Abstract
The changes in T regulatory cell (Treg) and T helper cell (Th) 17 ratios holds paramount importance in ensuring internal homeostasis and disease progression. Recently, novel subsets of Treg and Th17, namely IL-17-producing Treg and IL-10-producing Th17 have been identified. IL-17-producing Treg and IL-10-producing Th17 are widely considered as the intermediates during Treg/Th17 transformation. These “bi-functional” cells exhibit plasticity and have been demonstrated with important roles in multiple physiological functions and disease processes. Yin and Yang represent opposing aspects of phenomena according to the ancient Chinese philosophy “Yin-Yang” theory. Furthermore, Yin can transform into Yang, and vice versa, under specific conditions. This theory has been widely used to describe the contrasting functions of immune cells and molecules. Therefore, immune-activating populations (Th17, M1 macrophage, etc.) and immune overreaction (inflammation, autoimmunity) can be considered Yang, while immunosuppressive populations (Treg, M2 macrophage, etc.) and immunosuppression (tumor, immunodeficiency) can be considered Yin. However, another important connotation of “Yin-Yang” theory, the conversion between Yin and Yang, has been rarely documented in immune studies. The discovery of IL-17-producing Treg and IL-10-producing Th17 enriches the meaning of “Yin-Yang” theory and further promotes the relationship between ancient “Yin-Yang” theory and modern immunology. Besides, illustrating the functions of IL-17-producing Treg and IL-10-producing Th17 and mechanisms governing their differentiation provides valuable insights into the mechanisms underlying the dynamically changing statement of immune statement in health and diseases.
Similar content being viewed by others
Introduction
As a crucial component of the adaptive immune system, T lymphocytes play pivotal roles in various physiological processes, including pathogen defense and the maintenance of internal homeostasis. Among these processes, the dynamic equilibrium between T regulatory cell (Treg) and T helper cell (Th) 17 holds paramount importance in ensuring internal homeostasis [1]. Both Treg and Th17 subsets are CD4+ T cells, with distinct characteristics and functions. Tregs express CD25, forkhead box P3 (Foxp3), and interleukin (IL)-10 and differentiate from CD4+ T cells upon transforming growth factor (TGF)-β1 stimulation [2]. Tregs produce immunosuppressive factors such as IL-10 and TGF-β1, which are involved in dampening inflammatory responses, promoting tissue repair, and maintaining immune tolerance [3]. Conversely, Th17s are characterized by the expression of transcription factor retinoic acid-related orphan nuclear receptor γt (RORγT) and the production of interleukin IL-17. Their differentiation from native CD4+ T cells is induced by TGF-β1 and IL-6 stimulation [4]. Th17 cells secrete several cytokines, including IL-17, IL-21, and IL-23, which contribute to pro-inflammatory responses [5].
Imbalances in the Treg/Th17 ratio are implicated in the progression of various inflammatory responses [6], autoimmune diseases [6], and tumors [7]. In inflammatory and autoimmune diseases, an increase in Th17 differentiation and inadequate Treg differentiation leads to excessive release of pro-inflammatory factors, resulting in overactive immune responses [3]. In the context of tumor development, a significant portion of CD4+ T cells differentiates into Tregs, enabling tumor cell immune evasion [8]. Concurrently, Th17-produced inflammatory factors in tumor tissues can influence the tumor microenvironment and impact tumor progression through various mechanisms [9].
Notably, recent studies have identified novel subsets of Tregs and Th17 cells, namely IL-17-producing Treg [10] and IL-10-producing Th17 [11]. These “bi-functional” cells exhibit plasticity and play significant roles in multiple physiological functions and disease processes.
IL-17-producing Treg
IL-17-producing Tregs were initially reported by Kui Shin Voo et al. from the University of Texas in 2009 [10]. These cells were first identified in human peripheral blood and lymphoid tissue, accounting for 0.32% and 2.4% of CD4+ T cells in human peripheral blood and tonsils, respectively. Functional assessments of these cells revealed that both IL-17-producing Tregs and conventional Tregs (Foxp3+ IL-17−) were capable of significantly inhibiting T cell activation induced by anti-CD3 and anti-CD28. In contrast, conventional Th17 (Foxp3−IL-17+ T cells) or Foxp3−IL-17− T cells did not exhibit this inhibitory function, highlighting that IL-17-producing Tregs share immunosuppressive properties with Tregs [10, 12].
The characteristics and physiological function of IL-17-producing Treg
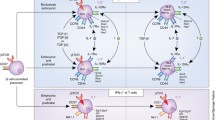
IL-17-producing Tregs have been identified not only in peripheral blood and lymphoid tissue but also in various organs, including the intestine [13], spleen [14], lymph nodes [14], lung [15], liver [15], kidney [16], and other tissues. In contrast to conventional Tregs, IL-17-producing Tregs exhibit distinct phenotypic characteristics, characterized by high levels of RORγT, inducible co-stimulator (ICOS), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), and CD103 expression. Notably, they show lower expression levels of Foxp3 and B-lymphocyte-inducing maturation protein 1 (Blimp-1), along with moderate levels of C-C chemokine receptor 6 (CCR6), CD25, and glucocorticoid induced TNF receptor (GITR) [14]. Additionally, these cells exhibit reduced expression of Th1-related markers, including interferon gamma (IFN-γ) and C-X-C motif chemokine receptor 3 (CXCR3) [17]. Consequently, IL-17-producing Tregs are often described as RORγT+ Tregs (Fig. 1). Currently, immunofluorescence, flow cytometry, and single-cell sequencing are commonly used to investigate IL-17-producing Treg in multiple tissues. The detailed information for investigating IL-17-producing Treg is shown in Table 1.
Regulation of IL-17-producing Treg differentiation. Tregs could convert into IL-17-producing Tregs, releasing both IL-10 and IL-17 A. Subsequently, IL-17-producing Treg could further convert into Th17 cells. IL-1β, IL-6 and IL-23 could induce IL-17-producing Treg differentiation through activating STAT3, whereas IL-7, IL-15, IL-35 and IL-2 may inhibit IL-17-producing Treg differentiation due to their active roles on STAT5. Activation of Wnt signaling pathway contributed to IL-17-producing Treg differentiation through upregulating Rorc expression and HIF-1α enhanced the IL-10-producing capacity in IL-17-producing Tregs. Akkermansia muciniphila induced IL-17-producing Treg differentiation through the activation of TLR4, whereas tryptophan metabolites-producing bacteria inhibit IL-17-producing Treg differentiation through AhR. In infants, a newly discovered APC (Thetis cell IV) in gut induced IL-17-producing Treg differentiation and ILC3 induced IL-17-producing Treg differentiation in adults
Functionally, IL-17-producing Tregs secrete a combination of cytokines, including IL-17, IL-10, and IL-35 [14]. Under physiological conditions, IL-17-producing Tregs play a crucial role in safeguarding organs from external stimuli and inflammatory injury, as evidenced by their anti-bacterial infection and inflammation repression functions (Fig. 1) [10]. Commensal bacteria, such as Helicobacter, have been shown to induce the differentiation of IL-17-producing Tregs, promoting immune tolerance and preventing excessive inflammation. Notably, the absence of IL-17-producing Tregs renders individuals more susceptible to microbially induced mucosal inflammation [30]. Comparison of the gene expression profiles of IL-17-producing Treg, Treg, and Th17 cells through transcriptomic analysis has revealed that IL-17-producing Tregs exhibit a gene expression pattern more akin to that of Treg cells. Furthermore, demethylation occurs in the CpG regions of Treg-related genes, such as Foxp3, Ctla-4, and Helios, within the IL-17-producing Treg genome. Importantly, IL-17-producing Tregs exert immune inhibitory effects when co-cultured with effector T cells [17].
A recent report in Immunity delves into the role of gut-derived IL-17-producing Tregs in regulating muscle regeneration [15]. Employing single-cell sequencing, researchers discovered that IL-17-producing Tregs are rarely present in normal skeletal muscle tissue, but their proportion significantly increases in muscle tissue following cardiotoxin (CTX)-induced muscle injury. This increase in IL-17-producing Tregs peaks three days after CTX injection, gradually declining and disappearing by day seven. Transplanting colon-derived CD4+ T cells into Tcrb−/− mice revealed that CTX injection led to a notable increase in IL-17-producing Tregs in skeletal muscle, while transplantation of spleen-derived CD4+ T cells did not yield this effect, signifying the colon as the origin of IL-17-producing Tregs in injured skeletal muscle tissue. Additionally, IL-17-producing Tregs exhibit the ability to migrate to injured muscle tissue under the influence of C-C chemokine ligand 2 (CCL2) and major histocompatibility complex class II (MHC-II). Neutralizing antibodies targeting CCL2 and MHC-II effectively block the migration of intestinal IL-17-producing Tregs to skeletal muscle. During the early stages of muscle injury, IL-17 and other pro-inflammatory cytokines stimulate muscle stem cell differentiation, promoting muscle regeneration [15]. However, in later stages, these pro-inflammatory cytokines induce muscle fibrosis, hindering muscle regeneration [31, 32]. Therefore, the increased presence of IL-17-producing Tregs in the early stage of injury (Day 3) contributes to IL-17 production and facilitates muscle regeneration. Subsequently, their numbers gradually diminish and disappear by Day 7, serving to control IL-17 levels and promote muscle repair. The removal of IL-17-producing Tregs in mice significantly impairs muscle repair ability [15].
Regulatory factors involved in IL-17-producing Treg differentiation
Presently, the consensus among researchers is that IL-17-producing Tregs represent an intermediate stage in the differentiation of Tregs into Th17 cells [33]. Notably, peripheral blood IL-17-producing Tregs can differentiate from thymic Tregs, and substantial differences in gene expression patterns exist between IL-17-producing Tregs in the gut and those in peripheral blood, implying that IL-17-producing Tregs can originate from multiple organs [19, 20]. It is essential to note, however, that one study contradicts this perspective by suggesting that IL-17-producing Tregs are not intermediates in the conversion of Tregs into Th17 cells [14]. These varying findings may stem from differences in the sources of IL-17-producing Tregs. Multiple regulatory factors have been identified as influencing the differentiation of IL-17-producing Tregs, including STAT signaling pathways, cytokines, TGF-β, autophagy, Wnt/β-catenin, hypoxia-inducible factor-1 alpha (HIF-1α), gut microbiota, costimulatory molecules, microRNA (miRNA), and others. These factors collectively contribute to the complex and finely tuned process of IL-17-producing Treg differentiation (Fig. 1).
Opposite functions of STAT3 and STAT5 in regulating IL-17-producing Treg differentiation
Key transcription factors, Foxp3 and RORγT, dictate the differentiation of CD4+ T cells into Tregs or Th17s, respectively [34]. Foxp3 plays a pivotal role in the maturation, development, and execution of immunosuppressive functions by Tregs [35, 36]. It upregulates the expression of immunosuppressive factors such as IL-10 and TGF-β1, inhibits the transcriptional activity of RORγT, and represses the expression of the pro-inflammatory factor gene IL-17 [37,38,39,40]. Conversely, RORγT acts as a critical transcription factor in Th17 differentiation, driving the upregulation of IL-17 expression while diminishing Foxp3 transcription and suppressing IL-10 and TGF-β1 expression [41]. Changes in the expression levels of Foxp3 and RORγT serve as crucial indicators of cell differentiation towards IL-10 and IL-17 secretion.
Within the realm of regulating Foxp3 and RORγT, the STAT3 and STAT5 transcription factors play pivotal roles during IL-17-producing Treg differentiation. STAT3 and STAT5 are composed of an N-terminal interaction domain, a coiled helix domain, a DNA-binding domain, a ligand domain, an Src homology 2 (SH2) domain, and a C-terminus transcriptional activation domain [42]. Upon tyrosine kinase induction, STAT3 and STAT5 undergo phosphorylation, leading to the formation of phosphorylated STAT3 or STAT5 homodimers through the N-terminal interaction domain, subsequently translocating to the nucleus. STAT3 binds to the promoter region of the Rorc gene, promoting Rorc transcription and inducing IL-17 production [43]. Conversely, STAT5 binds to the conserved non-coding sequence 2 (CNS2) region of the Foxp3 gene, enhancing Foxp3 expression and facilitating IL-10 production [44]. Additionally, STAT3 can promote RORγT activation by facilitating the dissociation of RORγT from Foxp3 and relieving the inhibitory effect of Foxp3 on RORγT [45]. Maintaining a balance between STAT3 and STAT5 signaling is a crucial factor in Treg/IL-17-producing Treg differentiation (Fig. 1). Elevated STAT3 phosphorylation levels in IL-17-producing Tregs enable these cells to express RORγT and possess the ability to produce IL-17. Promoting STAT3 phosphorylation while inhibiting STAT5 phosphorylation can induce the differentiation of Tregs into IL-17-producing Tregs [20]. Mice with conditional STAT3 knockout (Cd4creStat3fl/fl) in CD4+ cells fail to produce IL-17-producing Tregs [20].
Promotive roles of IL-2, IL-1β, IL-6, IL-21, IL-23 and suppressive roles of IL-7, IL-15, IL-35 in IL-17-producing Treg differentiation
The differentiation of IL-17-producing Tregs is subject to regulation by an array of cytokines. IL-2, IL-1β, IL-6, IL-21, and IL-23 have been identified as promoters of IL-17-producing Treg differentiation [46,47,48,49,50,51]. Among these cytokines, both IL-1β and IL-6 activate STAT3, leading to the induction of RORγT expression and the stimulation of T cell production of IL-17 A [12, 46, 52]. Additionally, IL-23 stabilizes IL-17 production through STAT4 [53]. IL-2 can induce CCR6+CD4+CD25high T cells to differentiate into IL-17-producing Tregs, while the combination of IL-2 with IL-1β, IL-6, IL-21, or IL-23 can induce CCR6−CD4+CD25high T cells to differentiate into IL-17-producing Tregs [10]. However, it is noteworthy that there is conflicting evidence regarding the impact of IL-2 on IL-17-producing Treg differentiation, possibly attributed to its role in activating STAT5 and inducing Foxp3 expression [54, 55]. The influence of IL-2 on IL-17-producing Treg differentiation remains an area of ongoing research. Furthermore, cytokines such as IL-7 [56], IL-15 [57], and IL-35 [58] induce STAT5 phosphorylation and upregulate Foxp3 expression in Treg cells, thereby maintaining Treg homeostasis, which may hinder IL-17-producing Treg differentiation (Fig. 1). Nevertheless, the potential effects of these cytokines on IL-17-producing Tregs warrant further investigation.
Intricate mechanism of TGF-β in inducing IL-17-producing Treg differentiation
TGF-β plays a crucial role in the differentiation of both Treg and Th17 [59]. While the role of TGF-β in promoting Foxp3 expression and inducing Treg differentiation has been extensively studied [2], its role in Th17 differentiation remains enigmatic, despite the antagonistic functions of Foxp3 and RORγT [60]. Notably, IL-6 alone is insufficient to induce IL-17 secretion from T cells lacking suppressor of mothers against decapentaplegic (Smad)4, underscoring the crucial role of TGF-β in Th17 differentiation [61].
As previously mentioned, TGF-β is not only essential for inducing Th17 cells to produce IL-10 but also plays a pivotal role in IL-17-producing Treg differentiation. The combination of TGF-β and IL-1β can induce CCR6−CD4+CD25high T cells to differentiate into IL-17-producing Tregs [10]. TGF-β promotes Foxp3 expression through the Arkadia-v-ski avian sarcoma viral oncogene homolog (Ski) / Ski-related novel protein N (SnoN) pathway, facilitating Treg differentiation. Ski/SnoN serve as inhibitors of Smads, recruiting transcriptional repressor Smad2/3/4 activity by binding to Smad2/3/4. Upon receptor binding, TGF-β activates the E3 ubiquitin ligase Arkadia, which ubiquitinates Ski/SnoN, leading to their degradation. This process derepresses Smad2/3/4 transcription and upregulates Foxp3 expression (Fig. 1) [62, 63]. Intriguingly, CD4+ T cells with specific Arkadia deletion (Cd4creArkadiafl/fl) display reduced Treg differentiation capacity. Remarkably, these mice exhibit a significant reduction in IL-17-producing Treg differentiation but no changes in Th17 cell differentiation compared to wild-type mice [61]. This observation suggests that Arkadia has no effect on RORγT expression. The mechanisms underlying Smad signaling pathways’ regulation of RORγT expression remain unclear. Moreover, variations in TGF-β sources, environmental factors such as cytokines and temperature, and intricate interactions between TGF-β and other signaling pathways may contribute to this phenomenon [61]. Future research is expected to unveil the mechanistic basis for TGF-β induction of IL-17-producing Treg differentiation.
Autophagy: an essential factor in IL-17-producing Treg differentiation
Autophagy, a vital intracellular metabolic process, plays a crucial role in maintaining cellular function and homeostasis by facilitating the degradation and recycling of damaged proteins, organelles, and other cellular structures [64]. Currently, there is extensive research on the mechanism by which autophagy regulates Treg differentiation [65,66,67,68]. Autophagy contributes to Treg differentiation and the preservation of Treg function by regulating cellular metabolism [68]. Deletion of autophagy-regulated (Atg)7 and Atg5 in Tregs has been shown to decrease Foxp3 expression, leading to apoptosis. Additionally, inhibiting autophagy in Tregs can elevate cellular glycolysis level, which is the primary metabolic pathway in Th17. This metabolic shift ultimately results in impaired Treg differentiation and function [68, 69].
Similarly, Atg5 has been identified as a necessity for IL-17-producing Treg differentiation. In Treg specific Atg5-deficient mice (Foxp3creAtg5fl/fl), the expression of IL-17-producing Tregs in the gut is nearly undetectable. However, further investigations are required to elucidate how Atg5 regulates IL-17-producing Treg differentiation and the role of glycolysis in this process [13]. Techniques such as metabolomics may provide valuable insights into the metabolic regulatory mechanisms governing IL-17-producing Treg differentiation.
Promotive role of Wnt/β-catenin signaling pathway in IL-17-producing Treg differentiation
Previous studies have affirmed the regulatory role of the Wnt/β-catenin signaling pathway in Treg and Th17 differentiation [70,71,72,73,74]. When Wnt is inactive, a cytoplasmic scaffold protein (Axin), casein kinase 1 alpha (CK1α), glycogen synthase kinase-3 beta (GSK-3β), and the colorectal adenomatous polyposis gene (APC) form a degradation complex that targets β-catenin for degradation in T cells, maintaining it at a low level [75]. This, in turn, enables T-cell factor 1 (TCF-1, a transcription factor that binds to activated β-catenin in the nucleus) and Foxp3 to bind jointly to the enhancer region of Th17-related genes [76]. This reduces the chromatin accessibility of Th17-related genes, thereby restraining the expression of genes associated with Th17 differentiation and promoting Treg differentiation [77,78,79]. Conversely, in the presence of Wnt protein ligands, Wnt becomes activated and forms a co-receptor complex with the Frizzled protein (Fzd) and low-density lipoprotein receptor 5/6 (LRP5/6). This complex blocks the formation of the Axin/CK1α/GSK-3β/APC complex, thus inhibiting β-catenin degradation and leading to its accumulation in the cytoplasm. GSK-3β and CK1α also induce β-catenin dephosphorylation by phosphorylating LRP5/6 [80, 81]. This results in the translocation of β-catenin from the cytoplasm into the nucleus, where it binds to the transcription factor TCF-1/lymphoid enhancer factor 1 (LEF1), ultimately inducing the transcription of RORγT and other genes and promoting Th17 differentiation [82,83,84,85].
In a mouse model, stable expression of β-catenin in Tregs (Foxp3YFP−CreCtnnb1fl(ex3) mice) significantly increased the expression of RORγT in Tregs, leading to a decrease in Treg immunosuppressive activity. This suggests that activation of the Wnt/β-catenin signaling pathway may serve as a crucial mechanism promoting IL-17-producing Treg differentiation [86,87,88]. Subsequent investigations employed Foxp3-Chip-Seq and ATAC sequencing technology to shed light on the mechanism by which β-catenin promotes IL-17-producing Treg differentiation through epigenetic pathways. Compared to their wild-type counterparts, Foxp3YFP−CreCtnnb1fl(ex3) mice exhibited significantly enhanced chromatin accessibility in genes related to Th17 differentiation (genes inhibited by TCF-1-Foxp3 co-binding) in Tregs. These findings imply that Wnt/β-catenin signaling pathway activation in Tregs disrupts the inhibitory TCF-1-Foxp3 co-binding, enhancing chromatin accessibility of Th17-related genes and promoting the expression of genes associated with Th17 differentiation (Fig. 1) [87].
Maintenance of IL-10 expression in IL-17-producing Treg by HIF-1α
The effects of HIF-1α on IL-17 and IL-10 expression in T cells have been well-documented [89,90,91,92,93,94,95]. Increased glycolysis is observed during CD4+ T cell differentiation into Th17 [96,97,98]. HIF-1α, activated under hypoxia, enhances the expression of glycolytic-related genes in cells, thereby promoting Th17 differentiation [99, 100]. However, HIF-1α in Th1 cells can induce IL-10 production under hypoxic conditions, conferring a certain degree of immunosuppressive function to Th1 [101].
Given the dual regulatory potential of HIF-1α on T cells, its impact on IL-17-producing Treg differentiation and function has been studied. It has been confirmed that HIF-1α is one of the factors promoting IL-10 production in IL-17-producing Tregs [102]. High levels of HIF-1α are expressed in IL-17-producing Tregs within the lamina propria of the intestinal mucosa. Deletion of Hif1a in IL-17-producing Tregs leads to decreased IL-10 production, although it does not affect RORγT expression. Through Chip-Seq analysis, it was discovered that HIF-1α can bind to the Il10 gene promoter region, as well as 26 kb upstream in the IL-17-producing Tregs distal enhancer region. Additionally, the RNA-binding protein DEAD box helicase 5 (DDX5) serves as a transcriptional corepressor by binding to the intronic region of Hif1a exon 1. This induces the disassembly of the R-loop of Hif1a, impedes the accumulation of RNA Pol2, and hinders histone H3K4 trimethylation on Hif1a. Consequently, Hif1a transcription is repressed, inhibiting HIF-1α-induced IL-10 production in IL-17-producing Tregs (Fig. 1) [102].
Multiple roles of gut microbiota in regulating IL-17-producing Treg differentiation
Numerous studies have underscored the pivotal influence of gut microbiota and its metabolites on the differentiation of Treg and Th17 [103,104,105]. Certain bacteria such as Lactobacillus reuteri [106, 107], Bacteroides fragilis [108,109,110], Bacteroides thetaiotaomicron [111], Clostridium [112,113,114,115], and Faecalibacterium prausnitzii [116,117,118,119] have been shown to promote Treg differentiation within the intestine. They play a crucial role in maintaining intestinal immune homeostasis and inhibiting the occurrence of inflammatory responses. Conversely, segmented filamentous bacteria [120,121,122] and Bifidobacterium adolescentis [123] have the unique ability to induce intestinal Th17 differentiation. Metabolites generated by the intestinal flora constitute one of the mechanisms by which they regulate Treg and Th17 differentiation [124, 125]. Of particular note are short-chain fatty acids (SCFAs), significant metabolites produced by the microbial breakdown of cellulose (e.g., acetate, propionate, and butyrate) [126, 127]. SCFAs have been extensively studied for their role in modulating Treg and Th17 differentiation [128,129,130,131,132]. Through various pathways including G-protein-coupled receptor signaling and histone deacetylase (HDAC) modulation, SCFAs can induce Treg differentiation while inhibiting Th17 differentiation [133,134,135]. Furthermore, gut microbiota impacts Treg and Th17 differentiation by modulating bile acid metabolism, a subject extensively reviewed by Su et al. in the latest publication in Frontiers in Immunology 2023 [125].
Notably, gut microbiota also plays a crucial role in IL-17-producing Treg differentiation. Comparative analyses of mice raised in a specific-pathogen-free (SPF) environment and germ-free (GF) environment have revealed a significant reduction in IL-17-producing Treg differentiation in GF mice compared to SPF mice. Additionally, IL-17-producing Treg levels in the intestines of GF mice increased following fecal transplantation from healthy humans. Subsequent investigations identified Clostridium ramosum as an inducer of IL-17-producing Treg differentiation in the gut of GF mice, whereas Peptostreptococcus magnus failed to elicit such a response [15, 136]. Due to its vital role in maintaining immune tolerance towards gut microbiota, some studies have referred to IL-17-producing Treg as “microbiota-dependent RORγT+Foxp3+ Treg” [15, 137].
Akkermansia muciniphila, through Toll like receptor 4 (TLR4) signaling, induces intestinal IL-17-producing Treg differentiation to preserve intestinal homeostasis [137]. TLR4, as an immune receptor, stands as a pivotal molecule essential for shaping colonic ecology and maintaining intestinal homeostasis [138]. Notably, TLR4-knockout mice (Tlr4−/−) exhibited disrupted intestinal homeostasis, a marked reduction in Akkermansia muciniphila abundance, and decreased IL-17-producing Treg ratios within the gut when compared to wild-type mice. In this context, wild-type mice could induce IL-17-producing Treg differentiation through Akkermansia muciniphila transplantation, whereas Tlr4−/− mice displayed no significant alterations in intestinal IL-17-producing Treg levels before and after Akkermansia muciniphila transplantation [137]. Moreover, beyond inducing IL-17-producing Treg differentiation, gut microbiota can also curtail excessive IL-17-producing Treg differentiation, thereby maintaining intestinal immune homeostasis. Tryptophan metabolites produced by the intestinal flora can inhibit the excessive differentiation of IL-17-producing Treg in the intestine through activation of the AhR [139]. Tryptophan can be metabolized by the intestinal flora into compounds such as indole and its derivatives, including indole lactic acid, indole acrylic acid, and Indole-3-carbinol (I3C) [140]. These indole derivatives, by activating AhR in various immune cells, regulate immune responses effectively [141, 142]. Notably, the ratio of IL-17-producing Tregs in the intestinal lamina propria (si-LP) and mesenteric lymph nodes (mLN) significantly increased in mice subjected to a tryptophan-deficient diet (TDD). Moreover, the TDD diet failed to alter the intestinal IL-17-producing Treg levels after the removal of intestinal flora with antibiotics. However, the administration of the AhR ligand I3C in TDD diet mice effectively inhibited the excessive differentiation of IL-17-producing Tregs and preserved intestinal homeostasis (Fig. 1) [139].
Induction of IL-17-producing Treg differentiation by different types of antigen-presenting cells (APC)s
APCs encompass various cell types, including dendritic cells (DCs), mononuclear phagocytes, and B cells, among others. These cells play a pivotal role in capturing and processing antigens, subsequently presenting them to T cells to initiate specific immune responses [143, 144]. Notably, among APCs, DCs exhibit the most robust antigen-presenting capabilities [145]. Extensive research has elucidated the regulatory influence of DCs on the differentiation of Treg and Th17 [146,147,148,149,150]. Activation of interferon regulatory factor 4 (IRF4) in human intestinal DCs has been found to induce the production of IL-6 and IL-23, thereby promoting T cell secretion of IL-17 [151, 152]. Furthermore, DCs can induce Th17 differentiation through the production of Integrin αVβ8, which activates TGF-β [153]. Langerhans cells (LCs) residing in the skin have demonstrated the ability to induce IL-6 production by activating Dectin-1, particularly in response to stimuli from microorganisms like Candida albicans or Staphylococcus aureus. This IL-6 production subsequently promotes Th17 differentiation [154,155,156,157]. Additionally, specific DC subsets can drive Treg differentiation by producing retinoic acid (RA) [158], indoleamine 2, 3-dioxygenase (IDO) [159, 160], IL-10 [161], and TGF-β [162]. This is facilitated through the presence of immunomodulatory molecules such as programmed death-ligand (PD-L)1, PD-L2 [163, 164], B and T lymphocyte attenuator (BTLA) [165], and ICOS ligand (ICOSL) [166] on the DC cell membrane. A comprehensive review of the regulatory mechanisms employed by DCs in Treg and Th17 differentiation has been conducted by Yin et al. [150].
On September 7, 2022, Nature published three articles detailing the mechanisms behind APC-induced IL-17-producing Treg differentiation, a process crucial for establishing tolerance to gut microbiota in the body [21, 167, 168]. These studies collectively revealed the existence of distinct APC-induced IL-17-producing Treg differentiation pathways in neonatal and adult mice. At birth, a novel APC termed “Thetis cells” was identified as responsible for inducing IL-17-producing Treg differentiation, thus facilitating neonatal mouse tolerance to gut microbiota. Single-cell sequencing analyses indicated a significant surge in IL-17-producing Treg numbers within the long intestinal lymph nodes of mice ten days post-birth. This IL-17-producing Treg differentiation was found to be regulated by APCs expressing RORγT. Subsequent isolation and analysis of RORγT-expressing APCs (RORγT+MHC+) using single-cell sequencing and single-cell ATAC sequencing (scATAC-Seq) led to the identification of Thetis cells as cells as a novel APC subset. Thetis cells were further categorized into four subtypes based on gene expression patterns. Specifically, Type I and III Thetis cells expressed the thymus epithelial cell marker autoimmune regulator (AIRE), Type II Thetis cells expressed CD11c and CCR6, and Type IV Thetis cells expressed CD11c and CD11b. Comparative gene expression analysis of these four Thetis cell subtypes with DCs and group 3 innate lymphoid cell (ILC3)s, as well as an examination of Thetis cell ontogeny, confirmed their unique identity. Since the TGF-β pathway plays a pivotal role in inducing IL-17-producing Treg differentiation, it was noteworthy that Type IV Thetis cells expressed multiple genes associated with the TGF-β pathway, including integrin alpha V (Itgav) and integrin beta 8 (Itgb8). Notably, Itgb8 was uniquely expressed by Type IV Thetis cells among RORγT+ cells. Conditional removal of DC and ILC3 in vivo did not affect IL-17-producing Treg differentiation in neonatal mice, indicating that Type IV Thetis cells are essential for inducing IL-17-producing Treg at birth. This assertion was substantiated by creating Itgb8-specific gene knockout (Itgb8ΔRORγT) mice, which exhibited significantly lower post-birth IL-17-producing Treg levels compared to wild-type controls. Furthermore, Thetis cells were found to be abundant within three weeks post-birth but gradually disappeared by the sixth week, affirming the role of Type IV Thetis cells as the APCs responsible for IL-17-producing Treg induction during neonatal birth [21]. Another study reported that in the adult gut, ILC3s play a crucial role in presenting antigens from intestinal microbiota to Tregs via MHC-II molecules on their surface. Simultaneously, they secrete alpha V integrin to induce IL-17-producing Treg differentiation, thus enabling tolerance to Helicobacter hepaticus. Single-cell sequencing revealed that RORγT− expressing cells in the follicular region of adult mouse lymph nodes primarily consisted of ILC3s and IL-17-producing Tregs. Specific deletion of MHC-II in mouse ILC3s (MHCIIΔILC3 mice) resulted in a substantial reduction in gut IL-17-producing Tregs. Moreover, when Helicobacter hepaticus was transplanted into MHCIIΔILC3 mice, IL-17-producing Treg differentiation was not induced, leading to the loss of intestinal immune tolerance and the emergence of enteritis. Inhibition of ILC3 secretion of αV integrin effectively nullified the induction of IL-17-producing Treg differentiation by ILC3s [168]. It’s noteworthy that while Helicobacter hepaticus can induce IL-17-producing Treg differentiation and immune tolerance, excessive proliferation of Helicobacter hepaticus can also trigger Th17 responses, leading to intestinal inflammation [169]. Another study elucidated the mechanism by which Helicobacter hepaticus induces differentiation of IL-17-producing Treg or Th17. It was confirmed that Helicobacter hepaticus can trigger the differentiation of IL-17-producing Tregs in APCs, including ILC3s and Janus cells, which express RORγT. This process is facilitated by the chemokine CCR7 and the TGF-β agonist αV integrin. Colonization of Helicobacter hepaticus in wild-type mice not only induces IL-17-producing Treg differentiation but also establishes immune tolerance to Helicobacter hepaticus. However, when Helicobacter hepaticus lacks antigen-presenting ability (MHCIIΔCD11c mice), it fails to induce IL-17-producing Treg differentiation upon colonization, leading to Th17-mediated intestinal inflammation. This observation underscores the crucial role of APCs in facilitating Helicobacter hepaticus-induced IL-17-producing Treg differentiation. Further investigations utilizing Cellular Indexing of Transcriptomes and Epitopes by Sequencing (CITE-seq) identified specialized APC cells, namely ILC3s expressing RORγT and MHC-II, as well as Janus cells (RORγT+ APCs), which actively promote IL-17-producing Treg differentiation. In mice lacking antigen-presenting capabilities in ILC3s and Janus cells (MHCIIΔRORγT), colonization with Helicobacter hepaticus fails to induce IL-17-producing Treg differentiation. This model revealed that CCR7 and αV integrin are essential for initiating IL-17-producing Treg differentiation through RORγT+ APCs. Strikingly, both CCR7ΔRORγT and ItgavΔCD11c mice, which lack these necessary components, are unable to induce IL-17-producing Treg differentiation in response to Helicobacter hepaticus. Consequently, Th17-mediated intestinal inflammation ensues [167]. These two studies collectively shed light on the intricate mechanism by which Helicobacter hepaticus elicits resistance through the induction of IL-17-producing Treg differentiation mediated by antigen-presenting ILC3s (Fig. 1).
Other factors (costimulatory molecules, zinc finger protein and microRNAs) involved in IL-17-producing Treg differentiation
T cell activation necessitates the engagement of dual signals, comprising the interaction between MHC molecules on the APC surface and TCR (the first signal), as well as the activation of co-stimulatory molecules (the second signal). IL-17-producing Treg expresses a range of co-stimulatory/co-inhibitory molecules that exert both positive and negative regulatory effects on IL-17-producing Treg differentiation and proliferation. In the presence of MHC-II, the co-stimulatory molecules CD28 and ICOS promote IL-17-producing Treg differentiation, whereas the co-inhibitory molecule CTLA-4 inhibits IL-17-producing Treg proliferation. The application of CD28 and ICOS neutralizing antibodies can decrease the IL-17-producing Treg ratio in the lamina propria of the mouse intestine, whereas CTLA-4 neutralizing antibody can enhance IL-17-producing Treg proliferation [170].
Zinc finger protein 362 (ZFP362), a member of the Cys2/His2 (C2H2) zinc finger protein family, possesses transcriptional factor and transcriptional regulatory factor activities [171]. While ZFP362 expression is increased in Th17 and can interact with RORγT to promote Th17 differentiation [172], recent investigations have unveiled its regulatory role in IL-17-producing Treg differentiation. Notably, the level of IL-17-producing Tregs in the mesenteric lymph nodes of Zfp362−/− mice surpasses that in wild-type mice [173]. Nevertheless, further exploration is warranted to fully elucidate the mechanisms by which ZFP362 regulates IL-17-producing Treg differentiation.
MicroRNAs (miRNAs), a class of non-coding RNAs approximately 20–25 bp in length, have the capability to bind to the 3’ UTR of specific mRNA molecules, subsequently leading to mRNA degradation or the inhibition of translation. Extensive research has elucidated the role of miRNAs in the differentiation of Treg and Th17, as comprehensively reviewed by Liu et al. [174]. It has also come to light that certain miRNAs possess the potential to regulate IL-17-producing Treg differentiation. For instance, the transfection of miR-182 has been shown to promote the differentiation of Jurkat cells into IL-17-producing Treg [54].
IL-10-producting TH17
The instability and plasticity of Th17 cells enable their transformation from a pro-inflammatory phenotype, characterized by the secretion of pro-inflammatory cytokines like IL-17, into an anti-inflammatory phenotype that produces immunosuppressive factors, such as IL-10, in specific environmental conditions [175]. The discovery of IL-10-producing Th17 cells, referred to as IL-10-producing Th17, was initially reported in a study published in Nature in 2011 [26]. The anti-CD3-induced colitis model serves as a research tool to investigate intestinal immune tolerance. Typically, mice develop transient colitis after anti-CD3 stimulation, followed by gradual recovery [176]. When the mucosal immune system’s tolerance to gut microbiota diminishes, mice stimulated with anti-CD3 displayed prolonged inflammatory responses in the gut [11, 177, 178]. Interestingly, the authors observed that anti-CD3 stimulation induced a substantial activation of Th17 cells in the intestine. Surprisingly, these Th17s did not undergo apoptosis as expected after the resolution of intestinal inflammation but instead persisted. Upon isolation, it was evident that these Th17s not only expressed IL-17 but also exhibited high levels of IL-10 expression, demonstrating significant immunosuppressive activities in vitro [26]. A subsequent study published in Nature in 2012 revealed that repeated stimulation of T cells by Staphylococcus aureus resulted in their differentiation into Th17 with IL-17 production. However, upon continued stimulation with Staphylococcus aureus, these cells shifted their cytokine profile towards IL-10 secretion, indicating that Staphylococcus aureus stimulation can induce Th17 to acquire anti-inflammatory properties. Notably, the IL-10-producing capability of these Th17s diminished, and they reverted to secreting only IL-17 after the removal of Staphylococcus aureus stimulation [26]. Moreover, Th17 has the potential to differentiate into Treg and Treg type 1 (Tr1) [179, 180]. In a study published in Nature in 2015, it was further confirmed that IL-10-producing Th17 represents an intermediate state of Th17s transitioning into Tr1s. This study showed that Th17 could be stimulated to become IL-10-producing Th17, co-expressing both IL-10 and IL-17 A. Subsequently, IL-10-producing Th17 lose their IL-17 A expression and exclusively produce IL-10, referred to as Tr1exTh17 cells. Tr1exTh17 cells exhibit gene expression patterns and immunological characteristics highly similar to those of Tr1s [25].
The characteristics and physiological function of IL-10-producing Th17
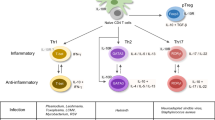
It has been established that IL-10-producing Th17s are capable of simultaneously secreting IL-10, IL-17, and IFN-γ [26]. When comparing the gene expression and cell surface markers of IL-10-producing Th17s with that of Th17, Tr1exTh17, Treg, and Tr1, it becomes evident that IL-10-producing Th17s exhibit low expression of Rorc but express Il17a. In contrast to Tr1exTh17 cells, IL-10-producing Th17s express Il17a, and unlike Treg cells, IL-10-producing Th17s do not express Foxp3. Furthermore, IL-10-producing Th17s express certain levels of Tr1 markers, such as lymphocyte activating 3 (LAG-3), as well as integrin alpha 2 (CD49B) and aryl hydrocarbon receptor (AhR). Notably, the gene expression pattern of Tr1exTh17 cells is more similar to that of IL-10-producing Th17 and Tr1 than to Th17s, further substantiating the notion that Tr1exTh17 cells represent an advanced form of IL-10-producing Th17s transitioning into Tr1s (Fig. 2) [25]. Given that IL-10-producing Th17s predominantly reside in the gut, one study investigated their distribution compared to Tr1exTh17 and Th17 in various parts of the gastrointestinal tract. The findings revealed that over 60% of IL-10-producing Th17s are distributed in the ileum, with around 30% residing in Peyer’s patches, and the remaining 10% distributed in the jejunum, duodenum, and colon [27]. Flow cytometry is widely used to investigate IL-10-producing Th17 in multiple tissues. The detailed information for investigating IL-10-producing Th17 is shown in Table 1.
Regulation of IL-10-producing Th17 differentiation. Th17 cells could convert into IL-10-producing Th17, co-expressing both IL-10 and IL-17 A. Subsequently, IL-10-producing Th17 lose their IL-17 A expression and exclusively produce IL-10, referred to as Tr1exTh17 cells. Tr1exTh17 cells could further differentiate into Tr1s. TGF-β, IL-2 and IL-27 have been shown to induce IL-10-producing Th17 differentiation, whereas IL-1β, IL-6, IL-12 and IL-23 could inhibit IL-10-producing Th17 differentiation
In physiological conditions, IL-10-producing Th17s play a crucial role in maintaining immune tolerance to the gut microbiota. In comparison to wild-type mice, mice with specific Il10 gene knockout in Th17 (Il17acreIl10fl/fl mice) exhibited significantly elevated expression of pro-inflammatory cytokines such as Il17f, Il1b, interferon gamma (Ifng), chemokine (C-X-C motif) ligand 11 (Cxcl1), and tumor necrosis factor a (Tnfa) in the gut, along with an increase in Th1 cell levels. Additionally, IL-10-producing Th17s have been identified as key players in maintaining immune homeostasis in the intestinal mucosa. Moreover, Il17acreIl10fl/fl mice subjected to anti-CD3 stimulation developed severe intestinal inflammation in comparison to their wild-type counterparts [27].
Regulatory factors involved in IL-10-producing Th17 differentiation
There is currently a limited body of literature exploring the regulatory mechanisms governing IL-10-producing Th17 differentiation. Given that IL-10-producing Th17 represents an intermediate state between Th17 and Tr1, it is pertinent to consider pathways known to regulate the transformation from Th17 to Tr1, including the TGF-β signaling pathway and certain cytokines, which have been identified as inducers of IL-10-producing Th17 differentiation.
Induction of IL-10-producing Th17 cell differentiation by TGF-β signaling pathway
TGF-β plays a pivotal role as a regulatory factor in guiding the differentiation of CD4+ T cell into both Th17 and Treg [59]. In the context of Th17, TGF-β interacts with TGF-β receptor (TGFβR) II on the cell surface, triggering the secretion of IL-10 by Th17. Notably, Th17 with specific Tgfbr2 knockout exhibited a significant reduction in IL-10 production compared to their wild-type counterparts [27]. TGFβRII is a transmembrane protein comprised of 567 amino acids, featuring an extracellular domain, a transmembrane domain, and an intracellular domain. TGF-β binding to the extracellular domain of TGFβRII induces phosphorylation of the extracellular domain. Subsequently, the phosphorylated TGFβRII-TGF-β complex activates TGFβRI on the cell membrane, thereby promoting Smad3 phosphorylation. Phosphorylated Smad3 forms a transcription complex with Smad4, facilitating the transcription of the Il10 gene [59] (Fig. 2). Mice with Smad4-specific knockout in Th17 cells exhibited significantly reduced IL-10 production in Th17. Moreover, using a dual-luciferase reporter gene technique, it was confirmed that the activation of Smad3/4 in Th17 binds to the promoter region of the Il10 gene, thereby inducing IL-10 secretion by Th17 [27]. Additionally, TGF-β can induce IL-10 secretion by activating AhR in Th17 [25].
Promotive role of IL-27 and suppressive roles of IL-1β, IL-6, IL-12, IL-23, IL-2 in IL-10-producing Th17 cell differentiation
The differentiation of IL-10-producing Th17 is subject to regulation by a range of cytokines. Notably, IL-1β, IL-6, IL-12, and IL-23 have been identified as inhibitors of IL-10 production by IL-10-producing Th17. Among these, IL-1β exerts the most pronounced inhibitory effect on IL-10 production by IL-10-producing Th17, potentially linked to its activation of the nuclear factor-kappa B (NF-κB) signaling pathway [26, 181]. IL-2 inhibits RORγT expression in Th17 through activating signal transducer and activator of transcription 5 (STAT5) [26, 182]. Conversely, IL-27 has been shown to promote IL-10 production by IL-10-producing Th17 [29]. IL-27 binds to IL-27 receptor subunit alpha (IL-27Ra) on the surface of Th17, activating activator protein-1 (AP-1) family transcription factors, which, in turn, promote the expression of musculoaponeurotic fibrosarcoma (Maf) [183]. Additionally, IL-27 induces B-lymphocyte-inducing maturation protein 1 (Blimp1) expression through early growth response gene 2 (Egr-2) Subsequently, the Maf/RORγT/Blimp-1 complex forms, inhibiting Il17 expression driven by RORγT alone. Moreover, the Maf/RORγT/Blimp-1 complex translocates into the nucleus, promoting IL-10 production by Th17. Furthermore, studies have corroborated that IL-27 counteracts the inhibitory effects of IL-12 on IL-10-producing Th17 differentiation through Blimp-1 (Fig. 2) [29].
The roles of IL-17-producing Treg and IL-10-producing TH17 in disease
The imbalance between the Treg and Th17 ratio plays a pivotal role in the pathogenesis of a wide a wide array of inflammatory diseases [184], tumors [185], autoimmune disorders [186, 187], and metabolic conditions [188,189,190]. A sour understanding of the contributions of IL-17-producing Treg and IL-10-producing Th17 in these diseases continues to expand, their associations with conditions such as inflammatory bowel disease (IBD), cancer, nephritis, autoimmune disorders, and others are gradually being unveiled.
IBD
As a vital component of the intestinal mucosal immune system, the equilibrium between Treg and Th17 stands as a pivotal immunological mechanism in the onset of IBD [191, 192]. Both clinical observations and animal experiments have consistently shown that, in comparison to healthy controls, IBD patients exhibit an elevated proportion of Th17 in the peripheral blood and gut, which secrete substantial quantities of pro-inflammatory cytokines. Concurrently, there is a decrease in Treg proportion or a relative deficiency in Treg function, resulting in a reduction or relative deficiency of immunosuppressive factors like IL-10. This imbalance, characterized by a higher Treg/Th17 ratio, fosters the development of intestinal inflammatory reactions [193, 194]. The mechanisms underlying the Treg/Th17 imbalance in IBD have been thoroughly reviewed by Iacomino et al. [195]. Given their significant presence in the gut, the relationship between IL-17-producing Treg and IL-10-producing Th17 and IBD has attracted extensive discussion.
Promotive role of IL-17-producing Treg in the development of IBD
In peripheral blood and intestinal inflammatory areas of IBD patients, the IL-17-producing Treg ratio was found to be increased in comparison to healthy controls. In particular, IL-17-producing Treg in the peripheral blood of IBD patients was found to possess pro-inflammatory properties, associated with sustained activation of β-catenin cells [87]. Furthermore, recent single-cell sequencing revealed a substantial infiltration of IL-17-producing Treg in the colonic mucosa of patients with ulcerative colitis (UC) and Crohn’s disease (CD) [24]. Compared to the remission period of UC patients, the peripheral blood IL-17-producing Treg levels in patients in the active phase were significantly elevated and positively correlated with the levels of pro-inflammatory factors such as IL-21 in the serum. Drug therapy-induced remission resulted in a significant decrease in peripheral blood IL-17-producing Treg levels in patients with active-phase UC [23]. In a mouse model of Giardia-induced diarrhea, the proportion of intestinal IL-17-producing Tregs increased, thereby enhancing mouse susceptibility to Giardia [196]. Tryptophan deficiency in the diet may lead to excessive proliferation of intestinal IL-17-producing Tregs and promote the progression of intestinal inflammation [139].
The amelioration of IBD by promoting IL-17-producing Treg differentiation
One study found that despite the increased proportion of IL-17-producing Tregs in the ileum, colon, and rectum of patients with Crohn’s disease compared to healthy individuals, these cells secrete IL-17. However, in vitro experiments have demonstrated their significant immunosuppressive activity [197]. Transplantation of Rag−/− mice with IL-17-producing Tregs can mitigate dextran sodium sulfate (DSS)-induced colitis [17]. The transplantation of healthy human feces can ameliorate DSS-induced UC in GF mice and increase the number of IL-17-producing Tregs, illustrating that gut probiotics can enhance UC by promoting IL-17-producing Treg differentiation [136]. Moreover, mice lacking Arkadia or Atg5-mediated differentiation of IL-17-producing Tregs display intestinal inflammation [13, 61]. It has also been reported that in the context of inflammatory bowel disease, ILC3s can alleviate intestinal inflammation by promoting IL-17-producing Treg differentiation [168]. Environmental enteric dysfunction (EED) is an intestinal inflammatory disease that can lead to stunted growth and a weakened response to oral vaccines in children [198, 199]. A study found that in an EED model, the level of IL-17-producing Tregs in the gut significantly increased, which suppressed intestinal inflammation and improved stunted growth caused by EED. However, IL-17-producing Tregs also suppressed CD4+ T cells in the gut that respond to oral vaccines, resulting in reduced vaccine effectiveness. Mice lacking IL-17-producing Tregs (Foxp3cre−ETR2Rorcfl/fl) displayed more severe stunting after the induction of EED compared to wild-type controls, but they exhibited a stronger response to oral vaccines [200].
Anti-inflammatory role of IL-10-producing Th17 in IBD
Notably, IL-10-producing Th17s were initially found to possess anti-inflammatory properties and induce tolerance to secondary gut bacterial infections, such as Staphylococcus aureus, thereby ameliorating intestinal inflammation, as reported in Nature [25]. Similarly, in a mouse model of colitis induced by CD3 monoclonal antibody + tamoxifen, the induction of IL-10-producing Th17 was found to inhibit inflammatory reactions [25]. However, there are currently conflicting conclusions regarding the relationship between IL-17-producing Tregs and IBD.
These findings suggest that IL-17-producing Tregs may have multifaceted roles in both promoting and suppressing inflammation in IBD. However, it is evident from these contrasting conclusions that different immune properties may be mediated by different pathways. For instance, β-catenin [87] and tryptophan deficiency [139] can augment the pro-inflammatory activity of IL-17-producing Tregs, while Arkadia [61], Atg5 [13], ILC3s [168], and certain probiotics can enhance the anti-inflammatory activity of IL-17-producing Tregs. Understanding the mechanisms governing the pro-inflammatory/anti-inflammatory immune activities of IL-17-producing Tregs represents a future research direction aimed at elucidating their dual role in IBD. Although there is evidence supporting the anti-inflammatory properties of IL-10-producing Th17 in IBD, it is also imperative to investigate whether IL-10-producing Th17 may exhibit pro-inflammatory activity in IBD and under what circumstances such activity may occur.
Tumor
The infiltration of Treg and Th17 into the tumor microenvironment can exert influence on tumor progression through various mechanisms [201]. The impact of Th17 on tumors is bidirectional. IL-17, secreted by Th17, contributes to the immunosuppressive environment within tumors by inducing vascular endothelial growth factor (VEGF) expression and promoting the differentiation of myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment. This inflammatory response is often referred to as promoting a pro-tumor inflammatory response [202,203,204,205]. Conversely, IL-17 has also been reported to enhance the recruitment of anti-tumor immune cells within tumor tissues, consequently elevating IFN-γ levels within the tumor microenvironment, which plays a role in inhibiting tumor progression [206]. This contrasting effect may be influenced by the type and stage of the tumor, as reviewed by Gorczynski [207]. Tregs are generally believed to drive tumor development by suppressing immune responses, making targeted inhibition of Treg differentiation an important approach in tumor treatment [208,209,210,211]. Currently, there is evidence suggesting that IL-17-producing Treg and IL-10-producing Th17 are associated with tumor development.
Induction of cancer-initiating cell differentiation and immunosuppression by IL-17-producing Treg
In comparison to healthy controls, the quantity of IL-17-producing Tregs in tumor tissues of colon cancer and breast cancer patients has been found to increase [54, 212, 213]. Studies have verified that IL-17-producing Tregs can induce the differentiation of colon cancer-initiating cells by secreting IL-17. After co-culturing IL-17-producing Tregs isolated from colon cancer patients’ tumors with Sphere cells derived from normal human bone marrow, Sphere cells exhibited increased expression of multiple colon cancer-initiating cell-related phenotypes, such as CD133, CD44s, CD166, epithelial cell adhesion molecule (EpCAM), and aldehyde dehydrogenase 1 (ALDH1). Notably, the addition of recombinant IL-17 A neutralizing antibody to the co-culture system impeded the inductive effect of IL-17-producing Tregs on colon cancer-initiating cell differentiation [22]. Furthermore, IL-17-producing Treg has also been shown to involve in the immunosuppression within the tumor. Inhibition of IL-17-producing Treg differentiation can enhance anti-tumor immune responses [212].
Promotive role of IL-17-producing Treg in the development of colitis-associated colorectal cancer (CAC)
Given the diverse immune activities of IL-17-producing Treg and IL-10-producing Th17, coupled with their role as intermediaries in the conversion between Treg and Th17, the involvement of IL-17-producing Treg and IL-10-producing Th17 in the carcinogenesis of colon tumors has garnered considerable attention. Colon polyposis serves as the primary precancerous lesion leading to colon cancer, and IL-17-producing Tregs have been observed to be extensively infiltrated in colon polyposis in the mouse model of Adenomatous polyposis coli (ApcΔ). Inhibition of IL-17-producing Treg differentiation in these mice can reduce the incidence of colon polyposis [212]. Similarly, research has shown that IL-17-producing Tregs are highly expressed in the spleen and tumor tissues of ApcΔ mice treated with DSS to induce IBD-associated CAC. These IL-17-producing Tregs express elevated levels of cell proliferation-related phenotypes, such as CD44 and Ki67 [87]. Subsequent studies have demonstrated that IL-17-producing Tregs promote the development of colorectal cancer by influencing DCs within the tumor microenvironment, resulting in heightened inflammatory responses and CAC. Moreover, mice with a conditional knockout of RORγT in Tregs (Foxp3GFP−creRorcfl/fl mice) exhibit lower susceptibility to colorectal cancer induction by azoxythane (AOM)/DSS treatment compared to wild-type mice. This can be attributed to the low expression of CTLA-4 by IL-17-producing Tregs, which leads to Forkhead box O 3 (FoxO3) inactivation in DCs and the activation of the IL-6 production-STAT3 signaling pathway in tumor cells, ultimately promoting their proliferation. The activation of STAT3 subsequently induces tumor cell proliferation. Expression of STAT3 and IL-6 by DCs is diminished in Foxp3GFP−creRorcfl/fl mice, while CTLA-4 expression is elevated in Tregs compared to wild-type mice. Co-culturing DCs with Tregs from Foxp3GFP−creRorcfl/fl mice results in reduced IL-6 expression in DCs, an effect that is abolished following FoxO3 knockdown or CTLA-4 neutralization antibody treatment. Silencing FoxO3 in Foxp3GFP−creRorcfl/fl mice accelerates tumor development, accompanied by increased IL-6 and STAT3 expression within the tumor tissue [213].
Increase of IL-10-producing Th17 differentiation in acute myeloid leukemia (AML) patients
A substantial presence of IL-10-producing Th17 has been observed in the peripheral blood of AML patients. In vitro experiments have revealed that the presence of IL-10-producing Th17 diminishes the immune response of T cells against bacterial infections. Induction of IL-10-producing Th17 differentiation can be accomplished by co-culturing peripheral blood CD33+ myeloid cells from AML patients with normal CD4+ T cells. However, this phenomenon is not observed with CD33+ myeloid cells from healthy individuals [28].
Although the aforementioned evidence collectively indicates the promotional effect of IL-17-producing Tregs on intestinal tumors, it is important to acknowledge that IL-17-producing Tregs can induce both pro-inflammatory reactions that promote tumorigenesis (pro-inflammatory properties) and immunosuppression that facilitates tumor evasion (immunosuppressive properties). These seemingly contradictory findings parallel the dual promoting and inhibitory effects of IL-17-producing Tregs observed in IBD. This underscores the importance of investigating the regulatory mechanisms governing IL-17-producing Tregs’ manifestation of both immune activation and inhibition, which will be a crucial avenue for researchers to better comprehend the functionality of IL-17-producing Tregs.
Autoimmune diseases
The association between the disruption of the Treg and Th17 balance and autoimmune diseases has been the focus of extensive research. On one hand, the excessive differentiation of Th17 can trigger autoimmune reactions, while the relative scarcity of Treg results in the loss of immune tolerance within the body [214,215,216,217]. Multiple signaling pathways are implicated in the imbalance between Treg and Th17 in autoimmune diseases, as comprehensively reviewed by Lee [6]. IL-17-producing Tregs have also been closely linked to various autoimmune diseases, including rheumatoid arthritis (RA), autoimmune encephalomyelitis (EAE), systemic lupus erythematosus (SLE), and more. Available evidence suggests that suggests that IL-17-producing Tregs exhibit diverse roles in these diseases.
Inhibition of RA inflammation by IL-17-producing Treg
The heightened infiltration of IL-17-producing Tregs in the joint cavity of collagen-induced arthritis (CIA) mice implies their involvement in RA-related inflammatory responses. Further investigations have revealed that IL-17-producing Tregs in the joint cavity of CIA mice express elevated levels of CD25, CTLA4, GITR, ICOS, and secrete substantial quantities of IL-10. Notably, these IL-17-producing Tregs also express CCR6, suggesting their potential migration to the joint cavity under the influence of CCR6. Mice from which IL-17-producing Tregs were removed (Foxp3creRorcfl/fl mice) developed more severe RA compared to wild-type mice [18], and the injection of IL-17-producing Tregs resulted in increased IL-10 levels and diminished inflammatory responses in RA mice [218].
Suppression of EAE autoimmune responses by IL-17-producing Treg
In the experimental autoimmune EAE model, induced by myelin oligodendrocyte glycoprotein (MOG) and complete Freund’s adjuvant (CFA), there is an increase in CCR6+ICOShigh IL-17-producing Tregs within lymph nodes. Isolation and subsequent culture of these CCR6+ICOShigh IL-17-producing Tregs with effector T cells revealed their capacity to inhibit effector T cell activation, suggesting their immunosuppressive activity. Additionally, in the Th17-mediated EAE model established in Rag1−/− mice through the Rag1−/− mice through the co-transfer of Th17 and CCR6+ICOShigh IL-17-producing Tregs, the latter effectively suppressed the autoimmune response induced by Th17 [20].
Exacerbation of kidney damage in SLE by IL-17-producing Treg
In a Pristane-induced SLE mouse model, IL-17-producing Treg infiltration in kidney tissue gradually increased with disease progression. Additionally, after Pristane induction, Foxp3creRorcfl/fl mice exhibited less kidney damage and decreased IL-17 levels compared to wild-type mice [16].
Other diseases
IL-17-producing Treg has also been demonstrated to involve in the progression of nephrotoxic nephritis (NTN), type 2 diabetes mellitus (T2DM) and non-alcoholic steatohepatitis (NASH). Inducing IL-17-producing Treg differentiation has shown with a mitigating effect in crescentic glomerulonephritis. In a mouse model of NTN-induced crescentic glomerulonephritis, a significant increase in IL-17-producing Treg levels within renal tissue occurred four days prior to NTN induction, followed by a gradual decline. Further analysis revealed that IL-17-producing Tregs secrete both pro-inflammatory and anti-inflammatory factors, and transplantation of IL-17-producing Tregs into crescentic glomerulonephritis mice substantially improved kidney damage. Interestingly, the mechanism by which IL-17-producing Tregs alleviate kidney injury in crescentic glomerulonephritis mice differs from Treg cells [219]. However, a comprehensive understanding of how IL-17-producing Tregs ameliorate kidney injury in crescentic glomerulonephritis mice remains to be elucidated. Furthermore, there is an elevated IL-17-producing Treg ratio in the peripheral blood of patients with T2DM, and this ratio is positively correlated with body mass index (BMI) and glycosylated hemoglobin type A1c (HbA1c), implying the potential involvement of IL-17-producing Tregs in the progression of T2DM [220]. Elevated IL-17-producing Treg levels have also been detected in the liver tissues of NASH mice induced by a high-fat diet. Nevertheless, upon the removal of IL-17-producing Tregs, more severe liver fibrosis occurred in high-fat diet mice. Furthermore, the population of γδT cells expressing Th17 and IL-17 increased significantly within liver tissues. This suggests that IL-17-producing Tregs play a role in inhibiting liver inflammation and fibrosis. Interestingly, the enrichment of IL-17-producing Tregs in liver tissue appears to depend on the presence of intestinal flora, as their quantity decreased following antibiotic-induced removal of intestinal flora in NASH model mice [15].
IL-10-producing Th17 contributed to the progression of endometriosis (EMS). In patients with EMS, a notable increase in the number of IL-10-producing Th17 has been observed within the endometriotic milieu, and this elevation escalates as the disease progresses. Promotion of IL-10-producing Th17 differentiation exacerbates EMS. IL-27 has been identified as a key factor inducing IL-10-producing Th17 differentiation in EMS [29].
The association between IL-17-producing Treg and IL-10-producing TH17 and Chinese traditional “Yin-Yang” theory
Ancient Chinese philosophy includes the significant concept of “Yin-Yang” theory. In brief, Yin and Yang represent opposing aspects of phenomena. In medicine, Yin pertains to substances and functions with a condensing, moistening, and inhibitory effect on the body, often depicted as the black “fish.” Conversely, Yang encompasses substances and functions that exert a stimulating, warming, and excitatory influence, represented by the white “fish.” Yin and Yang interact in a mutually restraining and complementary manner, with transitions between them. Yin can transform into Yang, and vice versa, under specific conditions, such as when Yin/Yang substances increase to a certain point and overcomes a limit. Researchers have employed the Yin-Yang theory to discuss the characteristics and functions of vital activities. As such, we will use the Yin-Yang theory to describe how IL-17-producing Treg and IL-10-producing Th17 both hold immunosuppressive (Yin) and immunostimulant (Yang) potentials, much like how a black Yang circle exists in Yin, and vice versa, in the Yin-Yang image (Fig. 3) [221, 222].
“Yin-Yang” theory and immune system. According to the opposing attributes of Yin and Yang, immune-activating cells and molecules, such as M1 macrophages, Th17s, and pro-inflammatory cytokines like IL-1β and IL-17, as well as immune activation-related molecules such as TLR4 and CD80, along with the transcription factor RORγT, are associated with Yang, represented by the white “fish”. Immunosuppressive cells and molecules such as M2 macrophages, Treg, immunosuppressive cytokines such as IL-10, inhibitory receptors PD-1 and CTLA-4, and the transcription factor Foxp3, are associated with Yin, represented by the black “fish”. Furthermore, Yin and Yang are mutually inclusive, represented by the black circle in Yang (Yin within Yang) and the white circle in Yin (Yang within Yin). Therefore, IL-10-producing Th17 can be viewed as a manifestation of Yang within Yin, whereas IL-17-producing Treg can be seen as Yin within Yang. Besides, the transformation of Yin and Yang are also reflected in the study of IL-17-producing Treg and IL-10-producing Th17 as IL-17-producing Treg and IL-10-producing Th17 represent intermediate forms in the transformation between Treg and Th17
The Chinese “Yin-Yang” theory and immune system
In light of the contrasting functions of immune cells and molecules, those that typically promote immune reactions are considered Yang, while those that inhibit immune reactions are considered Yin [223]. Under normal physiological conditions, the immune system maintains a delicate balance, where immunosuppressive cells and molecules like M2 macrophages, Treg, immunosuppressive cytokines such a IL-10, inhibitory receptors PD-1 and CTLA-4, and the transcription factor Foxp3, are in equilibrium with immune-activating cells and molecules, such as M1 macrophages, Th17s, and pro-inflammatory cytokines like IL-1β and IL-6, as well as immune activation-related molecules such as TLR4 and CD80, along with the transcription factor RORγT, respectively.
However, when there is an overexpression of immune-activating cells and molecules, coupled with a relative insufficiency of immunosuppressive cells and molecules, the body may develop inflammatory and autoimmune diseases, signifying an excess of Yang over Yin [224,225,226]. Conversely, when immunosuppressive cells and molecules surpass immune activation counterparts, the body may be prone to tumor development and immunodeficiency diseases, indicating a dominance of Yin over Yang [227,228,229]. Treating various immune-related diseases by modulating immune cells to restore immunological balance aligns with the principles of traditional Chinese medicine, which aims to harmonize the Yin and Yang forces (Fig. 4).
The relationship between “Yin-Yang” theory and the role IL-17-producing Treg and IL-10-producing Th17 differentiation during disease progression. Under inflammatory and autoimmune state, overexpression of immune-activation factors, coupled with a relative insufficiency of immunosuppressive factors can be signified as an excess of Yang over Yin. In tumor and immunodeficiency diseases, immunosuppressive factors surpass immune activation counterparts, indicating a dominance of Yin over Yang. During the transformation process from colitis to cancer, where there is an initial state of immune overreaction (Yang), dominated by Th17 cells during colitis, followed by a later immunosuppressive state (Yin) in colon tumors, where Tregs prevail. The yin-yang attributes of IL-17-producing Treg and IL-10-producing Th17 may offer a better understanding of the transition from immune hyperactivity to immunosuppression
The association between IL-17-producing Treg and IL-10-producing Th17 and “Yin-Yang”
Current discussions regarding the relationship between Yin and Yang and the immune system revolve around their opposing attributes, drawing parallels with the mutually inhibitory functions of immune cells and molecules [230,231,232]. In light of the immune functions of Treg and Th17, Treg, which secrete IL-10 to inhibit immune reactions, are linked to Yin, while Th17, which secrete IL-17 to promote immune reactions, are associated with Yang. Intriguingly, as described above, IL-17-producing Treg can be seen as Yin within Yang, whereas IL-10-producing Th17 can be viewed as a manifestation of Yang within Yin. This duality of “Yin-Yang " also corresponds to their documented dual roles in promoting and inhibiting immune reactions. Furthermore, there is evidence to suggest that IL-17-producing Treg and IL-10-producing Th17 represent intermediate forms in the transformation between Treg and Th17. The discovery and examination of IL-17-producing Treg and IL-10-producing Th17 enhance the alignment between Yin-Yang theory and the immune system. In addition to their opposing characteristics, the mutual interdependence and transformation of Yin and Yang are also reflected in the study of IL-17-producing Treg and IL-10-producing Th17, deepening our understanding of the connection between Yin-Yang philosophical thought and the immune system (Fig. 3).
IL-17-producing Treg and IL-10-producing TH17: current knowledge and unanswered questions
Understanding the “multiple roles” of IL-17-producing Treg and IL-10-producing Th17
Given the functional similarity of IL-17-producing Treg and IL-10-producing Th17, one may wonder if they are essentially the same cell type. Existing research indicates that they differ due to their distinct origins; IL-17-producing Treg is more likely an intermediate during the transformation of Treg to Th17 [33], while IL-10-producing Th17 arises as an intermediate during the conversion of Th17 to Tr1 [25]. Additionally, both IL-17-producing Treg and IL-10-producing Th17 participate in activating or suppressing immune responses across various physiological and pathological contexts. This seemingly contradictory outcome raises questions about the factors responsible for such dual roles. It remains to be explored whether the relative balance of Foxp3 (representing Yin) and RORγT (representing Yang) within the cells determines their distinct identities. Moreover, since IL-17-producing Treg and IL-10-producing Th17 share similar markers (IL-10+IL-17A+), it is conceivable that some of the IL-17-producing Tregs in previous studies might actually be IL-10-producing Th17 or vice versa. Various methods have been employed for detecting IL-17-producing Treg and IL-10-producing Th17, often involving modified Treg and Th17-related animal and cell models. The animal models in IL-17-producing Treg and IL-10-producing Th17 studies were summarized in Table 2. Furthermore, in vitro models including the amplification of IL-17-producing Treg [17], the induction of IL-17-producing Treg [22], IL-10-producing Th17 [27], and Tr1exTh17 [29] differentiation, sorting of IL-10-producing Th17 [25] have also been established by previous studies. However, there is currently no research that simultaneously detects IL-17-producing Treg and IL-10-producing Th17, necessitating further investigation to distinguish between them.
Illustrating the detailed mechanisms involved in IL-17-producing Treg and IL-10-producing Th17 differentiation
TGF-β plays a pivotal role in inducing the differentiation of IL-17-producing Treg and IL-10-producing Th17 [12, 61], promoting both Treg and Th17 differentiation simultaneously [59]. While the mechanism of TGF-β inducing Treg differentiation is well understood, its role in further inducing IL-17-producing Treg and IL-10-producing Th17 differentiation remains to be clarified. Interestingly, from a Yin-Yang perspective, an excess of Yin may generate Yang. TGF-β-induced Treg differentiation falls within the Yin category, and TGF-β, building upon the Treg foundation, may lead to the emergence of IL-17-producing Treg, aligning with the philosophical idea of Yin and Yang transformation. Further studies are required to explain this phenomenon, and the distinct roles of various downstream Smad proteins following TGF-β induction need in-depth investigation. Additionally, various factors such as STAT, epigenetic modifications, and co-stimulatory molecules have been shown to regulate IL-17-producing Treg and IL-10-producing Th17 differentiation. Whether these pathways interact with each other and their potential relationships necessitate further exploration.
Disease treatment by regulating the differentiation of IL-17-producing Treg and IL-10-producing Th17
The body’s immune system is in a constant state of flux, and under normal circumstances, immune cells and molecules that both activate and inhibit the immune system interact to maintain a dynamic immune balance, often described as a Yin-Yang equilibrium. Disease development disrupts this equilibrium. During the initial stages of illness, immune cells in a hyperactive state become highly activated and release pro-inflammatory factors, resulting in an excess of Yang. However, prolonged immune hyperactivity can deplete immune cells that play a role in immune promotion or prompt their transformation into immune regulatory cells, ultimately shifting the body towards an immunosuppressive state, characterized by an excess of Yin.
Consider the transformation process from colitis to cancer as an example, where there is an initial state of immune overreaction (Yang), dominated by Th17 cells during colitis, followed by a later immunosuppressive state (Yin) in colon tumors, where Treg cells prevail (Fig. 4) [238,239,240]. The yin-yang attributes of IL-17-producing Treg and IL-10-producing Th17 may offer a better understanding of the transition from immune hyperactivity to immunosuppression. Encouragingly, some studies have revealed the role of IL-17-producing Treg in the colitis-cancer transformation. However, whether IL-10-producing Th17 is also involved in this transition remains uncertain.
The discovery of IL-17-producing Treg and IL-10-producing Th17 provides valuable insights into the mechanisms underlying the transition process from immune activation to suppression (or vice versa) within the body. These findings offer crucial perspectives for reducing the progression of diseases like colitis to colon cancer by modulating the behavior of IL-17-producing Treg and IL-10-producing Th17.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Park BV, Pan F. The role of nuclear receptors in regulation of Th17/Treg biology and its implications for diseases. Cell Mol Immunol. 2015;12(5):533–42.
Moreau JM, Velegraki M, Bolyard C, Rosenblum MD, Li Z. Transforming growth factor-beta1 in regulatory T cell biology. Sci Immunol. 2022;7(69):eabi4613.
Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259(1):115–39.
Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13(10):991–9.
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–76.
Lee GR. The Balance of Th17 versus Treg Cells in Autoimmunity. Int J Mol Sci. 2018;19(3):730.
Qianmei Y, Zehong S, Guang W, Hui L, Lian G. Recent advances in the role of Th17/Treg cells in tumor immunity and tumor therapy. Immunol Res. 2021;69(5):398–414.
Whiteside TL. FOXP3 + Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin Ther Targets. 2018;22(4):353–63.
Pan Y, Yang W, Tang B, Wang X, Zhang Q, Li W, et al. The protective and pathogenic role of Th17 cell plasticity and function in the tumor microenvironment. Front Immunol. 2023;14:1192303.
Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, et al. Identification of IL-17-producing FOXP3 + regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106(12):4793–8.
Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011;475(7357):514–8.
Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113(18):4240–9.
Plaza-Sirvent C, Zhao B, Bronietzki AW, Pils MC, Tafrishi N, Schuster M, et al. A Central Role for Atg5 in Microbiota-Dependent Foxp3(+) RORgammat(+) Treg Cell Preservation to maintain intestinal Immune Homeostasis. Front Immunol. 2021;12:705436.
Kluger MA, Meyer MC, Nosko A, Goerke B, Luig M, Wegscheid C, et al. RORgammat(+)Foxp3(+) cells are an Independent Bifunctional Regulatory T Cell Lineage and Mediate Crescentic GN. J Am Soc Nephrol. 2016;27(2):454–65.
Hanna BS, Wang G, Galvan-Pena S, Mann AO, Ramirez RN, Munoz-Rojas AR, et al. The gut microbiota promotes distal tissue regeneration via RORgamma(+) regulatory T cell emissaries. Immunity. 2023;56(4):829–46.
Kluger MA, Nosko A, Ramcke T, Goerke B, Meyer MC, Wegscheid C, et al. RORgammat expression in T(regs) promotes systemic lupus erythematosus via IL-17 secretion, alteration of T(reg) phenotype and suppression of Th2 responses. Clin Exp Immunol. 2017;188(1):63–78.
Yang BH, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M, et al. Foxp3(+) T cells expressing RORgammat represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 2016;9(2):444–57.
Furuyama K, Kondo Y, Shimizu M, Yokosawa M, Segawa S, Iizuka A, et al. RORgammat + Foxp3 + regulatory T cells in the regulation of autoimmune arthritis. Clin Exp Immunol. 2022;207(2):176–87.
Pratama A, Schnell A, Mathis D, Benoist C. Developmental and cellular age direct conversion of CD4+ T cells into RORγ+ or Helios+ colon Treg cells. J Exp Med. 2020;217(1):e20190428. https://doi.org/10.1084/jem.20190428.
Kim BS, Lu H, Ichiyama K, Chen X, Zhang YB, Mistry NA, et al. Generation of RORgammat(+) Antigen-Specific T Regulatory 17 cells from Foxp3(+) precursors in Autoimmunity. Cell Rep. 2017;21(1):195–207.
Akagbosu B, Tayyebi Z, Shibu G, Paucar IY, Deep D, Parisotto YF, et al. Novel antigen-presenting cell imparts T(reg)-dependent tolerance to gut microbiota. Nature. 2022;610(7933):752–60.
Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, et al. Foxp3 + IL-17 + T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. 2011;89(1):85–91.
Long Y, Zhao X, Xia C, Li X, Fan C, Liu C, et al. Upregulated IL-17A secretion and CCR6 co-expression in Treg subsets are related to the imbalance of Treg/Th17 cells in active UC patients. Scand J Immunol. 2020;91(6):e12842.
Mitsialis V, Wall S, Liu P, Ordovas-Montanes J, Parmet T, Vukovic M, et al. Single-cell analyses of Colon and blood reveal distinct Immune Cell signatures of Ulcerative Colitis and Crohn’s Disease. Gastroenterology. 2020;159(2):591–608.
Gagliani N, Amezcua VM, Iseppon A, Brockmann L, Xu H, Palm NW, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559):221–5.
Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484(7395):514–8.
Xu H, Agalioti T, Zhao J, Steglich B, Wahib R, Vesely M, et al. The induction and function of the anti-inflammatory fate of T(H)17 cells. Nat Commun. 2020;11(1):3334.
Musuraca G, De Matteis S, Napolitano R, Papayannidis C, Guadagnuolo V, Fabbri F, et al. IL-17/IL-10 double-producing T cells: new link between infections, immunosuppression and acute myeloid leukemia. J Transl Med. 2015;13:229.
Chang KK, Liu LB, Jin LP, Zhang B, Mei J, Li H, et al. IL-27 triggers IL-10 production in Th17 cells via a c-Maf/RORgammat/Blimp-1 signal to promote the progression of endometriosis. Cell Death Dis. 2017;8(3):e2666.
Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, et al. Author correction: c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature. 2019;566(7744):E7.
Panduro M, Benoist C, Mathis D. T(reg) cells limit IFN-gamma production to control macrophage accrual and phenotype during skeletal muscle regeneration. Proc Natl Acad Sci U S A. 2018;115(11):E2585–93.
Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol. 2017;17(3):165–78.
Omenetti S, Pizarro TT. The Treg/Th17 Axis: a dynamic balance regulated by the gut Microbiome. Front Immunol. 2015;6:639.
Deng G, Song X, Fujimoto S, Piccirillo CA, Nagai Y, Greene MI. Foxp3 post-translational modifications and Treg suppressive activity. Front Immunol. 2019;10:2486.
Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–41.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61.
Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, et al. Suppression of tumour-specific CD4(+) T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. 2012;61(8):1163–71.
Chang LY, Lin YC, Mahalingam J, Huang CT, Chen TW, Kang CW, et al. Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res. 2012;72(5):1092–102.
Liu Z, Huang Q, Liu G, Dang L, Chu D, Tao K, et al. Presence of FOXP3(+)Treg cells is correlated with colorectal cancer progression. Int J Clin Exp Med. 2014;7(7):1781–5.
Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3 + T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186–92.
Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing Y, et al. FOXP3 and RORgammat: transcriptional regulation of Treg and Th17. Int Immunopharmacol. 2011;11(5):536–42.
Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809.
Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–54.
Ogawa C, Tone Y, Tsuda M, Peter C, Waldmann H, Tone M. TGF-beta-mediated Foxp3 gene expression is cooperatively regulated by Stat5, Creb, and AP-1 through CNS2. J Immunol. 2014;192(1):475–83.
Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, et al. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179(7):4313–7.
Ichiyama K, Gonzalez-Martin A, Kim BS, Jin HY, Jin W, Xu W, et al. The MicroRNA-183-96-182 cluster promotes T Helper 17 cell pathogenicity by negatively regulating transcription factor Foxo1 expression. Immunity. 2016;44(6):1284–98.
Jung MK, Kwak JE, Shin EC. IL-17A-Producing Foxp3(+) Regulatory T cells and human diseases. Immune Netw. 2017;17(5):276–86.
Li L, Kim J, Boussiotis VA. IL-1beta-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J Immunol. 2010;185(7):4148–53.
Li L, Patsoukis N, Petkova V, Boussiotis VA. Runx1 and Runx3 are involved in the generation and function of highly suppressive IL-17-producing T regulatory cells. PLoS ONE. 2012;7(9):e45115.
Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134(3):392–404.
Seif F, Ghalehbaghi B, Aazami H, Mohebbi A, Ahmadi A, Falak R, et al. Frequency of CD4(+) and CD8(+) T cells in Iranian chronic rhinosinusitis patients. Allergy Asthma Clin Immunol. 2018;14:47.
Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15(1):23.
Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37(10):2695–706.
Soheilifar MH, Vaseghi H, Seif F, Ariana M, Ghorbanifar S, Habibi N, et al. Concomitant overexpression of mir-182-5p and mir-182-3p raises the possibility of IL-17-producing Treg formation in breast cancer by targeting CD3d, ITK, FOXO1, and NFATs: a meta-analysis and experimental study. Cancer Sci. 2021;112(2):589–603.
Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, et al. IL-2 regulates FOXP3 expression in human CD4 + CD25 + regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108(5):1571–9.
Dupont G, Demaret J, Venet F, Malergue F, Malcus C, Poitevin-Later F, et al. Comparative dose-responses of recombinant human IL-2 and IL-7 on STAT5 phosphorylation in CD4 + FOXP3- cells versus regulatory T cells: a whole blood perspective. Cytokine. 2014;69(1):146–9.
Tosiek MJ, Fiette L, El DS, Eberl G, Freitas AA. IL-15-dependent balance between Foxp3 and RORgammat expression impacts inflammatory bowel disease. Nat Commun. 2016;7:10888.
Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–9.
Wang J, Zhao X, Wan YY. Intricacies of TGF-beta signaling in Treg and Th17 cell biology. Cell Mol Immunol. 2023;20(9):1002–22.
Zhang S. The role of transforming growth factor beta in T helper 17 differentiation. Immunology. 2018;155(1):24–35.
Xu H, Wu L, Nguyen HH, Mesa KR, Raghavan V, Episkopou V, Littman DR. Arkadia-SKI/SnoN signaling differentially regulates TGF-β-induced iTreg and Th17 cell differentiation. J Exp Med. 2021;218(11):e20210777. https://doi.org/10.1084/jem.20210777.
Inoue Y, Imamura T. Regulation of TGF-beta family signaling by E3 ubiquitin ligases. Cancer Sci. 2008;99(11):2107–12.
Miyazono K, Koinuma D. Arkadia–beyond the TGF-beta pathway. J Biochem. 2011;149(1):1–3.
Vargas J, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol. 2023;24(3):167–85.
Cheng LS, Li J, Liu Y, Wang FP, Wang SQ, She WM, et al. HMGB1-induced autophagy: a new pathway to maintain Treg function during chronic hepatitis B virus infection. Clin Sci (Lond). 2017;131(5):381–94.
Liu Y, Zhang Y, Zhang M, Meng J, Ma Q, Hao Z, et al. Activated autophagy restored the impaired frequency and function of regulatory T cells in chronic prostatitis. Prostate. 2021;81(1):29–40.
Marcel N, Sarin A. Notch1 regulated autophagy controls survival and suppressor activity of activated murine T-regulatory cells. Elife. 2016;5.
Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17(3):277–85.
Tian Y, Liang X, Wu Y. The alternation of autophagy/apoptosis in CD4 + CD25 + Foxp3 + tregs on the developmental stages of atherosclerosis. Biomed Pharmacother. 2018;97:1053–60.
Cane S, Bronte V. Wnt-beta-catenin as an epigenetic switcher in colonic T(reg) cells. Nat Immunol. 2021;22(4):400–1.
Ding Y, Shen S, Lino AC, Curotto DLM, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14(2):162–9.
Karnam A, Bonam SR, Rambabu N, Wong S, Aimanianda V, Bayry J. Wnt-beta-catenin signaling in human dendritic cells mediates Regulatory T-Cell responses to Fungi via the PD-L1 pathway. Mbio. 2021;12(6):e0282421.
Sumida T, Lincoln MR, Ukeje CM, Rodriguez DM, Akazawa H, Noda T, et al. Activated beta-catenin in Foxp3(+) regulatory T cells links inflammatory environments to autoimmunity. Nat Immunol. 2018;19(12):1391–402.
Zhou M, Jiao L, Liu Y. sFRP2 promotes airway inflammation and Th17/Treg imbalance in COPD via Wnt/beta-catenin pathway. Respir Physiol Neurobiol. 2019;270:103282.
Park S, Lee J. Inhibitory effect of nordihydroguaiaretic acid on beta-catenin/Tcf signalling in beta-catenin-activated cells. Cell Biochem Funct. 2011;29(1):22–9.
Osman A, Yan B, Li Y, Pavelko KD, Quandt J, Saadalla A, et al. TCF-1 controls T(reg) cell functions that regulate inflammation, CD8(+) T cell cytotoxicity and severity of colon cancer. Nat Immunol. 2021;22(9):1152–62.
Ma J, Wang R, Fang X, Ding Y, Sun Z. Critical role of TCF-1 in repression of the IL-17 gene. PLoS ONE. 2011;6(9):e24768.
Ma J, Wang R, Fang X, Sun Z. beta-catenin/TCF-1 pathway in T cell development and differentiation. J Neuroimmune Pharmacol. 2012;7(4):750–62.
Zhang J, He Z, Sen S, Wang F, Zhang Q, Sun Z. TCF-1 inhibits IL-17 gene expression to restrain Th17 immunity in a stage-specific manner. J Immunol. 2018;200(10):3397–406.
Schulte G. Frizzleds and WNT/beta-catenin signaling–the black box of ligand-receptor selectivity, complex stoichiometry and activation kinetics. Eur J Pharmacol. 2015;763(Pt B):191–5.
Tsutsumi N, Hwang S, Waghray D, Hansen S, Jude KM, Wang N, et al. Structure of the wnt-Frizzled-LRP6 initiation complex reveals the basis for coreceptor discrimination. Proc Natl Acad Sci U S A. 2023;120(11):e2076729176.
Steinke FC, Xue HH. From inception to output, Tcf1 and Lef1 safeguard development of T cells and innate immune cells. Immunol Res. 2014;59(1–3):45–55.
Tiemessen MM, Baert MR, Schonewille T, Brugman MH, Famili F, Salvatori DC, et al. The nuclear effector of Wnt-signaling, Tcf1, functions as a T-cell-specific tumor suppressor for development of lymphomas. Plos Biol. 2012;10(11):e1001430.
Yang BH, Wang K, Wan S, Liang Y, Yuan X, Dong Y, et al. TCF1 and LEF1 control Treg competitive survival and Tfr Development to Prevent Autoimmune diseases. Cell Rep. 2019;27(12):3629–45.
Zhao X, Shan Q, Xue HH. TCF1 in T cell immunity: a broadened frontier. Nat Rev Immunol. 2022;22(3):147–57.
Keerthivasan S, Aghajani K, Dose M, Molinero L, Khan MW, Venkateswaran V, et al. beta-catenin promotes colitis and colon cancer through imprinting of proinflammatory properties in T cells. Sci Transl Med. 2014;6(225):225ra28.
Quandt J, Arnovitz S, Haghi L, Woehlk J, Mohsin A, Okoreeh M, et al. Wnt-beta-catenin activation epigenetically reprograms T(reg) cells in inflammatory bowel disease and dysplastic progression. Nat Immunol. 2021;22(4):471–84.
Zhou Y, Xu J, Luo H, Meng X, Chen M, Zhu D. Wnt signaling pathway in cancer immunotherapy. Cancer Lett. 2022;525:84–96.
Ambade A, Satishchandran A, Saha B, Gyongyosi B, Lowe P, Kodys K, et al. Hepatocellular carcinoma is accelerated by NASH involving M2 macrophage polarization mediated by hif-1alphainduced IL-10. Oncoimmunology. 2016;5(10):e1221557.
Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–84.
Fecher RA, Horwath MC, Friedrich D, Rupp J, Deepe GJ. Inverse correlation between IL-10 and HIF-1alpha in Macrophages infected with Histoplasma Capsulatum. J Immunol. 2016;197(2):565–79.
Meng X, Bozec A. New insights into the key role of HIF-1alpha in IL-10-producing B cells. Cell Stress. 2018;2(4):94–5.
Samarpita S, Doss HM, Ganesan R, Rasool M. Interleukin 17 under hypoxia mimetic condition augments osteoclast mediated bone erosion and expression of HIF-1alpha and MMP-9. Cell Immunol. 2018;332:39–50.
Talreja J, Talwar H, Bauerfeld C, Grossman LI, Zhang K, Tranchida P, Samavati L. HIF-1α regulates IL-1β and IL-17 in sarcoidosis. Elife. 2019;8:e44519. https://doi.org/10.7554/eLife.44519.
Wang B, Li K, Wang H, Shen X, Zheng J. Systemic chemotherapy promotes HIF-1alpha-mediated glycolysis and IL-17F pathways in cutaneous T-cell lymphoma. Exp Dermatol. 2020;29(10):987–92.
Cluxton D, Petrasca A, Moran B, Fletcher JM. Differential Regulation of Human Treg and Th17 cells by fatty acid synthesis and Glycolysis. Front Immunol. 2019;10:115.
Wu L, Hollinshead K, Hao Y, Au C, Kroehling L, Ng C, et al. Niche-selective inhibition of pathogenic Th17 cells by targeting metabolic redundancy. Cell. 2020;182(3):641–54.
Zhao Z, Wang Y, Gao Y, Ju Y, Zhao Y, Wu Z, et al. The PRAK-NRF2 axis promotes the differentiation of Th17 cells by mediating the redox homeostasis and glycolysis. Proc Natl Acad Sci U S A. 2023;120(19):e2082354176.
Chakraborty S, Khamaru P, Bhattacharyya A. Regulation of immune cell metabolism in health and disease: special focus on T and B cell subsets. Cell Biol Int. 2022;46(11):1729–46.
Zhang Q, Wang L, Jiang J, Lin S, Luo A, Zhao P, et al. Critical role of AdipoR1 in regulating Th17 cell differentiation through modulation of HIF-1alpha-Dependent glycolysis. Front Immunol. 2020;11:2040.
Shehade H, Acolty V, Moser M, Oldenhove G. Cutting Edge: Hypoxia-Inducible factor 1 negatively regulates Th1 function. J Immunol. 2015;195(4):1372–6.
Ma S, Yang Q, Chen N, Zheng A, Abbasi N, Wang G, et al. RNA binding protein DDX5 restricts RORgammat(+) T(reg) suppressor function to promote intestine inflammation. Sci Adv. 2023;9(5):eadd6165.
Calvo-Barreiro L, Zhang L, Abdel-Rahman SA, Naik SP, Gabr M. Gut Microbial-Derived Metabolites as Immune Modulators of T Helper 17 and Regulatory T Cells. Int J Mol Sci. 2023;24(2):1806. https://doi.org/10.3390/ijms24021806.
Chen P, Tang X. Gut microbiota as regulators of Th17/Treg Balance in patients with Myasthenia Gravis. Front Immunol. 2021;12:803101.
Sun CY, Yang N, Zheng ZL, Liu D, Xu QL. T helper 17 (Th17) cell responses to the gut microbiota in human diseases. Biomed Pharmacother. 2023;161:114483.
Liu Y, Tran DQ, Fatheree NY, Marc RJ. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3 + regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2014;307(2):G177–86.
Savino F, Galliano I, Garro M, Savino A, Dapra V, Montanari P, et al. Regulatory T cells and toll-like receptor 2 and 4 mRNA expression in infants with colic treated with Lactobacillus reuteri DSM17938. Benef Microbes. 2018;9(6):917–25.
Nutsch KM, Hsieh CS. T cell tolerance and immunity to commensal bacteria. Curr Opin Immunol. 2012;24(4):385–91.
Telesford KM, Yan W, Ochoa-Reparaz J, Pant A, Kircher C, Christy MA, et al. A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39(+)Foxp3(+) T cells and Treg function. Gut Microbes. 2015;6(4):234–42.
Zhang Y, Sun D, Zhao X, Luo Y, Yu H, Zhou Y, et al. Bacteroides fragilis prevents aging-related atrial fibrillation in rats via regulatory T cells-mediated regulation of inflammation. Pharmacol Res. 2022;177:106141.
Li K, Hao Z, Du J, Gao Y, Yang S, Zhou Y. Bacteroides thetaiotaomicron relieves colon inflammation by activating aryl hydrocarbon receptor and modulating CD4(+)T cell homeostasis. Int Immunopharmacol. 2021;90:107183.
Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–41.
Li H, Jia Y, Weng D, Ju Z, Zhao Y, Liu S, et al. Clostridium butyricum inhibits Fat Deposition via Increasing the Frequency of Adipose Tissue-Resident Regulatory T Cells. Mol Nutr Food Res. 2022;66(12):e2100884.
Li YN, Huang F, Cheng HJ, Li SY, Liu L, Wang LY. Intestine-derived Clostridium leptum induces murine tolerogenic dendritic cells and regulatory T cells in vitro. Hum Immunol. 2014;75(12):1232–8.
Nagano Y, Itoh K, Honda K. The induction of Treg cells by gut-indigenous Clostridium. Curr Opin Immunol. 2012;24(4):392–7.
Mohebali N, Weigel M, Hain T, Sutel M, Bull J, Kreikemeyer B, et al. Faecalibacterium prausnitzii, Bacteroides faecis and Roseburia intestinalis attenuate clinical symptoms of experimental colitis by regulating Treg/Th17 cell balance and intestinal barrier integrity. Biomed Pharmacother. 2023;167:115568.
Qiu X, Zhang M, Yang X, Hong N, Yu C. Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J Crohns Colitis. 2013;7(11):e558–68.
Touch S, Godefroy E, Rolhion N, Danne C, Oeuvray C, Straube M, Galbert C, Brot L, Alonso Salgueiro I, Chadi S, Ledent T, Chatel JM, Langella P, Jotereau F, Altare F, Sokol H. Human CD4+CD8α+ Tregs induced by Faecalibacterium prausnitzii protect against intestinal inflammation. JCI Insight. 2022;7(12):e154722. https://doi.org/10.1172/jci.insight.154722.
Zhou L, Zhang M, Wang Y, Dorfman RG, Liu H, Yu T, et al. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg Balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm Bowel Dis. 2018;24(9):1926–40.
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98.
Schnupf P, Gaboriau-Routhiau V, Sansonetti PJ, Cerf-Bensussan N. Segmented filamentous bacteria, Th17 inducers and helpers in a hostile world. Curr Opin Microbiol. 2017;35:100–9.
Wang Y, Yin Y, Chen X, Zhao Y, Wu Y, Li Y, et al. Induction of intestinal Th17 cells by Flagellins from Segmented filamentous Bacteria. Front Immunol. 2019;10:2750.
Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci U S A. 2016;113(50):E8141–50.
Haase S, Haghikia A, Wilck N, Muller DN, Linker RA. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology. 2018;154(2):230–8.
Su X, Gao Y, Yang R. Gut microbiota derived bile acid metabolites maintain the homeostasis of gut and systemic immunity. Front Immunol. 2023;14:1127743.
Harris HC, Morrison DJ, Edwards CA. Impact of the source of fermentable carbohydrate on SCFA production by human gut microbiota in vitro - a systematic scoping review and secondary analysis. Crit Rev Food Sci Nutr. 2021;61(22):3892–903.
Vince AJ, McNeil NI, Wager JD, Wrong OM. The effect of lactulose, pectin, arabinogalactan and cellulose on the production of organic acids and metabolism of ammonia by intestinal bacteria in a faecal incubation system. Br J Nutr. 1990;63(1):17–26.
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, DeRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5.
Du H, Yue SY, Niu D, Liu C, Zhang LG, Chen J, et al. Gut Microflora modulates Th17/Treg cell differentiation in experimental autoimmune prostatitis via the short-chain fatty acid propionate. Front Immunol. 2022;13:915218.
Geuking MB, McCoy KD, Macpherson AJ. Metabolites from intestinal microbes shape Treg. Cell Res. 2013;23(12):1339–40.
Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014;14(6):277–88.
Su X, Yin X, Liu Y, Yan X, Zhang S, Wang X, Lin Z, Zhou X, Gao J, Wang Z, Zhang Q. Gut Dysbiosis Contributes to the Imbalance of Treg and Th17 Cells in Graves' Disease Patients by Propionic Acid. J Clin Endocrinol Metab. 2020;105(11):dgaa511. https://doi.org/10.1210/clinem/dgaa511.
Huang S, Hu S, Liu S, Tang B, Liu Y, Tang L, et al. Lithium carbonate alleviates colon inflammation through modulating gut microbiota and Treg cells in a GPR43-dependent manner. Pharmacol Res. 2022;175:105992.
Kibbie JJ, Dillon SM, Thompson TA, Purba CM, McCarter MD, Wilson CC. Butyrate directly decreases human gut lamina propria CD4 T cell function through histone deacetylase (HDAC) inhibition and GPR43 signaling. Immunobiology. 2021;226(5):152126.
Schwarz A, Philippsen R, Piticchio SG, Hartmann JN, Hasler R, Rose-John S, et al. Crosstalk between microbiome, regulatory T cells and HCA2 orchestrates the inflammatory response in a murine psoriasis model. Front Immunol. 2023;14:1038689.
Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, et al. Microbiotas from humans with inflammatory bowel Disease alter the balance of gut Th17 and RORgammat(+) Regulatory T cells and exacerbate colitis in mice. Immunity. 2019;50(1):212–24.
Liu Y, Yang M, Tang L, Wang F, Huang S, Liu S, et al. TLR4 regulates RORgammat(+) regulatory T-cell responses and susceptibility to colon inflammation through interaction with Akkermansia muciniphila. Microbiome. 2022;10(1):98.
Bruning EE, Coller JK, Wardill HR, Bowen JM. Site-specific contribution of toll-like receptor 4 to intestinal homeostasis and inflammatory disease. J Cell Physiol. 2021;236(2):877–88.
Rankin LC, Kaiser KA, de Los SK, Park H, Uhlemann AC, Gray D, et al. Dietary tryptophan deficiency promotes gut RORgammat(+) Treg cells at the expense of Gata3(+) Treg cells and alters commensal microbiota metabolism. Cell Rep. 2023;42(3):112135.
Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the gut microbiota on intestinal immunity mediated by Tryptophan Metabolism. Front Cell Infect Microbiol. 2018;8:13.
Hezaveh K, Shinde RS, Klotgen A, Halaby MJ, Lamorte S, Ciudad MT, et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55(2):324–40.
Pernomian L, Duarte-Silva M, de Barros CC. The Aryl Hydrocarbon receptor (AHR) as a potential target for the Control of Intestinal Inflammation: insights from an Immune and Bacteria sensor receptor. Clin Rev Allergy Immunol. 2020;59(3):382–90.
Cannon JL, Burkhardt JK. The regulation of actin remodeling during T-cell-APC conjugate formation. Immunol Rev. 2002;186:90–9.
Shrager SH, Kiel C. SnapShot: APC/T cell Immune checkpoints. Cell. 2020;183(4):1142.
Melgoza-Gonzalez EA, Bustamante-Cordova L, Hernandez J. Recent advances in antigen targeting to antigen-presenting cells in veterinary medicine. Front Immunol. 2023;14:1080238.
Isaksson M, Ardesjo B, Ronnblom L, Kampe O, Lassmann H, Eloranta ML, et al. Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur J Immunol. 2009;39(10):2925–35.
Izumi G, Nakano H, Nakano K, Whitehead GS, Grimm SA, Fessler MB, et al. CD11b(+) lung dendritic cells at different stages of maturation induce Th17 or Th2 differentiation. Nat Commun. 2021;12(1):5029.
Li C, Bi Y, Li Y, Yang H, Yu Q, Wang J, et al. Dendritic cell MST1 inhibits Th17 differentiation. Nat Commun. 2017;8:14275.
Thumkeo D, Punyawatthananukool S, Prasongtanakij S, Matsuura R, Arima K, Nie H, et al. PGE(2)-EP2/EP4 signaling elicits immunosuppression by driving the mregDC-Treg axis in inflammatory tumor microenvironment. Cell Rep. 2022;39(10):110914.
Yin X, Chen S, Eisenbarth SC. Dendritic cell regulation of T Helper cells. Annu Rev Immunol. 2021;39:759–90.
Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38(5):958–69.
Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, et al. IRF4 transcription factor-dependent CD11b + dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38(5):970–83.
Zhou L, Yao L, Zhang Q, Xie W, Wang X, Zhang H, et al. REGgamma controls Th17 cell differentiation and autoimmune inflammation by regulating dendritic cells. Cell Mol Immunol. 2020;17(11):1136–47.
Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35(2):260–72.
Kashem SW, Igyarto BZ, Gerami-Nejad M, Kumamoto Y, Mohammed JA, Jarrett E, et al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity. 2015;42(2):356–66.
Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity. 2015;42(4):756–66.
Linehan JL, Dileepan T, Kashem SW, Kaplan DH, Cleary P, Jenkins MK. Generation of Th17 cells in response to intranasal infection requires TGF-beta1 from dendritic cells and IL-6 from CD301b + dendritic cells. Proc Natl Acad Sci U S A. 2015;112(41):12782–7.
Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35(1):13–22.
Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181(8):5396–404.
Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–8.
Alameddine J, Godefroy E, Papargyris L, Sarrabayrouse G, Tabiasco J, Bridonneau C, et al. Faecalibacterium prausnitzii Skews Human DC to Prime IL10-Producing T cells through TLR2/6/JNK signaling and IL-10, IL-27, CD39, and IDO-1 induction. Front Immunol. 2019;10:143.
Mansouri S, Katikaneni DS, Gogoi H, Pipkin M, Machuca TN, Emtiazjoo AM, et al. Lung IFNAR1(hi) TNFR2(+) cDC2 promotes lung regulatory T cells induction and maintains lung mucosal tolerance at steady state. Mucosal Immunol. 2020;13(4):595–608.
Maier B, Leader AM, Chen ST, Tung N, Chang C, LeBerichel J, et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580(7802):257–62.
Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13(9):888–99.
Jones A, Bourque J, Kuehm L, Opejin A, Teague RM, Gross C, et al. Immunomodulatory functions of BTLA and HVEM Govern Induction of Extrathymic Regulatory T Cells and tolerance by Dendritic Cells. Immunity. 2016;45(5):1066–77.
Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204(1):105–15.
Kedmi R, Najar TA, Mesa KR, Grayson A, Kroehling L, Hao Y, et al. A RORgammat(+) cell instructs gut microbiota-specific T(reg) cell differentiation. Nature. 2022;610(7933):737–43.
Lyu M, Suzuki H, Kang L, Gaspal F, Zhou W, Goc J, et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature. 2022;610(7933):744–51.
Chai JN, Peng Y, Rengarajan S, Solomon BD, Ai TL, Shen Z, et al. Helicobacter species are potent drivers of colonic T cell responses in homeostasis and inflammation. Sci Immunol. 2017;2:13.
Cruz-Morales E, Hart AP, Fossett GM, Laufer TM. Helios(+) and RORgammat(+) Treg populations are differentially regulated by MHCII, CD28, and ICOS to shape the intestinal Treg pool. Mucosal Immunol. 2023;16(3):264–74.
Mackeh R, Marr AK, Fadda A, Kino T. C2H2-Type zinc finger proteins: evolutionarily old and New partners of the Nuclear hormone receptors. Nucl Recept Signal. 2018;15:552070831.
Yang BH, Floess S, Hagemann S, Deyneko IV, Groebe L, Pezoldt J, et al. Development of a unique epigenetic signature during in vivo Th17 differentiation. Nucleic Acids Res. 2015;43(3):1537–48.
Herppich S, Hoenicke L, Kern F, Kruse F, Smout J, Greweling-Pils MC, et al. Zfp362 potentiates murine colonic inflammation by constraining Treg cell function rather than promoting Th17 cell differentiation. Eur J Immunol. 2023;53(10):e2250270.
Liu C, Yang H, Shi W, Wang T, Ruan Q. MicroRNA-mediated regulation of T helper type 17/regulatory T-cell balance in autoimmune disease. Immunology. 2018;155(4):427–34.
Cerboni S, Gehrmann U, Preite S, Mitra S. Cytokine-regulated Th17 plasticity in human health and diseases. Immunology. 2021;163(1):3–18.
Tang W, Saret S, Tian R, Wang H, Claudio E, Murphy PM, et al. Bcl-3 suppresses differentiation of RORgammat(+) regulatory T cells. Immunol Cell Biol. 2021;99(6):586–95.
Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7(8):622–32.
Rutz S, Kayagaki N, Phung QT, Eidenschenk C, Noubade R, Wang X, et al. Deubiquitinase DUBA is a post-translational brake on interleukin-17 production in T cells. Nature. 2015;518(7539):417–21.
Xu X, Wang Y, Wei Z, Wei W, Zhao P, Tong B, et al. Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPARgamma/AMPK/ACC1 pathway. Cell Death Dis. 2017;8(3):e2723.
Said SS, Barut GT, Mansur N, Korkmaz A, Sayi-Yazgan A. Bacterially activated B-cells drive T cell differentiation towards Tr1 through PD-1/PD-L1 expression. Mol Immunol. 2018;96:48–60.
Whitley SK, Balasubramani A, Zindl CL, Sen R, Shibata Y, Crawford GE, et al. IL-1R signaling promotes STAT3 and NF-kappaB factor recruitment to distal cis-regulatory elements that regulate Il17a/f transcription. J Biol Chem. 2018;293(41):15790–800.
Jones DM, Read KA, Oestreich KJ. Dynamic roles for IL-2-STAT5 Signaling in Effector and Regulatory CD4(+) T cell populations. J Immunol. 2020;205(7):1721–30.
Pot C, Apetoh L, Awasthi A, Kuchroo VK. Molecular pathways in the induction of interleukin-27-driven regulatory type 1 cells. J Interferon Cytokine Res. 2010;30(6):381–8.
Da SE, Yariwake VY, Alves RW, de Araujo DR, Andrade-Oliveira V. Crosstalk between incretin hormones, Th17 and Treg cells in inflammatory diseases. Peptides. 2022;155:170834.
Marshall EA, Ng KW, Kung SH, Conway EM, Martinez VD, Halvorsen EC, et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol Cancer. 2016;15(1):67.
Alunno A, Bartoloni E, Bistoni O, Nocentini G, Ronchetti S, Caterbi S, et al. Balance between regulatory T and Th17 cells in systemic lupus erythematosus: the old and the new. Clin Dev Immunol. 2012;2012:823085.
Kmiolek T, Rzeszotarska E, Wajda A, Walczuk E, Kuca-Warnawin E, Romanowska-Prochnicka K, et al. The interplay between transcriptional factors and MicroRNAs as an important factor for Th17/Treg balance in RA patients. Int J Mol Sci. 2020;21:19.
Croce S, Avanzini MA, Regalbuto C, Cordaro E, Vinci F, Zuccotti G, Calcaterra V. Adipose Tissue Immunomodulation and Treg/Th17 Imbalance in the Impaired Glucose Metabolism of Children with Obesity. Children (Basel). 2021;8(7):554. https://doi.org/10.3390/children8070554.
He B, Wu L, Xie W, Shao Y, Jiang J, Zhao Z, et al. The imbalance of Th17/Treg cells is involved in the progression of nonalcoholic fatty liver disease in mice. Bmc Immunol. 2017;18(1):33.
Zi C, He L, Yao H, Ren Y, He T, Gao Y. Changes of Th17 cells, regulatory T cells, Treg/Th17, IL-17 and IL-10 in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Endocrine. 2022;76(2):263–72.
Geng X, Xue J. Expression of Treg/Th17 cells as well as related cytokines in patients with inflammatory bowel disease. Pak J Med Sci. 2016;32(5):1164–8.
Zhu XM, Shi YZ, Cheng M, Wang DF, Fan JF. Serum IL-6, IL-23 profile and Treg/Th17 peripheral cell populations in pediatric patients with inflammatory bowel disease. Pharmazie. 2017;72(5):283–7.
Li J, Ueno A, Iacucci M, Fort GM, Jijon HB, Panaccione R, et al. Crossover subsets of CD4(+) T lymphocytes in the Intestinal Lamina Propria of patients with Crohn’s Disease and Ulcerative Colitis. Dig Dis Sci. 2017;62(9):2357–68.
Zhang C, Ju J, Wu X, Yang J, Yang Q, Liu C, et al. Tripterygium Wilfordii Polyglycoside ameliorated TNBS-Induced colitis in rats via regulating Th17/Treg Balance in Intestinal Mucosa. J Inflamm Res. 2021;14:1243–55.
Iacomino G, Rotondi AV, Iannaccone N, Melina R, Giardullo N, De Chiara G, et al. IBD: role of intestinal compartments in the mucosal immune response. Immunobiology. 2020;225(1):151849.
Yordanova IA, Cortes A, Klotz C, Kuhl AA, Heimesaat MM, Cantacessi C, et al. RORgammat(+) Treg to Th17 ratios correlate with susceptibility to Giardia infection. Sci Rep. 2019;9(1):20328.
Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140(3):957–65.
Budge S, Parker AH, Hutchings PT, Garbutt C. Environmental enteric dysfunction and child stunting. Nutr Rev. 2019;77(4):240–53.
Morais MB, Silva G. Environmental enteric dysfunction and growth. J Pediatr (Rio J). 2019;95(Suppl 1):85–94.
Bhattacharjee A, Burr A, Overacre-Delgoffe AE, Tometich JT, Yang D, Huckestein BR, et al. Environmental enteric dysfunction induces regulatory T cells that inhibit local CD4 + T cell responses and impair oral vaccine efficacy. Immunity. 2021;54(8):1745–57.
Najafi S, Mirshafiey A. The role of T helper 17 and regulatory T cells in tumor microenvironment. Immunopharmacol Immunotoxicol. 2019;41(1):16–24.
Guan X, Liu Z, Zhang J, Jin X. Myeloid-derived suppressor cell accumulation in renal cell carcinoma is correlated with CCL2, IL-17 and IL-18 expression in blood and tumors. Adv Clin Exp Med. 2018;27(7):947–53.
He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184(5):2281–8.
Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, et al. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407(2):348–54.
Yazawa T, Shibata M, Gonda K, Machida T, Suzuki S, Kenjo A, et al. Increased IL-17 production correlates with immunosuppression involving myeloid-derived suppressor cells and nutritional impairment in patients with various gastrointestinal cancers. Mol Clin Oncol. 2013;1(4):675–9.
Hamai A, Pignon P, Raimbaud I, Duperrier-Amouriaux K, Senellart H, Hiret S, et al. Human T(H)17 immune cells specific for the tumor antigen MAGE-A3 convert to IFN-gamma-secreting cells as they differentiate into effector T cells in vivo. Cancer Res. 2012;72(5):1059–63.
Gorczynski RM. IL-17 signaling in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1240:47–58.
Dixon ML, Luo L, Ghosh S, Grimes JM, Leavenworth JD, Leavenworth JW. Remodeling of the tumor microenvironment via disrupting Blimp1(+) effector Treg activity augments response to anti-PD-1 blockade. Mol Cancer. 2021;20(1):150.
Itahashi K, Irie T, Nishikawa H. Regulatory T-cell development in the tumor microenvironment. Eur J Immunol. 2022;52(8):1216–27.
Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, et al. Regulatory T cells in Tumor-Associated Tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity. 2015;43(3):579–90.
Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19(1):116.
Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, et al. Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4(164):164ra159.
Rizzo A, Di Giovangiulio M, Stolfi C, Franze E, Fehling HJ, Carsetti R, et al. RORgammat-Expressing Tregs drive the growth of Colitis-Associated Colorectal Cancer by Controlling IL6 in dendritic cells. Cancer Immunol Res. 2018;6(9):1082–92.
Eggenhuizen PJ, Ng BH, Ooi JD. Treg Enhancing Therapies to Treat Autoimmune Diseases. Int J Mol Sci. 2020;21(19):7015. https://doi.org/10.3390/ijms21197015.
Scheinecker C, Goschl L, Bonelli M. Treg cells in health and autoimmune diseases: new insights from single cell analysis. J Autoimmun. 2020;110:102376.
Yang J, Sundrud MS, Skepner J, Yamagata T. Targeting Th17 cells in autoimmune diseases. Trends Pharmacol Sci. 2014;35(10):493–500.
Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41(3):283–97.
Kondo Y, Yao Z, Tahara M, Iizuka M, Yokosawa M, Kaneko S, et al. Involvement of RORgammat-overexpressing T cells in the development of autoimmune arthritis in mice. Arthritis Res Ther. 2015;17(1):105.
Stangou M, Bantis C, Skoularopoulou M, Korelidou L, Kouloukouriotou D, Scina M, et al. Th1, Th2 and Treg/T17 cytokines in two types of proliferative glomerulonephritis. Indian J Nephrol. 2016;26(3):159–66.
Zhu L, Song H, Zhang L, Meng H. Characterization of IL-17-producing Treg cells in type 2 diabetes patients. Immunol Res. 2019;67(4–5):443–9.
Legge D. Yin and Yang surfaces: an evolutionary perspective. J Acupunct Meridian Stud. 2014;7(6):281–90.
Liu G, An R. Applying a Yin-Yang Perspective to the theory of Paradox: a review of Chinese Management. Psychol Res Behav Manag. 2021;14:1591–601.
Hu Z, Lu SH, Lowrie DB, Fan XY. Trained immunity: A Yin-Yang balance. MedComm (2020). 2022;3(1):e121.
Tang DC, Nguyen HH. The Yin-Yang arms of vaccines: disease-fighting power versus tissue-destructive inflammation. Expert Rev Vaccines. 2014;13(3):417–27.
Yan Q. Stress and systemic inflammation: Yin-Yang dynamics in Health and diseases. Methods Mol Biol. 2018;1781:3–20.
Zhang J. Yin and Yang interplay of IFN-gamma in inflammation and autoimmune disease. J Clin Invest. 2007;117(4):871–3.
Horton B, Spranger S. A Tumor Cell-Intrinsic Yin-Yang determining Immune Evasion. Immunity. 2018;49(1):11–3.
Kalvakolanu DV. The Yin-Yang of cytokines in cancer. Cytokine. 2019;118:1–2.
Meri S, Magrini E, Mantovani A, Garlanda C. The Yin Yang of Complement and Cancer. Cancer Immunol Res. 2023;11(12):1578-88. https://doi.org/10.1158/2326-6066.CIR-23-0399.
Gao W, Wu Y, Tian Y, Ni B. Yin-Yang regulation of RORgammat protein complex in Th17 differentiation. Int Rev Immunol. 2015;34(4):295–304.
Mousa HS, Jones JL. The Yin and Yang of intracellular reactive oxygen species following T-cell activation. Brain. 2021;144(10):2909–11.
Wei S. Yin-Yang regulating effects of cancer-associated genes, proteins, and cells: an ancient Chinese concept in vogue in modern cancer research. Biosci Trends. 2018;11(6):612–8.
Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–7.
Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–63.
Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25(6):941–52.
Tomura M, Yoshida N, Tanaka J, Karasawa S, Miwa Y, Miyawaki A, et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein Kaede transgenic mice. Proc Natl Acad Sci U S A. 2008;105(31):10871–6.
Morton AM, Sefik E, Upadhyay R, Weissleder R, Benoist C, Mathis D. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc Natl Acad Sci U S A. 2014;111(18):6696–701.
Levin B. Ulcerative colitis and colon cancer: biology and surveillance. J Cell Biochem Suppl. 1992;16G:47–50.
Shawki S, Ashburn J, Signs SA, Huang E. Colon Cancer: inflammation-Associated Cancer. Surg Oncol Clin N Am. 2018;27(2):269–87.
Slominski RM, Sarna T, Plonka PM, Raman C, Brozyna AA, Slominski AT. Melanoma, melanin, and Melanogenesis: the Yin and Yang Relationship. Front Oncol. 2022;12:842496.
Acknowledgements
Not applicable.
Funding
This study is supported by National Natural Science Foundation of China (82374320, 82074048).
Author information
Authors and Affiliations
Contributions
H.C. and N.W. wrote the manuscript with the help of H.L., Y.B., W.W., X.K. and F.W. All authors contributed to this manuscript. All authors read and approved the fnal manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, H., Wang, N., Li, H. et al. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: a crosstalk between ancient "Yin-Yang" theory and modern immunology. Cell Commun Signal 22, 99 (2024). https://doi.org/10.1186/s12964-024-01505-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-024-01505-0