Abstract
Background
The prevalence of Type 2 Diabetes is rising in Low- and Middle-Income Countries (LMICs), affecting all age categories and resulting in huge socioeconomic implications. Mobile health (mHealth) is a potential high-impact approach to improve clinical and patient-centered outcomes despite the barriers of cost, language, literacy, and internet connectivity. Therefore, it is valuable to examine the clinical and implementation outcomes of mHealth interventions for Type 2 Diabetes in LMICs.
Methods
The Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) guidelines were applied in framing and reporting the review criteria. A systematic search of Cochrane Library, Web of Science, PubMed, Scopus, and Ovid databases was performed through a combination of search terms. Randomized Controlled Trials (RCTs) and cohort studies published in English between January 2010 and August 2021 were included. Risk of bias for missing results in the included studies was assessed using the Cochrane risk-of-bias tool for randomized trials (RoB 2). Quantitative and qualitative methods were used to synthesize the results.
Results
The search identified a total of 1161 articles. Thirty studies from 14 LMICs met the eligibility criteria. On clinical outcomes, 12 and 9 studies reported on glycated hemoglobin (HbA1c )and fasting blood glucose (FBG) respectively. Text messages was the most commonly applied mHealth approach, used in 19 out of the 30 studies. Ten out of the 12 studies (83.3%) that reported on HbA1c had a percentage difference of <0.3% between the mHealth intervention and the comparison group. Additionally, studies with longer intervention periods had higher effect size and percentage difference on HbA1c (1.52 to 2.92%). Patient-centred implementation outcomes were reported variedly, where feasibility was reported in all studies. Acceptability was reported in nine studies, appropriateness in six studies and cost in four studies. mHealth evidence reporting and assessment (mERA) guidelines were not applied in all the studies in this review.
Conclusion
mHealth interventions in LMICs are associated with clinically significant effectiveness on HbA1 but have low effectiveness on FBG. The application of mERA guidelines may standardize reporting of patient-centered implementation outcomes in LMICs.
Trial registration
PROSPERO: Registration ID 154209.
Similar content being viewed by others
Introduction
Type 2 Diabetes is now a leading public health problem in Low-and Middle Income Countries (LMICs) [1] affecting all age categories and resulting in huge economic implications to healthcare [2, 3]. LMICs are home to 80 % of all people with type 2 diabetes (336 million) [4] and more than 80% of all undiagnosed people with diabetes [2]. It is projected that between 2019 and 2030, the prevalence of type 2 diabetes is likely to increase from 13.5% to 15.0% in LMICs compared to 10.4% to 11.4% in high-income countries [2]. Further, out of the total number of deaths related to diabetes globally, 41.8% and 58.2% occur in Low- and Middle- Income Countries, respectively [2]. The rising prevalence of type 2 diabetes in LMICs is attributed to the nutrition transition, and the increasing prevalence of overweight and obesity. The other factors include, urbanization, cultural and social changes, sedentary lifestyles, changes in diagnostic criteria and screening practices [5,6,7].
Optimal diabetes management requires a systematic approach, and the involvement of a coordinated, multidisciplinary team that is committed to patient-centered outcomes [8]. It is recommended that clinicians apply a patient-centered approach and minimum clinically important difference (MCID) treatment models by considering the statistical significance and clinical significance of research findings [9]. Essential guidelines for the patient-centered approach include individualized therapy and shared decision-making [10]. Additionally, effective patient-centered diabetes self-management requires the support and promotion of essential self-care behaviors [11]. These behaviors include healthy eating, physical activity, medication usage, monitoring and usage of patient-generated data, prevention, detection and treatment of acute and chronic complications, healthy coping with psychosocial issues and problem solving [12]. These behaviors have been described as Diabetes Self-Management Education and Support (DSMES) domains. Self-management education is linked to clinically important benefits on glycated hemoglobin (HbA1c), and cost of treatment [13,14,15,16,17,18]. This notwithstanding, self-care in most LMICs is not optimally attained due to disadvantaged access to healthacre and low-quality healthcare, poverty, low literacy levels and incorrect perceptions about diabetes [19,20,21].
The remarkable increase in ownership and use of mobile phones in LMICs provides a potential opportunity for the application of mobile health (mHealth) in self-care and behavior change interventions for type 2 diabetes [22,23,24]. mHealth is the medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants (PDAs), and other wireless devices [25]. Evidence shows that mHealth has the potential to facilitate accessibility and coverage of healthcare services as well as positively influencing clinical outcomes, compliance, self-care practices and quality of life for people with type 2 diabetes [26,27,28,29]. Whereas there is close similarity between mHealth and e-health, the later refers to an emerging field that links medical informatics, public health and business, that delivers or enhances health services and information via web-based technologies [30]. However, eHealth heavily relies on internet technology, which limits its applicability in LMICs, due to unreliable access to internet [31].
A recent metanalysis on mHealth interventions for diabetes in LMICs revealed promising but limited evidence on the effectiveness of mHealth interventions on glycemic control [32]. Further, a pooled effect on HbA1c from three studies on mobile phone–based interventions showed a larger effect of 25.46 mmol/mol or 20.50%; (95% CI 20.7 to 20.3%; I2 = 0%) [33]. mHealth interventions have also been found to be cost-effective [34] despite being criticized for having meager user satisfaction ratings coupled with usability challenges [35]. In LMICs, a few studies on mHealth have shown changes in clinical outcomes, adherence and improved communication with providers, decreased travel time, ease to receive expert advice and cost-effective education [36].
Further, evidence from LMICs reveal unique patient circumstances that hinder optimal utilization of mHealth approaches. Inadequate resources, low digital literacy and low health literacy and limited inclusion of motivation techniques hinder optimal utilization of mHealth in LMICs [37]. As such, distinguishing treatment effectiveness or clinical outcomes from implementation effectiveness is important for transferring interventions from experimental settings to the community [38, 39]. This distinction, to the best of our knowledge, has not been examined on mHealth interventions for type 2 diabetes in LMICs.
The objective of this systematic review therefore was to examine the clinical outcomes and patient-centered implementation outcomes of mHealth interventions with a focus on type 2 diabetes in LMICs.
Methods
Data sources and registration
This review applied the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) guidelines [40] with the PICOS framing. The review has been registered and amended in PROSPERO https://www.crd.york.ac.uk/prospero/#recordDetails (Registration ID 154209) and funded by VLIR-UOS
(Grant-number: KE2017IUC037A101)
Search strategy
The search strategy was applied on Cochrane and Web of Science Cochrane Library, Web of Science, PubMed, Scopus, and Ovid databases. (Supplementary File 1). These databases were systematically searched with Boolean combinations of key words and MeSH headings. An electronic search was conducted using the following terms and Boolean Operators: ((mobile health OR mHealth) AND (type 2 diabetes) AND/OR (DSMES) AND/OR (acceptability) AND/OR (appropriateness) AND/OR (feasibility) AND/OR (cost) AND/OR (sustainability)). Acceptability, appropriateness, feasibility, cost and sustainability were based on the definitions in the conceptual framework for implementation outcomes by Proctor et al. [38]. We searched for articles published in English between January 2010 and August 2021. Additional records were searched through citations from relevant reviews given that online data bases can be incomplete [41].
Study selection
This review included randomized controlled trials (RCTs), cluster randomized controlled trials, feasibility studies and prospective observational cohort studies from LMICs. The search also included cohort and follow-up studies of intervention studies that have been published in peer-reviewed journals. Our review was limited to studies that are designed for adults diagnosed with type 2 diabetes. We included studies in which the mHealth intervention was designed to be an enabler for delivery of DSMES for patients with type 2 diabetes [1]. mHealth or mobile health are medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants (PDAs), and other wireless devices as defined by WHO [25]. DSMES domains include diabetes pathophysiology and treatment options; healthy eating; physical activity; medication usage; monitoring and usage of patient generated health data; prevention, detection, and treatment of acute and chronic complications; healthy coping with psychosocial issues; and problem solving [42]. The selection of studies was conducted by MM and independently reviewed by FK, CM and RV. We excluded studies on children and adolescents, pregnant women, or any other forms of diabetes besides type 2 diabetes such as pre-diabetes, type 1 diabetes or gestational diabetes [43]. We also excluded studies where the mHealth intervention was designed for to support healthcare workers and those studies that did not target the patient.
Data collection process
Data from all eligible articles was summarized by the first author (MM) and reviewed by the second and third authors (FK & CM) using structured evidence tables (Table 1 & 2). A standardized criterion for data collection was designed by the authors to extract and tabulate relevant study characteristics. These characteristics include study location, study type, duration of study, clinical outcomes (HbA1c and FBG), mHealth intervention and function, DSMES domains and patient-centered implementation outcomes.
Quality of studies and risk of bias assessment
To assess quality of the articles, we applied the 2010 CONSORT (Consolidated Standards of Reporting Trials) guidelines [75] and the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [76]. This approach has been used elsewhere to assess the quality of studies [77,78,79]. An analysis of the quality of the studies included in this review is presented as heat maps (Supplementary File 2). A percentage quality score of >66.6% is rated as high, 50-66.6% as fair and <50% as low. Assessment of quality was conducted by two independent researchers, MM and ES. Additionally, the risk of bias in the included studies was assessed using the Cochrane risk of bias tool for randomized trials (RoB 2) (Supplementary File 3).
Summary measures
The primary outcome measures in this study are clinical outcomes and patient-centered implementation outcomes for type 2 diabetes mHealth interventions. Specifically, clinical outcomes were synthesized using quantitative methods based on effect sizes of HbA1c and FBG. HbA1c and FBG measure the effectiveness of interventions for the management of type 2 diabetes [80]. Additionally, the percentage difference between the mHealth intervention and the comparison group for HbA1c was analyzed. The patient centered implementation outcomes included acceptability, feasibility, appropriateness, cost and sustainability [38, 81,82,83,84]. Acceptability is the perception amongst implementation stakeholders that a particular treatment, service is agreeable, palatable, or satisfactory [33]. Appropriateness is the perceived fit, relevance or compatibility of the innovation for a given practice setting, provider or consumer; and/or perceived fit of the innovation to address a particular issue [33]. Cost is defined as the incremental, implementation or overall costs of delivery based on the settings [33]. Feasibility is the extent to which a new treatment, or an innovation, can be successfully applied or implemented in a specific setting [34]. Sustainability is the extent to which an implemented treatment is maintained within a service setting’s usual or stable operations, as defined by various authors [35,36,37].
Synthesis of results
To conduct a quantitative synthesis for clinical outcomes, standardized effect sizes were calculated using two-stage process [85]. In the first stage the effect size of HbA1c and FBG was calculated separately for each study from means and standard deviations. In the second stage, the combined effect size as a weighted average of the intervention effects was derived from the individual studies. The effect sizes were calculated using the formula d = (<post>-<pre>)/stdev to account for between group and within group comparisons. Cohen's d was calculated to derive standardized effect sizes and then converted into Hedges' g to correct for their upwards bias [86]. The magnitude of Hedges' g was interpreted using Cohen's convention where an effect size of < 0.20 is considered to be small, 0.50 to 0.80 as medium, while scores > 0.80 as large [87]. The five patient-centered implementation outcomes were analyzed in an excel spreadsheet and presented descriptively.
Results
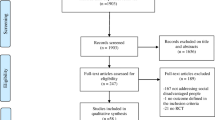
The search identified a total of 1,161 articles. After removal of duplicates, 1,116 titles of articles were screened and a total of 30 studies that met the eligibility criteria were included in this review (Fig. 1). The 30 eligible studies include 21 randomized controlled trials [44, 46, 47, 50, 51, 53,54,55,56, 58,59,60,61,62, 66, 68, 70,71,72,73,74] two feasibility randomized controlled trials [52, 63], three cluster randomized control trials [49, 57, 69] and five cohort studies [45, 48, 64, 65, 67]. The studies were conducted in 14 LMICs including nine in China [45,46,47, 53, 56, 57, 70, 73, 88], five in India [49, 58, 62, 65, 89], three in South Africa [60, 61, 67], three in Iran [51, 63, 66], two in Bangladesh [49, 54] and one each in Iraq [52], Lebanon [48], Pakistan [68], Mexico [44], Cambodia [69] and Thailand [64]. One other study was multicentre, conducted in Congo, Cambodia and Philippines [59].
Study quality
The overall mean rating based on these checklists for the randomized controlled trials and cohort studies was 81.8% and 87.7% respectively and categorized as high quality (Supplementary Files 2).
Study and sample characteristics
The 30 studies included a total of 27,142 participants (Mean =904.7 SD=2548.6) published between 2010-2021, with 66.7% published between 2017 to 2021. The mean duration of the studies was 8.9 months (SD=6.4 min-max: 3-24 months).
mHealth interventions
Table 3 below categorizes mHealth interventions based on the WHO categorization [25]. In summary text mobile phone text messages (MPTMs) was the most applied mHealth approach, applied in 19 studies. Mobile apps were applied in 10 studies while four studies [50, 56, 57, 70] applied wearable or portable monitoring devices to monitor blood glucose, physical activity or heartbeat rate.
Clinical outcomes of mHealth intervention
To examine clinical outcomes in this review, we examined changes in HbA1c and FBG. HbA1c (mean SD) was reported in 12 studies [44, 46, 47, 51, 53, 56, 63, 64, 68, 70, 71, 73] (Table 4). As summarized in Table 4, one study [47] had a large effect size (Cohen’s d =1.15) while three studies [44, 46, 73] reported a medium effect size (Cohen’s d =0.57). Five of the 12 studies that reported HbA1c had a small effect size (Table 4). Ten out of the twelve studies (83.3%) that reported on HbA1c had a percentage difference of <0.3% between the mHealth intervention and the comparison group. Pichayapinto et al [64] only reported the effect size (Cohen’s d= -0.5) and hence the percentage difference was not calculated. This review found a correlation between studies that used mobile applications approach with medium effect sizes, including Zhou et al. [73] (ES=0.57) Chao et al. [82], (ES=0.58), Anzaldo et al. [44] (ES=0.64). Three studies that used text messages had lower effect sizes, including Peimani et al. [63] (ES=0.28) Huo et al. [53] (ES=0.36) and Goodarzi et al. [51] (ES=0.40). The highest effect size (ES=1.16) in this review was reported by Dong et al. [47], a study that used a text messaging platform (WeChat). Studies that used wearable devices had mixed effect sizes, with Li et al. [56] reporting the least effect size (ES=0.12), while Sun et al. [70] had a medium effect size (ES=0.46).
Additionally, studies that had longer durations of the intervention [44, 46, 47] had higher effect size and percentage difference (2.83, 1.52 and 2.92) between the intervention group and the comparison group.
Table 5 shows FBG as reported in 9 studies [47, 51, 53, 55, 63, 66, 70, 71, 73]. In summary, this review revealed a small effect size of FBG in eight out of the nine studies. The highest effect size for FBG was reported by Zhou et al. [73] with a medium effect (Cohen’s d and Hedge’s g= 0.60). Two studies [47, 55] that had longer intervention durations had lower effect size (Cohen’s d 0.08 and 0.01) for FBG.
Patient-centred Implementation outcomes
Acceptability of mHealth
Acceptability was reported in nine studies [52, 53, 57, 58, 61, 62, 65, 70]. Three studies [52, 53, 61] reported acceptability as the users’ preferred time to receive text messages. Chao et al. [46] conducted a pre- and post-interventional assessments and used the interactive personalized management framework mobile application to assess the participants mental readiness to change behaviour. Fottrell et al. [49] used small group discussions prior to all interviews involving men and women attendees and with non-attenders to get consensus on desired community changes. Table 6 below describes various aspects of user-satisfaction reported in these studies. Most of the studies that assessed and reported on user-satisfaction provided scanty details on the findings.
Only two studies in this review [53, 61] assessed the usefulness of the messages with 94.1% and 90.7% of the participants respectively. Two studies [52, 53] showed that 90.5% and 97.1% of the participants respectively, found the content of the intervention to be understandable. In general, only three studies had included measurement of acceptability as a secondary outcome. Five [52, 53, 58, 61, 70] of the nine studies that assessed acceptability measured the willingness to continue using the mHealth intervention after the study. Willingness to continue using mHealth after the intervention was 100% in Haddad et al. [52], 93.7% in Huo et al. [53], 98.0% in Limaye et al [58], 95.9% in Owolabi et al. [61]. In Limaye et al. [58], 96% of the participants also acknowledged willingness to recommend the intervention to friends.
Feasibility of mHealth interventions
Feasibility of mHealth intervention was examined by the application of any DSMES. Table 7 below summarizes the DSMES applied in the studies. In summary, the most applied DSMES was healthy eating, in 26 studies (86.7%) and physical activity, in 24 studies (80.0%). The most applied combination of DSMES was healthy eating, physical activity, and medication usage, applied in 26, 24 and 23 studies respectively.
Appropriateness of mHealth interventions
Hermes et al. [91] describes objective measurement of appropriateness to be the perceived interventional technology fit with the specific context. Seven studies reported appropriateness variedly. As illustrated in Table 8 below.
Cost of mHealth interventions
Four studies [49, 52, 53, 58] analysed the cost of the mHealth intervention (Table 9). Various aspects of cost were reported targeting the patient, the program, or the general population.
Sustainability of mHealth intervention
As shown in Table 10 below, only two studies reported on sustainability of the mHealth interventions [58, 70]
Discussion
This systematic review found clinically significant effectiveness of mHealth interventions on HbA1c in most interventions for type 2 diabetes in LMICs. Ten out of 12 studies had a >0.3% difference for HbA1c between the mHealth intervention group and comparison group. There was however low effectiveness of mHealth on FBG in most interventions, with 8 out of 9 studies that reported FBG showing an effect size of <0.05. Mobile phone text messages (MPTMs) and mobile apps was the most common mHealth approach in 19 and 10 out of 30 studies respectively. Voice calls and wearable devices were used in five and two studies respectively. Despite the popularity of MPTMs in most interventions in our review, this mode of mHealth was associated with lower effectiveness on HbA1c and FBG. Among the patient centered outcomes, feasibility, based on DSMES domains was reported in all studies. There was substantial heterogeneity in reporting of acceptability, appropriateness, cost, and sustainability.
A change of 0.3% (3 mmol/mol) in HbA1c denotes a clinically significant margin and is generally considered to be an acceptable change [92]. Although this change seems to be relatively small, this difference in HbA1c has been associated with clinically significant effects, including reduction in the risk to diabetic complications, lower long-term risk to microvascular complications and all-cause mortality [92,93,94]. Despite the heterogeneity, our findings indicate that mHeath can be an effective tool to improve HbA1c. On the contrary, studies in this review revealed low effectiveness of mHealth on FBG. These findings concur with a recent metanalysis consisting of nine studies drawn from LMICs and high-income countries that reported a pooled effect size of −0.39; (P<.001) despite the different populations targeted [95]. FBG is known to be affected by numerous factors, that could be attributable to the low effectiveness found in our review [96]. Another interesting finding from this review is that studies with intervention durations of >10 months had a higher percentage change on HbA1c compared to those conducted for shorter periods of time. On the contrary, longer durations were associated with lower effect size for FBG. Longer interventions have been associated with increased engagement, and effectiveness of mHealth interventions [97]. The reasons for the lower effect sizes for FBG are unclear, but could equally be linked with intervening factors that cannot be controlled during the interventions [96].
Patient-centered implementation outcomes included in this review were acceptability, appropriateness, feasibility, cost, and sustainability. Acceptability is associated with user-satisfaction [98]. Studies in our review hardly reported on most patient centered outcomes. Reporting of these outcomes was also widely varied. None of the studies in this review applied the mHealth evidence reporting and assessment (mERA) guidelines [99] in reporting its findings. mERA guidelines provide a criteria to identify minimum sets of information needed to the define the mHealth intervention, where it is implemented, and how it is implemented to facilitate a possible replication of an intervention. mERA guidelines recommend that interventions report on appropriateness of the interventions, user opinions on content or user interface, perceptions about usability, access, cost assessment and connectivity. This review showed that only 4 out of the 30 studies (13.3%) reported on the cost aspects of the intervention. Further, six studies reported on various aspects of appropriateness including assessments on appropriate timing of messages, satisfaction, and convenience of the intervention. In this review, we described feasibility of the intervention based on the DSMES domain applied. Three DSMES domains, including healthy eating, physical activity, and medication usage in this review were associated with a difference of >0.3% for HbA1c . Similar findings have been reported in a review or reviews that linked the application of technology enabled DSME domains to significantly improvement of HbA1c [100]. Additionally, Muller et al. [101]. Found that mHealth interventions can be effective in promoting physical activity and healthy diets in low income settings.
Strengths and limitations
This review has the strength that we used clearly defined inclusion and exclusion criteria and conducted searches in two phases. However, this review has some limitations. First, most studies in this review did not report on HbA1c, which is considered as the gold standard clinical outcome in diabetes care. Secondly, patient centered implementation outcomes are mainly reported in grey literature, which were not included in our review. Thirdly, most of the studies in this review did not apply the mERA guidelines, hence reducing replication of the intervention. Finally, we only included articles published in the English language, which introduced the language bias.
Conclusion
mHealth interventions in LMICs are associated with clinically significant effectiveness on HbA1c but have low effectiveness on FBG. Interventions applying mobile apps have a high effect size on HbA1c compared to those that apply text messaging, voice calls or wearable devices. Percentage changes of >0.3% in HbA1c was correlated with three DSMES domains, including healthy eating, physical activity, and medication usage. The use of the mHealth evidence reporting and assessment (mERA) guidelines may standardize and improve reporting of patient-centered implementation outcomes in LMICs.
Implications for future research
Clinical and patient centered implementation outcomes should be considered in the planning, implementation and monitoring of mHealth interventions. This approach optimizes the individualization of care, which is vital in diabetes care. Additionally, mERA guidelines need to be applied in reporting so as to standardize and provide rigor in mHealth intervention globally.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- App:
-
Application
- AST:
-
aspartate transaminase
- BG:
-
Blood Glucose
- BMI:
-
Body Mass Index
- BP:
-
Blood pressure
- CONSORT:
-
Consolidated Standards of Reporting Trials
- DSME:
-
Diabetes Self-Management and Education
- DSMES:
-
Diabetes self-management education and support
- FBG:
-
Fasting blood glucose
- FU:
-
follow-up
- HbA1c:
-
Glycated haemoglobin
- HDL-c:
-
High Density Lipoprotein Cholesterol
- I1:
-
First Intervention Arm
- I2:
-
Second Intervention Arm
- IPMF:
-
Interactive personalized management framework
- LDL-c:
-
Low Density Lipoprotein Cholesterol
- LMICs:
-
Low- and middle-income countries
- Med:
-
Medication
- Mos:
-
Months
- NO:
-
Number of
- NP:
-
Not Provided
- OP:
-
Outpatient
- PA:
-
Physical Activity
- PBG:
-
Post-prandial Blood Glucose
- PCHR:
-
Personally Controlled Health Record
- PICOS:
-
Participants, interventions, comparisons and outcome(s) and study design
- PRISMA:
-
Preferred Reporting Items for Systematic review and Meta-Analysis
- PT:
-
Patient
- QA:
-
Question and answer
- RCTs:
-
Randomized controlled Trials
- SBP:
-
Systolic Blood pressure
- SD:
-
Standard Deviation
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- Wk:
-
Week
- Wt:
-
Weight
References
The World Bank. World Bank Country and Lending Groups [Internet]. Data. 2019. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
International Diabetes Federation. IDF Diabetes Atlas. IDF Diabetes Atlas 9th Ed. 2019.
Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, et al. The global economic burden of diabetes in adults aged 20–79 years: a cost-of-illness study. Lancet Diabetes Endocrinol [Internet]. 2017 1 [cited 2018 Dec 3];5(6):423–30. Available from: https://www.sciencedirect.com/science/article/pii/S2213858717300979
IDF. IDF Diabetes Atlas 8th Edition [Internet]. Internasional Diabetes Federation. 2017 [cited 2019 Feb 11]. Available from: https://reports.instantatlas.com/report/view/704ee0e6475b4af885051bcec15f0e2c/KEN
Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015 Jul 1;30(7):529–42.
Sami W, Ansari T, Butt N, Ah HM. Effect of diet on type 2 diabetes mellitus: A review Introduction. Int J Health Sci (Qassim). 2017;11(2).
Kennerly A-M, Kirk A. Physical activity and sedentary behaviour of adults with type 2 diabetes: a systematic review. Pract Diabetes [Internet]. 2018 May 1 [cited 2020 Feb 12];35(3):86-89g. Available from: http://doi.wiley.com/10.1002/pdi.2169
Powers MA, Bardsley J, Cypress M, Duker P, Funnell MM, Hess Fischl A, et al. Diabetes Self-management Education and Support in Type 2 Diabetes: A Joint Position Statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Care [Internet]. 2015 Jul 5 [cited 2019 Mar 10];38(7):1372–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26048904
Fleischmann M, Vaughan B. Commentary: Statistical significance and clinical significance - A call to consider patient reported outcome measures, effect size, confidence interval and minimal clinically important difference (MCID). J Bodyw Mov Ther [Internet]. 2019;23(4):690–4. https://doi.org/10.1016/j.jbmt.2019.02.009.
Brunton S. By what standard should we manage diabetes? Clin Diabetes. 2018;36(1):12–3.
Kent D, D’Eramo Melkus G, Stuart PMW, McKoy JM, Urbanski P, Boren SA, et al. Reducing the risks of diabetes complications through diabetes self-management education and support. Popul Health Manag. 2013;16(2):74–81.
American Diabetes Association. 5. Facilitating behavior change and wellbeing to improve health outcomes: Standards of Medical Care in Diabetes- 2020. Diabetes Care. 2020;43(Suppl. 1):S48–65.
Chodosh J, Morton SC, Mojica W, Maglione M, Suttorp MJ, Hilton L, et al. Annals of Internal Medicine Improving Patient Care Meta-Analysis : Chronic Disease Self-Management Programs for Older Adults. Ann Intern Med. 2005;143:427–38.
Worswick J, Wayne SC, Bennett R, Fiander M, Mayhew A, Weir MC, et al. Improving quality of care for persons with diabetes: an overview of systematic reviews - what does the evidence tell us? Syst Rev. 2013;2:26.
Minet L, Møller S, Vach W, Wagner L, Henriksen JE. Mediating the effect of self-care management intervention in type 2 diabetes: A meta-analysis of 47 randomised controlled trials. Patient Educ Couns. 2010;80(1):29–41.
Gary T, Genkinger J, Giallar E, Payrot M, Bracati F. Meta-analysis of Randomizes Education and Behavior Interventions in Type 2 Diabetes. Diabetes Educ. 2003;29(3):488–501.
Pillay J, Armstrong MJ, Butalia S, Donovan LE, Sigal RJ, Vandermeer B, et al. Behavioral programs for type 2 diabetes mellitus: A systematic review and network meta-Analysis. Vol. 163, Annals of Internal Medicine. American College of Physicians; 2015. p. 848–60.
Powers MA, Bardsley JK, Cypress M, Funnell MM, Harms D, Hess-Fischl A, et al. Diabetes Self-management Education and Support in Adults with Type 2 Diabetes: A Consensus Report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy. Diabetes Care. 2020;43(7):1636–49.
Bekele H, Asefa A, Getachew B, Belete AM. Barriers and Strategies to Lifestyle and Dietary Pattern Interventions for Prevention and Management of TYPE-2 Diabetes in Africa, Systematic Review. J Diabetes Res. 2020;2020.
U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition [Internet]. [cited 2019 Feb 1]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
Mogre V, Johnson N, Tzelepis F, Shaw J, Paul C. A systematic review of adherence to diabetes self-care behaviours: Evidence from low-and middle-income countries. J Adv Nurs [Internet]. 2019 [cited 2020 Jun 22];75:3374–89. Available from: https://publons.com/publon/10.1111/jan.14190
Chib A, Velthoven MH Van, Car J, Chib A, Helena M. mHealth Adoption in Low-Resource Environments : A Review of the Use of Mobile Healthcare in Developing Countries mHealth Adoption in Low-Resource Environments : A Review of the Use of Mobile Healthcare in Developing Countries. 2015;0730.
Blumenstock J, Cadamuro G, On R. Predicting poverty and wealth from mobile phone metadata. Science [Internet]. 2015 [cited 2019 Jun 19];350(6264):1073–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26612950
Milward J, Day E, Wadsworth E, Strang J, Lynskey M. Mobile phone ownership, usage and readiness to use by patients in drug treatment. Drug Alcohol Depend [Internet]. 2015 Jan 1 [cited 2019 Jun 19];146:111–5. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0376871614018894
Kay M. mHealth: New horizons for health through mobile technologies World Health Organization Global Observatory for eHealth. GSMA mHA Mob Heal Summit Cape T. 2011;(June).
Stephani V, Opoku D, Quentin W. A systematic review of randomized controlled trials of mHealth interventions against non-communicable diseases in developing countries. 2016;
Beratarrechea A, Moyano D, Irazola V, Rubinstein A. mHealth Interventions to Counter Noncommunicable Diseases in Developing Countries: Still an Uncertain Promise. Cardiol Clin [Internet]. 2017;35(1):13–30. https://doi.org/10.1016/j.ccl.2016.08.009.
Afable A, Karingula NS. Evidence based review of type 2 diabetes prevention and management in low and middle income countries. World J Diabetes [Internet]. 2016 [cited 2020 May 7];7(10):209–29. Available from: http://www.wjgnet.com/esps/HelpDesk:http://www.wjgnet.com/esps/helpdesk.aspxURL:http://www.wjgnet.com/1948-9358/full/v7/i10/209.htmDOI:http://dx.doi.org/10.4239/wjd.v7.i10.209
Hall AK, Cole-Lewis H, Bernhardt JM. Mobile Text Messaging for Health: A Systematic Review of Reviews. Annu Rev Public Heal [Internet]. 2015 [cited 2020 Jun 24];36:393–415. Available from: www.annualreviews.org
Eysenbach G. What is e-health? J Med Internet Res. 2001;3(2):1–5.
Bahia K, Suardi S. Connected Society: The State of Mobile Internet Connectivity 2019 [Internet]. Gsma. 2019. Available from: http://www.comscore.com/Press_Events/Press_Releases/2010/6/comScore_Reports_April_2010_U.S._Mobile_Subscriber_Market_Share
Nanditha A, Snehalatha C, Raghavan A, Vinitha R, Satheesh K, Susairaj P, et al. The post-trial analysis of the Indian SMS diabetes prevention study shows persistent beneficial effects of lifestyle intervention. Diabetes Res Clin Pract [Internet]. 2018;142:213–21. https://doi.org/10.1016/j.diabres.2018.05.042.
Pal K, Eastwood S V, Michie S, Farmer A, Barnard ML, Peacock R, et al. Computer-Based Interventions to Improve Self-management in Adults With Type 2 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care [Internet]. 2014 [cited 2020 Jun 25];37:1759–66. Available from: http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-1386/-/DC1.
Rinaldi G, Hijazi A, Haghparast-Bidgoli H. Cost and cost-effectiveness of mHealth interventions for the prevention and control of type 2 diabetes mellitus: A systematic review. Diabetes Res Clin Pract. 2020;162:1–14.
Fu H, McMahon SK, Gross CR, Adam TJ, Wyman JF. Usability and clinical efficacy of diabetes mobile applications for adults with type 2 diabetes: A systematic review. Diabetes Res Clin Pract [Internet]. 2017;131:70–81. https://doi.org/10.1016/j.diabres.2017.06.016.
Hurt K, Walker RJ, Campbell JA, Egede LE. mHealth Interventions in Low and Middle-Income Countries. A Systematic Review. Glob J Health Sci. 2016;8(9).
van Olmen J, Erwin E, Cristina García-Ulloa A, Meessen B, Jaime Miranda J, Bobrow K, et al. Implementation barriers for mHealth for non-communicable diseases management in low and middle income countries: a scoping review and field-based views from implementers . 2020 [cited 2020 Jun 30]; Doi: 10.12688/wellcomeopenres.15581.1
Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Heal Ment Heal Serv Res. 2011;38(2):65–76.
Chaudoir SR, Dugan AG, Barr CHI. Measuring factors affecting implementation of health innovations: A systematic review of structural, organizational, provider, patient, and innovation level measures. Implement Sci. 2013;8(1).
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ [Internet]. 2015;349(January):1–25. https://doi.org/10.1136/bmj.g7647.
Montori VM, Swiontkowski MF, Cook DJ. Methodologic Issues in Systematic Reviews and Meta-Analyses. Clin Orthop Relat Res. 2003;413:43–54.
Beck J, Greenwood DA, Blanton L, Bollinger ST, Butcher MK, Ellen Condon J, et al. 2017 National Standards for Diabetes Self-Management Education and Support. 2017 [cited 2020 Jun 19]; Doi: 10.2337/dci17-0025
Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women’s dietary patterns change little from before to during pregnancy. J Nutr [Internet]. 2009 Oct [cited 2019 Oct 14];139(10):1956–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19710161
Anzaldo-Campos MC, Contreras S, Vargas-Ojeda A, Menchaca-Díaz R, Fortmann A, Philis-Tsimikas A. Dulce wireless Tijuana: A randomized control trial evaluating the impact of project Dulce and short-term mobile technology on glycemic control in a family medicine clinic in Northern Mexico. Diabetes Technol Ther. 2016;18(4):240–51.
Chai S, Wang D, Yao B, Xu L, Ji L, Zhang X. The effect of education and mobile health management on improvement of blood glucose with type 2 diabetes mellitus. 2020;
Chao DYP, Lin TMY, Ma WY. Enhanced self-efficacy and behavioral changes among patients with diabetes: Cloud-based mobile health platform and mobile app service. J Med Internet Res. 2019;21(5).
Dong Y, Wang P, Dai Z, Liu K, Jin Y, Li A, et al. Increased self-care activities and glycemic control rate in relation to health education via Wechat among diabetes patients: A randomized clinical trial. Med (United States). 2018;97(50):1–5.
Doocy S, Paik KE, Lyles E, Hei Tam H, Fahed Z, Winkler E, et al. Guidelines and mHealth to Improve Quality of Hypertension and Type 2 Diabetes Care for Vulnerable Populations in Lebanon: Longitudinal Cohort Study. JMIR mHealth uHealth. 2017;5(10):e158.
Fottrell E, Ahmed N, Morrison J, Kuddus A, Shaha SK, King C, et al. Community groups or mobile phone messaging to prevent and control type 2 diabetes and intermediate hyperglycaemia in Bangladesh (DMagic): a cluster-randomised controlled trial. Lancet Diabetes Endocrinol [Internet]. 2019;7(3):200–12. Available from:. https://doi.org/10.1016/S2213-8587(19)30001-4.
Gunawardena KC, Jackson R, Robinett I, Dhaniska L, Eng B, Jayamanne S. The Influence of the Smart Glucose Manager Mobile Application on Diabetes Management. 2019;
Goodarzi M, Ebrahimzadeh I, Rabi A, Saedipoor B, Jafarabadi MA. Impact of distance education via mobile phone text messaging on knowledge, attitude, practice and self efficacy of patients with type 2 diabetes mellitus in Iran. J Diabetes Metab Disord. 2012;11(1):1–8.
Haddad NS, Istepanian R, Philip N, Khazaal FAK, Hamdan TA, Pickles T, et al. A feasibility study of mobile phone text messaging to support education and management of type 2 diabetes in Iraq. Diabetes Technol Ther. 2014;16(7):454–9.
Huo X, Krumholz HM, Bai X, Spatz ES, Ding Q, Horak P, et al. Effects of Mobile Text Messaging on Glycemic Control in Patients With Coronary Heart Disease and Diabetes Mellitus. Circ Cardiovasc Qual Outcomes. 2019;12(9):e005805.
Mohammed S, Islam S, Lechner A, Ferrari U, Seissler J, Holle R, et al. Mobile phone use and willingness to pay for SMS for diabetes in Bangladesh. 2015;38(1):163–9.
Kumar D, Sujeet R, Satya Bhushan S, Sunil Kumar R, Ashok Kumar B. Effectiveness of Randomized Control Trial of Mobile Phone Messages on Control of Fasting Blood Glucose in Patients with Type-2 Diabetes Mellitus in a Northern State of India. Indian J Public Health [Internet]. 2018;62:224–6. Available from: www.ijph.in
Li J, Wei D, Liu S, Li M, Chen X, Chen L, et al. Efficiency of an mHealth app and chest-wearable remote exercise monitoring intervention in patients with type 2 diabetes: A prospective, multicenter randomized controlled trial. JMIR mHealth uHealth. 2021;9(2).
Liao J, Xiao H, Li X, Sun S, Liu S, Yang Y. A Social Group-Based Information-Motivation-Behavior Skill Intervention to Promote Acceptability and Adoption of Wearable Activity Trackers Among Middle-Aged and Older Adults : Cluster Randomized Controlled Trial Corresponding Author. JMIR mHealth uHealth. 2020;8(4):1–13.
Limaye T, Kumaran K, Joglekar C, Bhat D, Kulkarni R, Nanivadekar A, et al. Efficacy of a virtual assistance-based lifestyle intervention in reducing risk factors for Type 2 diabetes in young employees in the information technology industry in India: LIMIT, a randomized controlled trial. Diabet Med. 2017;34(4):563–8.
Van Olmen J, Kegels G, Korachais C, de Man J, Van Acker K, Kalobu JC, et al. The effect of text message support on diabetes self-management in developing countries – A randomised trial. J Clin Transl Endocrinol [Internet]. 2017;7:33–41. Available from: http://dx.doi.org/10.1016/j.jcte.2016.12.005
Owolabi EO, Ter GD, Ajayi AI. Impact of mobile phone text messaging intervention on adherence among patients with diabetes in a rural setting. Med (United States). 2020;12(July 2019).
Owolabi EO, Ter GD, Ajayi AI. Efficacy, acceptability and feasibility of daily text-messaging in promoting glycaemic control and other clinical outcomes in a low-resource setting of South Africa: A randomised controlled trial. PLoS One. 2019;14(11):1–17.
Patnaik L, Panigrahi SK, Sahoo AK, Mishra D, Beura S, Muduli AK. Mobile health application based intervention for improvement of quality of life among newly diagnosed type 2 diabetes patients.
Peimani M, Rambod C, Omidvar M, Larijani B, Ghodssi-Ghassemabadi R, Tootee A, et al. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: A feasibility study. Prim Care Diabetes. 2016;10(4):251–8.
Pichayapinyo P, Saslow LR, Aikens JE, Marinec N, Sillabutra J, Rattanapongsai P, et al. Feasibility Study of Automated Interactive Voice Response Telephone Calls with Community Health Nurse Follow-up to Improve Glycaemic Control in Patients with Type 2 Diabetes. Int J Nurs Pr. 2020;25(6):1–19.
Pfammatter A, Spring B, Saligram N, Davé R, Gowda A, Blais L, et al. MHealth intervention to improve diabetes risk behaviors in India: A prospective, parallel group cohort study. J Med Internet Res. 2016;18(8):1–9.
Rasoul AM, Jalali R, Abdi A, Salari N, Rahimi M, Mohammadi M. The effect of self-management education through weblogs on the quality of life of diabetic patients. BMC Med Inform Decis Mak. 2019;19(1):1–12.
Rotheram-Borus MJ, Tomlinson M, Gwegwe M, Comulada WS, Kaufmann N, Keim M. Peer support through a mobile phone buddy system. Diabetes Educ. 2014;38(3):357–65.
Shahid M, Mahar SA, Shaikh S, Shaikh Z-U-D. Mobile phone intervention to improve diabetes care in rural areas of Pakistan: A randomized controlled trial. J Coll Physicians Surg Pakistan [Internet]. 2015;25(3):166–71. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84925009675&partnerID=40&md5=baee8225db308d5c3ea5075ac23f42af
Steinman L, Pelt M Van, Hen H, Chhorvann C, Lan CS, Te V, et al. Can mHealth and eHealth improve management of diabetes and hypertension in a hard-to-reach population ? — lessons learned from a process evaluation of digital health to support a peer educator model in Cambodia using the RE-AIM framework. 2020;
Sun C, Sun L, Xi S, Zhang H, Wang H, Feng Y, et al. Mobile phone–Based telemedicine practice in older chinese patients with type 2 diabetes mellitus: Randomized controlled trial. JMIR mHealth uHealth [Internet]. 2019;7(1). Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85060366180&doi=10.2196%2F10664&partnerID=40&md5=7b7e148c2a2cf469fd8a7fa10f36ef61
Wang Y, Li M, Zhao X, Pan X, Lu M, Lu J, et al. Effects of continuous care for patients with type 2 diabetes using mobile health application : A randomised controlled trial. 2019;1025–35.
Yasmin F, Yasmin F, Nahar N, Banu B, Ali L, Sauerborn R, et al. The influence of mobile phone-based health reminders on patient adherence to medications and healthy lifestyle recommendations for effective management of diabetes type 2: A randomized control trial in Dhaka. Bangladesh. BMC Health Serv Res. 2020;20(1):1–12.
Zhou W, Chen M, Yuan J, Sun Y. Welltang - A smart phone-based diabetes management application - Improves blood glucose control in Chinese people with diabetes. Diabetes Res Clin Pract [Internet]. 2016;116:105–10. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84965036413&doi=10.1016%2Fj.diabres.2016.03.018&partnerID=40&md5=60c67aa80e08774974e8e3b021c561b4
Islam SMS, Lechner A, Ferrari U, Froeschl G, Alam DS, Holle R, et al. Mobile phone intervention for increasing adherence to treatment for type 2 diabetes in an urban area of Bangladesh: Protocol for a randomized controlled trial. BMC Health Serv Res. 2014;14(1):1–9.
Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med [Internet]. 2010 Mar 24 [cited 2019 Oct 18];8:18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20334633
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. PLoS Med [Internet]. 2007 Oct 16 [cited 2020 Feb 3];4(10):e296. Available from: https://dx.plos.org/10.1371/journal.pmed.0040296
Schoeppe S, Alley S, Van Lippevelde W, Bray NA, Williams SL, Duncan MJ, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: A systematic review. Int J Behav Nutr Phys Act. 2016 Dec 7;13(1).
Maher CA, Lewis LK, Ferrar K, Marshall S, De Bourdeaudhuij I, Vandelanotte C. Are health behavior change interventions that use online social networks effective? A systematic review. J Med Internet Res [Internet]. 2014 Feb 14 [cited 2019 Oct 18];16(2):e40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24550083
Davies CA, Spence JC, Vandelanotte C, Caperchione CM, Mummery WK. Meta-analysis of internet-delivered interventions to increase physical activity levels. Int J Behav Nutr Phys Act. 2012;9.
Nagayama H, Inaba M, Okabe R, Emoto M, Ishimura E, Okazaki S, et al. Glycated albumin as an improved indicator of glycemic control in hemodialysis patients with type 2 diabetes based on fasting plasma glucose and oral glucose tolerance test. Biomed Pharmacother [Internet]. 2009;63(3):236–40. Available from: https://doi.org/10.1016/j.biopha.2008.04.002
Karsh BT. Beyond usability: Designing effective technology implementation systems to promote patient safety. Qual Saf Heal Care. 2004;13(5):388–94.
Johnson K, Hays C, Center H, Daley C. Building capacity and sustainable prevention innovations: A sustainability planning model. Eval Program Plann. 2004;27(2):135–49.
Turner KMT, Sanders MR. Dissemination of evidence-based parenting and family support strategies: Learning from the Triple P - Positive Parenting Program system approach. Aggress Violent Behav. 2006;11(2):176–93.
Dopp AR, Parisi KE, Munson SA, Lyon AR. A glossary of user-centered design strategies for implementation experts. Transl Behav Med. 2019;9(6):1057–64.
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Chapter 10: Analysing data and undertaking meta-analyses. In: Deeks JJ, Higgins J, Altman D, editors. Cochrane Handbook for Systematic Reviews of Interventions version 60 (updated July 2019) [Internet]. Cochrane; 2019. Available from: https://training.cochrane.org/handbook/current/chapter-10
Durlak JA. How to Select, Calculate, and Interpret Effect Sizes. J Pediatr Psychol [Internet]. 2009 [cited 2020 14];34(9):917–928. Available from: http://www.
Cohen J. Statistical Power Analysis for Behavioral Sciences. 2nd ed. Hillsdale: Erlbaum; 1988.
Wang Y, Min J, Khuri J, Xue H, Xie B, Kaminsky LA, et al. Effectiveness of mobile health interventions on diabetes and obesity treatment and management: Systematic review of systematic reviews. JMIR mHealth uHealth. 2020;8(4):1–12.
Kumar S, Shewade HD, Vasudevan K, Durairaju K, Santhi VS, Sunderamurthy B, et al. Effect of mobile reminders on screening yield during opportunistic screening for type 2 diabetes mellitus in a primary health care setting: A randomized trial. Prev Med Reports [Internet]. 2015;2:640–4. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84939810303&doi=10.1016%2Fj.pmedr.2015.08.008&partnerID=40&md5=7f9bce084928d33e416137491dca6b4b
Islam SMS, Niessen LW, Ferrari U, Ali L, Seissler J, Lechner A. Effects of mobile phone sms to improve glycemic control among patients with type 2 diabetes in Bangladesh: A prospective, parallel-group, randomized controlled trial. Diabetes Care. 2015;38(8):e112–3.
Hermes EDA, Lyon AR, Schueller SM, Glass JE. Measuring the implementation of behavioral intervention technologies: Recharacterization of established outcomes. J Med Internet Res. 2019;21(1):1–15.
Committee for Medicinal Products for Human Use (CHMP), Medicines Agency E. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus [Internet]. United Kingdom; 2012 [cited 2020 Oct 6]. Available from: www.ema.europa.eu
Zheng J, Cheng J, Wang T, Zhang Q, Xiao X. Does HbA1c Level Have Clinical Implications in Diabetic Patients Undergoing Coronary Artery Bypass Grafting? A Systematic Review and Meta-Analysis. 2017 [cited 2020 Oct 6]; Doi: 10.1155/2017/1537213
Campbell L, Pepper T, Shipman K. HbA1c: a review of non-glycaemic variables. J Clin Pathol [Internet]. 2019 [cited 2020 Oct 6];72:12–9. Available from: http://www.ngsp.org/interf.asp
Sequi-Dominguez I, Alvarez-Bueno C, Martinez-Vizcaino V, Fernandez-Rodriguez R, del Saz LA, Cavero-Redondo I. Effectiveness of mobile health interventions promoting physical activity and lifestyle interventions to reduce cardiovascular risk among individuals with metabolic syndrome: Systematic review and meta-analysis. J Med Internet Res. 2020;22(8).
American Diabetes Association. Good to know: Factors affecting blood glucose. Clin Diabetes. 2018;36(2):17.
Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: A systematic review of the effect on glycemic control. Patient Educ Couns [Internet]. 2016;99(6):926–43. https://doi.org/10.1016/j.pec.2015.11.003.
Donabedian A. The seven pillars of quality. Arch Pathol Lab Med. 1990;Nov;114(11):1115–8.
Agarwal S, LeFevre AE, Lee J, Mehl G, Sinha C, Labrique A. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ [Internet]. 2016 [cited 2020 Sep 22];352(i1174). Available from: http://dx.doi.org/10.1136/bmj.i1174http://www.bmj.com/
Greenwood DA, Gee PM, Fatkin KJ, Peeples M. A Systematic Review of Reviews Evaluating Technology-Enabled Diabetes Self-Management Education and Support. J Diabetes Sci Technol. 2017;11(5):1015–27.
Müller AM, Alley S, Schoeppe S, Vandelanotte C. The effectiveness of e-& mHealth interventions to promote physical activity and healthy diets in developing countries: A systematic review. Int J Behav Nutr Phys Act [Internet]. 2016;13(1). Available from: http://dx.doi.org/10.1186/s12966-016-0434-2
Acknowledgements
We thank members of Legumes Center for Excellence in Food and Nutrition Security (LCEFoNS) at Jomo Kenyatta University of Agriculture and Technology (Kenya) and KU Leuven (Belgium) for participating in the protocol development and review of the study. We also acknowledge the contribution of Eddah Saruni from Jomo Kenyatta University of Agriculture and Technology, who was a rater on quality assessment of the articles.
Funding
Moses Mokaya was supported by a Ph.D. scholarship from VLIR-UOS through the Legume Centre of Excellence in Food and Nutrition Security (LCEFoNS), Institutional University Cooperation (IUC) at Jomo Kenyatta University of Agriculture and Technology (grant-number: KE2017IUC037A101). VLIR-UOS (www.vliruos.be) was not involved in the conceptualization and interpretation of this manuscript.
Author information
Authors and Affiliations
Contributions
MM developed the protocol in collaboration with CM and FK. MM carried out the search, analyzed and wrote the manuscript. CM and FK were major contributors in writing the manuscript. RV interpreted the data from a clinical point of view. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mokaya, M., Kyallo, F., Vangoitsenhoven, R. et al. Clinical and patient-centered implementation outcomes of mHealth interventions for type 2 diabetes in low-and-middle income countries: a systematic review. Int J Behav Nutr Phys Act 19, 1 (2022). https://doi.org/10.1186/s12966-021-01238-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12966-021-01238-0