Abstract
Yes-associated protein (YAP) is a pivotal regulator in cellular proliferation, survival, differentiation, and migration, with significant roles in embryonic development, tissue repair, and tumorigenesis. At the maternal–fetal interface, emerging evidence underscores the importance of precisely regulated YAP activity in ensuring successful pregnancy initiation and progression. However, despite the established association between YAP dysregulation and adverse pregnancy outcomes, insights into the impact of aberrant YAP levels in fetal-derived, particularly trophoblast cells, and the ensuing dysfunction at the maternal–fetal interface remain limited. This review comprehensively examines YAP expression and its regulatory mechanisms in trophoblast cells throughout pregnancy. We emphasize its integral role in placental development and maternal–fetal interactions and delve into the correlations between YAP dysregulation and pregnancy complications. A nuanced understanding of YAP's functions during pregnancy could illuminate intricate molecular mechanisms and pave the way for innovative prevention and treatment strategies for pregnancy complications.
Video Abstract
Similar content being viewed by others
Background
The Yes-associated protein 1 (YAP1), hereafter referred to as YAP, is a pivotal effector of the Hippo signaling pathway. It is renowned for its essential roles in embryonic development, organ regeneration, and tissue homeostasis [1,2,3]. The YAP signaling dysregulation is associated with the onset of various diseases [4,5,6]. Recent studies have revealed that YAP can sense intrinsic biochemical and extrinsic biomechanical cues, including mechanical signals, cell polarity, and energy status, and convert them into soluble cellular signals and metabolic pathways, which are instrumental in determining cell fate [7]. This intricate process is meticulously regulated, with the phosphorylation of YAP protein being a cardinal step. Phosphorylated YAP is sequestered in the cytoplasm and degraded by the ubiquitin–proteasome system. In contrast, unphosphorylated YAP translocates to the nucleus, modulating the transcription of genes pivotal for cell proliferation, differentiation, survival, and migration [8].
A successful pregnancy is contingent upon a cascade of sequential and discrete events, encompassing fertilization, implantation, decidualization, placentation, and parturition, each integral to the process [9]. The meticulous regulation of cell proliferation and differentiation at specific spatial and temporal junctures is indispensable. The first cell lineage specification in mammalian development occurs at the compact morula stage, culminating in a blastocyst comprising an outer trophectoderm (TE) and an inner cell mass (ICM). Subsequently, the TE undergoes gradual proliferation and differentiation to give rise to the placenta and contribute to the amnion-chorion membrane (referred to as fetal membranes) formation, a process contingent upon the equilibrium between trophoblast stem/progenitor cell proliferation and differentiation into distinct lineages. Notably, abnormalities in these trophoblast stem/progenitor cell lineages are implicated in pregnancy complications [10, 11]. Given the centrality of trophoblast cells in these processes, aberrant YAP activity has been linked to pregnancy complications like placental dysfunction and pregnancy-induced hypertension [12]. Therefore, understanding the regulatory mechanisms of YAP activity during pregnancy is paramount for elucidating the complex processes involved and developing strategies to prevent and treat pregnancy complications.
This review provides an exhaustive insight into YAP signaling, delineating its activation, regulatory mechanisms, and conjectured role in pregnancy complications. We meticulously examine the extant literature, aiming to unveil the complex interactions between YAP and pregnancy-associated pathologies, potentially illuminating pathways for innovative therapeutic interventions.
YAP signaling pathway
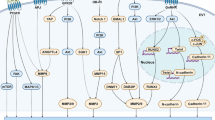
The YAP gene, located on chromosome 11q22, encodes a protein of 488 amino acids. This protein consists of various domains arranged from the NH2-terminus to the COOH-terminus, including a proline-rich domain, a TEA domain-containing sequence-specific transcription factor (TEAD-binding region), a 14–3-3 binding domain, one or two WW domains (YAP1 and YAP2 are two splicing variants, respectively), a Src homology domain 3- (SH3-) binding motif, a coiled-coil domain, a transcription activation domain (TAD), and a PDZ-binding motif [13, 14] (Fig. 1). YAP lacks a DNA binding domain, rendering it incapable of initiating gene transcription directly. It is initially known to interact with the transcription factor TEADs and serves as the primary effector of the Hippo signaling pathway [15, 16]. When the Hippo kinase module is activated, it phosphorylates the mammalian STE20-like protein kinase 1/2 (MST1/2)- Salvador homolog 1 (SAV1) complex or mitogen-activated protein kinase (MAP4Ks), resulting in the activation of the large tumor suppressor 1/2 (LATS1/2)-MOB kinase activator (MOB1A/B) complex or the nuclear Dbf2-related (NDR) kinases 1/2 (NDR1/2), respectively. Subsequently, the activated NDR1/2 and LATS1/2-MOB1A/B complex phosphorylates and inactivates YAP, leading to its retention in the cytoplasm and subsequent degradation [17,18,19]. Conversely, when the Hippo pathway is inactive, non-phosphorylated YAP translocates to the nucleus and stimulates gene expression by binding to the TEAD transcription factor family.
Regulatory domains of YAP. YAP protein is composed of a proline-rich domain (Pro-rich), a TEAD-binding region, a 14–3-3 binding domain, one or two WW domains (two splicing variants, YAP1 and YAP2, respectively), an Src homology domain 3- (SH3-) binding motif, a coiled-coil domain, a transcription activation domain, and a PDZ-binding motif. There are two ways in which the YAP protein is regulated, one is direct phosphorylation regulation and the other involves binding to its partner through a specific domain. S, Serine. T, Threonine
This review delves into the diverse upstream mechanisms that modulate YAP signaling activity, including the posttranslational modification of the YAP protein, the regulators that influence YAP signaling, and the interplay between YAP signaling and other signaling pathways.
Posttranslational modifications of the YAP protein
YAP activity is intricately modulated by posttranslational modifications, which dictate its intracellular distribution and abundance (Fig. 1). A plethora of research underscores the pivotal role of serine residue phosphorylation in the switch for YAP function. LATS1/2 and NDR1/2 orchestrate the phosphorylation of YAP at specific serine residues (Ser61, Ser109, Ser127, Ser164, and Ser381 for LATS1/2; Ser61, Ser109, and Ser127 for NDR1/2) within the HxRxxS motifs [19, 20]. The phosphorylation events at Ser127 and Ser381 are crucial for modulating YAP activity. For instance, the phosphorylation at Ser127 engenders a binding site for 14–3-3 proteins, culminating in the cytoplasmic sequestration of YAP and the subsequent inhibition of its transcriptional activity [21]. In contrast, NLK-mediated phosphorylation at Ser128 fosters YAP's nuclear localization by impeding its interaction with 14–3-3 proteins [22]. Moreover, the initial phosphorylation at Ser384 and Ser387 facilitates subsequent phosphorylation by casein kinase 1 (CK1), leading to the recruitment of SCFβ-TRCP E3 ligase, YAP ubiquitination, and its eventual proteasomal degradation [23]. Interestingly, YAP activity can also be inhibited by Akt (which phosphorylates YAP at Ser127) [24] and protein kinase C ζ (PKCζ, which phosphorylates YAP at Ser109 and Thr110) [25].
Beyond the realm of phosphorylation, the PDZ-binding motif emerges as another significant player in the regulatory landscape of YAP (Fig. 1). The tight junction protein zonula occludens 2 (ZO-2) can interact with YAP's PDZ-binding motif, promoting its nuclear translocation [26]. Similarly, Na + /H + exchanger regulatory factor 1 (NHERF1) exhibits a high affinity for YAP's PDZ-binding motif [27]. Evidence suggests that the abrogation of the PDZ-binding motif attenuates YAP/TEAD-mediated transcriptional activity, underscoring its significance in YAP function [28].
Key regulators and upstream signaling molecules of YAP
The regulation of the YAP signaling pathway is uniquely complex, given its absence of a dedicated cell surface receptor. Consequently, its modulation is intricately tied to the cellular context (Fig. 2).
Upstream regulators of YAP. MST1/2, LATS1/2, MAP4Ks and NDR1/2 are the core components of the kinase cascade of the hippo pathway. Besides, YAP activity can be regulated by cellular context (tight junctions, adherens junctions, and soluble factors (LPA, S1P, Glucagon, and epinephrine)) and crosstalk with other signaling pathways (WNT signaling pathway, Notch signaling pathway, and NF-κB signaling pathway)
Tight junction and adherens junction
Cellular contacts are instrumental in modulating YAP activity, a critical aspect for overseeing embryonic development and sustaining tissue architecture in mature organisms. An increase in cell density correlates with a rise in the number of tight junctions (TJs) and adherens junctions (AJs), leading to the suppression of YAP translocation from the cytosol to the nucleus. AJs, characterized by their composition of transmembrane cadherin-catenin complexes [29], facilitate the cytoplasmic retention of YAP via interactions with α-catenin [30]. In a similar vein, tight junction proteins, including the angiomotin family of proteins (AMOT), protein tyrosine phosphatase nonreceptor type 14 (PTPN14), and ZO-1/2, obstruct YAP function through their direct interactions [31,32,33].
Soluble factors
In mammals, soluble factors, encompassing hormones and growth factors, serve as conduits for transmitting organismal and distal signals from the extracellular environment, orchestrating cellular responses. G protein-coupled receptors (GPCRs), the largest family of cell surface receptors in the human genome, have the capacity to either amplify or attenuate YAP activity. This is achieved through signaling cascades initiated by heterotrimeric G-proteins, which are activated by GPCRs. Specifically, G12/13- and Gαq/11-coupled receptors, upon being stimulated by lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P), inhibit Lats1/2 kinase activity, leading to YAP activation. In contrast, molecules like glucagon and epinephrine, signaling through Gαs-coupled receptors, enhance Lats1/2 kinase activity, resulting in the suppression of YAP function [34]. The interplay between protein kinases, Rho GTPases, and the remodeling of the actin cytoskeleton is pivotal in mediating GPCR signaling and, consequently, YAP regulation [35]. The Gα12/13-RhoA-ROCK pathway augments YAP activity by inhibiting LATS1/2-induced phosphorylation of YAP and by modulating the assembly of F-actin and myosin [36]. However, the influence of protein kinases on YAP activity is nuanced and distinct. While GPCR-PKC/PKD signaling enhances YAP nuclear localization, PKA fosters YAP phosphorylation, leading to its cytoplasmic retention [37].
Crosstalk between YAP signaling and other pathways
Interactions between the YAP signaling pathway and other cellular signaling networks are pivotal in regulating various cellular functions. This section delineates the intricate crosstalk between YAP and other embryonic development-associated pathways, including WNT, Notch, and NF-κB signaling (Fig. 2).
WNT signaling pathway
The WNT signaling pathway is instrumental in orchestrating stem cell maintenance, cellular proliferation, and the determination of cell fate. In the absence of WNT ligands, β-catenin, a cytosolic protein, is targeted and phosphorylated by a heterotetrameric "destruction complex" consisting of Axin, APC, CK1, and GSK3β. This interaction facilitates the subsequent degradation of β-catenin by the β-Trcp ubiquitin ligase. However, the engagement of WNT ligands with lipoprotein receptor-related protein 5/6 (LRP-5/6) and frizzled protein (FZD) receptors on the cellular surface activates DVL, which in turn recruits the destruction complex to the receptor, elevating the cytosolic concentration of β-catenin. This accumulation of β-catenin facilitates its nuclear translocation, where it partners with T-cell-specific factor (TCF)/lymphoid enhancer-binding factor (LEF) to initiate the transcription of WNT target genes [38].
The crosstalk between YAP and WNT signaling is intricate and has been reported at various levels (Fig. 2). In the cytoplasm, YAP functions as an inhibitor of β-catenin, promoting its degradation mediated by β-Trcp through its association with the destruction complex. The presence of WNT ligands triggers the dissociation of YAP and β-catenin from the destruction complex, enabling their nuclear translocation and the subsequent activation of target genes [39, 40]. YAP also curtails enhanced WNT signaling independently of the APC/Axin/GSK3β complex, either by inhibiting DVL activity or by directly binding and sequestering β-catenin in the cytoplasm [41, 42].
In the nuclear compartment, YAP emerges as a key effector of WNT signaling. The β-catenin/TCF-4 complex augments YAP transcription by associating with its promoter [43]. There is evidence of the existence of YAP/β-catenin [44], YAP/β-catenin/TCF4 [45], and YAP/TEAD4/β-catenin [46] complexes, underscoring their substantial role in mediating cell proliferation through YAP or WNT signaling. These observations accentuate the functional overlap between YAP and β-catenin, necessitating further investigations to elucidate their shared and distinct biological roles.
Notch signaling pathway
The Notch signaling pathway, characterized by its evolutionary conservation, plays a pivotal role in orchestrating a myriad of developmental processes. It operates as a mechanotransduction pathway, which involves direct interactions between receptors and ligands to facilitate the transmission of cellular information between adjacent cells. The pathway comprises four distinct receptors (Notch1-4) and five ligands (Jagged 1 and 2, Delta-like 1, 3, and 4) in mammals. The activation of this pathway is initiated when a Notch receptor on one cell engages with a Notch ligand on an adjacent cell [47]. This interaction triggers the sequential cleavage of the Notch receptor by ADAM-family metalloproteases and γ-secretase, releasing the Notch intracellular domain (NICD). The NICD then migrates to the nucleus, where it associates with recombining binding protein suppressor of hairless (RBP-Jκ, also known as CSL or CBF1), instigating the transcription of Notch target genes, including those encoding basic helix-loop-helix (bHLH) transcriptional repressors like Hes/Hey [48].
The crosstalk between YAP and Notch signaling has been substantiated by numerous studies, underscoring their intertwined regulatory mechanisms (Fig. 2). YAP augments the Notch signaling pathway by enhancing the expression of Notch ligands and receptors, such as Notch1/2/3, Jagged 1/2, and Delta-like ligands 1/3 [49, 50]. However, the intricate molecular mechanisms underpinning this regulation are yet to be fully elucidated. A handful of studies have documented YAP’s capability to associate with the promoter of Notch2 and the distant enhancer of Delta-like ligands [49, 51]. Furthermore, YAP has been observed to interact with NICD physically, forming the YAP-NICD complex, which is recruited to chromatin by RBP-Jκ, contributing to the modulation of Notch signaling [52].
Reciprocally, Notch signaling exerts influence over YAP signaling [53]. A comprehensive mice genome-wide study utilizing ChIP-Seq and transcriptome analyses pinpointed YAP as a direct target of the RBPJ/N1ICD complex. Remarkably, the expression of YAP is potent enough to counteract the inhibition of the Notch pathway in neural stem cell self-renewal assays [54]. A recent study unveiled an activated Notch-YAP circuit that fosters stemness and tumorigenesis in embryonal rhabdomyosarcoma. In this context, Notch signaling elevates both YAP gene expression and activity, while YAP reciprocally boosts the transcription of JAG1, DLL1, and RBPJ mRNA levels [55]. These findings highlight the functional redundancy between YAP signaling and Notch signaling. Elucidating the primary and secondary relationships of these signaling pathways and the molecular mechanisms underlying their crosstalk under distinct physiological conditions holds a significant interest.
NF-κB signaling pathway
The NF-κB signaling pathway plays a crucial role in mediating inflammatory responses. In its inactive state, NF-κB is sequestered in the cytoplasm by inhibitor kappa B (IκB) proteins. Various stimuli, including proinflammatory cytokines like IL-1β and TNF-α, activate transforming growth factor-beta-activated kinase 1 (TAK1) and the IκB kinase complex, leading to IκBα phosphorylation, ubiquitination, and degradation. This process liberates NF-κB, allowing its translocation to the nucleus to regulate a plethora of target genes. The classic NF-κΒ family consists of NF-κB1 (p105/p50), NF-κB2 (p100/p52), p65 (RelA), c-rel, and RelB. Homodimers or heterodimers of these family members assemble into active NF-κΒ transcription factors [56].
The interaction between YAP and NF-κB signaling is multifaceted. Proinflammatory cytokines TNF-α and IL-1β have been shown to inhibit YAP expression in a concentration-dependent manner, a process potentially mediated by p65/NF-κB inhibiting ΔNp63 [57]. On the flip side, increased YAP levels can counteract inflammation by inhibiting NF-κB signaling [58]. YAP achieves this by enhancing the transcription of IκBa [59] and interacting with key upstream components of the NF-κB pathway, including TAK1 and TRAF6 [60, 61]. Moreover, YAP can suppress the transcription of NF-κB target genes like cyclooxygenase 2 (COX2), especially at low cell densities, by recruiting HDAC7 to the COX2 promoter region in conjunction with TEAD, even in the presence of IL-1β and TNF-α-induced NF-κB activity [62] (Fig. 2).
Interestingly, a positive regulatory relationship also exists between YAP and NF-κB signaling (Fig. 2). In adult T-cell leukemia/lymphoma cells, Tax-induced p65 activation interrupts the YAP-LATS1 interaction, preventing YAP phosphorylation. Activated p65 then associates with YAP to enhance the expression of YAP target genes [63]. YAP and p65 interaction also plays a pivotal role in modulating the macrophage inflammatory response to lipopolysaccharide (LPS) stimulation [64]. IKKβ/ε has emerged as a novel modulator of YAP phosphorylation, with activated YAP and NF-κB working in tandem to regulate the transcription of downstream genes [65]. Furthermore, YAP augments NF-κB signaling by inhibiting the expression of ubiquitin-specific peptidase 31 (USP31), a potent NF-κB inhibitor [66].
The role of YAP in trophoblast cells
The TE constitutes the outer layer of the human blastocyst, giving rise to both the placental and fetal membrane trophoblast post-implantation. However, due to ethical constraints and limited human models, our understanding of the TE's early developmental stages is limited. Early TE growth and differentiation have been delineated through descriptive studies of human embryonic material [67, 68], bulk transcriptome analyses, and in vitro research employing primary cells, cell lines, and villous explants.
Approximately 4–5 days post-fertilization, the TE emerges, marking the inaugural cell fate specification and distinguishing the TE from the ICM [67]. The TE is characterized by its polarity. The polar trophectoderm, a distinct segment of the trophectoderm adjacent to the ICM, binds to the receptive endometrial epithelium around 5–6 days post-fertilization, initiating human placental development. Notably, polar trophectoderm cells, at the onset of implantation, exhibit invasive and proliferative traits, unlike their distal trophoblast counterparts [69].
Upon establishing a stable connection with the maternal endometrium, the polar trophectoderm differentiates into the first trophoblast lineages: the multinucleated primitive syncytium (PS) [70]. Concurrently, the cytotrophoblast cells proliferate swiftly, forming projections that pierce the PS, leading to the creation of primary villi that delve into the maternal decidua, eroding its blood vessels and glands. The epithelial surface undergoes branching and expansion due to the continuous proliferation and fusion of emerging villous cytotrophoblast (CTB). This results in the formation of the outer syncytiotrophoblast (STB) layer, which interfaces directly with maternal blood, facilitating the exchange of oxygen, nutrients, and waste.
In addition to chorionic villi development, CTBs at distal locations spread laterally, constructing the trophoblastic shell. This structure serves as the origin for the second differentiated trophoblast cell type, the extravillous trophoblasts (EVT). Once mature villi are established, EVTs derive from the differentiation of CTBs at the tips of anchoring villi. In the cytotrophoblastic cell column, proximal cell column trophoblasts exhibit a proliferative phenotype, representing EVT lineage progenitors [71]. In contrast, distal cell column trophoblasts differentiate into EVTs, which widely lose their replicative capability, transitioning into a senescent state [72]. Functionally, EVTs can be further categorized into interstitial EVTs, which anchor the placenta by invading the uterine wall, and endovascular EVTs, which permeate maternal decidual arterioles and glands, enhancing nutrient and oxygen transport [73]. Trophoblast lineage differentiation persists throughout gestation and remains consistent until term. Any anomalies in trophoblastic differentiation can lead to pregnancy complications, such as miscarriages [74].
Conversely, insights into the trophoblast lineage in the smooth chorion are sparse. Smooth chorionic trophoblast cells are organized in an epithelial configuration. Prior research indicates that smooth chorionic CTBs penetrate the uterine wall, promoting the fusion of the smooth chorion with the parietal decidua [75]. Unlike placental EVTs, which remodel maternal arteries, smooth chorionic CTBs refrain from invading maternal blood vessels in the decidual tissue [75]. Recent single-cell RNA sequencing of the smooth chorion has identified CTBs, EVTs, and STBs subtypes. Notably, CTB 4 emerges as a unique subtype exclusive to the smooth chorion, distinct from the villous chorion [76], implying divergent functions between the placental chorion and the smooth chorion.
Recently, the advent of the human trophoblast stem cell model [77], trophoblast organoids [78], and stem cell embryo models, including blastoids [79], has facilitated investigations into the role of transcription factors in human trophoblast differentiation. In the human blastoids model, atypical protein kinase C (aPKC) and F-actin expression domains were observed to align in outer cells, which also exhibited nuclear YAP accumulation. TE specification and morphogenesis are contingent upon aPKC, Hippo pathway inhibition, YAP's nuclear translocation, and its binding affinity to TEAD transcription factors [79]. Furthermore, various studies have underscored the implications of aberrant YAP activity on trophoblast lineage development and cellular dysfunction.
This review delves into YAP's influence during trophoblast lineage evolution.
YAP in trophoblast differentiation
The initiation of the pre-implantation trophectoderm (TE) program occurs in the outer cells of the morula stage. These cells establish apical-basal cell polarity, a process governed by the activation of aPKC, which in turn modulates the expression and nuclear translocation of YAP and GATA-binding protein 3 (GATA3). This mechanism is conserved across human, bovine, and murine species [80]. By the blastocyst stage, the YAP/TEAD complex, localized in the nuclei of outer cells, induces the expression of caudal-type homeobox transcription factor 2 (CDX2), facilitating human TE specification. This observation aligns with previous findings in mouse blastocysts. In mouse ICM, YAP is phosphorylated and confined to the cytoplasm, a process yet to be thoroughly examined in humans [79, 81] (Fig. 3).
The dynamic changes of YAP activity in trophoblasts during the formation of human placental anchoring villus. A At the blastocyst stage, the trophectoderm has high YAP activity. At the beginning of embryo implantation, trophoblast located in the polar trophectoderm comes into contact with uterine epithelial cells and rapidly proliferates and fuses to form the invasive primitive syncytium, which is the first event in trophoblast specification. B The dynamic changes of YAP, NOTCH, and WNT/β-catenin signaling in the development of placental anchoring villus. CTB, cytotrophoblast. STB, syncytiotrophoblasts. pCCT, proximal cell column trophoblast. dCCT, distal cell column trophoblasts. iEVT, interstitial extravillous trophoblast
In mouse embryos, the YAP/TEAD4 complex is known to modulate the transcriptional activity of CDX2, in conjunction with the Notch signaling pathway [82]. Strawberry Notch1 (Sbno1), a highly conserved chromatin factor, has been identified in mice to influence the trophectoderm-enhancer (TEE) of CDX2, ensuring its robust activation by the YAP-TEAD4 and NICD-RBPJ complexes [83]. This differential YAP signaling is pivotal in determining the fate specification of trophoblast cells during TE formation.
The TE serves as a precursor to both placental villous trophoblast and the smooth chorionic trophoblast cells. Aberrations in trophoblast differentiation can precipitate placentation failures and subsequent fetal and maternal complications [84]. Differentiated human trophoblasts, encompassing chorion trophoblast cells (CTCs), CTBs, STBs, and EVTs, execute diverse functions throughout pregnancy. Evidence suggests that CTB growth and/or cell fusion are compromised in cultures derived from human placentas associated with preeclampsia (PE) or fetal growth restriction (FGR) [85, 86]. Moreover, CTBs sourced from preeclamptic placentas display defects in EVT formation in vitro [87].
The regulatory elements steering human placental differentiation remain elusive. Emerging research posits YAP as a potential regulator of trophoblast lineage formation and placental expansion. YAP exhibits varied expression across different trophoblast populations and interacts with a unique set of transcription factors in the early human placenta [88]. It is notably absent in hormone-producing STBs but is strongly expressed in CTBs and cell column trophoblasts (CCTs) of early human placenta, with muted expression in EVTs [89]. The YAP/TEAD complex is instrumental in preserving CTB stemness and also modulates the differentiation of CTBs into STBs and EVTs. Recent insights from studies utilizing primary cells, three-dimensional organoids, and CRISPR-Cas9 genome-edited JEG-3 clones have unveiled that human CTBs can spontaneously differentiate into syncytiotrophoblast-like cells. The inhibition of YAP/TEAD activity is integral to this differentiation process [89]. Further exploration of the early human placenta has highlighted the role of S100P and cAMP signaling-induced YAP activity inhibition in triggering the syncytialization of CTB-derived human TSCs [90, 91].
The modulation of WNT downstream effectors and NOTCH receptor expression in human first-trimester placental tissues is instrumental in directing the differentiation of EVT progenitors into EVTs [71, 92]. NOTCH signaling components, akin to the YAP expression pattern, display varied expression across distinct trophoblast subtypes within the placental villus. NOTCH1 ICD is identifiable in EVT progenitors, while NOTCH2 is predominantly expressed in EVTs [71, 93]. YAP, potentially through its interaction with Notch1 or Notch2, is implicated in the regulation of CTB differentiation into EVTs and EVT progenitor cell differentiation. The activation of canonical WNT signaling plays a crucial role in TSC and/or CTB progenitor expansion, as well as the regulation of EVT migration and differentiation [94]. It is speculated that the crosstalk between YAP transcription factors and WNT signaling is deemed essential for balancing TSC and/or CTB progenitor expansion and EVT differentiation. In murine TSCs derived from blastocysts, nuclear YAP accumulation is observed, while it is primarily localized in the cytoplasm in differentiated trophoblast cells [95].
YAP-mediated stemness maintenance and proliferation of trophoblast cells
YAP contributes to sustaining the stemness and proliferation of CTB progenitors, mediated through intricate genomic mechanisms. In the developing human placenta, YAP-TEAD4 complexes are pivotal in activating genes associated with the cell cycle and stemness. Concurrently, they repress genes implicated in trophoblast cell fusion, thus promoting CTBs growth and expansion [89]. The role of the YAP-TEAD4 complex can extend to being a vital regulator of murine TE development, where it activates CDX2 and other key TE regulators in the outer cells of preimplantation embryos [81]. In murine TSCs, the nuclear translocation of YAP facilitates its WW2 domain to interact with the PPQY motif of CDX2. This interaction is central to modulating trophoblast proliferation by downregulating CyclinD1 levels [95]. These findings indicate that the nuclear presence of YAP not only augments trophoblast cell expansion but also intrinsically moderates trophoblast proliferation. The equilibrium between pro-proliferative and inhibitory actions mediated by YAP is fundamental to trophoblast lineage differentiation and fostering a healthy pregnancy. Therefore, positioning YAP as a prospective target for interventions in hyperplastic trophoblast disorders, albeit necessitating further research.
The activation of the WNT signaling pathway may be necessary for in vitro TSC/CTB progenitor expansion. Yet, the principal components of this pathway in vivo are to be fully delineated. Nuclear recruitment of YAP in the Hippo-off state could destabilize the cytoplasmic β-catenin destruction complex, culminating in β-catenin's nuclear accumulation. YAP may also interact with TCF-1 to ensure the self-renewal of CTBs in cytotrophoblast organoids from the human placenta [96]. Moreover, Wnt3a has been implicated in maintaining bovine TSCs, modulating CDX2 expression via the WNT-YAP signaling pathway [97]. In essence, the interplay between WNT signaling and YAP is anticipated to be vital for TSC expandability. YAP underscores its significance in maintaining cell proliferation and stemness, warranting extensive studies to comprehensively unravel its intricate mechanisms and broader implications.
YAP in trophoblast invasion
Abnormal placental development has profound implications for both maternal and fetal health, with inadequate trophoblast invasion often linked to severe conditions such as PE and FGR [87, 98]. A notable reduction in YAP expression in preeclamptic placentas underscores the integral role of YAP signaling in the etiology of PE, particularly in modulating trophoblast invasion [99, 100].
Empirical studies elucidate that YAP overexpression enhances cell invasion capabilities in BeWo, HTR-8/SVneo which is a heterogeneous population consisted by trophoblast and stromal/mesenchymal cells, and JAR cells [100]. In a nuanced interaction, miR-326 suppresses trophoblast cell (HTR-8/SVneo and JEG-3 cells) growth, invasion, and migration by targeting PAX8-mediated YAP expression [101]. The activation of the Hippo/YAP signaling pathway in human trophoblasts from PE-complicated pregnancies is linked to inhibiting trophoblast invasion and migration. This inhibition is mediated by the upregulation of miR21, which impedes PP2A β function, leading to LATS1-YAP phosphorylation and the subsequent cytoplasmic retention of YAP, thereby restraining EVT invasion and migration [102].
It has been evident in the crosstalk between Notch and WNT signaling in cell migration and invasion. Notch2 signaling, for instance, has been implicated in attenuating trophoblast migration in human distal cell column trophoblasts. Conversely, Wnt3A enhances the migration and invasion of trophoblast cells isolated from the early placenta by magnetic bead sorting, an effect that can be mitigated by Dickkopf-1 [93, 103]. The complex interactions among Notch, WNT, and YAP signaling pathways are anticipated to be central in trophoblast invasion, necessitating comprehensive research to unravel their synergistic and antagonistic mechanisms.
YAP and pregnancy complications
The intricate balance of trophoblast cell proliferation and differentiation is fundamental to the development of the placenta [104]. The human placental STB are instrumental in fostering maternal immune tolerance through secreting immunosuppressive proteins such as PD-L1 and type III IFNs and exhibit an enhanced resistance to infection, being 20-fold more resilient than CTBs [105,106,107]. In parallel, research involving rodents and HTR-8/SVneo cells indicates that EVTs possess a unique ability to induce a regulatory phenotype in decidual immune cells, promoting fetal tolerance [108].
Beyond its immune functions, the chorion, as a barrier, is integral in modulating intrauterine prostaglandin (PG) concentrations and metabolism. This is particularly evident in the role of human amniotic membrane-derived PGs in initiating labor through the facilitation of cervical ripening and myometrial contractions [109, 110]. NAD-dependent 15-hydroxy-PG dehydrogenase (15-PGDH), an enzyme responsible for converting active PGs into inactive forms, is predominantly expressed in the chorionic trophoblast layer. Notably, lower expression levels of 15-PGDH are observed in spontaneous labor at term compared to elective caesarean sections, with a further reduction noted in preterm labor without infection [111]. Moreover, the intensity and number of 15-PGDH-positive cells are markedly reduced in the chorionic trophoblast layer of preterm patients with diagnosed infection compared to idiopathic preterm patients without a diagnosed infection [112].
Mounting evidence suggests that dysregulation of biochemical, endocrine, and immunological processes at the maternal–fetal interface, particularly chorionic dysfunction, is linked to adverse pregnancy outcomes. The restricted proliferation of human trophoblast stem cells (TSCs) in early pregnancy correlates with recurrent spontaneous abortion (RSA) [113]. Concurrently, anomalies in the proliferation, migration, and invasion of trophoblast cells are implicated in severe pregnancy complications, including FGR and PE [114, 115]. Notably, abnormal trophoblast differentiation is also a key factor in pregnancy complications, as CTBs extracted from the placentae of preeclamptic patients have been observed to exhibit defects in EVTs formation [87], and impairments in CTBs growth and cell fusion are noted in cases of PE and FGR [89].
To enhance the understanding of YAP's role in these complications, future research should focus on elucidating the specific mechanisms through which YAP signaling influences trophoblast cell functions, immune interactions at the maternal–fetal interface, and the onset of labor, providing insights for potential therapeutic interventions.
Spontaneous miscarriage
Miscarriage, defined as the spontaneous loss of a pregnancy before 24 weeks of gestation, is a common complication, affecting approximately 15% of all pregnancies [9]. During the first trimester, a significant proportion of early miscarriages are attributed to impaired placentation due to developmental or functional anomalies in the trophoblastic lineage and chromosomal abnormalities [116, 117]. A subset of placentae from patients with idiopathic recurrent pregnancy loss (RPL) displays compromised CTBs/STBs bilayer formation and defective trophoblastic column formation [118].
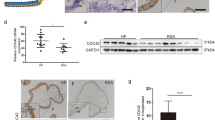
A marked reduction in YAP activity has been observed in the villi tissue of miscarriage samples. In healthy pregnancies (HP) around 6–8 weeks of gestation, YAP and Ki-67 are primarily localized in the nucleus of CTBs within the villous tissue. In contrast, in RSA cases of similar gestational age, phosphorylated YAP (p-YAP) is predominantly found in the cytoplasm of CTBs. This elevation in p-YAP levels is facilitated by the suppression of CDC42/EZRIN signaling, leading to the human TSCs differentiation and the inhibition of their proliferation [113]. Additionally, evidence suggests that the absence of TEAD4 curtails the self-renewal of both mouse and human CTB progenitors by downregulating essential cell cycle gene expression [118], underscoring YAP's role in modulating CTB proliferation through the activation of TEAD4-mediated gene expression.
Increased YAP activity in CTBs is also associated with RPL. Patients experiencing RPL have been found to have reduced serum S100P levels compared to healthy individuals. The inadequate levels of S100P are unable to inhibit YAP/TEAD signaling effectively, resulting in the sustained progenitor status of CTBs and hampering the trophoblast syncytialization of CTBs-derived human TSCs [91]. However, the specific mechanism through which S100P inhibits YAP/TEAD signaling in the human placenta remains to be elucidated.
Excessive inflammation is recognized as a contributing factor to miscarriage [119]. Elevated levels of TNF-α have been detected in the serum of women undergoing miscarriage [120]. Rodent studies have shown that mouse blastocysts, when pre-treated with TNF-α in vitro, exhibit increased mortality rates upon transfer into pseudopregnant mice. Additionally, the nucleoplasmic ratio of NF-κB p65 in villous stromal cells of the human placenta from early spontaneous abortions is significantly elevated compared to controls [121]. It has been reported that NF-κB activation augments CXCL8 expression and triggers the release of TNF-α and IL-1β, instigating unexplained RPL through the activation of the inflammatory response in human placental trophoblasts [122]. Moreover, a result of immunological staining has reported higher levels of tumor necrosis factor receptor 1 (TNFR1) in villous stromal cells from early spontaneous abortion samples compared to those from normal pregnancies of matched gestational age, while TNFR1 levels in placental trophoblasts did not exhibit significant variations between the two groups [123]. These insights underscore the role of the activated TNF-α/TNFR1/NF-κB signaling pathway in villous stromal cells and placental trophoblasts in precipitating immunological pregnancy loss.
Preeclampsia
Preeclampsia is a complex disorder diagnosed by the onset of hypertension (BP > 140/90 mmHg) and proteinuria (> 300 mg/24 h) post the 20 weeks of gestation. It is often accompanied by renal and liver dysfunction, uteroplacental insufficiency, and FGR [115]. The condition is partly attributed to impaired trophoblast invasion, along with insufficient spiral arterial remodeling, oxidative stress, and trophoblast dysfunction [124, 125].
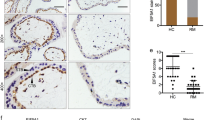
YAP has been implicated in PE development. A significant reduction in both mRNA and protein levels of YAP has been observed in placentas from patients with severe PE (sPE) compared to those from normal pregnancies [100]. Further investigation revealed a notable decrease in the number of CTBs and EVTs with high YAP nuclear expression in PE placentas compared to age-matched controls, suggesting inactive YAP signaling at the onset of PE [102, 114]. The elevated expression levels of MST1/2 in sPE placentas further suggest that YAP signaling inactivation occurs through the dual mechanisms of mRNA level reduction and YAP protein degradation induced by Hippo signaling [100].
YAP dysfunction is increasingly recognized for its role in PE development, particularly by inhibiting cytotrophoblast invasion. In HTR-8/SVneo cells, 17β-estradiol (E2) stimulates G protein-coupled estrogen receptor (GPER) activation, promoting YAP nuclear translocation and subsequently enhancing trophoblast cell invasion via the upregulation of angiopoietin-like 4 (ANGPTL4) [114]. Given the reported lower serum E2 levels in PE patients, a deficiency in E2 synthesis or signaling is suggested as a contributing factor to PE development [126]. Consequently, downregulation of the GPER/YAP/ANGPTL4 axis due to decreased estrogen levels exacerbates trophoblast cell invasion impairment, intensifying PE pathogenesis [114].
Sphingosine-1-phosphate (S1P), another GPCR ligand, is implicated in regulating YAP activity in trophoblasts affected by PE. While S1P synthesis and expression are typically abundant in normal trophoblasts, the placentas of PE mice exhibit reduced S1P levels. Sphingosine-1-phosphate receptor-2 (S1PR2), a GPCR located on the membrane, acts as a known receptor of S1P [127]. S1P enhances HTR8/SVneo cell invasion in a YAP-dependent manner, stimulated by S1PR2 and downstream Rho/ROCK-induced actin polymerization. However, diminished S1P expression in PE mice placentas attenuates this YAP activity, resulting in compromised EVTs invasion [12].
The role of microRNAs (miRNAs) in modulating YAP activity is also evident. Elevated levels of microRNA let-7a in human early-onset sPE placentas inhibit YAP levels by directly binding to its 3' UTR, leading to increased apoptosis in JEG-3 cells [115]. Concurrently, enhanced miR21 levels in EVTs from human PE pregnancies are associated with reduced trophoblast invasion. In vitro, miR21 suppresses HTR-8/SVneo cell invasion and migration by elevating cytoplasmic p-YAPSer127 and LATS1Thr1079 levels, mediated by the reduction of PP2A β levels [102].
During PE, the placenta is exposed to excessive oxidative stress and inflammation due to insufficient spiral arterial remodelling, leading to increased NF-κB activity [128]. NF-κB protein levels in the placenta of women with PE were significantly higher than those in normotensive pregnant women [129]. In first-trimester human villous explants, LPS treatment upregulates IL-6, IL-1β, IL-8, RANTES, and TNF-α levels, inducing trophoblast cell apoptosis [130]. Targeting NF-κB can ameliorate LPS-induced trophoblast dysfunction [131], and antagonizing the TLR4 signaling pathway can improve the LPS-induced PE-like phenotype in rodents associated with NF-κB inactivation in the placenta [132]. Thus, inhibiting the TLR4/NF-κB signaling pathway emerges as a promising therapeutic strategy for PE, mitigating inflammation-induced pyroptosis [133].
Preterm birth
Preterm birth, characterized by deliveries occurring between 28 and 37 weeks of gestation, is a complex condition with multifactorial etiologies. The involvement of YAP, though not directly established, can be inferred through its interaction with molecules and pathways implicated in preterm birth.
NF-κB, a pivotal player in inflammatory responses, has been studied in the context of fetal membranes. Research utilizing an ex vivo model of perfused full-thickness term fetal membranes revealed augmented nuclear translocation of p65 in human CTCs following LPS treatment on the decidual surface [134]. NHERF1, a negative regulator of YAP activity, is implicated in escalating the release of proinflammatory cytokines mediated by NF-κB across various cell types [135, 136]. In a comparative study between preterm and term births focusing on the human fetal membrane's amnion and chorion layers, elevated NHERF1 protein levels were discerned in the preterm group. This observation is corroborated by in vitro findings where LPS treatment escalated NHERF1 levels in human primary CTCs. An inflammation-associated preterm labor mouse model, subjected to LPS treatment, exhibited preterm delivery within 24 h and heightened NHERF1 levels in fetal membranes, underscoring the potential link between YAP inactivity, NHERF1 elevation, and NF-κB signaling activation in inflammation-induced preterm birth [137]. However, this hypothesis requires further support from direct data.
Tight junctions and adherens junctions play crucial roles in maintaining the physical barrier function of epithelial cells. YAP participates in tight junction formation by interacting with the tight junction-related protein ZO-1, which subsequently regulates cell migration [138]. ZO-1 deficiency in mouse embryos precipitates a lethal phenotype marked by impaired yolk sac angiogenesis and increased embryonic cell apoptosis [139]. During pregnancy, ZO-1 participates in human trophoblast differentiation and placental defense mechanisms [140, 141]. Predominantly found in human placental CTBs, reduced ZO-1 expression is associated with the transition of CTBs to STBs, mirroring YAP's inhibitory effect on trophoblastic syncytialization [89, 141]. In normal pregnancies, ZO-1 expression is evident in both human amniotic epithelial cells and chorionic trophoblast cells. However, a negative correlation emerges between the severity of intrauterine infection and ZO-1 expression in chorionic trophoblast cells, not in the amniotic epithelium [142]. The intricate dance between YAP, NF-κB, NHERF1, and ZO-1 in the context of preterm birth underscores the necessity for comprehensive investigations.
Fetal growth restriction
Fetal growth restriction, also known as intrauterine growth restriction (IUGR), refers to the failure of a fetus to achieve its genetic growth potential. It is a common pregnancy complication associated with multiple adverse perinatal outcomes. While various factors contribute to the pathophysiology of FGR, impaired placentation is considered the primary and most prevalent cause due to the crucial role of the placenta in providing optimal conditions for fetal growth in utero [143]. Deficiencies in extravillous trophoblast invasion and maternal arterial remodeling are central to placental dysfunction in FGR [144].
YAP expression dynamics are notable in the context of FGR. While YAP is expressed in CTBs, it is markedly downregulated or degraded in STBs. The human FGR placentas exhibit increased YAP phosphorylation compared to their normal counterparts. A study utilizing an ERK inhibitor-induced FGR mouse model revealed elevated placental p-YAP levels and diminished expression of YAP target genes, including CTGF, CYR61, and AMOTL2. In vitro experiments underscored the potential of upregulated YAP to mitigate the ERK inhibition-induced impairment of HTR-8/SVneo cells invasion and migration [145]. The role of maternal vitamin D deficiency (VDD) in IUGR is highlighted by its association with enhanced YAP phosphorylation. Mice subjected to a VDD diet exhibited a series of placental abnormalities, including a thinner labyrinth, trophoblast necrosis, and cytotrophoblast vacuolar degeneration. These pathological changes were concomitant with increased YAP phosphorylation. In vitro studies further elucidated VDD's role in suppressing human trophoblast cell invasion and promoting EVT apoptosis, mediated by the activation of the Hippo-YAP signaling pathway [146]. These findings underscore the intricate relationship between YAP signaling and FGR.
Conclusion and perspectives
The integrity of the maternal–fetal interface is pivotal for a healthy pregnancy, yet its disruption is associated with a range of pregnancy complications, including miscarriage [147], PE [148], and PTB [148]. This disruption is often linked to placental and fetal membrane dysfunction, characterized by the abnormal proliferation and differentiation of trophoblast cells [149]. In this review, we highlighted the intricate role of Yes-associated protein (YAP) in this context, illuminating its influence on trophoblast cell dynamics and subsequent pregnancy outcomes (Fig. 4). In studies on placental tissues from abortion cases, we encountered conflicting data on YAP expression in CTBs. While one study associated reduced nuclear YAP levels with inhibited CTB proliferation leading to abortion, another attributed increased nuclear YAP levels to disease occurrence by impeding cell differentiation. These contrasting findings underscore the complex and multifaceted role of YAP in the placenta of pregnancy complications. Based on the dynamic shifts in YAP signaling during CTB to EVT differentiation, we hypothesize that the deregulation of YAP activity, which inhibits EVT differentiation, maybe a secondary effect stemming from its influence on CTB and trophoblast stem cell proliferation. Given that YAP affects the proliferation of CTB and trophoblast stem cells, and deregulation of the proliferation rate contributes to the dysfunction of the progenitor pool and the development of pregnancy complications. However, ethical constraints and the lack of comprehensive human models render the progenitor pool during this differentiation a "black box," leaving the mechanisms by which YAP disrupts this pool largely unexplored.
Aberrant YAP function in trophoblasts is involved in the pathological mechanism of pregnancy complications. The schematic diagram shows the association of abnormal YAP activity in trophoblasts with pregnancy complications such as preeclampsia, fetal growth restriction, miscarriage, and preterm birth. Lines indicate proven. Dotted lines indicate presumed
Inflammation plays a dual role in pregnancy, being both a necessity for embryo implantation and pregnancy maintenance and a potential threat when dysregulated [150]. Our review identified activated NF-κB signaling in villous CTBs associated with spontaneous miscarriage and PE. The intricate interplay between YAP and inflammation is underscored by their mutual regulatory dynamics and the diverse cellular responses elicited. Evidence from various studies underscores YAP's protective role in cellular and tissue contexts under inflammatory conditions. A case in point is a mouse model of bacterial pneumonia, where YAP facilitated the proliferation and differentiation of alveolar epithelial cells’ stem/progenitor cells post-infection, aiding in tissue repair and regeneration [59]. Similarly, in hepatocytes, YAP's role is concentration-dependent; low TNF-α levels promote YAP nuclear translocation and cell proliferation, whereas higher concentrations induce YAP phosphorylation and inactivation, culminating in apoptosis [151]. While these insights are not directly derived from studies on placental or fetal membrane trophoblast cells, the structural similarities these cells share with the studied tissues warrant the extrapolation of these findings. The protective role of YAP against inflammation, particularly in placental trophoblast cells, emerges as a critical area for further investigation. In the context of LPS-induced vascular injury, the dual role of YAP is again evident. While LPS induces endothelial cell pyroptosis, it also inhibits the proliferation of surviving cells by promoting YAP phosphorylation and inactivation, a process contributing to inflammatory lung injury [152]. These findings suggest that even under inflammatory conditions, some cells remain viable but exhibit altered activity compared to normal cells, which can manifest as impaired YAP-induced cell proliferation-mediated repair capacity. The ambiguity extends to the realm of pregnancy, where the impact of inflammation on YAP-regulated functions in surviving trophoblast cells is yet to be fully elucidated. Early pregnancy is characterized by a delicate balance, where a moderated inflammatory environment is conducive to trophoblast lineage differentiation and the establishment of the maternal–fetal interface. However, an excessive inflammatory response precipitates trophoblast cell pyroptosis [133, 150]. The nuanced modulation of YAP activity in response to varying inflammatory concentrations emerges as a pivotal aspect warranting comprehensive investigation. Unraveling this complexity could illuminate targeted interventions to mitigate the adverse impacts of inflammation on pregnancy outcomes.
Excitingly, several drugs in clinical use can already restrict YAP activity, and several novel YAP inhibitors are under development. Broadly categorized based on their target pathways, YAP inhibitors can be classified into three groups: those targeting upstream regulators of YAP activity, those targeting YAP/TAZ or TEADs and disrupting their interaction, and those targeting downstream YAP transcriptional target genes with oncogenic effects [153]. Several YAP inhibitors have progressed to the first phase of clinical trials, marking a significant milestone in this field. These include the antisense oligonucleotide inhibitor ION537 (NCT04659096) by Ionis Pharmaceuticals, VT3989 by Vivace (NCT04665206) for solid tumors, and Novartis' IAG933 (NCT04857372), a proprietary compound delineated in patent WO2021186324A1, for neuromas. Among the FDA-approved drugs, statins have emerged as potent YAP inhibitors [154]. These 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors are known to suppress YAP activity by inhibiting its nuclear translocation and augmenting cellular sensitivity to other inhibitors, especially in the context of solid tumors [155]. In the realm of pregnancy complications like PE, statins have been employed to manage hypercholesterolemia, with recent studies attesting to their safety and dispelling concerns over potential teratogenic effects [156,157,158]. However, a meta-analysis encompassing nine studies revealed an uptick in spontaneous abortion rates among women subjected to statin therapy during pregnancy [159]. Another study correlated statin exposure to increased incidences of preterm labor and low birth weight [160]. These adverse outcomes are hypothesized to be tethered to the attenuation of YAP activity at the maternal–fetal interface, accentuating the vulnerability of trophoblast cells. In this context, the adjunctive use of a YAP agonist could potentially counterbalance the effects of statins, preserving the integrity of the pregnancy. However, the clinical landscape is yet bereft of a specific YAP agonist. XMU-MP-1 has been a staple in laboratory settings as a YAP agonist [161], underscoring the imperative for the development and clinical validation of novel, efficacious YAP-activating compounds.
The functional integrity of fetal membranes is crucial for maintaining a pregnancy. Fetal membranes consist of two distinct tissues: the amnion and the chorion. The amnion is believed to govern the mechanical behavior of fetal membranes. It acts as a structural barrier [162], while the chorion acts as an immune barrier, protecting the fetus from the maternal immune system and preventing degradation of the amnion [163]. Recent research underscores the pivotal role of the chorion layer, especially CTCs, in facilitating maternal–fetal communication and material exchange. Notably, the intricate regulation of endogenous calcium channel inhibitor activity and the prostaglandin E2 metabolic pathway within CTCs is instrumental in thwarting maternal uterine activation triggered by fetal-derived signals [109, 164]. However, the perturbation of progesterone levels and utero inflammation can lead to preterm premature rupture of membranes (pPROM) by impairing the functions of the chorion layer [165, 166]. It has been reported that pPROM is associated with a thin chorion layer, with an estimated 37% of cases presenting an indiscernible chorionic layer, a phenomenon linked to chorionic cell senescence and apoptosis [167, 168]. CTCs are the primary source of progesterone in fetal membranes. A decline in progesterone levels and signaling is implicated in instigating a proinflammatory state within the uterus [165, 169]. Existing literature attests to the role of progesterone in augmenting cardiomyocyte proliferation, mediated by the stimulation of YAP's transcriptional activity. This raises the imperative for extensive research to elucidate the potential influence of progesterone on the senescence and apoptosis of CTCs through YAP regulation. YAP-induced cell proliferation has been heralded for its reparative effects on barriers across various tissues and organs [170]. The attenuation of the chorion layer, observable in pPROM cases, is potentially associated with diminished YAP-mediated barrier repair mechanisms, but more direct experimental evidence is needed.
Progesterone, a steroid hormone crucial for implantation and maintenance of pregnancy, is associated with a high risk of miscarriage when present at low levels [171, 172]. Mifepristone, a progesterone receptor antagonist, is frequently administered alongside prostaglandins for the medical termination of early pregnancies [173]. Notably, the use of mifepristone within the initial 39 days of gestation has been linked to an increase in apoptotic cells within the chorionic villi [174]. The potential association between this phenomenon and YAP dysfunction warrants comprehensive investigation.
In this review, we have summarized studies highlighting the role of YAP dysfunction in trophoblast cells and its implication in the onset of pregnancy complications. A limited yet insightful number of research have also unveiled the intricate involvement of proteins that modulate YAP activity, accentuating their role in fetal membrane dysfunction. In light of these findings, we advocate for strategically employing YAP agonists or antagonists as potential therapeutic interventions to ameliorate pregnancy complications. The exigency for extensive research to ascertain the optimal timing and specific types of YAP modulators for efficacious and safe application is emphatically underscored.
Availability of data and materials
Not data availability.
Abbreviations
- 15-PGDH:
-
NAD + -dependent 15-hydroxyprostaglandin dehydrogenase
- AJs:
-
Adherens junctions
- AMOT:
-
Angiomotin family of proteins
- ANGPTL4:
-
Angiopoietin-like 4
- APC:
-
Adenomatous polyposis coli
- CCTs:
-
Cell column trophoblasts
- CDX2:
-
Caudal-type transcription factor
- CK1:
-
Casein kinase 1
- COX2:
-
Cyclooxygenase 2
- CTBs:
-
Cytotrophoblasts
- CTCs:
-
Chorion trophoblast cells
- dCCT:
-
Distal cell column trophoblasts
- E2:
-
17β-Estradiol
- EVTs:
-
Extravillous trophoblasts
- FGR:
-
Fetal growth restriction
- FZD:
-
Frizzled protein
- GPCRs:
-
G protein-coupled receptors
- GPER:
-
G protein-coupled estrogen receptor
- GSK3β:
-
Glycogen synthase kinase 3β
- HDAC7:
-
Histone deacetylase 7
- ICM:
-
Inner cell mass
- iEVT:
-
Interstitial extravillous trophoblast
- IL-1β:
-
Interleukin-1β
- IUGR:
-
Intrauterine growth restriction
- IκB:
-
Inhibitor kappa B
- LATS1/2:
-
Large tumor suppressor 1/2
- LEF:
-
Lymphoid enhancer-binding factor
- LPA:
-
Lysophosphatidic acid
- LPS:
-
Lipopolysaccharide
- LRP-5/6:
-
Lipoprotein receptor-related protein 5/6
- MAP4Ks:
-
Mitogen-activated protein kinase kinase kinase kinase
- MOB1A/B:
-
MOB kinase activator
- MST1/2:
-
STE20-like protein kinase 1/2
- NDR1/2:
-
The nuclear Dbf2-related (NDR) kinases 1/2
- NHERF1:
-
Na + /H + exchanger regulatory factor 1
- NICD:
-
Notch intracellular domain
- pCCT:
-
Proximal cell column trophoblast
- PE:
-
Preeclampsia
- PG:
-
Prostaglandin
- PKCζ:
-
Protein kinase C ζ
- pPROM:
-
Preterm premature rupture of membranes
- PS:
-
Primitive Syncytium
- PTPN14:
-
Protein tyrosine phosphatase nonreceptor type 14
- RBP-Jκ:
-
Recombining binding protein suppressor of hairless
- RPL:
-
Recurrent pregnancy loss
- RSA:
-
Recurrent spontaneous abortion
- S1P:
-
Sphingosine 1-phosphate
- S1PR2:
-
Sphingosine-1-phosphate receptor-2
- SAV1:
-
Salvador homolog 1
- sPE:
-
Severe preeclampsia
- STBs:
-
Syncytiotrophoblasts
- TAD:
-
Transcription activation domain
- TAK1:
-
Transforming growth factor-beta-activated kinase 1
- TCF:
-
T-cell-specific factor
- TE:
-
Trophectoderm
- TEAD:
-
TEA Domain Transcription Factor
- TJs:
-
Tight junctions
- TNFR1:
-
Tumor necrosis factor receptor 1
- TNF-α:
-
Tumor necrosis factor-alpha
- TSCs:
-
Trophoblast stem cells
- USP31:
-
Ubiquitin-specific peptidase 31
- VDD:
-
Vitamin D deficiency
- YAP:
-
Yes-associated protein
- ZO-1/2:
-
Zonula occludens 1/2
References
Hong AW, Meng Z, Guan KL. The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol. 2016;13:324–37.
Russell JO, Camargo FD. Hippo signalling in the liver: role in development, regeneration and disease. Nat Rev Gastroenterol Hepatol. 2022;19:297–312.
Wu Z, Guan KL. Hippo Signaling in Embryogenesis and Development. Trends Biochem Sci. 2021;46:51–63.
Sun T, Chi JT. Regulation of ferroptosis in cancer cells by YAP/TAZ and Hippo pathways: The therapeutic implications. Genes Dis. 2021;8:241–9.
Huang Z, Zhou J, Leung WT, Gober HJ, Pan X, Li C, Li L, Wang L. The novel role of Hippo-YAP/TAZ in immunity at the mammalian maternal-fetal interface: Opportunities, challenges. Biomed Pharmacother. 2020;126:110061.
Szulzewsky F, Holland EC, Vasioukhin V. YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Dev Biol. 2021;475:205–21.
Heng BC, Zhang X, Aubel D, Bai Y, Li X, Wei Y, Fussenegger M, Deng X. An overview of signaling pathways regulating YAP/TAZ activity. Cell Mol Life Sci. 2021;78:497–512.
Guo Y, Luo J, Zou H, Liu C, Deng L, Li P. Context-dependent transcriptional regulations of YAP/TAZ in cancer. Cancer Lett. 2022;527:164–73.
Mendes S, Timoteo-Ferreira F, Almeida H, Silva E. New Insights into the Process of Placentation and the Role of Oxidative Uterine Microenvironment. Oxid Med Cell Longev. 2019;2019:9174521.
Lee BK, Jang YJ, Kim M, LeBlanc L, Rhee C, Lee J, Beck S, Shen W, Kim J. Super-enhancer-guided mapping of regulatory networks controlling mouse trophoblast stem cells. Nat Commun. 2019;10:4749.
Lawless L, Qin Y, Xie L, Zhang K. Trophoblast Differentiation: Mechanisms and Implications for Pregnancy Complications. Nutrients. 2023;15(16):3564.
Liao J, Zheng Y, Hu M, Xu P, Lin L, Liu X, Wu Y, Huang B, Ye X, Li S, et al. Impaired Sphingosine-1-Phosphate Synthesis Induces Preeclampsia by Deactivating Trophoblastic YAP (Yes-Associated Protein) Through S1PR2 (Sphingosine-1-Phosphate Receptor-2)-Induced Actin Polymerizations. Hypertension. 2022;79:399–412.
Reggiani F, Gobbi G, Ciarrocchi A, Sancisi V. YAP and TAZ Are Not Identical Twins. Trends Biochem Sci. 2021;46:154–68.
Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145–52.
Noland CL, Gierke S, Schnier PD, Murray J, Sandoval WN, Sagolla M, Dey A, Hannoush RN, Fairbrother WJ, Cunningham CN. Palmitoylation of TEAD Transcription Factors Is Required for Their Stability and Function in Hippo Pathway Signaling. Structure. 2016;24:179–86.
Chen L, Chan SW, Zhang X, Walsh M, Lim CJ, Hong W, Song H. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev. 2010;24:290–300.
Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357.
Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade. Dev Cell. 2015;34:642–55.
Hergovich A. The Roles of NDR Protein Kinases in Hippo Signalling. Genes (Basel). 2016;7(5):21.
Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–74.
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61.
Moon S, Kim W, Kim S, Kim Y, Song Y, Bilousov O, Kim J, Lee T, Cha B, Kim M, et al. Phosphorylation by NLK inhibits YAP-14-3-3-interactions and induces its nuclear localization. EMBO Rep. 2017;18:61–71.
Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24:72–85.
Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23.
Llado V, Nakanishi Y, Duran A, Reina-Campos M, Shelton PM, Linares JF, Yajima T, Campos A, Aza-Blanc P, Leitges M, et al. Repression of Intestinal Stem Cell Function and Tumorigenesis through Direct Phosphorylation of beta-Catenin and Yap by PKCzeta. Cell Rep. 2015;10:740–54.
Oka T, Remue E, Meerschaert K, Vanloo B, Boucherie C, Gfeller D, Bader GD, Sidhu SS, Vandekerckhove J, Gettemans J, Sudol M. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem J. 2010;432:461–72.
Mohler PJ, Kreda SM, Boucher RC, Sudol M, Stutts MJ, Milgram SL. Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J Cell Biol. 1999;147:879–90.
Shimomura T, Miyamura N, Hata S, Miura R, Hirayama J, Nishina H. The PDZ-binding motif of Yes-associated protein is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity. Biochem Biophys Res Commun. 2014;443:917–23.
Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–61.
Robinson BS, Moberg KH. Cell-cell junctions: alpha-catenin and E-cadherin help fence in Yap1. Curr Biol. 2011;21:R890-892.
Oka T, Schmitt AP, Sudol M. Opposing roles of angiomotin-like-1 and zona occludens-2 on pro-apoptotic function of YAP. Oncogene. 2012;31:128–34.
Xu J, Kausalya PJ, Ong AGM, Goh CMF, Mohamed Ali S, Hunziker W. ZO-2/Tjp2 suppresses Yap and Wwtr1/Taz-mediated hepatocyte to cholangiocyte transdifferentiation in the mouse liver. NPJ Regen Med. 2022;7:55.
Wang W, Huang J, Wang X, Yuan J, Li X, Feng L, Park JI, Chen J. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26:1959–71.
Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–91.
Luo J, Yu FX. GPCR-Hippo Signaling in Cancer. Cells. 2019;8(5):426.
Regue L, Mou F, Avruch J. G protein-coupled receptors engage the mammalian Hippo pathway through F-actin: F-Actin, assembled in response to Galpha12/13 induced RhoA-GTP, promotes dephosphorylation and activation of the YAP oncogene. BioEssays. 2013;35:430–5.
Chen X, Yuan W, Li Y, Luo J, Hou N. Role of Hippo-YAP1/TAZ pathway and its crosstalk in cardiac biology. Int J Biol Sci. 2020;16:2454–63.
Schunk SJ, Floege J, Fliser D, Speer T. WNT-beta-catenin signalling - a versatile player in kidney injury and repair. Nat Rev Nephrol. 2021;17:172–84.
Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–70.
Chen C, Zhu D, Zhang H, Han C, Xue G, Zhu T, Luo J, Kong L. YAP-dependent ubiquitination and degradation of beta-catenin mediates inhibition of Wnt signalling induced by Physalin F in colorectal cancer. Cell Death Dis. 2018;9:591.
Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–10.
Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31:1109–22.
Guillermin O, Angelis N, Sidor CM, Ridgway R, Baulies A, Kucharska A, Antas P, Rose MR, Cordero J, Sansom O, et al. Wnt and Src signals converge on YAP-TEAD to drive intestinal regeneration. EMBO J. 2021;40:e105770.
Zhang Y, Xu H, Cui G, Liang B, Chen X, Ko S, Affo S, Song X, Liao Y, Feng J, et al. beta-Catenin Sustains and Is Required for YES-associated Protein Oncogenic Activity in Cholangiocarcinoma. Gastroenterology. 2022;163:481–94.
Deng F, Peng L, Li Z, Tan G, Liang E, Chen S, Zhao X, Zhi F. YAP triggers the Wnt/beta-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis. 2018;9:153.
Quinn HM, Vogel R, Popp O, Mertins P, Lan L, Messerschmidt C, Landshammer A, Lisek K, Chateau-Joubert S, Marangoni E, et al. YAP and beta-Catenin Cooperate to Drive Oncogenesis in Basal Breast Cancer. Cancer Res. 2021;81:2116–27.
Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33.
Sprinzak D, Blacklow SC. Biophysics of Notch Signaling. Annu Rev Biophys. 2021;50:157–89.
Totaro A, Castellan M, Battilana G, Zanconato F, Azzolin L, Giulitti S, Cordenonsi M, Piccolo S. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat Commun. 2017;8:15206.
Hao B, Chen X, Cao Y. Yes-associated protein 1 promotes the metastasis of U251 glioma cells by upregulating Jagged-1 expression and activating the Notch signal pathway. Exp Ther Med. 2018;16:1411–6.
Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–38.
Manderfield LJ, Aghajanian H, Engleka KA, Lim LY, Liu F, Jain R, Li L, Olson EN, Epstein JA. Hippo signaling is required for Notch-dependent smooth muscle differentiation of neural crest. Development. 2015;142:2962–71.
Hu S, Molina L, Tao J, Liu S, Hassan M, Singh S, Poddar M, Bell A, Sia D, Oertel M, et al. NOTCH-YAP1/TEAD-DNMT1 Axis Drives Hepatocyte Reprogramming Into Intrahepatic Cholangiocarcinoma. Gastroenterology. 2022;163:449–65.
Li Y, Hibbs MA, Gard AL, Shylo NA, Yun K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells. 2012;30:741–52.
Slemmons KK, Crose LES, Riedel S, Sushnitha M, Belyea B, Linardic CM. A Novel Notch-YAP Circuit Drives Stemness and Tumorigenesis in Embryonal Rhabdomyosarcoma. Mol Cancer Res. 2017;15:1777–91.
Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 2006;25:6706–16.
Li Y, Kong F, Shao Q, Wang R, Hu E, Liu J, Jin C, He D, Xiao X. YAP Expression and Activity Are Suppressed by S100A7 via p65/NFkappaB-mediated Repression of DeltaNp63. Mol Cancer Res. 2017;15:1752–63.
Yang B, Sun H, Xu X, Zhong H, Wu Y, Wang J. YAP1 inhibits the induction of TNF-alpha-stimulated bone-resorbing mediators by suppressing the NF-kappaB signaling pathway in MC3T3-E1 cells. J Cell Physiol. 2020;235:4698–708.
LaCanna R, Liccardo D, Zhang P, Tragesser L, Wang Y, Cao T, Chapman HA, Morrisey EE, Shen H, Koch WJ, et al. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J Clin Invest. 2019;129:2107–22.
Deng Y, Lu J, Li W, Wu A, Zhang X, Tong W, Ho KK, Qin L, Song H, Mak KK. Reciprocal inhibition of YAP/TAZ and NF-kappaB regulates osteoarthritic cartilage degradation. Nat Commun. 2018;9:4564.
Lv Y, Kim K, Sheng Y, Cho J, Qian Z, Zhao YY, Hu G, Pan D, Malik AB, Hu G. YAP Controls Endothelial Activation and Vascular Inflammation Through TRAF6. Circ Res. 2018;123:43–56.
Zhang Q, Han X, Chen J, Xie X, Xu J, Zhao Y, Shen J, Hu L, Xu P, Song H, et al. Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) mediate cell density-dependent proinflammatory responses. J Biol Chem. 2018;293:18071–85.
Zhao T, Wang Z, Fang J, Cheng W, Zhang Y, Huang J, Xu L, Gou H, Zeng L, Jin Z, Matsuoka M. HTLV-1 activates YAP via NF-kappaB/p65 to promote oncogenesis. Proc Natl Acad Sci U S A. 2022;119(9):e2115316119.
Yang K, Xu J, Fan M, Tu F, Wang X, Ha T, Williams DL, Li C. Lactate Suppresses Macrophage Pro-Inflammatory Response to LPS Stimulation by Inhibition of YAP and NF-kappaB Activation via GPR81-Mediated Signaling. Front Immunol. 2020;11:587913.
Gao Y, Yang Y, Yuan F, Huang J, Xu W, Mao B, Yuan Z, Bi W. TNFalpha-YAP/p65-HK2 axis mediates breast cancer cell migration. Oncogenesis. 2017;6:e383.
Qiao X, Zhang Y, Sun L, Ma Q, Yang J, Ai L, Xue J, Chen G, Zhang H, Ji C, et al. Association of human breast cancer CD44(-)/CD24(-) cells with delayed distant metastasis. Elife. 2021;10:e65418.
Hamilton WJ, Boyd JD. Development of the human placenta in the first three months of gestation. J Anat. 1960;94:297–328.
Hertig AT, Rock J, Adams EC. A description of 34 human ova within the first 17 days of development. Am J Anat. 1956;98:435–93.
Enders AC. Cytology of human early implantation. Res Reprod. 1976;8:1–2.
Knofler M, Haider S, Saleh L, Pollheimer J, Gamage T, James J. Human placenta and trophoblast development: key molecular mechanisms and model systems. Cell Mol Life Sci. 2019;76:3479–96.
Haider S, Meinhardt G, Saleh L, Fiala C, Pollheimer J, Knofler M. Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proc Natl Acad Sci U S A. 2016;113:E7710–9.
Velicky P, Meinhardt G, Plessl K, Vondra S, Weiss T, Haslinger P, Lendl T, Aumayr K, Mairhofer M, Zhu X, et al. Genome amplification and cellular senescence are hallmarks of human placenta development. PLoS Genet. 2018;14:e1007698.
Gauster M, Moser G, Wernitznig S, Kupper N, Huppertz B. Early human trophoblast development: from morphology to function. Cell Mol Life Sci. 2022;79:345.
Wang XH, Xu S, Zhou XY, Zhao R, Lin Y, Cao J, Zang WD, Tao H, Xu W, Li MQ, et al. Low chorionic villous succinate accumulation associates with recurrent spontaneous abortion risk. Nat Commun. 2021;12:3428.
Genbacev O, Vicovac L, Larocque N. The role of chorionic cytotrophoblasts in the smooth chorion fusion with parietal decidua. Placenta. 2015;36:716–22.
Marsh B, Zhou Y, Kapidzic M, Fisher S, Blelloch R. Regionally distinct trophoblast regulate barrier function and invasion in the human placenta. Elife. 2022;11:e78829.
Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T. Derivation of Human Trophoblast Stem Cells. Cell Stem Cell. 2018;22(50–63):e56.
Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, McWhinnie A, Esposito L, Fernando R, Skelton H, et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature. 2018;564:263–7.
Kagawa H, Javali A, Khoei HH, Sommer TM, Sestini G, Novatchkova M, Scholte Op Reimer Y, Castel G, Bruneau A, Maenhoudt N, et al. Human blastoids model blastocyst development and implantation. Nature. 2022;601:600–5.
Gerri C, McCarthy A, Alanis-Lobato G, Demtschenko A, Bruneau A, Loubersac S, Fogarty NME, Hampshire D, Elder K, Snell P, et al. Initiation of a conserved trophectoderm program in human, cow and mouse embryos. Nature. 2020;587:443–7.
Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410.
Rayon T, Menchero S, Nieto A, Xenopoulos P, Crespo M, Cockburn K, Canon S, Sasaki H, Hadjantonakis AK, de la Pompa JL, et al. Notch and hippo converge on Cdx2 to specify the trophectoderm lineage in the mouse blastocyst. Dev Cell. 2014;30:410–22.
Watanabe Y, Miyasaka KY, Kubo A, Kida YS, Nakagawa O, Hirate Y, Sasaki H, Ogura T. Notch and Hippo signaling converge on Strawberry Notch 1 (Sbno1) to synergistically activate Cdx2 during specification of the trophectoderm. Sci Rep. 2017;7:46135.
Than NG, Romero R, Tarca AL, Kekesi KA, Xu Y, Xu Z, Juhasz K, Bhatti G, Leavitt RJ, Gelencser Z, et al. Integrated Systems Biology Approach Identifies Novel Maternal and Placental Pathways of Preeclampsia. Front Immunol. 2018;9:1661.
Costa MA. Scrutinising the regulators of syncytialization and their expression in pregnancy-related conditions. Mol Cell Endocrinol. 2016;420:180–93.
Sheridan RM, Stanek J, Khoury J, Handwerger S. Abnormal expression of transcription factor activator protein-2alpha in pathologic placentas. Hum Pathol. 2012;43:1866–74.
Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, Fisher SJ. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am J Pathol. 1997;151:1809–18.
Soncin F, Parast MM. Role of Hippo signaling pathway in early placental development. Proc Natl Acad Sci U S A. 2020;117:20354–6.
Meinhardt G, Haider S, Kunihs V, Saleh L, Pollheimer J, Fiala C, Hetey S, Feher Z, Szilagyi A, Than NG, Knofler M. Pivotal role of the transcriptional co-activator YAP in trophoblast stemness of the developing human placenta. Proc Natl Acad Sci U S A. 2020;117:13562–70.
Mizutani T, Orisaka M, Miyazaki Y, Morichika R, Uesaka M, Miyamoto K, Yoshida Y. Inhibition of YAP/TAZ-TEAD activity induces cytotrophoblast differentiation into syncytiotrophoblast in human trophoblast. Mol Hum Reprod. 2022;28(10):gaac032.
Zhou H, Pan Y, Yang W, Zhao C, Sun X, Hong B, Jin X, Zhang T, Zhang Y, Liu N, et al. S100P promotes trophoblast syncytialization during early placenta development by regulating YAP1. Front Endocrinol (Lausanne). 2022;13:860261.
Meinhardt G, Haider S, Haslinger P, Proestling K, Fiala C, Pollheimer J, Knofler M. Wnt-dependent T-cell factor-4 controls human etravillous trophoblast motility. Endocrinology. 2014;155:1908–20.
Plessl K, Haider S, Fiala C, Pollheimer J, Knofler M. Expression pattern and function of Notch2 in different subtypes of first trimester cytotrophoblast. Placenta. 2015;36:365–71.
Dietrich B, Haider S, Meinhardt G, Pollheimer J, Knofler M. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell Mol Life Sci. 2022;79:292.
Basak T, Ain R. Molecular regulation of trophoblast stem cell self-renewal and giant cell differentiation by the Hippo components YAP and LATS1. Stem Cell Res Ther. 2022;13:189.
Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, Ellinger A, Burkard TR, Fiala C, Pollheimer J, et al. Self-Renewing Trophoblast Organoids Recapitulate the Developmental Program of the Early Human Placenta. Stem Cell Reports. 2018;11:537–51.
Wang C, Han X, Zhou Z, Uyunbilig B, Huang X, Li R, Li X. Wnt3a Activates the WNT-YAP/TAZ Pathway to Sustain CDX2 Expression in Bovine Trophoblast Stem Cells. DNA Cell Biol. 2019;38:410–22.
Chaddha V, Viero S, Huppertz B, Kingdom J. Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med. 2004;9:357–69.
Sun M, Na Q, Huang L, Song G, Jin F, Li Y, Hou Y, Kang D, Qiao C. YAP Is Decreased in Preeclampsia and Regulates Invasion and Apoptosis of HTR-8/SVneo. Reprod Sci. 2018;25:1382–93.
Liu R, Wei C, Ma Q, Wang W. Hippo-YAP1 signaling pathway and severe preeclampsia (sPE) in the Chinese population. Pregnancy Hypertens. 2020;19:1–10.
Zang J, Yan M, Zhang Y, Peng W, Zuo J, Zhou H, Gao G, Li M, Chu Y, Ye Y. MiR-326 inhibits trophoblast growth, migration, and invasion by targeting PAX8 via Hippo pathway. Reprod Biol Endocrinol. 2022;20:38.
Hu M, Zheng Y, Liao J, Wen L, Cheng J, Huang J, Huang B, Lin L, Long Y, Wu Y, et al. miR21 modulates the Hippo signaling pathway via interference with PP2A Bbeta to inhibit trophoblast invasion and cause preeclampsia. Mol Ther Nucleic Acids. 2022;30:143–61.
Pollheimer J, Loregger T, Sonderegger S, Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P, Knofler M. Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol. 2006;168:1134–47.
Xiao Z, Yan L, Liang X, Wang H. Progress in deciphering trophoblast cell differentiation during human placentation. Curr Opin Cell Biol. 2020;67:86–91.
Williams MM, Richer JK. Revealing Molecular Mechanisms Supporting Trophoblast-Mediated Maternal Immune Tolerance. Endocrinology. 2022;163(8):bqac099.
Megli C, Morosky S, Rajasundaram D, Coyne CB. Inflammasome signaling in human placental trophoblasts regulates immune defense against Listeria monocytogenes infection. J Exp Med. 2021;218(1):e20200649.
Johnson LJ, Azari S, Webb A, Zhang X, Gavrilin MA, Marshall JM, Rood K, Seveau S. Human Placental Trophoblasts Infected by Listeria monocytogenes Undergo a Pro-Inflammatory Switch Associated With Poor Pregnancy Outcomes. Front Immunol. 2021;12:709466.
Li M, Sun F, Qian J, Chen L, Li D, Wang S, Du M. Tim-3/CTLA-4 pathways regulate decidual immune cells-extravillous trophoblasts interaction by IL-4 and IL-10. FASEB J. 2021;35:e21754.
McCoshen JA, Hoffman DR, Kredentser JV, Araneda C, Johnston JM. The role of fetal membranes in regulating production, transport, and metabolism of prostaglandin E2 during labor. Am J Obstet Gynecol. 1990;163:1632–40.
Casciani V, Premyslova M, Luo D, Marinoni E, Moscarini M, Di Iorio R, Challis JR. Effect of calcium ionophore A23187 on prostaglandin synthase type 2 and 15-hydroxy-prostaglandin dehydrogenase expression in human chorion trophoblast cells. Am J Obstet Gynecol. 2008;199(554):e551-558.
Sangha RK, Walton JC, Ensor CM, Tai HH, Challis JR. Immunohistochemical localization, messenger ribonucleic acid abundance, and activity of 15-hydroxyprostaglandin dehydrogenase in placenta and fetal membranes during term and preterm labor. J Clin Endocrinol Metab. 1994;78:982–9.
Van Meir CA, Sangha RK, Walton JC, Matthews SG, Keirse MJ, Challis JR. Immunoreactive 15-hydroxyprostaglandin dehydrogenase (PGDH) is reduced in fetal membranes from patients at preterm delivery in the presence of infection. Placenta. 1996;17:291–7.
Shilei B, Lizi Z, Lijun H, Weixu M, Nan M, Weinan D, Yulian L, Yingyu L, Minshan H, Pei X, et al. Downregulation of CDC42 inhibits the proliferation and stemness of human trophoblast stem cell via EZRIN/YAP inactivation. Cell Tissue Res. 2022;389:573–85.
Cheng JC, Fang L, Li Y, Thakur A, Hoodless PA, Guo Y, Wang Z, Wu Z, Yan Y, Jia Q, et al. G protein-coupled estrogen receptor stimulates human trophoblast cell invasion via YAP-mediated ANGPTL4 expression. Commun Biol. 2021;4:1285.
Zha W, Guan S, Liu N, Li Y, Tian Y, Chen Y, Wang Y, Wu F. Let-7a inhibits Bcl-xl and YAP1 expression to induce apoptosis of trophoblast cells in early-onset severe preeclampsia. Sci Total Environ. 2020;745:139919.
Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction. 2010;140:803–13.
Menasha J, Levy B, Hirschhorn K, Kardon NB. Incidence and spectrum of chromosome abnormalities in spontaneous abortions: new insights from a 12-year study. Genet Med. 2005;7:251–63.
Saha B, Ganguly A, Home P, Bhattacharya B, Ray S, Ghosh A, Rumi MAK, Marsh C, French VA, Gunewardena S, Paul S. TEAD4 ensures postimplantation development by promoting trophoblast self-renewal: An implication in early human pregnancy loss. Proc Natl Acad Sci U S A. 2020;117:17864–75.
Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Semin Fetal Neonatal Med. 2006;11:302–8.
Liu Y, Chen H, Feng L, Zhang J. Interactions between gut microbiota and metabolites modulate cytokine network imbalances in women with unexplained miscarriage. NPJ Biofilms Microbiomes. 2021;7:24.
Wang LQ, Yu XW, Yan CF, Wang X. Nuclear translocation of nuclear factor Kappa B in first trimester deciduas and chorionic villi in early spontaneous miscarriage women. Int J Mol Sci. 2010;11:521–31.
Huang Z, Du G, Huang X, Han L, Han X, Xu B, Zhang Y, Yu M, Qin Y, Xia Y, et al. The enhancer RNA lnc-SLC4A1-1 epigenetically regulates unexplained recurrent pregnancy loss (URPL) by activating CXCL8 and NF-kB pathway. EBioMedicine. 2018;38:162–70.
Yu X, Wang L, Yan C, Li X. Expression and localization of tumor necrosis factor receptor 1 protein in the chorionic villi in early normal and spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2007;132:58–63.
Jung E, Romero R, Yeo L, Gomez-Lopez N, Chaemsaithong P, Jaovisidha A, Gotsch F, Erez O. The etiology of preeclampsia. Am J Obstet Gynecol. 2022;226:S844–66.
Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:1690–702.
Berkane N, Liere P, Oudinet JP, Hertig A, Lefevre G, Pluchino N, Schumacher M, Chabbert-Buffet N. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr Rev. 2017;38:123–44.
O’Sullivan C, Dev KK. The structure and function of the S1P1 receptor. Trends Pharmacol Sci. 2013;34:401–12.
Li FH, Wang Y, Liu XL, Xu Q. The silencing of ApoC3 suppresses oxidative stress and inflammatory responses in placenta cells from mice with preeclampsia via inhibition of the NF-kappaB signaling pathway. Biomed Pharmacother. 2018;107:1377–84.
Silva Carmona A, Mendieta Zeron H. NF-kappaBeta and SOD expression in preeclamptic placentas. Turk J Med Sci. 2016;46:783–8.
Kadam L, Kilburn B, Baczyk D, Kohan-Ghadr HR, Kingdom J, Drewlo S. Rosiglitazone blocks first trimester in-vitro placental injury caused by NF-kappaB-mediated inflammation. Sci Rep. 2018;2019:9.
Yin A, Chen Q, Zhong M, Jia B. MicroRNA-138 improves LPS-induced trophoblast dysfunction through targeting RELA and NF-kappaB signaling. Cell Cycle. 2021;20:508–21.
Gong P, Liu M, Hong G, Li Y, Xue P, Zheng M, Wu M, Shen L, Yang M, Diao Z, Hu Y. Curcumin improves LPS-induced preeclampsia-like phenotype in rat by inhibiting the TLR4 signaling pathway. Placenta. 2016;41:45–52.
Zhang Y, Liu W, Zhong Y, Li Q, Wu M, Yang L, Liu X, Zou L. Metformin Corrects Glucose Metabolism Reprogramming and NLRP3 Inflammasome-Induced Pyroptosis via Inhibiting the TLR4/NF-kappaB/PFKFB3 Signaling in Trophoblasts: Implication for a Potential Therapy of Preeclampsia. Oxid Med Cell Longev. 2021;2021:1806344.
Keelan JA, Khan S, Yosaatmadja F, Mitchell MD. Prevention of inflammatory activation of human gestational membranes in an ex vivo model using a pharmacological NF-kappaB inhibitor. J Immunol. 2009;183:5270–8.
Leslie KL, Song GJ, Barrick S, Wehbi VL, Vilardaga JP, Bauer PM, Bisello A. Ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) and nuclear factor-kappaB (NF-kappaB): a feed-forward loop for systemic and vascular inflammation. J Biol Chem. 2013;288:36426–36.
Georgescu MM, Gagea M, Cote G. NHERF1/EBP50 Suppresses Wnt-beta-Catenin Pathway-Driven Intestinal Neoplasia. Neoplasia. 2016;18:512–23.
Kammala AK, Sheller-Miller S, Radnaa E, Kechichian T, Subramanian H, Menon R. Sodium Hydrogen Exchanger Regulatory Factor-1 (NHERF1) Regulates Fetal Membrane Inflammation. Int J Mol Sci. 2020;21(20):7747.
Kim SY, Park SY, Jang HS, Park YD, Kee SH. Yes-Associated Protein Is Required for ZO-1-Mediated Tight-Junction Integrity and Cell Migration in E-Cadherin-Restored AGS Gastric Cancer Cells. Biomedicines. 2021;9(9):1264.
Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, et al. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell. 2008;19:2465–75.
Li J, Zhou J, Tian B, Chu Y, Zhang N, Hu X, Wan X, Ye Y. Activation of HO-1 protects placental cells function in oxidative stress via regulating ZO-1/occludin. Biochem Biophys Res Commun. 2019;511:903–9.
Pidoux G, Gerbaud P, Gnidehou S, Grynberg M, Geneau G, Guibourdenche J, Carette D, Cronier L, Evain-Brion D, Malassine A, Frendo JL. ZO-1 is involved in trophoblastic cell differentiation in human placenta. Am J Physiol Cell Physiol. 2010;298:C1517-1526.
Li J, Liu Y, Xue R, Shen H, Wu Y, Quinn M, Zhang H, Wu W. Inflammation-related downregulation of zonula Occludens-1 in fetal membrane contributes to development of prelabor rupture of membranes. Placenta. 2020;99:173–9.
Nowakowska BA, Pankiewicz K, Nowacka U, Niemiec M, Kozlowski S, Issat T. Genetic Background of Fetal Growth Restriction. Int J Mol Sci. 2021;23(1):36.
Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218:S745–61.
Wang H, Xu P, Luo X, Hu M, Liu Y, Yang Y, Peng W, Bai Y, Chen X, Tan B, et al. Phosphorylation of Yes-associated protein impairs trophoblast invasion and migration: implications for the pathogenesis of fetal growth restrictiondagger. Biol Reprod. 2020;103:866–79.
Wang J, Qiu F, Zhao Y, Gu S, Wang J, Zhang H. Exploration of fetal growth restriction induced by vitamin D deficiency in rats via Hippo-YAP signaling pathway. Placenta. 2022;128:91–9.
Zhu D, Zou H, Liu J, Wang J, Ma C, Yin J, Peng X, Li D, Yang Y, Ren Y, et al. Inhibition of HMGB1 Ameliorates the Maternal-Fetal Interface Destruction in Unexplained Recurrent Spontaneous Abortion by Suppressing Pyroptosis Activation. Front Immunol. 2021;12:782792.
Couture C, Brien ME, Boufaied I, Duval C, Soglio DD, Enninga EAL, Cox B, Girard S: Proinflammatory changes in the maternal circulation, maternal-fetal interface, and placental transcriptome in preterm birth. Am J Obstet Gynecol 2022.
Illsley NP, DaSilva-Arnold SC, Zamudio S, Alvarez M, Al-Khan A. Trophoblast invasion: Lessons from abnormally invasive placenta (placenta accreta). Placenta. 2020;102:61–6.
Nadeau-Vallee M, Obari D, Palacios J, Brien ME, Duval C, Chemtob S, Girard S. Sterile inflammation and pregnancy complications: a review. Reproduction. 2016;152:R277–92.
Zhao S, Jiang J, Jing Y, Liu W, Yang X, Hou X, Gao L, Wei L. The concentration of tumor necrosis factor-alpha determines its protective or damaging effect on liver injury by regulating Yap activity. Cell Death Dis. 2020;11:70.
Huang LS, Hong Z, Wu W, Xiong S, Zhong M, Gao X, Rehman J, Malik AB. mtDNA Activates cGAS Signaling and Suppresses the YAP-Mediated Endothelial Cell Proliferation Program to Promote Inflammatory Injury. Immunity. 2020;52(475–486):e475.
Pobbati AV, Hong W. A combat with the YAP/TAZ-TEAD oncoproteins for cancer therapy. Theranostics. 2020;10:3622–35.
Santos DM, Pantano L, Pronzati G, Grasberger P, Probst CK, Black KE, Spinney JJ, Hariri LP, Nichols R, Lin Y, et al. Screening for YAP Inhibitors Identifies Statins as Modulators of Fibrosis. Am J Respir Cell Mol Biol. 2020;62:479–92.
Xia H, Dai X, Yu H, Zhou S, Fan Z, Wei G, Tang Q, Gong Q, Bi F. EGFR-PI3K-PDK1 pathway regulates YAP signaling in hepatocellular carcinoma: the mechanism and its implications in targeted therapy. Cell Death Dis. 2018;9:269.
Costantine MM, Cleary K, Hebert MF, Ahmed MS, Brown LM, Ren Z, Easterling TR, Haas DM, Haneline LS, Caritis SN, et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol. 2016;214:720 e721-720 e717.
Smith DD, Costantine MM. The role of statins in the prevention of preeclampsia. Am J Obstet Gynecol. 2022;226:S1171–81.
Maierean SM, Mikhailidis DP, Toth PP, Grzesiak M, Mazidi M, Maciejewski M, Banach M. The potential role of statins in preeclampsia and dyslipidemia during gestation: a narrative review. Expert Opin Investig Drugs. 2018;27:427–35.
Vahedian-Azimi A, Bianconi V, Makvandi S, Banach M, Mohammadi SM, Pirro M, Sahebkar A. A systematic review and meta-analysis on the effects of statins on pregnancy outcomes. Atherosclerosis. 2021;336:1–11.
Chang JC, Chen YJ, Chen IC, Lin WS, Chen YM, Lin CH. Perinatal Outcomes After Statin Exposure During Pregnancy. JAMA Netw Open. 2021;4:e2141321.
Zhao X, Qin W, Jiang Y, Yang Z, Yuan B, Dai R, Shen H, Chen Y, Fu J, Wang H. ACADL plays a tumor-suppressor role by targeting Hippo/YAP signaling in hepatocellular carcinoma. NPJ Precis Oncol. 2020;4:7.
Chowdhury B, David AL, Thrasivoulou C, Becker DL, Bader DL, Chowdhury TT. Tensile strain increased COX-2 expression and PGE2 release leading to weakening of the human amniotic membrane. Placenta. 2014;35:1057–64.
Oyen ML, Calvin SE, Landers DV. Premature rupture of the fetal membranes: is the amnion the major determinant? Am J Obstet Gynecol. 2006;195:510–5.
Carroll EM, Gianopoulos JG, Collins PL. Abnormality of calcium channel inhibitor released from fetal membranes in preterm labor. Am J Obstet Gynecol. 2001;184:356–62.
Talati AN, Hackney DN, Mesiano S. Pathophysiology of preterm labor with intact membranes. Semin Perinatol. 2017;41:420–6.
Menon R, Behnia F, Polettini J, Richardson LS. Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin Immunopathol. 2020;42:431–50.
Canzoneri BJ, Feng L, Grotegut CA, Bentley RC, Heine RP, Murtha AP. The chorion layer of fetal membranes is prematurely destroyed in women with preterm premature rupture of the membranes. Reprod Sci. 2013;20:1246–54.
Feng L, Allen TK, Marinello WP, Murtha AP. Roles of Progesterone Receptor Membrane Component 1 in Oxidative Stress-Induced Aging in Chorion Cells. Reprod Sci. 2019;26:394–403.
Lozovyy V, Richardson L, Saade G, Menon R. Progesterone receptor membrane components: key regulators of fetal membrane integrity. Biol Reprod. 2021;104:445–56.
Deng F, Yan J, Lu J, Luo M, Xia P, Liu S, Wang X, Zhi F, Liu D. M2 Macrophage-Derived Exosomal miR-590-3p Attenuates DSS-Induced Mucosal Damage and Promotes Epithelial Repair via the LATS1/YAP/ beta-Catenin Signalling Axis. J Crohns Colitis. 2021;15:665–77.
Ku CW, Zhang X, Zhang VR, Allen JC, Tan NS, Ostbye T, Tan TC. Gestational age-specific normative values and determinants of serum progesterone through the first trimester of pregnancy. Sci Rep. 2021;11:4161.
Coomarasamy A, Devall AJ, Brosens JJ, Quenby S, Stephenson MD, Sierra S, Christiansen OB, Small R, Brewin J, Roberts TE, et al. Micronized vaginal progesterone to prevent miscarriage: a critical evaluation of randomized evidence. Am J Obstet Gynecol. 2020;223:167–76.
Chu JJ, Devall AJ, Beeson LE, Hardy P, Cheed V, Sun Y, Roberts TE, Ogwulu CO, Williams E, Jones LL, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770–8.
Tian F, Han H, Jia L, Zhang J, Chu Z, Li J, Zhang Y, Yan P. The effects of mifepristone on the structure of human decidua and chorion and Bax and Bcl-2 expression at early stage of pregnancy. BMC Pharmacol Toxicol. 2022;23:55.
Acknowledgements
We wish to thank Professor Xu Chen (Tianjin Key Laboratory of Human Development and Reproductive Regulation, Tianjin Central Hospital of Obstetrics and Gynecology & Nankai University Affiliated Maternity Hospital, Tianjin 300100, China) and Professor Yang Yu (Beijing Key Laboratory of Reproductive Endocrinology and Assisted Reproductive Technology and Key Laboratory of Assisted Reproduction, Ministry of Education, Center of Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing 100191, China) for their valuable discussion.
Funding
This study was supported by the National Clinical Key Discipline Cohort Study Project, China (GJZDZKZBDL2022-04 to YC), the Natural Science Foundation of Tianjin, China (21JCYBJC00100 to JSC), and the Open Project of Tianjin Key Laboratory of Human Development and Reproductive Regulation (2021XH05 to JSC).
Author information
Authors and Affiliations
Contributions
QML and JSC conceived, designed, and drafted the manuscript; JY, YZ, and YMS participated in the data investigation and analysis; SQW, YXW, and ZL assisted in the preparation of the figures; YC edited and revised the manuscript; all authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, Q., Cao, J., Yu, J. et al. YAP-mediated trophoblast dysfunction: the common pathway underlying pregnancy complications. Cell Commun Signal 21, 353 (2023). https://doi.org/10.1186/s12964-023-01371-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-023-01371-2