Abstract
Background
Multiple health behaviour change (MHBC) interventions that promote healthy lifestyles may be an efficient approach in the prevention or treatment of chronic diseases in primary care. This study aims to evaluate the cost-utility and cost-effectiveness of the health promotion EIRA intervention in terms of MHBC and cardiovascular reduction.
Methods
An economic evaluation alongside a 12-month cluster-randomised (1:1) controlled trial conducted between 2017 and 2018 in 25 primary healthcare centres from seven Spanish regions. The study took societal and healthcare provider perspectives. Patients included were between 45 and 75 years old and had any two of these three behaviours: smoking, insufficient physical activity or low adherence to Mediterranean dietary pattern. Intervention duration was 12 months and combined three action levels (individual, group and community). MHBC, defined as a change in at least two health risk behaviours, and cardiovascular risk (expressed in % points) were the outcomes used to calculate incremental cost-effectiveness ratios (ICER). Quality-adjusted life-years (QALYs) were estimated and used to calculate incremental cost-utility ratios (ICUR). Missing data was imputed and bootstrapping with 1000 replications was used to handle uncertainty in the modelling results.
Results
The study included 3062 participants. Intervention costs were €295 higher than usual care costs. Five per-cent additional patients in the intervention group did a MHBC compared to usual care patients. Differences in QALYS or cardiovascular risk between-group were close to 0 (− 0.01 and 0.04 respectively). The ICER was €5598 per extra health behaviour change in one patient and €6926 per one-point reduction in cardiovascular risk from a societal perspective. The cost-utility analysis showed that the intervention increased costs and has no effect, in terms of QALYs, compared to usual care from a societal perspective. Cost-utility planes showed high uncertainty surrounding the ICUR. Sensitivity analysis showed results in line with the main analysis.
Conclusion
The efficiency of EIRA intervention cannot be fully established and its recommendation should be conditioned by results on medium-long term effects.
Trial registration
Clinicaltrials.gov NCT03136211. Registered 02 May 2017 – Retrospectively registered
Similar content being viewed by others
Introduction
Chronic diseases are one of the main challenges for health systems. These diseases account for a high proportion of morbidity and mortality worldwide [1]. The cost of these diseases, when they are considered alone, has a relevant impact but it is even higher when comorbidities are present [2]. It is estimated that 10 million deaths attributed to chronic diseases can be prevented by adopting healthy behaviours while not adopting them has a significant economic impact [3, 4]. Against this background, the primary health care (PHC) system offers comprehensive, continuous care as the optimal context for the promotion of healthy lifestyles [5]. Health promotion does not have to be focused on specific health risk, but it can address several of them together. In this context, multiple health behaviour changes (MHBC) interventions approach several risk factors within the same intervention [6], which might increase their efficiency and effectiveness.
However, as far as we know, few economic evaluations have assessed the impact of MHBC interventions [7]. McRobbie et al showed that a diet and physical activity intervention addressing weight management in obese patients had an incremental cost-utility ratio (ICUR) of £7400 per quality-adjusted life-year (QALY) gained using a health system perspective [7], while van Keulen et al showed that a tailored print intervention aiming to improve physical activity and/or diet in adults between 45 and 70 years cost €2867 per QALY gained and €160 per improved behaviour [8]. Similar evidence is available on the assessment of single behaviour interventions. Systematic reviews focused on cardiovascular risk (CVR) or physical activity did not find a great number of economic evaluations based on randomized clinical trials [9, 10]. Indeed, some authors have pointed out that more robust, real-world evidence focused on MHBC intervention is needed, especially in light of the resource requirements to implement them [9].
The EIRA study contained an MHBC intervention focused on smoking cessation, physical activity and adherence to the Mediterranean dietary pattern in PHC patients [11]. This study aimed to evaluate the cost-utility and cost-effectiveness, in terms of MHBC and CVR reduction, of the EIRA intervention through a cluster-randomized controlled trial.
Methods
This was an economic evaluation alongside a 12-month cluster-randomized control trial conducted between 2017 and 2018 in 25 PHC centres from seven Spanish regions. The cost perspectives taken were societal and healthcare providers. The study protocol (NCT03136211.R), with details on the study design and intervention, was published elsewhere [11].
Setting
The Spanish public healthcare system provides universal coverage for citizens and foreign nationals. It is funded through taxes and free of charge at the point of use, with some exceptions such as medication. Although it is a decentralised system, where each of the 17 Spanish regions controls health planning, public health and the management of health services (including tariffs publication), PHC is the most accessible point of contact within the public system [12].
Participants
PHC centres
The following inclusion criteria were used for selection: 1) internet access; 2) possibility of developing community activities; 3) not being located in areas with high cultural or linguistic diversity or tourist areas and; 4) having a signed commitment document from the management team. Healthcare professionals and administrative staff were involved in the study and signed a collaboration commitment form.
Participants were people aged between 45 and 75 years old who engaged in at least two of these behaviours: smoking, insufficient physical activity or low adherence to Mediterranean dietary pattern. Participants had to be registered in the health system and have an assigned healthcare professional. They needed to provide signed informed consent. The exclusion criteria set were: advanced serious illnesses, dependence in daily life activities, being included in long-term home health programme and planning to move to another area in the current year.
Randomisation
Twenty-six PHC centres were computer randomized 1:1 based on each region at a central location (IDIAP Jordi Gol, Barcelona, Spain). After randomisation, one intervention centre dropped out due to administrative reasons, leaving 12 centres in the intervention group. Participants were assigned to the intervention or control group based on their PHC centre.
Interventions
EIRA was a health promotion intervention designed following Medical Research Council methodology [13] (including systematic reviews [14,15,16,17,18,19,20,21,22], citizen and professional participation through qualitative research [23,24,25] and a previous pilot study, among other techniques) and was based on the Transtheoretical Model [26]. Physicians and nurses applied the intervention in their routine practice in the PHC centre. It consisted of a first screening visit where the PHC professionals assessed the potential risk behaviours. Subsequently, the PHC professionals advised the participant, reached an agreement with them on achievable goals, developed a specific plan taking the three potential risks and stage of change into account, assisted with anticipation of barriers and arranged follow-up support (i.e., 2–3 visits). The duration of the intervention was 12 months and it combined three action levels (i.e., individual, group and community). The individual intervention consisted of a face-to-face intervention to increase awareness of the need for a behaviour change or making a plan for this change. The group intervention consisted of two health education workshops about healthy diet and physical activity while the community intervention consisted of social prescribing of resources available in the catchment area.
Participants in the control PHC centres received usual care, which integrates Spanish preventive protocols involving lifestyle recommendations and preventive activities based on screening and brief advice. These recommendations are focused on lifestyles, cardiovascular and mental diseases, cancer and vaccination [27,28,29,30,31].
Outcomes and data collection
Information was recorded at baseline and 12-month follow-up.
Use of resources, loss of productivity and other costs
Resources included healthcare utilisation in PHC (general practitioner (GP) and nurse home or centre visits, laboratory test, social worker visits, emergency visits) and hospital (admissions and length of admission, emergency visits, diagnostic tests), medication use, sick leave and intervention-related costs such as tobacco use and cost of activities (group activities were managed by a nurse but they varied in attendees and/or duration; cost assumed was one nurse visit at the centre per patient). As described in Supplementary Table, information on healthcare utilisation and sick leave were collected from electronic health records, individual clinical record review and/or a Case Report Form (CRF) depending on the region. Intervention-related cost (tobacco use and cost of activities) were obtained from CRF. The information recorded at baseline referred to the previous year while information recorded at 12-month follow-up referred to the year of the intervention.
Unit costs
Healthcare services unit costs were based on public health service tariffs published in Regional Government Official Bulletins, which were updated to 2019 using the specific regional healthcare Consumer Price Index. Subsequently, the mean tariff was calculated for each health service. A social worker visit tariff was not found, and it was assumed to be the same as that of the Nurse centre visit. Medication cost was obtained from administrative databases. The cost of productivity loss was calculated based on the human capital approach using the minimum daily wage in Spain. All costs were expressed in euros 2019. Table 1 shows unit costs used in the study. The time horizon was one year and no discount rate was applied.
Clinical outcomes
The European Quality of Life instrument (EuroQol-5D-3L; EQ5D) was used to measure health-related quality of life [32, 33]. QALYs were calculated by linearly interpolating baseline and one-year follow-up utility score based on EQ5D Spanish tariffs. CVR (expressed in %) was calculated using the REGICOR function chart based on sex, age, total cholesterol, HDL, diagnosis of diabetes, smoker status and blood pressure [34,35,36]. MHBC, which was the primary outcome of the intervention was defined as a change in at least two unhealthy behaviours. This variable was recorded by the research team in the CRF and, lately, it was dichotomized between change in at least two unhealthy behaviours and no change or change in only one unhealthy behaviour.
Other sociodemographic and clinical variables including participant age, gender, civil status, education level, diagnosis of hypertension, the presence of comorbidities and Body Mass Index (BMI) was also recorded on the CRF.
Statistical analysis
We assumed that the variables with missing values are Missing At Random (MAR). MAR assumption can be made more plausible by collecting more explanatory variables and including them in the analysis, and we have included almost all the possible explanatory variables (excluding duplicate variables, very similar variables and highly correlated variables to avoid collinearity). Imputation was used to deal with missing data. Missing values varied from 0 to 13% at baseline and from 1 to 44% at follow-up. Multiple imputation by chained equations (MICE) using 50 imputed databases was applied to all variables. This number of the database was based on the fraction of missing information. To perform MICE, we used predictive mean matching in continuous variables, logistic regression in dichotomous variables and polytomous regression in categorical variables (more than two categories). Societal perspective included all costs recorded while healthcare perspective included all costs except productivity losses and tobacco costs. Cost-utility in terms of extra cost per QALY gain and cost-effectiveness in terms of extra cost per a one-point reduction in CVR or per MHBC in one extra participant was estimated by obtaining incremental cost-utility (ICUR) and incremental cost-effectiveness ratios (ICER), respectively. ICUR and ICER were calculated as the difference in the cost between the intervention and the control group, divided by the difference in QALYs, the difference in % CVR points and the probability of modifying behaviours. The base-case analysis was an intention-to-treat analysis. Differences in cost and effects were estimated using adjusted generalised linear models with gamma (for QALYs and cost) or Gaussian (CVR) distributions and estimating marginal means using margins STATA command and adjusted logistic regression for behaviour change. These adjustments consisted of baseline cost or effects and those variables that showed statistically significant differences at baseline (i.e., BMI) as was indicated in the study protocol [37]. All analyses clustered participants in PHC centres using the “vce” STATA option. We used bootstrapping with 1000 replications (20 replications in each of the 50 imputed databases) to assess uncertainty and to construct the cost-utility planes and acceptability curves.
We performed six sensitivity analyses: 1) a complete-case analysis (only considering those participants who attended the follow-up visit); 2) an analysis considering mean wage for productivity losses; 3) an analysis considering the maximum regional tariff for each of the healthcare services and 4) the minimum; 5) an analysis adjusted only by baseline costs and effects (not adjusted by BMI) and 6) an analysis using seemingly unrelated regressions. The first four sensitivity analyses were pre-specified [11].
Imputation analyses were performed with R and all other analyses were performed with Stata MP 13.1.
Results
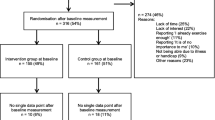
Overall, 3062 participants (1481 in the intervention group) were included in the study. Figure 1 shows the study flowchart and intervention adherence rates. Lost participants in the follow-up assessment were around 22%. Some 41% of intervention participants were adherent to all MHBC interventions (79% to at least one). Table 2 presents the sample characteristics. There were statistically significant differences between groups at baseline in the BMI, with a higher proportion of the control group in the overweight category and a higher proportion of the intervention group in the obese category.
Cost-utility and cost-effectiveness of EIRA intervention
The intervention mean cost, including individual visits, workshops and community activities, was €49.33 per person (minimum €0 and maximum €1188). Table 3 shows each mean cost by group at baseline and follow-up and the unadjusted difference between groups at each point. Table 4 shows unadjusted costs for both groups at baseline and follow-up. The intervention effectively promoted MHBC. There were no statistically significant differences between groups in costs, QALYs or cardiovascular risk in the main analyses. Tables 5 and 6 shows the results of the cost-utility and cost-effectiveness analyses. Unadjusted effects for both groups are detailed in Table 4.
Usual care dominated (decreased cost and is more effective) the intervention in the cost-utility analysis. When MHBC is considered in the cost-effectivity analysis, ICERs from the societal and healthcare perspectives were €5598 and €3932 per additional change in one patient, respectively. Considering the cardiovascular risk, ICERs from the societal and healthcare perspectives were €6926 and €4864 per one-point reduction in cardiovascular risk, respectively. Figure 2 shows cost-utility and cost-effectiveness planes. Cost-utility planes showed high uncertainty surrounding the ICUR. Most bootstrapped incremental cost-utility pairs fell in the north-west (intervention increased costs and is less effective than usual care) or south-west quadrants (intervention decreased costs and is less effective than usual care) of the cost-utility plane. In terms of cardiovascular risk reduction and MHBC, the intervention fell in the north-east (intervention increased costs and is more effective than usual care) and south-east (intervention decreased costs and is more effective than usual care) quadrants of the cost-utility place, although, in the healthcare perspective, most pairs are distributed over the cost axis (€0). The intervention was cost-saving in 32 and 49% of the pairs from the societal and healthcare perspectives while it was more effective in 9% of the pairs in terms of QALYS; 81% of the pairs in terms of CVR and all pairs in terms of MHBC. Acceptability curves are shown in Supplementary Files.
Sensitivity analysis
Table 5 shows the results of sensitivity analysis. All sensitivity analyses showed similar results to those of the main analyses. The scenarios where the differences in cost were smaller were the complete case and that using seemingly unrelated regressions. The scenario with the largest differences in cost was that considering the mean wage as unit cost for sick leave. In terms of cost-effectiveness, the best scenario for both outcomes was the complete case (both ICER were around €2200 per extra MHBC in one participant or REGICOR reduction), while the worse scenario was that considering the mean wage as unit cost for sick leave.
Table 4 also shows the unadjusted mean cost and effects of the sensitivity analysis.
Discussion
Summary
The cost-effectiveness of the EIRA intervention measured in terms of MHBC remains unclear. However, although the intervention was shown to be no more costly than usual care and it promoted MHBC, the probabilistic analysis showed high uncertainty surrounding cost differences and intervention did not affect quality of life or cardiovascular risk reduction. Based on the results of this economic evaluation, the EIRA intervention should be reformulated before being translated to clinical practice or it should be assessed with a longer follow-up period.
Strengths and limitations
The large, representative sample is the main strength of this study. This was a multisite study evaluating an MHBC intervention implemented in a complex context such as PHC. Participants from 25 PHC centres in 7 Spanish regions were included and this represents one of the first economic evaluations that has been developed under these circumstances. The pragmatism of this trial is one of the key points of its design as it was developed with the aim of an immediate implementation in the PHC system throughout the modification and elimination of potential barriers. We intended to develop a flexible intervention that could be adapted to different PHC settings, and the intervention’s design was based on the results of previous phases of the study. These features led to adherence being measured in a permissive way and the content of the intervention itself in each of the centres was not fully documented. This fact together with a low fidelity of the intervention could have limited our capacity to detect differences between groups [38]. Similarly, not all cost information was gathered using the same protocols (each region has combined electronic health record download, individual clinical record review and/or CRF based on availability) and this may introduce some bias. However, it increased the external validity of the results of the study. The follow-up period may have limited the impact of the intervention in outcomes such as QALYs and CVR reduction considering that intervention is focused on health promotion.
Comparison with existing literature
There were no differences in costs between groups. As suggested previously, health promotion interventions have a very low cost or, at least, a similar cost compared to usual care [7, 8, 39,40,41]. Similarly, the intervention had no effect on QALYs at one-year follow-up. Although QALYs are the standard effect measure in cost-utility analysis, health promotion interventions are not expected to produce improvements in QALYs in the short term. Similar results have been observed in economic evaluations of health promotion interventions in PHC [7, 8, 39,40,41,42]. However, some of these studies showed positive ICURs with a clear dominance of the intervention [7, 40]. Although the effect of these interventions was small (0.01 for QALYS and 80£ for costs [7] or 0.02 for QALYS and €-16 for cost [40]), the uncertainty regarding the cost-effectiveness of the intervention was also small. The ICUR dominance in some of these preventive interventions could be related to the targeted population (obese) [7] or the risk approached (major depression) [40] while the EIRA intervention is focused on health behaviours in a general population sample where differences in QALYs after 1 year is hardly seen.
MHBC was more sensitive to the intervention in the short term. The EIRA intervention presented statistical differences in this outcome and showed a societal ICER of €5600 per one extra patient changing two or three unhealthy behaviours. This ICER was reduced to €3900 when the healthcare perspective was considered. Previous studies showed lower ICERs, up to £986 (€1098 at November 2020), per one extra patient behaviour change [8, 10]. However, most of these interventions only focus on one behaviour. Although previous authors have suggested proposals of transforming unit changes of different outcomes into comparable metrics, currently, there is no national or international threshold in terms of behaviour change to consider an intervention efficient. In any case, the EIRA intervention would seem to exceed this virtual threshold; especially when the national threshold to consider an intervention efficient in terms of QALYs is €20,000 per QALYs [43] and given the difficulty in translating healthy behaviours into gains in QALYs [44]. A suggested alternative to thresholds is the Relative Value Index, being the formula for this index: RVI = (usual care mean costs/usual care mean effects)/ICER)) [45]. A Relative Value Index greater than 1 means that a new intervention would provide better outcomes at a lower incremental cost per outcome than the comparator. However, values below 1 and close to 0, as EIRA intervention shows (taking into account mean cost and effects, in terms of MHBC, in the usual care group) would mean that the additional outcome associated with the new intervention would cost more than the previously “accepted value” of a cost per outcome.
Partially similar results were observed when CVR was considered as the outcome. The EIRA intervention showed a societal ICER of €6900 per one-point reduction in CVR and a healthcare ICER of 4900. In this situation, and considering that usual care already involves preventive protocols, it is very difficult to observe substantial changes in these outcomes, and consequently, CVR in the short-medium term. Furthermore, changes in the medium-long term can be preceded by promotion interventions on healthy lifestyles which have an impact on CVR [46].
The largest effect in CVR and MHBC was observed in the complete-case analysis; this might suggest that higher levels of intervention fidelity would have produced better results. However, this effect is still clearly lower than previous preventive PHC interventions aiming to reduce CVR [47], other related outcomes such as weight [7], or behaviour change [8, 10].
A technical but crucial issue is the model and assumption choice. In this paper, we considered generalized linear models as the main choice due to previous recommendations related to checking non-normality distribution in cost and effects after a visual inspection [37]. However, it is important to emphasise that seemingly unrelated regression, a choice that led to correlate the error terms across regression models in cost and effects but assuming normality, was the second most favourable scenario to the intervention in terms of cost-utility.
Implication for research and practice
In a globalized world, it is increasingly important to develop complex healthcare actions that can be adapted to each patient and implemented in a large number of settings. At this point, developing hybrid trials is a very good option. This is particularly important in the context of PHC where systemic interventions predominate and professionals approach more than one health or risk behaviour. However, researchers should not overlook the importance of monitoring the specific actions in each setting. This would ensure that clear information is available to determine whether these actions are genuinely (cost)effective or not.
Developing cost-effective interventions that promote healthy lifestyles are crucial to support the healthcare system. A healthy population should be an optimal target for these interventions although, due to patient health status, intervention assessment needs to be extended into the medium-long term. Conducting economic models which assess the long-term cost-effectiveness using mortality and QALYS as outcomes would give relevant information for health decision-makers. Similarly, standardizing the use of other generalizable outcomes that could be sensitive to health promotion interventions would help decision making in this field. A healthy population is usually located in very heterogeneous and complex contexts, a fact that complicated the registry and cost definition, key factors for economic evaluations. In this line, efforts should be taken in standardization without leaving the complexity of the context out of consideration [48].
Conclusions
A health promotion PHC intervention such as EIRA effectively impact MHBC in the Spanish context but its efficiency cannot be fully established under current circumstances. The recommendation of this intervention should be conditioned by positive results in the medium-long term clinical assessment or by modification of the intervention.
Availability of data and materials
The data that support the findings of this study are available from IDIAP Jordi Gol but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of IDIAP Jordi Gol.
Abbreviations
- MHBC:
-
Multiple health behaviour changes
- QALY:
-
Quality-adjusted life years
- ICUR:
-
Cost-utility ratio
- ICER:
-
Incremental cost-effectiveness ratio
- PHC:
-
Primary health care
- CVR:
-
Cardiovascular risk
- GP:
-
General practitioner
- CRF:
-
Case report form
- EQ5D:
-
EuroQol-5D-3L
- BMI:
-
Body mass index
References
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1789–858. https://doi.org/10.1016/S0140-6736(18)32279-7.
Cortaredona S, Ventelou B. The extra cost of comorbidity: multiple illnesses and the economic burden of non-communicable diseases. BMC Med. 2017;15:1–11.
Scarborough P, Bhatnagar P, Wickramasinghe KK, Allender S, Foster C, Rayner M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: an update to 2006-07 NHS costs. J Public Health (Bangkok). 2011;33(4):527–35. https://doi.org/10.1093/pubmed/fdr033.
Violán C, Foguet-Boreu Q, Roso-Llorach A, Rodríguez-Blanco T, Pons-Vigués M, Pujol-Ribera E, et al. Burden of multimorbidity, socioeconomic status and use of health services across stages of life in urban areas: a cross-sectional study. BMC Public Health. 2014;14(1):530. https://doi.org/10.1186/1471-2458-14-530.
World Health Organization. Noncommunicable diseases: country profiles 2018. Geneva: World Health Organization; 2018. https://apps.who.int/iris/bitstream/handle/10665/274512/9789241514620-eng.pdf?sequence=1&isAllowed=y
Prochaska JJ, Spring B, Nigg CR. Multiple health behavior change research: an introduction and overview. Prev Med (Baltim). 2008;46(3):181–8. https://doi.org/10.1016/j.ypmed.2008.02.001.
McRobbie H, Hajek P, Peerbux S, Kahan BC, Eldridge S, Trépel D, et al. Randomised controlled trial and economic evaluation of a task-based weight management group programme. BMC Public Health. 2019;19:1–10.
van Keulen HM, Bosmans JE, van Tulder MW, Severens JL, de Vries H, Brug J, et al. Cost-effectiveness of tailored print communication, telephone motivational interviewing, and a combination of the two: results of an economic evaluation alongside the Vitalum randomized controlled trial. Int J Behav Nutr Phys Act. 2010;7:1–12.
Lee JT, Lawson KD, Wan Y, Majeed A, Morris S, Soljak M, et al. Are cardiovascular disease risk assessment and management programmes cost effective? A systematic review of the evidence. Prev Med (Baltim). 2017;99:49–57. https://doi.org/10.1016/j.ypmed.2017.01.005.
GC V, Wilson ECF, Suhrcke M, Hardeman W, Sutton S. Are brief interventions to increase physical activity cost-effective? A systematic review. Br J Sports Med. 2016;50:408–17.
Zabaleta-Del-Olmo E, Pombo H, Pons-Vigues M, Casajuana-Closas M, Pujol-Ribera E, Lopez-Jimenez T, et al. Complex multiple risk intervention to promote healthy behaviours in people between 45 to 75 years attended in primary health care (EIRA study): study protocol for a hybrid trial. BMC Public Health. 2018;18(1):874. https://doi.org/10.1186/s12889-018-5805-y.
Bernal E, Sandra D, Juan G-A, Fernando O, Sánchez Martínez I, Ramón J, et al. Spain: health system review. Health Systems in Transition. 2018;20:1–179 www.healthobservatory.eu.
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655.
Bully P, Sánchez Á, Zabaleta-del-Olmo E, Pombo H, Grandes G. Evidence from interventions based on theoretical models for lifestyle modification (physical activity, diet, alcohol and tobacco use) in primary care settings: a systematic review. Prev Med (Baltim). 2015;76(S):S76–93. https://doi.org/10.1016/j.ypmed.2014.12.020.
March S, Torres E, Ramos M, Ripoll J, García A, Bulilete O, et al. Adult community health-promoting interventions in primary health care: a systematic review. Prev Med (Baltim). 2015;76(S):S94–104. https://doi.org/10.1016/j.ypmed.2015.01.016.
Sanchez A, Bully P, Martinez C, Grandes G. Effectiveness of physical activity promotion interventions in primary care: a review of reviews. Prev Med (Baltim). 2015;76(S):S56–67. https://doi.org/10.1016/j.ypmed.2014.09.012.
Maderuelo-Fernandez JA, Recio-Rodríguez JI, Patino-Alonso MC, Pérez-Arechaederra D, Rodriguez-Sanchez E, Gomez-Marcos MA, et al. Effectiveness of interventions applicable to primary health care settings to promote Mediterranean diet or healthy eating adherence in adults: a systematic review. Prev Med (Baltim). 2015;76(S):S39–55. https://doi.org/10.1016/j.ypmed.2014.12.011.
Cantera CM, Puigdomènech E, Ballvé JL, Arias OL, Clemente L, Casas R, et al. Effectiveness of multicomponent interventions in primary healthcare settings to promote continuous smoking cessation in adults: a systematic review. BMJ Open. BMJ Open. 2015;5(10):e008807.
Zabaleta-del-Olmo E, Bolibar B, Garcia-Ortiz L, Garcia-Campayo J, Llobera J, Bellon JA, et al. Building interventions in primary health care for long-term effectiveness in health promotion and disease prevention. A focus on complex and multi-risk interventions. Prev Med. 2015;76(Suppl):S1–4.
Moreno-Peral P, Conejo-Ceron S, Fernandez A, Berenguera A, Martinez-Andres M, Pons-Vigues M, et al. Primary care patients’ perspectives of barriers and enablers of primary prevention and health promotion-a meta-ethnographic synthesis. PLoS One. 2015;10(5):e0125004. https://doi.org/10.1371/journal.pone.0125004.
Rubio-Valera M, Pons-Vigues M, Martinez-Andres M, Moreno-Peral P, Berenguera A, Fernandez A. Barriers and facilitators for the implementation of primary prevention and health promotion activities in primary care: a synthesis through meta-ethnography. PLoS One. 2014;9(2):e89554. https://doi.org/10.1371/journal.pone.0089554.
Gómez-Gómez I, Bellón J, Resurrección DM, Cuijpers P, Moreno-Peral P, Rigabert A, et al. Effectiveness of universal multiple-risk lifestyle interventions in reducing depressive symptoms: systematic review and meta-analysis. Prev Med (Baltim). 2020;134(March):106067. https://doi.org/10.1016/j.ypmed.2020.106067.
Gil-Girbau M, Pons-Vigués M, Rubio-Valera M, Murrugarra G, Masluk B, Rodríguez-Martín B, et al. Modelos Teóricos De Promoción De La Salud En La Práctica Habitual En Atención Primaria De Salud. Gac Sanit. 2021;35(1):48–59. https://doi.org/10.1016/j.gaceta.2019.06.011.
Pons-Vigués M, Berenguera A, Coma-Auli N, Pombo-Ramos H, March S, Asensio-Martínez A, et al. Health-care users, key community informants and primary health care workers’ views on health, health promotion, health assets and deficits: qualitative study in seven Spanish regions. Int J Equity Health. 2017;16:1–16.
Berenguera A, Pons-Vigues M, Moreno-Peral P, March S, Ripoll J, Rubio-Valera M, et al. Beyond the consultation room: proposals to approach health promotion in primary care according to health-care users, key community informants and primary care Centre workers. Heal Expect an Int J public Particip Heal Care Heal Policy. 2017;20:896–910.
Prochaska JO, Velicer WF. The Transtheoretical model of health behavior change. Am J Health Promot. 1997;12(1):38–48. https://doi.org/10.4278/0890-1171-12.1.38.
Brotons-Cuixart C, Alemán-Sánchez JJ, Banegas-Banegas JR, Fondón-León C, Lobos-Bejarano JM, Martín-Rioboó E, et al. Recomendaciones preventivas cardiovasculares. Actualización PAPPS 2018. Aten Primaria. 2018;50(Supl 1):4–28. https://doi.org/10.1016/S0212-6567(18)30360-3.
Buitrago Ramírez F, Ciurana Misol R, Chocrón Bentata L, Del CFAM, García Campayo J, Montón Franco C, et al. Prevención de los trastornos de la salud mental en atención primaria. Actualización PAPPS 2018. Aten Primaria. 2018;50(Supl 1):83–108. https://doi.org/10.1016/S0212-6567(18)30364-0.
Córdoba-García R, Camarelles-Guillem F, Muñoz-Seco E, Gómez-Puente JM, San-José-Arango J, Ramírez-Manent JI, et al. Recomendaciones sobre el estilo de vida. Actualizacón PAPPS 2018. Aten Primaria. 2018;50(Supl 1):29–40.
Marzo-Castillejo M, Vela-Vallespín C, Bellas-Beceiro B, Bartolomé-Moreno C, Melús-Palazón E, Vilarrubí-Estrella M, et al. Recomendaciones de prevención del cáncer. Actualización PAPPS 2018. Aten Primaria. 2018;50(Supl 1):41–65. https://doi.org/10.1016/S0212-6567(18)30362-7.
Aldaz-Herce P, Morató-Agustí ML, Gómez-Marco JJ, Javierre-Miranda AP, Martín-Martín S, Moreno-Millán N, et al. Prevención de las enfermedades infecciosas. Actualización PAPPS en vacunas 2018. Aten Primaria. 2018;50(Supl 1):66–82. https://doi.org/10.1016/S0212-6567(18)30363-9.
Badia X, Schiaffino A, Alonso J, Herdman M. Using the EuroQoI 5-D in the Catalan general population: feasibility and construct validity. Qual Life Res. 1998;7(4):311–22. https://doi.org/10.1023/A:1008894502042.
The EuroQol Group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
Marrugat J, Subirana I, Comín E, Cabezas C, Vila J, Elosua R, et al. Validity of an adaptation of the Framingham cardiovascular risk function: the VERIFICA study. J Epidemiol Community Health. 2007;61(1):40–7. https://doi.org/10.1136/jech.2005.038505.
Ramos R, Solanas P, Cordón F, Rohlfs I, Elosua R, Sala J, et al. Comparación de la función de Framingham original y la calibrada del REGICOR en la predicción del riesgo coronario poblacional. Med Clin (Barc). 2003;121:521–6.
Marrugat J, Solanas P, D’Agostino R, Sullivan L, Ordovas J, Cordón F, et al. Estimación del riesgo coronario en España mediante la ecuación de Framingham calibrada. Rev Esp Cardiol. 2003;56(3):253–61. https://doi.org/10.1016/S0300-8932(03)76861-4.
Franklin M, Lomas J, Walker S, Young T. An educational review about using cost data for the purpose of cost-effectiveness analysis. Pharmacoeconomics. 2019;37(5):631–43. https://doi.org/10.1007/s40273-019-00771-y.
Zabaleta-Del-Olmo E, Casajuana-Closas M, López-Jiménez T, Pombo H, Pons-Vigués M, Pujol-Ribera E, et al. Multiple health behaviour change primary care intervention for smoking cessation, physical activity and healthy diet in adults 45 to 75 years old (EIRA study): a hybrid effectivenessimplementation cluster randomised trial. 2020. PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-52192/v1].
Broekhuizen K, Simmons D, Devlieger R, van Assche A, Jans G, Galjaard S, et al. Cost-effectiveness of healthy eating and/or physical activity promotion in pregnant women at increased risk of gestational diabetes mellitus: economic evaluation alongside the DALI study, a European multicenter randomized controlled trial. Int J Behav Nutr Phys Act. 2018;15:1–12.
Fernández A, Mendive JM, Conejo-Cerón S, Moreno-Peral P, King M, Nazareth I, et al. A personalized intervention to prevent depression in primary care: cost-effectiveness study nested into a clustered randomized trial. BMC Med. 2018;16(1):28. https://doi.org/10.1186/s12916-018-1005-y.
Osborn D, Burton A, Hunter R, Marston L, Atkins L, Barnes T, et al. Clinical and cost-effectiveness of an intervention for reducing cholesterol and cardiovascular risk for people with severe mental illness in English primary care: a cluster randomised controlled trial. Lancet Psychiatry. 2018;5(2):145–54. https://doi.org/10.1016/S2215-0366(18)30007-5.
Tsiachristas A, Burgers L, Rutten-Van Mölken MPMH. Cost-effectiveness of disease management programs for cardiovascular risk and COPD in the Netherlands. Value Heal. 2015;18(8):977–86. https://doi.org/10.1016/j.jval.2015.07.007.
Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Heal Econ (United Kingdom). 2018;27:746–61.
Tate DF, Finkelstein EA, Khavjou O, Gustafson A. Cost effectiveness of internet interventions: review and recommendations. Ann Behav Med. 2009;38(1):40–5. https://doi.org/10.1007/s12160-009-9131-6.
Hyewon HL, Levine M. Determing the threshold of acceptability of an ICER when natural health units are used. J Popul Ther Clin Pharmacol. 2012;19(2):e234–8.
World Health Organization. Prevention of cardiovascular disease: Guidelines for assessment and management of cardiovascular risk. Geneva: World Health Organization; 2007. https://apps.who.int/iris/bitstream/handle/10665/43685/9789241547178_eng.pdf?sequence=1&isAllowed=y
Näslund U, Ng N, Lundgren A, Fhärm E, Grönlund C, Johansson H, et al. Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA): a pragmatic, open-label, randomised controlled trial. Lancet. 2019;393(10167):133–42. https://doi.org/10.1016/S0140-6736(18)32818-6.
Byford S, Sefton T. Economic evaluation of complex health and social care interventions. Natl Inst Econ Rev. 2003;186:98–108.
Acknowledgements
We are grateful to Stephen Kelly for his contribution to English language editing.
Funding
This study was supported by a grant from the “Instituto de Salud Carlos III, Ministerio de Economía y Competitividad” (Institute of Health Carlos III, Ministry of Economy and competitiveness), Spain (FIS PI15–00114 & FIS PI15–00519); a grant from the Department of Health of the government of Catalonia, Spain (SLT002/16/00112); and a grant awarded by the “Pla Estratègic de Recerca i Innovació en Salut” (PERIS) (Strategic Plan for Health Research and Innovation) at the Ministry of Health (Government of Catalonia) (SLT002/16/00190). We thank the CIBERESP (CIBER in Epidemiology and Public Health, CB16/02/00322) the redIAPP “Red de Investigación en Actividades Preventivas y Promoción de la Salud” Research Network Prevention and Health Promotion in Primary Care (RD12/0005/0006 & RD12/0005/0008) and the European Union ERDF funds for support in the development of this study. MRV has a “Miguel Servet” research contract from the Instituto de Salud Carlos III (ISCIII) at the Ministry of Economy and Competitiveness (Spain) (CP19/00029). ASB has a personal grant funded by the PERIS programme (SLT006/17/68), Generalitat de Catalunya (Spain), to partially dedicate his time to research during the time the study was conducted.
Author information
Authors and Affiliations
Contributions
ASB, BB and EZDO obtained the finances for this study. BB, EZDO, MCC, HPR, MVMM, RMB, JAMF, EM, RML, and JB developed and executed the study. IAL, MRV and ASB advised on the conceptions of data collection, analyses and execution. TL contributed statistical expertise. IAL and MRV performed the analyses. IAL significantly contributed to the writing of the manuscript, while EPR, ASV, EZDO, MCC, MRV and ASB were involved in revising it. All authors have seen and approved of the version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee at the Jordi Gol i Gurina Foundation (P18/70). Patients needed to provide signed informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
Authors declare no conflict of interest. The funding bodies did not play any role in the design of the study and collection, analysis, or interpretation of data and writing.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure.
Acceptability curves for cost-utility analysis.
Additional file 2: Supplementary Table.
Source of information of cost and effects.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Aznar-Lou, I., Zabaleta-Del-Olmo, E., Casajuana-Closas, M. et al. Cost-effectiveness analysis of a multiple health behaviour change intervention in people aged between 45 and 75 years: a cluster randomized controlled trial in primary care (EIRA study). Int J Behav Nutr Phys Act 18, 88 (2021). https://doi.org/10.1186/s12966-021-01144-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12966-021-01144-5