and adult PAGs in late adolescence (age 18 years) to early adulthood (age 22 years).
Methods
The study sample included 2,099 participants (1,113 females) from the 1993 Pelotas (Brazil) Study. At ages 18 and 22 years, MVPA was measured using wrist-worn accelerometry and fat mass was measured using dual-energy X-ray absorptiometry. MVPA at age 18 was categorized into two groups: 0–59 or ≥ 60 min/day (no [N] or yes [Y] for meeting the youth recommendation, respectively). MVPA at age 22 was categorized into three groups: 0–21, 22–59, or ≥ 60 min/day (N, Y22, or Y60 for not meeting the adult recommendation, meeting the adult recommendation, or meeting the youth recommendation, respectively). The combination of these groups created six MVPA groups (N&N, N&Y22, N&Y60, Y&N, Y&Y22, and Y&Y60). Sex-specific multivariable linear regression analyses were conducted to estimate change in fat mass index (FMI) from age 18 to 22 years in the six MVPA groups.
Results
Among males, compared to Y&Y60 (FMI increase = 1.2 kg/m2 [95% CI = 1.0, 1.4]), Y&Y22 and Y&N had larger FMI increases (1.9 [1.6, 2.1] and 1.9 [1.2, 2.5], respectively). Among females, Y&Y60 and Y&Y22 had an equal FMI increase (1.6 [1.4, 1.9] for both groups), while Y&N had a larger FMI increase (2.4 [1.8, 3.0]).
Conclusions
These findings suggest that among those who were active in late adolescence, engaging in ≥ 22 min/day of MVPA in adulthood is associated with lower body fat gain for females, but not for males.
Similar content being viewed by others
Background
Physical activity (PA) provides numerous health benefits across the life course [1, 2]. However, physical inactivity is prevalent globally [3, 4]. To promote and support healthy levels of PA across various populations, the World Health Organization (WHO) [1] as well as several developed countries, such as Australia [5], Canada [6], United Kingdom [7], and United States [2], have established physical activity guidelines (PAG). The guidelines are organized using a lifecourse approach with specific recommendations for youth, adults, and older adults. The recommendations for moderate- and vigorous-intensity PA (MVPA) are overall consistent across the guidelines, which recommend ≥ 60 min/day for youth age 5 to 17 or 18 years (youth PAG) and ≥ 150 min/week (≥ 22 min/day on average) for adults (adult PAG). Notably, the transition in recommended MVPA level abruptly changes from late adolescence to emerging adulthood (18–25 years of age) [8], dropping from ≥ 60 min/day to ≥ 22 min/day [9, 10].
The emerging adulthood population is considered to be one of the healthiest age subgroups, and as such, they have often been overlooked in studied of physical health and health behaviors [11]. However, recent statistics are alarming: one in four U.S. young adults age 19 to 34 years is estimated to have prediabetes, with males having a two-fold higher prevalence than females [12]. Obesity plays a major role in the development of prediabetes and the later development of type 2 diabetes and cardiovascular diseases [12]. During emerging adulthood, obesity incidence is higher compared to the adolescent period [13,14,15] or any other adulthood periods [16].
A knowledge gap exists regarding the dose–response relationship between MVPA and adiposity during this transitional period. Oh et al. [17] examined the relationship between MVPA patterns from age 15 to 23 years and fat mass at age 23 years and found that participants with consistently higher MVPA (approximately 100 min/day) from age 15 to 23 years had lower fat mass at age 23 years compared to those with lower and declining MVPA (approximately 65 min/day at age 15 years to approximately 50 min/day at age 23 years). The study [17] provided valuable insight that maintaining higher MVPA during the transitional period is associated with lower body fat in early adulthood. However, the study was limited by a relatively small sample size and did not examine adiposity development separately for those who meet the youth PAG and/or the adult PAG and for those who do not.
Few studies have prospectively examined the associations between PA and body fat during the transition from adolescence to young adulthood, a period when the current PAG changes abruptly [9]. Although obesity is a health risk shared by low-, middle-, and high-income countries [18], studies in low- and middle-income countries (LMIC) are particularly limited [19]. The data that are available suggest that the influences of societal factors on PA and adiposity are different in LMIC compared to high income countries [20]. The aim of this study was to compare changes in body fat mass according to whether meeting the youth and adult PAGs from late adolescence to early adulthood (age 18 to 22 years) in a large sample of the Brazilian population. There was no prespecified hypothesis.
Methods
Study sample
A study design was a prospective cohort study design. The study sample were participants of the 1993 Pelotas (Brazil) Study [21]. The 1993 Pelotas study was a birth cohort study that recruited 5,249 newborns (99.7% of all births) in the city of Pelotas, Brazil, in 1993 and followed them over time. For an assessment at age 18 years (2011 and 2012), 4,563 cohort members were located, of whom 4,106 (90.0%) attended the clinic visit. For an assessment at age 22 years (2015 and 2016), 4,933 cohort members were located, of whom 3,810 (77.2%) attended the clinic visit. Participants were ineligible for accelerometry assessment if participants did not live in Pelotas at the time of data collection, had physical disabilities, or were unable to wear an accelerometer due to work restriction. Participants were ineligible for dual-energy X-ray absorptiometry (DXA) assessment if participants used a wheelchair or had osteoarticular deformities, implanted metal pins, screws, plates and non-removable metallic objects (body piercings and/or chains), or were extreme obesity, height over 1.92 m, or pregnant [22]. The current report used the data from 1993 Pelotas study participants who completed accelerometer assessments and DXA assessments at 18 and 22 years of age. All study protocols were approved by the Ethics Committee of the Federal University of Pelotas Medical School (register number 05/2011 and 1.250.366). Written informed consent was obtained from individual participants.
Exposure
The exposure of interest was change in accelerometry-measured MVPA from age 18 to 22 years. Because of device availability, different brands of accelerometers were utilized: GENEActiv Accelerometers (range of ± 8 g; 85.7 Hz; 5-s epoch; ActivInsights, Kimbolton, UK) at age 18 and ActiGraph GT3X + (range of ± 8 g; 60 Hz; 5-s epoch; ActiGraph Inc., Pensacola, FL, USA) at age 22. A prior study [23] showed a high agreement for an acceleration magnitude metric (intraclass correlation coefficient for Euclidian Norm Minus One [ENMO] = 0.99) between the two devices. During clinic visits at age 18 and 22 years, participants were asked to wear an accelerometer on the non-dominant wrist for 24 h and for 4 to 7 consecutive days, including at least 1 weekend day. Detailed information regarding the protocol can be found elsewhere [19, 24].
Accelerometer data were analyzed using the R-package GGIR [25]. Briefly, the GGIR data processing includes automatic calibration, detection of sustained abnormally high values, detection of non-wear, detection of wake/sleep, and quantification of dynamic acceleration magnitude. Data were analyzed per 24 h from midnight to midnight (MM windows). To be included in data analysis, at least 3 wear days and at least 16 wear hours per day were required [26]. The activity-related acceleration metric, ENMO, was calculated. ENMO (mg) is one omnidirectional measure of body acceleration, calculated by subtracting the value of gravity from vector magnitude [√(x2 + y2 + z2)-1] [27]. MPA minute was defined as a minute with 100 mg ≤ ENMO ≤ 400 mg for ≥ 48 s (80% of 60 s) [19]. VPA minute was defined as a minutes with ENMO ≥ 400 mg for ≥ 48 s [19]. MVPA minutes were calculated by summing MPA minutes and VPA minutes. In addition, because in the adult PAG (i.e., ≥ 150 min/week of MPA, ≥ 75 min/week of VPA, or an equivalent combination of MPA and VPA) [1, 2], one minute of VPA is considered to be equivalent to two minutes of MPA, we calculated MPA-equivalent minutes (minutes/day) at age 22 years by summing MPA minutes and twice VPA minutes (MPA minutes + 2 × VPA minutes) [28].
MVPA at age 18 years was categorized into two groups: 0–59 or ≥ 60 min/day (no [N] or yes [Y] for meeting the youth PAG). MVPA at age 22 years was categorized into three groups: 0–21, 22–59, or ≥ 60 min/day (N, Y22, or Y60 for not meeting the adult PAG, meeting the adult PAG, or meeting the youth PAG, respectively). The combination of these groups created six MVPA groups (N&N, N&Y22, N&Y60, Y&N, Y&Y22, or Y&Y60). In addition, we also created six weighted-MVPA (wMVPA) groups using MPA-equivalent minutes, instead of MVPA minutes, at age 22 years.
Outcomes
The primary outcome was change in fat mass index (FMI) from age 18 to 22 years (∆FMI). The secondary outcome was change in fat mass from age 18 to 22 years (∆FM). We also explored change in body mass index (BMI) from age 18 to 22 years (∆BMI) as an outcome. During clinic visits at age 18 and 22 years, participants underwent a whole-body DXA scan (GE Lunar Prodigy, USA). Detailed study procedures have been described elsewhere [29]. Scan images were analyzed using the in-built GE Lunar enCore software. Fat mass (kg) was derived from the image analysis. FMI was calculated by dividing fat mass (kg) by height squared (height2).
Confounders
Several potential confounders were considered: the exact age at the age 22 assessment, wealth index quintiles at age 18 years, education level at age 22 years (0–8, 9–11, or ≥ 12 years of schooling), energy intake quintiles at ages 18 and 22, and FMI at age 18. Wealth index, education level, and energy intake were grouped to allow for non-linear trends in association between the confounders and the outcome. Wealth index was calculated using a principal component analysis based on a set of goods and assets, from which the first component was extracted and then divided into quintiles [30]. Missing data for wealth index quintile (n = 2) was imputed to the middle category. Daily energy intake (kcal/day) at ages 18 and 22 years was estimated using a food frequency questionnaire [31]. Daily energy intake (kcal/day) was categorized into five groups based on sex-specific quintile cut-points. Missing data for the energy intake quintile at age 22 years (n = 1) was imputed with the energy intake quintile at age 18 years. Energy intake quintile at age 18 years and change in energy intake quintile from age 18 to 22 years were used to account for the energy intake effects.
Statistical analysis
All analyses were conducted separately by sex using SAS 9.4 (Cary, NC). To address potential selection bias, we conducted Chi-square analyses to compare the frequencies of the key characteristics (i.e., sex, birthweight, maternal education level, and family income level) between those who were included in the analysis and those who were excluded. Descriptive analyses, including means and standard deviations of the exposure and outcome variables, were conducted. Analysis of variance (ANOVA) was conducted to compare the exposure and outcome variables among the six MVPA groups. A multivariable linear regression model was used to estimate ∆FMI by the MVPA group variable (reference group = Y&Y60), adjusting for age, family wealth index quintile at age 18, education level at age 22 years, energy intake quintile at age 18 years, change in energy intake quintile from age 18 to 22 years, and FMI at age 18 years. The analysis was repeated for the ∆FM outcome. The statistical significance level was set at 0.05.
Results
At age 18 years, 3,308 participants completed the accelerometry assessment and 3,851 completed the DXA assessment. At age 22 years, 2,803 participants completed the accelerometry assessment and 3,318 completed the DXA assessment. A total of 2,099 participants (1,090 females; 40.0% of the original cohort) who had both accelerometer and DXA data at ages 18 and 22 years were included in this data analysis. As presented in Supplementary Table 1, the included sample was similar to the excluded sample in the distributions of sex, birthweight, maternal education level, and family income level. In the included sample, 30.0% had ≥ 12 years of schooling, 36.9% were enrolled in school, 67.3% were employed, and 54.8% lived with parent(s) at age 22 years.
Among males, there were trends that as wealth index and education level were higher, MVPA was lower and FMI was higher (Supplementary Table 2). Among females, there were trends that as wealth index and education level were higher, MVPA were lower (Supplementary Table 3). However, females in the highest wealth index quintile and ≥ 12 years of schooling tended to have lower FMI. Males and females who remained in school from age 18 to 22 years showed a significantly smaller FMI increase than their counterparts who were no longer in school at age 22 years. However, we found no significant difference in ∆FMI between those who changed from living with a parent at age 18 to living without a parent at age 22 years and those living with a parent at both 18 and 22 years of age. Change in height between 18 years (mean = 173.5 cm for males and 160.8 cm for females) and 22 years of age (mean = 174.1 cm for males and 161.0 cm for females) was < 1 cm and was not significantly different across the six MVPA groups. Mean VPA minutes were low at age 22 years: 2 min for both males and females.
Among males, during the 4-year period from age 18 and 22 years, fat mass increased 4.0 kg for Y&Y60, while it increased 6.1 kg for both Y&Y22 and Y&N (Table 1). Among females, fat mass increased 4.6 kg for Y&Y60, 4.3 kg for Y&Y22, and 6.1 kg for Y&N (Table 2).
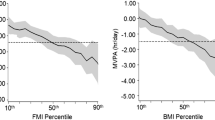
After adjusting for the covariates among males (Fig. 1), Y&Y22 had a significantly larger increase in FMI compared to Y&Y60. However, ∆FMI did not differ between Y&Y22 and Y&N. Among males who did not meet the youth PAG at age 18 years, FMI increase was the smallest in N&Y60, followed by N&Y22 and N&N. After adjusting for the covariates among females (Fig. 2), ∆FMI did not differ between Y&Y22 and Y&Y60. However, Y&Y22 had a significantly smaller increase in FMI compared to Y&N. Among females who did not meet the youth PAG at age 18 years, FMI increase was the smallest in N&Y60, followed by N&Y22 and N&N.
Adjusted means of FMI change (kg/m.2) from age 18 to 22 years in six MVPA groups among males. The means of change in FMI were adjusted for age in years, wealth index quintile at age 18 years, years of schooling at age 22 years, energy intake quintile at age 18 years, change in energy intake quintile from age 18 to 22 years, and FMI at age 18 years. FMI, fat mass index; MVPA, moderate- and vigorous-intensity physical activity; PA, physical activity
Adjusted means of FMI change (kg/m.2) from age 18 to 22 years in six MVPA groups among females. The means of change in FMI were adjusted for age in years, wealth index quintile at age 18 years, years of schooling at age 22 years, energy intake quintile at age 18 years, change in energy intake quintile from age 18 to 22 years, and FMI at age 18 years. FMI, fat mass index; MVPA, moderate- and vigorous-intensity physical activity; PA, physical activity
When ∆FM was examined as an outcome, we found consistent results: males in the Y&Y22 and Y&N group gained 2.0 kg more fat mass compared to males in the Y&Y60 group (Supplementary Figs. 1). Females in the Y&N group gained 1.6 kg more fat mass compared to females in the Y&Y60 or Y&Y22 group (Supplementary Fig. 2). The ∆BMI outcome also showed consistent results: males in the Y&Y22 group had higher ∆BMI than males in the Y&Y60 group and females in the Y&Y60 and Y&Y22 groups had similar ∆BMI (Supplementary Figs. 3 and 4). When wMVPA groups were used as the exposure variable, we found consistent results: larger ∆FMI in Y&Y22 and Y&N than Y&Y60 among males and larger ∆FMI in Y&N than Y&Y22 or Y&Y60 among females (Supplementary Figs. 5 and 6).
Discussion
We found that among males who were active ≥ 60 min/day of MVPA in late adolescence (age 18 years), those maintaining ≥ 60 min/day of MVPA in early adulthood (age 22 years) had less fat gain compared with those engaged in MVPA 22–59 min/day (meeting the adult PAG). Males who reduced MVPA to 22–59 min/day and those who reduced MVPA 0–21 min/day (not meeting the adult PAG) in early adulthood showed the same amount of fat gain. Among females who were active in late adolescence, those who engaged in ≥ 22 min/day of MVPA in early adulthood (meeting the adult PAG) experiences less fat gain compared with females who reduced MVPA to 0–21 min/day in early adulthood. In addition, for males and females who were inactive in late adolescence, higher MVPA in adulthood was associated with lower fat gain. After accounting for the intensity of PA at age 22 years (wMVPA groups), the findings remained the same.
The substantial difference in recommended MVPA levels between youth and adults might reflect different goals between the groups, i.e., achieving optimal health benefits for youth versus preventing non-communicable diseases for adults [9, 10]. Although these goals may overall be appropriate, the abrupt PAG change for populations in emerging adulthood is poorly justified with little evidence of a dose–response relationship between MVPA and health outcomes during the emerging adulthood period. The present study is one of only a few prospective studies to examine the relationship between MVPA and adiposity during emerging adulthood in a large sample. This study suggests that the currently recommended level of MVPA for adults may be insufficient for lowering fat gain in emerging adulthood among males, while it may be sufficient among females. These findings call for a re-assessment of the current MVPA recommendations for populations in emerging adulthood. As an individual at the end of adolescence is not the same as an adult in terms of physical, physiological, intellectual, social, emotional, and behavioral measures [32], experts in adolescent medicine as well as the United Nations have (re)defined the “youth” period to include up to 24 years of age [32, 33], which adds the emerging adulthood period. Applying the current youth MVPA recommendations to the population in emerging adulthood could be considered. Ultimately, prospective dose–response relationship studies across the emerging adulthood period will help identify how much MVPA is optimal for specific health outcomes to inform future PAG.
In emerging adulthood, most individuals stop growing taller while body fat continues to accumulate. Katsoulis et al. [16] showed that in comparisons of weight gain across U.K. adults from age 18 to 75 years, young adults (18–24 years of age) were identified as having the highest risk of weight gain. The present study is one of the few to examine adiposity development in emerging adulthood in a middle-income country. In the 1993 Pelotas study sample, we found that fat mass continued to increase on average 1.0 kg per year in both males and females throughout the 4 years between age 18 and 22 years. Emerging adulthood is a unique life stage when many significant life events occur, such as leaving the parental home, starting postsecondary education, and/or entering the labor market. Although evidence is lacking to explain why such a large increase in adiposity occurs during emerging adulthood [34], studies in high-income countries found that such life events were associated with obesogenic behaviors. For example, leaving the parental home and moving to college were associated with less healthy diet and PA behaviors [35, 36]. These life events could serve as key opportunities for interventions in young adults, such as improving PA and dietary environment in college [16]. However, the impact of life events that occur during emerging adulthood could be somewhat different in LMICs compared to high-income countries. In the present study sample, those who remained in school from age 18 to 22 years showed lower fat gain than their counterparts who were no longer in school at age 22 years, while there was no difference in fat gain between those who changed from living with a parent at age 18 to living without a parent at age 22 years and those living with a parent at both 18 and 22 years of age, as well as between those employed and unemployed. Future research is warranted to better understand fat gain during emerging adulthood, particularly in LMICs.
Although empirical data are limited from LMICs, it appears that the development of body fat is differently influenced by societal factors in high-income countries vs. middle income countries [20]. For example, a prior study reported a negative association between socioeconomic status and fat mass in high-income countries, but a positive or no association in middle-income countries [20]. The present study demonstrated that higher socioeconomic status was associated with higher body fat among young Brazilian men. This positive association could partly be explained by lower MVPA among young Brazilian men in a higher socioeconomic position. However, the relationships were somewhat inconsistent among young Brazilian women: females the highest wealth index quintile had the smallest fat gain between age 18 and 22 years and the lowest absolute fat mass at age 22 years, while they were also the least physically active. Nonetheless, after accounting for socioeconomic status in our analysis, we found that achieving ≥ 60 min/day of MVPA at ages 18 and 22 years was associated with a lower gain in fat mass compared to those who reduced MVPA to < 22 min/day at age 22 years. The benefits of MVPA on body fat gain should be widely communicated and advocated in the general population.
Some limitations should be acknowledged. First, despite an overall large sample size, the Y&N group accounted for only a small fraction of the sample (≤ 50 participants; ≤ 2.4%), producing wide CIs. Second, we cannot rule out that higher fat mass could have influenced lower MVPA (“reverse causation”) [37]. Third, use of two different types of accelerometers and different sampling frequencies could have provided different MVPA estimates. Further, wrist accelerometer-measured PA metrics may not be comparable with hip accelerometer-measured PA metrics. Fourth, this study did not account for potential seasonal effects. Lastly, the results may not be generalizable to the entire 1993 Pelotas study sample, because only 40% of the original sample was included in the current data analysis, which may have introduced bias in our findings. In addition, the results may not be generalizable young adults from other populations in different settings.
Conclusions
Our findings suggest that among individuals who were active in late adolescence, engaging in ≥ 22 min/day of MVPA in adulthood (meeting the adult PAG) is associated with lower body fat gain for females, but not for males.
Availability for data and materials
Data may be available upon request to Ana MB Menezes (anamene.epi@gmail.com) at Federal University of Pelotas, Brazil.
Abbreviations
- ∆BMI:
-
Change in body mass index
- ∆FM:
-
Change in fat mass
- ∆FMI:
-
Change in fat mass index
- BMI:
-
Body mass index
- DXA:
-
Dual-energy X-ray absorptiometry
- FM:
-
Fat mass
- FMI:
-
Fat mass index
- LMIC:
-
Low- to middle-income country
- MPA:
-
Moderate-intensity physical activity
- MVPA:
-
Moderate- and vigorous-intensity physical activity
- N&N:
-
Not meeting the youth MVPA guideline at age 18 years (< 60 min/day) and the adult MVPA guideline at age 22 years (< 22 min/day)
- N&Y22:
-
Not meeting the youth MVPA guideline at age 18 years (< 60 min/day) and meeting the adult MVPA guideline at age 22 years (≥ 22 min/day)
- N&Y60:
-
Not meeting the youth MVPA guideline at age 18 years (< 60 min/day) and meeting the youth MVPA guideline at age 22 years (≥ 60 min/day)
- Y&N:
-
Meeting the youth MVPA guideline at age 18 years (≥ 60 min/day) and not meeting the adult MVPA guideline at age 22 years (< 22 min/day)
- Y&Y22:
-
Meeting the youth MVPA guideline at age 18 years (≥ 60 min/day) and the adult MVPA guideline at age 22 years (≥ 22 min/day)
- Y&Y60:
-
Meeting the youth MVPA guideline at age 18 years (≥ 60 min/day) and meeting the youth MVPA guideline at age 22 years (≥ 60 min/day)
- PA:
-
Physical activity
- PAG:
-
Physical activity guidelines
- VPA:
-
Vigorous-intensity physical activity
- WHO:
-
World Health Organization
- wMVPA:
-
Weighted moderate- and vigorous-intensity physical activity
References
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62.
Office of Disease Prevention and Health Promotion. Physical activity guidelines [Available from: https://health.gov/paguidelines/.
Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health. 2018;6(10):e1077–86.
Guthold R, Stevens GA, Riley LM, Bull FC. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child Adolesc Health. 2020;4(1):23–35.
Australian Government Department of Health. Physical activity and exercise guidelines for all Australians [Available from: https://www.health.gov.au/health-topics/physical-activity-and-exercise/physical-activity-and-exercise-guidelines-for-all-australians.
Canadian Society for Exercise Physiology (CSEP). Canadian 24-hour movement guidelines [Available from: https://csepguidelines.ca/.
Department of Health and Social Care. Physical Activity Guidelines. London: UK Chief Medical Officers’ Report; 2019.
Arnett JJ, Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000;55(5):469–80.
Janz KF. Transition from youth to adult physical activity guidelines. Mineapolis: American College of Sports Medicine; 2018.
van Sluijs EMF, Ekelund U, Crochemore-Silva I, Guthold R, Ha A, Lubans D, et al. Physical activity behaviours in adolescence: current evidence and opportunities for intervention. Lancet. 2021;398(10298):429–42.
Nelson MC, Story M, Larson NI, Neumark-Sztainer D, Lytle LA. Emerging adulthood and college-aged youth: an overlooked age for weight-related behavior change. Obesity (Silver Spring). 2008;16(10):2205–11.
Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of Prediabetes Among Adolescents and Young Adults in the United States, 2005-2016. JAMA Pediatr. 2020;174(2):e194498. https://doi.org/10.1001/jamapediatrics.2019.4498.
Gordon-Larsen P, The NS, Adair LS. Longitudinal trends in obesity in the United States from adolescence to the third decade of life. Obesity (Silver Spring). 2010;18(9):1801–4.
Harris KM, Perreira KM, Lee D. Obesity in the transition to adulthood: predictions across race/ethnicity, immigrant generation, and sex. Arch Pediatr Adolesc Med. 2009;163(11):1022–8.
Gordon-Larsen P, Adair LS, Nelson MC, Popkin BM. Five-year obesity incidence in the transition period between adolescence and adulthood: the national longitudinal study of adolescent health. Am J Clin Nutr. 2004;80(3):569–75.
Katsoulis M, Lai AG, Diaz-Ordaz K, Gomes M, Pasea L, Banerjee A, Denaxas S, Tsilidis K, Lagiou P, Misirli G, Bhaskaran K, Wannamethee G, Dobson R, Batterham RL, Kipourou DK, Lumbers RT, Wen L, Wareham N, Langenberg C, Hemingway H. Identifying adults at high-risk for change in weight and BMI in England: a longitudinal, large-scale, population-based cohort study using electronic health records. Lancet Diabetes Endocrinol. 2021;9(10):681-94. https://doi.org/10.1016/S2213-8587(21)00207-2.
Oh M, Zhang D, Whitaker KM, Letuchy EM, Janz KF, Levy SM. Moderate-to-vigorous intensity physical activity trajectories during adolescence and young adulthood predict adiposity in young adulthood: the Iowa Bone Development Study. J Behav Med. 2021;44(2):231–40.
World Health Organization. Noncommunicable diseases country profiles 2018. World Health Organization. 2018. https://apps.who.int/iris/handle/10665/274512.
Mielke GI, Menezes AMB, BGC DAS, Ekelund U, Crochemore-Silva I, Wehrmeister FC, et al. Associations between device-measured physical activity and cardiometabolic health in the transition to early adulthood. Med Sci Sports Exerc. 2021;53(10):2076–85.
Bridger Staatz C, Kelly Y, Lacey RE, Blodgett JM, George A, Arnot M, et al. Life course socioeconomic position and body composition in adulthood: a systematic review and narrative synthesis. Int J Obes (Lond). 2021;45(11):2300–15.
Reichert FF, Menezes AM, Kingdom Wells JC, Ekelund E, Rodrigues FM, Hallal PC. A methodological model for collecting high-quality data on physical activity in developing settings-the experience of the 1993 Pelotas (Brazil) Birth Cohort study. J Phys Act Health. 2009;6(3):360–6.
Muniz LC, Menezes AM, Assunção MC, Martínez-Mesa J, Wehrmeister FC, Howe LD, et al. Body mass index at 11 years and bone mass at age 18: path analysis within the 1993 Pelotas (Brazil) birth cohort study. BMC Musculoskelet Disord. 2015;16:71.
Rowlands AV, Yates T, Davies M, Khunti K, Edwardson CL. Raw accelerometer data analysis with GGIR R-package: does accelerometer brand matter? Med Sci Sports Exerc. 2016;48(10):1935–41.
da Silva IC, van Hees VT, Ramires VV, Knuth AG, Bielemann RM, Ekelund U, et al. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int J Epidemiol. 2014;43(6):1959–68.
Migueles JH, Rowlands AV, Huber F, Sabia Sv, van Hees VT. GGIR: a research community-driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J Measur Phys Behav. 2019;2(3):188–96.
Ricardo LIC, Wendt A, Galliano LM, de Andrade MW, Niño Cruz GI, Wehrmeister F, et al. Number of days required to estimate physical activity constructs objectively measured in different age groups: findings from three Brazilian (Pelotas) population-based birth cohorts. PLoS One. 2020;15(1):e0216017.
van Hees VT, Gorzelniak L, Dean León EC, Eder M, Pias M, Taherian S, et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS One. 2013;8(4):e61691.
Kwon S, Letuchy EM, Levy SM, Janz KF. Youth Sports Participation Is More Important among Females than Males for Predicting Physical Activity in Early Adulthood: Iowa Bone Development Study. Int J Environ Res Public Health. 2021;18(3):1328. https://doi.org/10.3390/ijerph18031328.
Ramires VV, Dumith SC, Wehrmeister FC, Hallal PC, Menezes AM, Gonçalves H. Physical activity throughout adolescence and body composition at 18 years: 1993 Pelotas (Brazil) birth cohort study. Int J Behav Nutr Phys Act. 2016;13(1):105.
Rutstein S, Johnson K, The DHS wealth Index. DHS Comparative Reports No. 6. Calverton: ORC Macro; 2004.
Schneider BC, Motta JV, Muniz LC, Bielemann RM, Madruga SW, Orlandi SP, et al. Design of a digital and self-reported food frequency questionnaire to estimate food consumption in adolescents and young adults: birth cohorts at Pelotas, Rio Grande do Sul. Brazil Rev Bras Epidemiol. 2016;19(2):419–32.
Hochberg ZE, Konner M. Emerging adulthood, a pre-adult life-history stage. Front Endocrinol (Lausanne). 2019;10:918.
Sawyer SM, Afifi RA, Bearinger LH, Blakemore SJ, Dick B, Ezeh AC, et al. Adolescence: a foundation for future health. Lancet. 2012;379(9826):1630–40.
Mooyaart JE, Liefbroer AC, Billari FC. Becoming obese in young adulthood: the role of career-family pathways in the transition to adulthood for men and women. BMC Public Health. 2019;19(1):1511.
Winpenny EM, van Sluijs EMF, White M, Klepp KI, Wold B, Lien N. Changes in diet through adolescence and early adulthood: longitudinal trajectories and association with key life transitions. Int J Behav Nutr Phys Act. 2018;15(1):86.
Wengreen HJ, Moncur C. Change in diet, physical activity, and body weight among young-adults during the transition from high school to college. Nutr J. 2009;8:32.
Kwon S, Janz KF, Burns TL, Levy SM. Effects of adiposity on physical activity in childhood: Iowa Bone Development Study. Med Sci Sport Exercise. 2011;43(3):443–8.
Acknowledgements
We acknowledge our 1993 Pelotas (Brazil) Birth Cohort co-investigators and project staff for assistance with survey development, project management, data coding and cleaning, and data management. We also thank all participants for their cooperation during data collection.
Funding
This article is based on data from the study “Pelotas Birth Cohort, 1993” conducted by the Postgraduate Program in Epidemiology at Universidade Federal de Pelotas with the collaboration of the Brazilian Public Health Association (ABRASCO). From 2004 to 2013, the Wellcome Trust supported the 1993 birth cohort study. The European Union, National Support Program for Centers of Excellence (PRONEX), the Brazilian National Research Council (CNPq), and the Brazilian Ministry of Health supported previous phases of the study. The 22-year follow-up was supported by the Science and Technology Department / Brazilian Ministry of Health, with resources transferred through the Brazilian National Council for Scientific and Technological Development (CNPq), grant 400943/2013–1. This study was also financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The role of these funding bodies was to financially support various study activities, including data collection and the time and effort of researchers and staff. This article was also partly funded by U.S. National Institute of Child Health and Development grant R03-HD100657 to support the time and effort of SK, KJ, and UE.
Author information
Authors and Affiliations
Contributions
SK analyzed and interpreted the data and drafted the manuscript. UE, FC, HG, AM, BG, and KJ contributed to writing the manuscript and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Federal University of Pelotas Medical School Ethics Committee approved the study protocol (register number 05/2011 and 1.250.366). Written informed consent was obtained from individual participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. Comparisons of characteristics of the included sample and the excluded sample. Supplementary Table 2. Means of MVPA and FMI by wealth index and education level among males. Supplementary Table 3. Means of MVPA and FMI by wealth index and education level among females. Supplementary Figure 1. Adjusted means of the change in fat mass (kg) from age 18 to 22 years by six MVPA groups among males. Supplementary Figure 2. Adjusted means of the change in fat mass (kg) from age 18 to 22 years by six MVPA groups among females. Supplementary Figure 3. Adjusted means of the change in BMI from age 18 to 22 years by six MVPA groups among males. Supplementary Figure 2. Adjusted means of the change in BMI from age 18 to 22 years by six MVPA groups among females. Supplementary Figure 5. Adjusted means of the change in fat mass (kg) from age 18 to 22 years by six wMVPA groups among males. Supplementary Figure 6. Adjusted means of the change in fat mass (kg) from age 18 to 22 years by six wMVPA groups among females.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kwon, S., Menezes, A.M.B., Ekelund, U. et al. Longitudinal change in physical activity and adiposity in the transition from adolescence to early adulthood: the 1993 Pelotas cohort study. Int J Behav Nutr Phys Act 19, 83 (2022). https://doi.org/10.1186/s12966-022-01321-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12966-022-01321-0