Abstract
Until the advent of immune checkpoint inhibitors (ICIs), definitive radiotherapy (RT) concurrently with chemotherapy was recommended for unresectable, locally advanced non-small cell lung cancer (LA-NSCLC). The trimodality paradigm with consolidation ICIs following definitive concurrent chemoradiotherapy has been the standard of care since the PACIFIC trial. Preclinical evidence has demonstrated the role of RT in the cancer-immune cycle and the synergistic effect of RT combined with ICIs (iRT). However, RT exerts a double-edged effect on immunity and the combination strategy still could be optimized in many areas. In the context of LA-NSCLC, optimized RT modality, choice, timing, and duration of ICIs, care for oncogenic addicted tumors, patient selection, and novel combination strategies require further investigation. Targeting these blind spots, novel approaches are being investigated to cross the borders of PACIFIC. We discussed the development history of iRT and summarized the updated rationale for the synergistic effect. We then summarized the available research data on the efficacy and toxicity of iRT in LA-NSCLC for cross-trial comparisons to eliminate barriers. Progression during and after ICIs consolidation therapy has been regarded as a distinct resistance scenario from primary or secondary resistance to ICIs, the subsequent management of which has also been discussed. Finally, based on unmet needs, we probed into the challenges, strategies, and auspicious orientations to optimize iRT in LA-NSCLC. In this review, we focus on the underlying mechanisms and recent advances of iRT with an emphasis on future challenges and directions that warrant further investigation. Taken together, iRT is a proven and potential strategy in LA-NSCLC, with multiple promising approaches to further improve the efficacy.
Video Abstract
Similar content being viewed by others

Background
Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases, and nearly one-third of patients have stage III, locally advanced (LA) disease at diagnosis [1]. Encompassing a heterogeneous group of tumor presentations, a multidisciplinary approach to define the resectability of stage III LA-NSCLC is mandatory [2]. Indeed, most LA-NSCLC patients lose the opportunity for curative resection at diagnosis. Radiotherapy (RT) has been used to cure malignant cancers in the past century, and approximately half of the cancer patients are treated with RT, which involves curative and palliative interventions [3]. The anti-tumor effects of RT have historically been regarded as radiation-induced deoxyribonucleic acid (DNA) deadly functional and structural changes that result in direct local cancerous cell apoptosis, senescence, and autophagy [3,4,5]. For more than two decades, the standard treatment for unresectable NSCLC has been thoracic RT [6].
Based on improved survival, definitive RT with concurrent platinum-based chemotherapy (concurrent chemoradiotherapy, cCRT) has become the standard of care (SoC) for unresectable LA-NSCLC [7, 8]. However, the outcome is still unsatisfactory, with the overall survival (OS) rate of 15–25% [2]. After the combination of targeted or chemotherapy consolidation treatment failed to bring survival benefits, the PACIFIC trial, like a huge "tsunami,” completely revolutionized the treatment of unresectable LA-NSCLC, accomplishing the success of RT combined with immune checkpoint inhibitors (ICIs) in stage III NSCLC [9]. ICIs combined with RT (immunoradiotherapy, iRT) have doubled the objective response rate (ORR) of advanced NSCLC in the PEMBRO-RT trial compared to ICI monotherapy [10]. It is believed that iRT can play a role of ‘1 + 1 > 2’ in treating tumors, for the synergistic anti-tumor effect of RT and immunotherapy. To date, a series of preclinical and clinical trials have been conducted to explore the theoretical basis and maximize the efficacy of iRT. Notably, for patients with unresectable LA-NSCLC, more novel patterns of combining RT and ICIs following the PACIFIC pattern are ongoing.
It is well known that RT should be administered in doses and fractionations suitable to ignite tumor-targeting immune responses, and innovative combination therapies can further improve outcomes in patients with unresectable LA-NSCLC. Herein, we review the underlying mechanisms and recent advances in iRT, summarize the current status and unmet needs of iRT in unresectable LA-NSCLC, and provide an overview of novel strategies for optimizing therapeutic effects.
The history and development of iRT
Routinely, RT is a treatment against local lesions, bringing damage to both tumor cells and normal cells; research in this area has focused on the biological effects on tumor cells induced by RT. Accidently, a special “abscopal effect” was put forward by Mole in 1953 to describe the inhibition of metastatic diseases distant from the irradiated field [11]. In other words, apart from the role of local control, RT is also a weapon that provokes a systemic response. Naturally, changes in the surrounding stroma and tumor microenvironment (TME) triggered by damaged or necrotic tumor cells have gradually attracted the attention of researchers [12]. Meanwhile, efforts to eliminate resistance to RT have never ceased, which is closely related to the original TME and dynamic alterations in response to RT [13]. Moreover, in addition to novel RT modalities such as stereotactic body radiation therapy (SBRT) and charged particle RT, combining other treatments has been an effective strategy. For decades, RT has been successfully integrated with surgery, chemotherapy, and molecular-targeted therapy.
Antibodies against programmed cell death 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen 4 (CTLA-4) aim to block negative checkpoints of immune homeostasis and reinvigorate exhausted CD8 + T cells [14]. These ICIs have reshaped the treatment landscape of multiple solid tumors, including NSCLC [15]. Clearly, there is considerable interest in combination regimens of RT and ICIs [16]. PEMBRO-RT, the first phase II randomized trial in advanced NSCLC by Welsh et al., validated the safety of combining RT with pembrolizumab, but the benefits in survival deserve further exploration [10]. Subsequently, a pooled analysis of PEMBRO-RT and MDACC (phase I/II) by the team further demonstrated that pembrolizumab combined with RT significantly improved the efficacy and survival of patients with advanced NSCLC and confirmed that iRT enhanced the abscopal effect for the first time, raising the ORR of lesions out of the irradiated field from 19.7% to 41.7% [17]. In addition, a secondary analysis of the KEYNOTE-001 study also found that patients who had previously received RT achieved longer progression-free survival (PFS) and OS after treatment with pembrolizumab, with acceptable safety [18]. However, no large-scale phase III clinical studies have verified the efficacy of RT combined with ICIs in metastatic NSCLC, which requires further validation.
The PACIFIC trial verified the benefits of ICIs consolidation after definitive cCRT [9], initiating the application of iRT in LA-NSCLC [8]. Of course, no matter what type of tumor and stage, to achieve an effect of “1 + 1 ≥ 2” of iRT, it is necessary to determine optimal dose and fractionation, irradiated site and field, RT modality, sequence of RT and ICIs, type of combined ICI, and suitable patients. In addition, attention should be paid to the safety of combined therapy, secondary resistance, and other promising combination strategies. We depict the history and development of iRT along with shifts in the SoC of unresectable LA-NSCLC in Fig. 1.
The history and development of iRT, along with shifts in SoC of unresectable LA-NSCLC. RT has experienced several technological revolutions though more than 100 years of development, from first use of radiation to to SBRT and image-guided RT. Cancer immunotherapy also has a history of more than 100 years, until PD-1/PD-L1 inhibitors were approved. On the other hand, RT has been the cornerstone of unresectable LA-NSCLC, while the amazing result from PACIFIC trial opened a new era of iRT in LA-NSCLC. Abbreviations: RT Radiotherapy, ICIs Immune checkpoint inhibitors, PD-1 Programmed cell death 1, PD-L1 Programmed cell death-ligand 1, iRT ICIs combined with RT, SoC Standard of care, LA-NSCLC Locally advanced non-small cell lung cancer, cCRT Concurrent chemoradiotherapy, mPFS, Median progression-free survival, OS Overall survival, mOS Median overall survival, yr Year, SBRT Stereotactic body radiation therapy
Current cognitions of the effects of RT on immunity
RT participates in the cancer-immune cycle to exert a systemic anti-tumor effect
Although known for decades, abscopal responses induced by RT are rare and there is a lack of adequate explanation for this phenomenon. An estimated 46 cases of abscopal effects were reported between 1969 and 2014 [19]. A literature analysis reported that 7 (14%) cases of primary tumors and 41% of metastases were recorded in the lungs, among 51 cases of the abscopal effect at various locations [20]. After entering the era of immunotherapy, this phenomenon was confirmed to be mediated by immunity, as no abscopal effect was observed in immunodeficient mice [21]. RT is believed to induce a systemic, immune-mediated anti-tumor effect by participating in the cancer-immunity cycle, as illustrated in Fig. 2 In general, the series of reactions induced by RT provides a more supportive immune microenvironment for anti-tumor immunity, turning a “cold” tumor into a “hot” tumor, and the immunoregulatory effect of RT is exactly the theoretical basis of its abscopal effect [13].
Essential immune-associated pathways activated by RT
RT activates multiple immune-associated pathways by damaging tumor cells, including cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (Cyclic GMP-AMP synthase, cGAS), and stimulator of interferon genes (STING) protein (cGAS-STING pathway) [22]. The cross-priming capacity of DCs induced by RT requires the activation of cGAS-STING and subsequent type I interferon signaling [23]. It is widely believed that the accumulation of cytoplasmic DNA, notably in the form of micronuclei, is essential for the cGAS-STING sensor [24]. Recently, Deng et al. found that released mitochondrial DNA (mtDNA), induced by the ZBP1-MLKL necroptotic signaling cascade, played a parallel role in triggering the cGAS-STING pathway in response to RT [25]. However, the latest research has also shown that cGAS-STING activation facilitated breast tumor progression in mouse xenograft models [26, 27], and knockdown of cGAS or STING expression prevents tumor metastasis [27]. Activation of cGAS-STING signaling may differentially affect diverse cell types in the TME, but how cGAS-STING activation mediates immunosuppression, conveying a tumor-promoting effect, remains poorly defined. Moreover, TLR 9, absent in melanoma 2 (AIM2), interferon-inducible protein 16 (IFI16), and others, could also sense the accumulation of DNA (extracellular or intracellular) and TLR 9 could heighten the downstream interferon regulatory factor (IRF) pathway, while AIM2 and IFI16 induced the secretion of inflammatory cytokines such as IL-1β and IL-18 [13].
Double-edged sword function of RT on immunity
Both clinical and experimental observations suggest that RT may stimulate cancer cell metastasis and induce cancer-promoting effects. The double-edged sword immunomodulatory function of RT in TME is presented in Fig. 3. Myeloid-derived cells include tumor-associated macrophages (TAMs), DCs, polymorphonuclear neutrophils (PMNs), and myeloid-derived suppressor cells (MDSCs) [28, 29]. They are essential for facilitating anti-tumor immunity; however, a plastic immunomodulatory phenotype can be influenced by certain treatment factors. MDSCs are increased in the TME following RT in mouse models, the accumulation of which is through CCL2 – CCR2 signaling and CCL2 may be derived from tumor cells [30]. TAMs generally exhibit pro-tumor (M2-phenotype) properties, inducing angiogenesis and secreting immunosuppressive mediators such as IL-10 and TGF-β. They inhibit T cell function and anti-tumor immunity, and promote a radioresistant phenotype [31]. Stromal-derived factor 1 (SDF-1), colony stimulating factor-1 (CSF-1), and C-X-C chemokine receptor type 4 mediate the recruitment of TAMs and MDSCs, which are upregulated in the irradiated TME [32,33,34]. The regulatory T cells (Tregs) are also increased in the TME following RT, inducing immunosuppression by CTLA-4 expression, IL-10 release, and adenosine production by CD39 and CD73 ectonucleotidases [35]. Increased TGF-β and hypoxia-inducible factor 1-α (HIF-1α) levels can inhibit DCs maturation and induce radioresistance in endothelial cells. TGF-β can not only affect CD8 + T cell proliferation and function, but also induce CD4 + T cells to adopt a regulatory phenotype (Treg), thus dampening the radiation-induced anti-tumor immune response. Evidence indicates that hypofractionated radiation can result in a significant increase in TGF-β [36]. Typically, increased TAMs promote tumor growth, invasion, and metastasis by negatively regulating anti-tumor immunity, thus leading to worse tumor suppression [37]. Moreover, evidence has shown that SBRT with a single dose of 12 Gy or 15 Gy can result in the recruitment of CD4 + T cells mainly composed of Foxp3 + Tregs [36]. Furthermore, a study indicated that the increase in Tregs induced by radiation was dose-dependent, with a single dose of 20 Gy doubling that of a single dose of 2 Gy [38]. The accumulation of Tregs in the TME significantly abrogated anti-tumor immune responses, which confers surviving tumor cells with potent resistance to RT.
The double-edged sword immunomodulatory function of RT on TME. RT can exert a potent antitumor immune response by influencing almost all steps in the cancer-immunity cycle, from the first step of releasing antigens to the final immunomodulatory response. Some essential immune-associated pathways are activated by RT in the process, such as cGAS-STING pathway. However, RT may also induce a suppressed TME in the presence of overactivated MDSCs, TAMs, CAFs and Tregs. Such double-edged sword role would determing the final effect of combining RT and ICIs. Abbreviations: RT radiotherapy, TME Tumor microenvironment, ICD Immunogenic cell death, GMP Cyclic guanosine monophosphate, AMP Adenosine monophosphate, cGAS Cyclic GMP-AMP synthase, STING Stimulator of interferon genes, MDSCs Myeloid-derived suppressor cells, DCs Dendritic cells, Tregs The regulatory T cells, CTLs Cytotoxic lymphocytes, TAMs Tumor-associated macrophages, CAFs Cancer-associated fibroblasts
The synergia of RT and ICIs
Most patients currently cannot benefit from ICIs, as they either do not respond to ICIs at all (innate or primary resistance) or acquire secondary resistance (acquired or secondary) after an initial period of response [39, 40]. Additionally, a fixed treatment duration for ICIs is being increasingly utilized in adjuvant and neoadjuvant settings. In parallel, the progression arising from resistance to ICIs during and after adjuvant therapy could have elements of either primary or secondary resistance, which is difficult to define. After considerable discussion, progression or resistance after treatment discontinuation for any reason has recently been distinguished from the above two resistance scenarios by the Society for Immunotherapy of Cancer [41]. The timeframe between the last dose of adjuvant therapy and disease progression further defined resistance in this setting. The taskforce ultimately agreed on 12 weeks as a cutoff to classify resistant disease in the adjuvant setting into “adequate treatment exposure” and “inadequate treatment exposure [41].”
The response rate is limited to 8–30% in unselective NSCLC patients with treatment of ICI monotherapy [42, 43]. RT participates in the cancer-immunity cycle by activating the immune system and providing a more supportive immune microenvironment for anti-tumor immunity, which alleviates primary resistance and delays the development of secondary resistance to ICIs. In addition, RT can be used to trigger an immune response after ICIs resistance. Elevated levels of PD-L1 can promote T cell exhaustion, a state characterized by dysfunction in T cell proliferation and effector function, related to immune escape and tolerance [44]. It has been observed in multiple cancer types that upregulated PD-L1 on tumor cells can be a dominant resistance mechanism to RT and CTLA4, demonstrating persistent T cell exhaustion and rapid progression [45]. Several studies have shown that PD-L1 upregulation can be detected after RT, which contributes to an explanation for tumor resistance to RT [23, 46, 47]. However, the addition of PD-L1 blockade can reverse T cell exhaustion to mitigate depression in the CD8-to-regulatory T cell ratio and further promote response and immunity through distinct mechanisms [23, 45, 47, 48]. Notably, it is generally accepted that as a leading biomarker, a relatively high expression of PD-L1 indicates a better response to ICIs, which also supports the use of ICIs to overcome resistance to RT [42, 43]. Thus, it can be assumed that PD-L1 upregulation induced by RT gives rise to an immunosuppressive tumor microenvironment, which, on the one hand, impairs the efficacy of RT but supports the use of ICIs in RT to overcome resistance. Similarly, the PEMBRO-RT trial showed that patients with low levels of PD-L1 expression benefit more from the combination of RT and ICIs in OS compared with patients with higher PD-L1 expression levels [10].
Many types of T cells, especially CD8 + T cells, which play a major role in the anti-tumor immune response, are activated and begin to reproduce via initiation of the cancer-immune cycle induced by RT. This assumption has been demonstrated in numerous studies and has been used to explain the abscopal effect of RT, a rare but promising phenomenon. Theoretically, the abscopal effect will be amplified with ICIs, which has also been shown in several preclinical studies [23, 25, 47]. The number of cases reporting abscopal effects at the beginning of the twentieth century increased significantly with the advent of immunotherapy [20]. Besides, the time of manifestation of the abscopal effect with regression of distant metastases was also reduced from 5.4 to 3.3 months, although statistically insignificant [20]. In addition, a study conducted by Deng et al. also found that RT alone cannot produce sustained anti-tumor immune effects, but a combination with PD-1/PD-L1 inhibitors can induce an increase in memory CD8 + T cells, resulting in long-lasting immune memory effects [23]. In general, RT combined with ICIs can overcome resistance to RT and ICIs, enhance the abscopal effect of RT, and strengthen the immune memory effect, generating a synergistic role in the anti-tumor response.
IRT in unresectable LA-NSCLC
Therapeutic efficacy
Consolidation ICIs after CRT
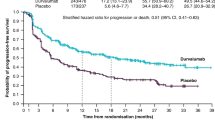
The traditional SoC for unresectable LA-NSCLC is definitive cCRT, with an unsatisfactory 5-year OS rate of 15%–25% [2]. However, the end of the patient’s survival curve was relatively flat, suggesting that there is still hope for a cure. After the combination of targeted or chemotherapy consolidation treatment failed to bring conspicuous survival benefits, the PACIFIC trial, like a huge “tsunami”, completely revolutionized the treatment of unresectable stage III NSCLC, and durvalumab as consolidation therapy for patients whose disease had not progressed after cCRT quickly became the new standard of care [9]. The last update with 5-year survival outcomes demonstrated that consolidation treatment with durvalumab brought robust and sustained OS and PFS benefits after cCRT compared with placebo, with median OS of 47.5 months (vs. 29.1) and 5-year OS rate of 42.9% (vs. 33.4%) [49]. In addition, the risk of death or distant metastasis was also reduced by 41% compared to placebo (stratified hazard ratio [HR], 0.59). However, an unplanned post hoc analysis showed that OS did not improve in tumors with PD-L1 expression ≤ 1% (HR, 1.15) [50]. The ongoing international retrospective PACIFIC-R study is assessing the real-world efficacy of durvalumab in patients from an early access program, which has also allowed sequential chemoradiotherapy (sCRT) in some countries [51]. The preliminary analysis of 1399 patients who received at least one cycle of durvalumab presented a median PFS of 21.7 months in the full population, with 23.7 months in patients treated with cCRT (77%) versus 19.3 months in patients treated with sCRT (14%). Notably, mirroring PACIFIC, the PFS was numerically longer among patients with PD-L1 expression ≥ 1% versus < 1% (22.4 vs. 15.6 months). LUN 14–179 is a phase II study aimed at evaluating the safety and efficacy of pembrolizumab as consolidation therapy after cCRT [52]. The results showed that consolidation pembrolizumab after cCRT prolonged the time to metastatic disease or death, PFS, and OS in comparison with historical controls of chemoradiotherapy (CRT) alone, and it did not increase the rates of grade 3–5 pneumonitis.
However, only half of the patients with stage III NSCLC are treated with radical intent in clinical practice, and only 2/3 receive cCRT [53]. Many patients are unable to tolerate cCRT owing to substantial toxicities and a high rate of treatment-related mortality [6, 54]. Thus, sCRT is widely used in clinical practice around the world as an option for patients who cannot tolerate or access cCRT. Whether PD-1 or PD-L1 inhibitors can prolong the survival of these patients is of great concern. GEMSTONE-301 is the first randomized, double-blind, multicenter, phase III trial to report adjuvant single-agent ICI for patients with stage III NSCLC whose disease had not progressed after sequential or concurrent CRT [55]. Updated data showed that the median PFS was significantly longer with sugemalimab as consolidation treatment than with placebo (10.5 vs. 6.2 months, P = 0.0012) [56]. The median PFS was 8.1 and 15.7 months in the sCRT and cCRT arms, respectively. The results demonstrated that sugemalimab is an effective consolidation therapy for patients with unresectable LA-NSCLC without disease progression after either cCRT or sCRT. In addition, the survival curves of PACIFIC-R for patients treated with sCRT also suggested a plateau and long-term benefit, similar to PACIFIC [51]. The primary safety and secondary efficacy analyses from the single-arm phase II open-label PACIFIC-6 trial were recently reported, and the primary endpoint was the incidence of grade ≥ 3 treatment-related adverse events (TRAEs) within 6 months [57]. Overall, 117 patients with ECOG PS ≤ 2 who did not have any progressive disease after sCRT received adjuvant durvalumab for up to 2 years. As a result, 22 (18.8%) patients developed grade 3 or 4 adverse events (AEs), and only 5 (4.3%) experienced grade 3 or 4 possibly related AEs within 6 months of starting treatment, revealing that consolidation durvalumab was well tolerated following sCRT. In terms of survival, median PFS and OS were 10.9 and 25 months, respectively. In general, cCRT followed by ICIs consolidation remains the first choice; however, consolidation after sCRT is also a priority treatment option in frailer populations if cCRT cannot be tolerated.
The combination of monoclonal antibodies has demonstrated a sustained long-term response and survival in patients with stage IV disease [58,59,60]. In theory, if tolerable, a dual combination of immune strategies in the consolidation setting with complementary mechanisms of action may also overcome resistance to anti-PD-(L)-1 antibodies and further enhance the benefits of immunotherapy in LA-NSCLC. CTLA-4 suppresses T cell activity and inhibits immune responses by inhibiting the binding of the costimulatory molecules CD80 or CD86, found on the surface of antigen-presenting cells (APCs) in the tumor-draining lymph nodes (TDLNs), to the coactivation receptor CD28 [61]. It has been found that RT to a metastatic site of NSCLC could act as an in situ vaccine and synergize with anti-CTLA-4 antibodies [62]. Furthermore, anti-CTLA-4 inhibitors might mitigate the immunosuppressive effects exerted by irradiation on the TDLNs, thus of particular attention [63]. RT and dual checkpoint blockade, which is expected to cross the borders of PACIFIC, have gained attention. The open-label, randomized, phase II BTCRC-LUN 16–081 trial was designed to explore the combination of nivolumab plus ipilimumab for a shorter treatment duration of 6 months as consolidation treatment compared to nivolumab alone after cCRT [64]. A total of 115 patients were randomized to receive nivolumab (arm A) or nivolumab plus ipilimumab (arm B) after completion of CRT. The percentage of patients completing the full 6 months of treatment was 70.4% in arm A and 56.9% in arm B (P = 0.15). Despite a shortened interval of ICIs treatment, an improved 18-month PFS was achieved in both arms (63.7% in arm A and 67.6% in arm B) compared with historical controls (18-month PFS of 30%). The median PFS and 2-year OS rates were both similar in arm A and arm B. However, the incidence of grade 3 AEs (52.9% vs. 38.9%), grade 3 TRAEs (27.5% vs. 18.5%), and grade 3 pneumonitis (17.6% vs. 9.3%) were higher in arm B than in arm A. It appears that the combination shows no additive value in this setting. The phase III CheckMate 73 L trial will compare the PFS and OS of nivolumab plus cCRT, nivolumab plus ipilimumab consolidation (arm A), nivolumab plus cCRT, nivolumab consolidation (arm B), and the standard PACIFIC strategy (arm C) in unresectable stage III NSCLC [65].
Monalizumab is an immunoglobulin that targets NKG2A receptors. The COAST trial was a three-arm randomized (1:1:1) phase II study of consolidation durvalumab alone (control, arm C) or in combination with the anti-CD73 monoclonal antibody oleclumab (arm A) or the antiNKG2A monoclonal antibody monalizumab (arm B) in LA-NSCLC patients without disease progression after cCRT [66]. After a limited median follow-up of 11.5 months, ORR was 17.9%, 30.0% and 35.5% in the control arm, arm A, and arm B, respectively. PFS was significantly prolonged in both combinations compared to durvalumab alone. The incidence of serious TRAEs, all-cause grade ≥ 3 AEs, and all-grade pneumonitis were similar between the treatment arms. The clinical benefit of the combinations appeared to be persistent in an exploratory subgroup analysis, regardless of PD-L1 status, which certainly needs to be further verified in more patients. Indeed, the durvalumab arm in the COAST trial heavily underperformed as compared to that in the PACIFIC trial, which may be mainly related to different patient characteristics. In general, such combination approaches are feasible, safe, and may have the potential to improve the prognosis of patients with LA-NSCLC. Notably, the currently recruiting phase III PACIFIC-9 trial will further evaluate these combinations. The T-cell immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain (TIGIT) is a novel inhibitory immune checkpoint expressed on CD8 + T cells and NK and T regulatory cells in multiple cancers [67]. Cancer cells and cancer antigen-presenting cells express CD155 and CD112, which bind TIGIT, decreasing T cell activity, and coordination with PD-1 or PD-L1 inhibitors shows promising early results [68]. Based on the improved ORR with atezolizumab plus the anti-TIGIT antibody tiragolumab, compared to atezolizumab and placebo, in the metastatic NSCLC setting, two randomized phase II clinical trials, the PACIFIC-8 with domvanalimab (AB154) plus durvalumab and the Skyscraper-03 with tiragolumab plus atezolizumab, are testing this approach as consolidation treatment after cCRT in unresectable stage III NSCLC. However, as the combination of atezolizumab plus tiragolumab has neither been reported to improve the PFS in the first-line setting in PD-L1 ⩾50% metastatic NSCLC (phase III RCT, SKYSCRAPER-01), PFS, and OS in advanced small cell lung cancer (phase III RCT, SKYSCRAPER-02), the anti-TIGIT enthusiasm has now decreased [69]. In our view, anti-TIGIT in stage III NSCLC is still unclear, as patients with stage III NSCLC represent a different patient population.
Concurrent ICIs with CRT
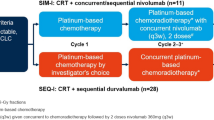
Upon the success of ICIs consolidation following CRT, a series of upcoming clinical trials are investigating novel approaches to explore the best mode of application of ICIs in patients with LA-NSCLC. Representative completed and ongoing clinical trials investigating RT combined with ICIs are shown in Table 1.
Nearly half of the patients with unresectable stage III NSCLC who received CRT did not meet the PACIFIC criteria for durvalumab eligibility [78, 79]. The most common reason for durvalumab ineligibility was disease progression during CRT followed by therapy-related pneumonitis [78]. Concurrent ICIs with CRT would offer all patients eligible for cCRT the opportunity to receive ICIs and may also exploit the potential synergism between chemotherapy and ICIs [80, 81]. In a phase I trial to study the safety and efficacy of using pembrolizumab concurrently with CRT in LA-NSCLC, the 12-month PFS rate was 69.7%, higher than the 55.7% reported in the PACIFIC trial, and the median OS and 1-year OS rate were 29.4 months and 85.2%, respectively [82]. The incidence of irAEs above grade 2 was 67% and the incidence of pneumonia above grade 2 was 33%. The phase II, nonrandomized, 2-cohort (arm A, squamous and non-squamous histology; arm B, non-squamous histology), open-label KEYNOTE-799 study showed that pembrolizumab plus cCRT provided robust anti-tumor activity (ORR, 70.5%) with a manageable safety profile for patients with previously untreated, stage III, unresectable LA-NSCLC, regardless of tumor histologic type and PD-L1 expression [71]. In the most recent two-year update, a median PFS of 30.6 months was reported in cohort A with 2-year OS of 64.3%, and the median PFS was not reached in cohort B, with 2-year OS of 71.2% [72]. DETERRED is a phase II study designed to evaluate the efficacy and safety of atezolizumab combined with cCRT in patients with LA-NSCLC [75]. Part 1, with 10 evaluable patients, was administered conventionally fractionated cCRT followed by two cycles of chemotherapy plus atezolizumab, followed by consolidation atezolizumab for up to 1 year. Part 2, with 30 evaluable patients, involved the administration of cCRT concurrently with atezolizumab followed by the same maintenance therapies as in part 1. The median OS in part 2 has not yet been reached, suggesting better efficacy than combining atezolizumab sequentially with cCRT in part 1. The single-arm phase II NICOLAS trial evaluated the use of nivolumab concomitant with cCRT in 79 LA-NSCLC patients [73]. After receiving one cycle of chemotherapy, the patients were treated with two cycles of nivolumab concurrently with cCRT, and then treated with nivolumab as consolidation therapy for 12 months. Overall, nine (11.7%) patients experienced grade 3 pneumonitis. The 1-year PFS was 53.7% (95% CI, 42.0–64.0) with a median PFS of 12.7 months (95% CI, 10.1–22.8), and the median OS was 38.8 months (95% CI, 26.8–NR) [74]. The results of the three non-randomized phase II trials cannot be compared directly with PACIFIC (randomization after cCRT) due to the design and small sample sizes. However, these data suggest the feasibility and safety of the concurrent administration of ICIs and cCRT in LA-NSCLC. Except for KEYNOTE-799, the 1-year PFS was approximately equal to that of PACIFIC. Of course, the actual efficacy of the triplet regimen should be compared with the PACIFIC status in phase III randomized controlled trials.
Further insight is expected from the ongoing phase III KEYNOTE-012 study comparing pembrolizumab plus cCRT followed by pembrolizumab with or without the poly adenosine diphosphate-ribose polymerase (PARP) inhibitor olaparib with cCRT followed by durvalumab in patients with unresectable stage III LA-NSCLC. KEYVIBE-006 evaluated MK-7684A (co-formulation of vibostolimab-anti-TIGIT plus pembrolizumab) plus cCRT, followed by MK-7684 versus cCRT followed by durvalumab. Other phase III clinical trials, including the CheckMate 73 L (concurrent nivolumab with or without ipilimumab followed by nivolumab), ECOG-ACRIN EA5181 (concurrent and consolidation durvalumab), and PACIFIC2 trial (concurrent and consolidation durvalumab) are also ongoing. The results of these trials will elucidate whether more intensive treatment improves outcomes without compromising safety.
Induction ICIs followed by CRT
Similarly, induction ICIs followed by CRT also enable more patients to benefit from ICIs treatment. Moreover, both RT and chemotherapy play a double-edged role in the immune system. Effective T cell infiltration triggered by RT or chemotherapy only occurs when the immune system is not destroyed. The direct killing effect of RT and chemotherapy on circulating lymphocytes and stem cells cannot be disregarded [5, 83]. Hence, the approach of using ICIs before CRT has the advantage of an intact and healthy immune system. The AFT-16 trial is the first phase II trial to explore induction ICIs before CRT in stage III NSCLC [76]. Enrolled patients first received two cycles of atezolizumab and were then restaged. Two more atezolizumab treatments were delivered if not progressive, followed by standard cCRT and consolidation atezolizumab for up to 1 year. Patients who had progressed at the first restaging point immediately received cCRT. The primary endpoint was the disease control rate at the end of induction atezolizumab, and an inspiring result of 77.4% was reported. A remarkable median PFS of 23.7 months was observed. The PFS at 12 months after the completion of cCRT was 78%, which is impressive compared with PACIFIC (12 months PFS = 55%). Although the AFT-16 population was highly selected, the study did not limit the eligibility to responders to cCRT, as in the PACIFIC trial [9, 49, 70]. Similarly, the ongoing phase II SPRINT trial is evaluating a chemotherapy-free strategy in PD-L1 ⩾50% tumors (n = 25), with sequential three cycles of induction pembrolizumab followed by risk-adapted thoracic RT and followed by 12 additional cycles of pembrolizumab [77]. The trial also enrolled patients with tumors with PD-L1 expression < 50% who were treated with standard cCRT to serve as a non-randomized comparison group (n = 38). In the first interim analysis of patients with PD-L1 ⩾50%, 48% achieved partial response (PR), with 1-year PFS and OS rates of 73% and 91%, respectively. Intriguingly, after three cycles of pembrolizumab induction, patients with PR at the restaging positron emission tomography/computed tomography (PET-CT) (n = 12) had a 1-year PFS of 100%, compared to 61% in patients with stable or progressive disease. Thus, the response observed by PET following pembrolizumab induction may be useful for identifying patients who can be successfully treated without chemotherapy. Similarly, the NRG-LU004 trial assesses the combination of durvalumab concomitantly with RT followed by durvalumab for 1 year in patients with PD-L1 ⩾50% NSCLC. The phase II APOLO trial assessed neoadjuvant atezolizumab plus chemotherapy followed by cCRT and the maintenance of atezolizumab for 12 months. Another phase II trial evaluated the same treatment strategy as nivolumab. The difference is that a comparator arm without nivolumab maintenance was included. Moreover, three single-arm phase II trials (NCT05128630, NCT04765709, and PACIFIC-BRAZIL) evaluated the induction of durvalumab plus chemotherapy, followed by RT (cCRT in PACIFIC-BRAZIL) concurrently with durvalumab followed by durvalumab consolidation. We are conducting a phase II, multicenter, randomized, open-label, controlled trial comparing induction treatment with camrelizumab combined with chemotherapy following cCRT and maintenance camrelizumab with standard CRT for LA-NSCLC. It is expected that ICIs and chemotherapy can work synergistically to better play the role of neoadjuvant therapy. In addition, it has been proven in resectable NSCLC that ICIs combined with chemotherapy can achieve tumor downstaging and provide a more supportive immune microenvironment [84]. Thus, a reasonable hypothesis would be that induction ICIs and chemotherapy in unresectable LA-NSCLC can not only downsize initial tumors, but also reduce resistance to RT. The preliminary results demonstrated that induction ICIs with chemotherapy followed by radical cCRT yielded an inspiring median PFS of 20.4 months for unresectable LA-NSCLC, which was markedly superior to most of the results in the aforementioned studies with other treatment regimens.
Toxicity and safety
Enhanced anti-tumor immune surveillance upon treatment with ICIs is inherently at the expense of immune-related adverse events (irAEs), which affect virtually every organ system and are a proposed Achilles’ heel of this class of therapeutic agents [85, 86]. It is estimated that 50% of patients treated with ICIs will experience some form of irAEs [85, 87]. Compared to other irAEs, ICIs-related pneumonitis (CIP) is characterized by rapid onset and high fatality, warranting early detection. CIP is defined as the development of dyspnea and/or other respiratory symptoms in the presence of new infiltrates on chest imaging without the presence of new infections[88]. Chest CT scans currently play a major role in the diagnosis of CIP because of the difficulty in predicting the development of irAEs prior to starting therapy [89]. A meta-analysis reported that the incidence of all-grade CIP during PD-1 inhibitor monotherapy for NSCLC was 4.1%, which is higher than the overall incidence of multiple advanced cancers [90]. Similarly, the reported incidence of any grade pneumonitis without RT was 3.8% in the largest pooled analysis to date of AEs risk associated with the use of RT prior to ICIs [91]. Moreover, patients who received RT before ICIs had similar rates of various AEs, including pneumonitis. However, the incidence of CIP was found in 10% of the entire population from a single institute retrospective study, higher than that reported for other irAEs (1–5% of all malignancies) [88]. The population included 151 lung cancer patients and the incidence of CIP was even higher (12.6%) in these patients. This discrepancy could mainly arise from the drawbacks of the small sample. On the other hand, a higher incidence in lung cancer patients could arise from the dominance of male lung cancer patients, a large proportion of whom are smokers [92]. Additionally, combination immunotherapy (vs. monotherapy), the use of PD-1 inhibitors (vs. PD- L1 inhibitors), and the use of ICI as a first-line therapy (vs. second-line or further) are also associated with a higher risk of CIP [93]. Notably, the incidence of other irAEs was also significantly higher in patients with patients who did not develop CIP [88]. In general, due to the variety of local or systemic treatments that act together on lung tissue, CIP in NSCLC is worthy of close attention.
A phase II study with multisite SBRT and pembrolizumab treatment, as well as the PEMBRO-RT phase 2 randomized trial, showed concordant results with tolerable irAEs [10, 94]. As reported in the PACIFIC trial, grade 3 or 4 AEs occurred in 29.9% of patients who received durvalumab after cCRT; the most common grade 3 or 4 AE was pneumonia, with an incidence of 4.4% [9]. A total of 15.4% of the patients discontinued durvalumab because of AEs. In the GEMSTONE-301 trial, grade 3 or 4 treatment-related irAEs occurred in 22 (9%) of 255 patients in the sugemalimab group compared with seven (6%) of 126 patients in the placebo group [55]. The most common, pneumonitis or immune-mediated pneumonitis, occurred in 7 (3%) patients. The rate of grade 2 or higher pneumonitis was 10% in the DETERRED trial, demonstrating good tolerance [75]. However, a secondary analysis by KEYNOTE 001 proposed that the treatment-induced pulmonary toxicity rate differed between the two groups (13% with combination vs. 1% with ICIs only, P = 0.046) [95]. Another multicenter analysis of safety and toxicity reported that the rates of related subacute grade ≥ 3 irAEs in the SBRT combined with ICIs and SBRT alone groups were 26.8% and 2.9%, respectively, and the rates of grade ≥ 3 pneumonitis were 10.7% vs. 0 with P < 0.01 [96]. IrAEs can also induce fatal outcomes, reminding us of cautious surveillance. Many factors could contribute to the disparate results, such as prior lung disease, prior treatment, previous or current smoking, age > 70 years, type of inhibitor, and histological type [97]. Moreover, the timing of RT may be important, and the sequence of combining RT and ICIs remains controversial.
Interestingly, several publications have proposed that irAEs might be related to significantly better ORR, PFS, and OS in NSCLC patients who received PD-1/PD-L1 inhibitor monotherapy [87, 98, 99]. In combination therapy, this finding was also observed. In a retrospective study of 201 patients with nivolumab combined with prior thoracic RT, longer mPFS and lower disease progression rates were found in those who experienced therapy-associated pneumonitis compared with those who didn’t (3.6 vs. 2.3 months, P = 0.023; 29.4% vs. 47.9%, P = 0.059) [100]. Hwang et al. proposed that patients with grade 2 or higher irAEs, especially pneumonitis, had better survival benefits [101]. Based on preclinical theory, some studies have speculated that the occurrence of irAEs might reflect a much more active immune response, indicating a strong anti-tumor immunity function under combination treatment [89]. Therefore, the observation of irAEs may not only be induced by overlapping toxicity, but also contribute to outcome prediction.
Progression during and after ICIs consolidation therapy
Resistance to ICIs remains a key clinical barrier to further improving the outcomes of patients with advanced or metastatic lung cancer. Approximately 80% of patients with unselected advanced NSCLC do not respond to single-agent nivolumab [102]. As mentioned above, progression after ICIs discontinuation is classified into a distinct resistance scenario, from primary or secondary resistance [41]. Progression during ICIs therapy can be defined based on the presence of more than 6 months of disease control. In terms of LA-NSCLC, more than half of the patients would progress within 2 years of the start of treatment [49, 55]. Updated data from the PACIFIC trial showed that 49.0% of patients completed 12 months of ICIs treatment, and 31.3% discontinued owing to disease progression [49]. In the PACIFIC trial, 7.1% of patients in the ICIs arm received durvalumab retreatment and completed the initial 12 months of durvalumab with disease control and progressed during follow-up, and the median time to second progression measured from the random assignment was 48.0 months [49]. Subsequent ICIs were less commonly used among patients randomly assigned to the durvalumab arm than those in the placebo arm (12.6% vs. 29.1%). A real-world multicenter retrospective study of 116 patients with unresectable stage III NSCLC treated with CRT followed by at least one dose of durvalumab reported no significant difference in response and time of treatment with combined chemotherapy and ICIs vs. chemotherapy alone, which was posted in 2022 European Lung Cancer Congress [103]. Another phase II, single-arm, multi-center trial of consolidation pembrolizumab for up to one year following concurrent chemo-RT in unresectable stage III NSCLC reported in the 2019 World Lung Cancer Congress that response rates with chemotherapy were similar to what is expected in the second-line setting for patients with disease progression after consolidation pembrolizumab, and only 1 of 6 patients rechallenged with ICIs responded [104]. Apart from these, no other data were available on rechallenge with ICIs at progression after completion of 12 months of durvalumab treatment in LA-NSCLC. Therefore, rechallenge with ICIs or immunochemotherapy was less encouraging at progression during and after the ICIs consolidation therapy in a locally advanced setting.
The relative prevalence of oligometastatic disease is estimated to range from 30 to 50% in advanced NSCLC [105]. Oligoprogression, a more specific concept, is increasingly encountered in patients treated with ICIs, which may be a common pattern of acquired resistance to ICIs [106, 107]. Unlike systemic treatment options, RT not only eradicates local lesions but also plays a role in overcoming resistance to ICIs. In a retrospective study of 26 patients with acquired resistance to anti-PD-1 inhibitors, 88% had recurrence limited to one (54%) or two (35%) sites, and local RT to oligo-progression with the continuation of ICIs achieved superior survival [108]. Similarly, local RT plus continued ICIs led to significantly longer PFS and OS in patients with oligo-progression from ICIs treatment compared with those who received no local RT [109]. In theory, chemo-RT provides a supportive TME for ICIs consolidation treatment, reducing primary or acquired resistance to ICIs to some extent, while the addition of RT during or after ICIs helps overcome the developed resistance. Promising clinical evidence highlights the superiority of iRT in LA-NSCLC and the role of RT in oligo-progressive NSCLC after ICIs treatment.
Challenges, strategies, and auspicious orientations
Optimal dose and fractionation of RT
Regarding RT, the dose and fraction scheme are essential in the lesion local control and outcome, while enhanced immunity probably plays a mediating role. Hyperfractionation RT (HyperRT) and hypofractionated RT (HypoRT) are the concepts of conventional fractionation. SBRT is a typical representation of hypo-RT, consisting of the administration of high doses of RT with a narrow margin and a strong gradient to protect the surrounding healthy tissues, also known as stereotactic ablative radiotherapy (SABR). Siva et al. proposed that conventional fractionation was detrimental to RT-induced anti-tumor immune responses, as irradiation intervention would frequently purge local immune lymphocytes [110]. Their research demonstrated that a single high-dose RT could release more TAAs without depleting immunocytes, shielding CD8 + T cells and NK cells to a certain extent [110]. Chen et al. also confirmed that SBRT can better protect lymphocytes than conventional fractionation [111]. However, a higher dose in a single fraction is not always preferable. However, an excessive dose in a single fraction may induce a suppressive TME. It has been reported that high dose irradiation such as 20 Gy induced expression of three prime repair exonuclease 1 (Trex1), which bears the function of degrading the dsDNA in the cytoplasm, and thereby weakened the immunomodulatory effects of RT [112]. Therefore, SBRT has been the standard therapy for early NSCLC patients who are not suitable for surgery, allowing the delivery of high doses to relatively small target lesions [8, 113]. Notably, the latest revised STARS study provided a higher level of evidence for its use in patients with operable early NSCLC [114]. In the randomized phase I/II MDACC trial for metastatic NSCLC with lung and liver lesions, compared to traditional RT with 45 Gy in 15 fractions, 50 Gy in 4 fractions led to better out-of-field ORRs (38% vs. 10%) and longer median PFS (20.8 vs. 6.8 months) when combined concurrently with pembrolizumab [115]. The pooled analysis of the PEMBRO-RT (24 Gy in 3 fractions, sequential with pembrolizumab) and MDACC trials showed that pembrolizumab plus RT with 50 Gy in 4 fractions corresponded to the best PFS [17]. However, better survival over 24 Gy in three fractions may mostly derive from the concurrent delivery of RT and ICIs. In conclusion, these data indicate that SBRT and HypoRT are not only prominent in local control but can also better coordinate the effect of ICIs. In addition, it seems that a high single dose of 8–10 Gy is the optimal dose to activate the anti-tumor immune response, in contrast to conventional fractionation [5, 37]. This hypothesis warrants further corroboration in a dedicated, large-volume, phase III, randomized trial.
The current standard of CRT for unresectable LA-NSCLC consists of 6–7 weeks of RT with a dose of 60–70 Gy in 2 Gy daily fractions and chemotherapy administered at a reduced dose, as opposed to the systemic dose when chemotherapy is administered by itself [8]. The RTOG 0617 trial reported the highest OS (5-year OS rate, 32.1%) of any phase III trial without the addition of ICIs for stage III NSCLC patients, strongly supporting a SoC RT with 60 Gy given to a target volume directed at the tumor plus margin on the basis of CT and PET/CT, excluding elective nodal irradiation (ENI) [116]. Secondary analysis suggested that a higher RT dose to immune cells correlated with worse tumor control and OS [117]. However, the controversy regarding the benefits of dose escalation remains open. These poor results may be attributed to the prolongation of the global treatment time, which leads to an accelerated repopulation of cells. A meta-analysis examining different RT schemes, including regimens with splits, hypoRT, hyperRT, and dose escalation with conventional fractionation, found that an increased biologically effective dose administered without chemotherapy improved survival [118]. Therefore, the role of SBRT in LA-NSCLC has become an area of great interest. Several studies have examined a combination of conventional and SBRT boost for locally advanced disease, but there are limited data regarding SBRT as a complete replacement for conventional radiation. Recently, safety results of NRG-LU004 reported that chemotherapy-free thoracic accelerated fractionated RT (60 Gy/15F) was safe when administered with concurrent durvalumab in LA-NSCLC patients with high PD-L1 expression [119]. Another single-arm phase II study showed that a combination of SBRT and systemic dose chemotherapy was a safe and effective treatment for LA-NSCLC [120]. Of course, the results warrant further investigation, owing to the small sample size.
SBRT may increase local control in patients with LA-NSCLC with an acceptable safety profile, although the level of evidence is still deficient. Further, given the immunomodulatory role of RT, especially SBRT, it is presumable that novel treatment schemes for LA-NSCLC integrating hypoRT or SBRT with ICIs will be proposed in the coming years. Moreover, low-dose irradiation (LDI) ranging from 0.5 Gy to 2 Gy has recently been proposed, which can reshape the TME, including the polarization of M1 macrophages and homing of T cells. The results of a clinical trial of SBRT combined with ipilizumab for advanced malignant tumors found that tumors exposed to low-dose scattered radiation (close to the SBRT-targeted region) were more likely to respond to therapy than lesions far from the target [121]. Based on these, a novel treatment modality with LDI combined with SBRT was proposed, in which SBRT irradiates primary lesions to ignite the “in situ vaccine” effect and LDI reshapes the stroma of other metastases [122]. Recently, a novel strategy of high- and low-dose RT combined with anti-TIGIT and anti-PD1 monoclonal antibodies in lung adenocarcinoma cell lines revealed further improved efficacy, providing a new treatment alternative for cases refractory to other checkpoints [123]. Hence, a high-plus low-dose RT strategy for LA-NSCLC may also be worth exploring. Notably, although controversial, single-fraction SBRT has also been evaluated for negative regulation of TME in peripheral early-stage NSCLC and metastatic lesions [124, 125]. Of course, there is a long way to go for the use of SBRT or even one-stop SBRT in LA-NSCLC.
RT target and target volume
It is certain that a consensus on the definition of the target volume is key to avoiding excess toxicity due to large volumes. As mentioned before, the RTOG 0617 trial excluded ENI without targeting areas of high FDG uptake and denied dose escalation [116]. TDLNs are important sites for the activation and accumulation of anti-tumor T lymphocytes; therefore, ENI may affect the adaptive immune response to some extent. Accordingly, by irradiating TDLNs, the adaptive immune response was attenuated in a transplantable mouse model treated with SBRT and ENI, especially when RT and ICIs were combined [126]. A multicenter open-label, randomized, controlled trial PET-Plan (ARO-2009–09) suggested that [18] F-FDG PET-based planning could potentially improve local control without increasing CRT-related toxicity in patients with LA-NSCLC. Our phase II randomized trial also found that [18] F-FDG PET/CT adaptive shrinking field and simultaneous integrated boost RT technique can improve ORR, OS, and PFS without increasing the risk of RT-related toxicity [127]. However, van Diessen et al. reported higher rates of acute and late toxicity in a randomized phase II dose escalation trial that used PET boost. The results of the RTOG 1106 trial were also reported, in which a mid-treatment PET/CT was used to allow a hypofractionated boost over the last 2 weeks to escalate the RT dose to residual disease, failing to achieve improved local control and OS [128]. In general, randomized data support the omission of ENI from PET information, but whether to boost the RT dose deserves further investigation.
Charged particle therapy
Charged particle therapies, such as protons and heavy ions, have rapidly developed to play a vital role in tumor therapy. Compared with photon RT, the most notable feature of charged-particle therapy is the sharper dose distribution derived from the spread-out Bragg peak, which could significantly reduce the radiation beam on adjoint normal tissues. Both early stage and LA-NSCLC are suitable for proton RT [129]. However, there is a lack of strong evidence to prove the superiority of proton RT over photon RT. Some phase I/II trials are attempting to hypofractionate RT dose by proton RT, with a focus on the ability of protons to limit the normal tissue dose [130]. Another ongoing phase III trial, RTOG 1308, comparing photons to protons, allows a higher dose of 70 Gy to be delivered to the appropriate arm when normal tissue dose constraints are met. Carbon-ion RT (CIRT), a type of heavy ion RT, not only has the Bragg peak character but also influences the immune response differently from photon RT. Preclinical studies have proposed that carbon-ion beams render complex and difficult-to-repair DNA double-strand breaks in irradiated tumor cells [131]. This could enhance the release of HMGB1 by increasing linear energy transfer in tumor cells, indicating that the combination of ICIs and CIRT is a promising point to investigate.
Choice, timing, and duration of ICIs
Although numerous trials have confirmed that combined ICIs and CRT would benefit patients with LA-NSCLC, the optimum mode of combination therapy remains controversial. The first question was which type of ICI could match better with CRT. In metastatic NSCLC, two single-institution prospective trials showed a significantly better PFS with anti-PD-1 combined with SBRT than with anti-CTLA4 (6-month PFS, 87% vs. 52%; 18-month PFS, 63% vs. 23%; P = 0.02) [132]. In terms of LA-NSCLC, all published and ongoing trials have selected anti-PD-(L)-1 antibodies as monotherapy for efficacy intensification. Interestingly, the optimal timing of anti-CTLA4 combined with RT was different from that of anti-PD-(L)-1 antibodies. The stimulatory effect of RT on the TME can be exploited when anti-PD-(L)-1 antibodies are used concurrently with or after RT because they function to limit T cell activity, whereas anti-CTLA4 targeting Tregs should be administered before RT to assist antigen presentation [133, 134]. For patients with LA-NSCLC, ICIs may be administered before, after, or concurrently with RT. PACIFIC has laid a framework for administering ICIs after RT in this setting [9]. Subgroup analysis of the PACIFIC study showed that receiving ICIs within 0–14 days after the end of cCRT correlated with better PFS and OS than patients receiving ICIs between 15–42 days [9]. Similarly, a retrospective observational study of patients with stage III unresectable NSCLC who received durvalumab after cCRT from 2018 to 2021 was recently reported; patients who received durvalumab 30–60 days after cCRT had lower OS rate at 30 months compared to those who started durvalumab before 30 days (44% vs. 90%) [135]. However, this difference was not statistically significant (P = 0.45). Additionally, as the TROG1937 (DATE study, jRCTs031190117) reported, a phase II study, durvalumab can be safely administered immediately after completion of cCRT for patients with unresectable stage III NSCLC, with no additional or unexpected toxicity as a reference to PACIFIC [136]. In contrast, a retrospective analysis of 371 patients treated with ICIs after SBRT showed that administration of ICIs for at least 21 days after SBRT had longer OS [133]. However, this was a retrospective study with several confounding factors. In contrast, the pooled analysis mentioned before of AEs associated with the use of RT prior to ICIs demonstrated that patients receiving RT prior to ICI generally had similar rates of AEs compared with those who did not receive prior RT [91]. The administration of ICI within 90 days generated a slightly numerically higher rate of AEs, and this difference was attributed to low-grade AEs. Thus, they concluded that it would appear safe to administer ICI within 90 days of receiving RT. It appears that the administration of ICIs after RT is generally safe for both locally and advanced NSCLC patients. Nevertheless, a retrospective analysis of patients with prior irAEs found that thoracic RT resulted in a very high risk of clinically significant and persistent RP [137]. However, it remains unclear whether pneumonitis is caused by RT. With regard to ICIs concurrent with CRT, data from three main non-randomized phase II trials suggested tolerable toxicity and at least comparable efficacy in LA-NSCLC [71,72,73,74,75].
Taken together, both preclinical and clinical evidence tends to support RT prior to or concurrently with anti-PD-(L)-1 antibodies, and in consolidation schemes, it seems that early addition after RT improves survival. However, as elaborated previously, induction ICIs before RT also have unique advantages, such as the potential to mitigate resistance to RT and retain intact immunity. Concerns regarding toxic effects have focused on the synchronous administration of ICIs and CRT. There may be no difference in toxicities between the two sequential schemes before or after CRT. Which strategy is superior or inferior warrants a detailed comparison in prospective head-to-head trials. A comprehensive evaluation and consideration of the efficacy, side effects, and actual conditions of patients should be adopted in clinical practice. Specifically, when combined with anti-CTLA4, the mainstream view supports the delivery of ICIs prior to RT. Strategies with dual checkpoint blockade could consider using anti-CTLA4 first, which needs to be validated in randomized clinical trials.
The optimal treatment duration for ICIs consolidation remains to be determined. In the PACIFIC trial, durvalumab was scheduled to be administered every 2 weeks for up to 12 months; however, only 43% of the enrolled patients completed the planned therapy [9]. In the first-line advanced setting, PD-1 or PD-L1 inhibitor treatment often lasted up to 2 years (KEYNOTE-024, KEYNOTE-189) or until disease progression (IMpower150) [55]. Accordingly, in the GEMSTONE-301 trial, the patient received sugemalimab treatment for up to 24 months [55]. Nevertheless, the percentage of patients completing the 2 years of therapy is still unknown, as at the data cutoff, 43% of patients in the sugemalimab arm were still on treatment. We need to know whether a longer treatment duration correlates with a higher benefit from ICIs or whether a shorter duration of treatment is also feasible. A retrospective study of 1006 patients with stage III NSCLC who received cCRT and at least one dose of adjuvant durvalumab suggested that PFS was similar for 9 months versus 12 months of durvalumab treatment, and PFS for 6 months was inferior versus 12 months [138]. The most common reasons for early discontinuation were tumor progression (22%), irAEs (15%), and non-immune-related toxicities (6.0%). In the absence of conclusive evidence from prospective randomized controlled studies, further consideration is required to make clinical decisions. For example, opinions were divided on whether ICIs consolidation should be used in PD-L1 negative patients. Therefore, we may choose to administer a shortened course of ICIs treatment, especially when toxicities are severe. The role of the dynamic circulating tumor DNA (ctDNA), other than PD-L1 expression, is also of relevance. Growing evidence has demonstrated that ctDNA minimal residual disease (MRD) following treatment for solid tumors can predict relapse [139]. According to the MRD status after CRT, early intervention may be feasible in patients at high risk of progression, and the dynamic evolution of ctDNA carrying more information may facilitate personalization of the duration of ICIs. For NSCLC, a study of patients with metastatic NSCLC receiving pembrolizumab or a combination of pembrolizumab and chemotherapy indicated that serial monitoring of ctDNA may serve as a non-invasive predictor of response [140]. In LA-NSCLC, ctDNA has also been shown to predict significantly better clinical outcomes, which may allow for personalized adjuvant treatment [141]. In terms of RT, Bi et al. performed targeted next-generation sequencing (NGS) of serial plasma samples from NSCLC patients who received front-line CRT or RT and found that ctDNA collected 1 month after treatment was optimal for predicting patient survival [142]. As mentioned before, the BTCRC LUN 16–081 trial investigated a shortened interval (6 months) of treatment with nivolumab or nivolumab plus ipilimumab, and improved 18-month PFS was achieved in both arms [64]. Analysis of ctDNA in the BTCRC LUN 16–081 trial found that MRD-positive patients after completion of CRT were strongly associated with inferior PFS compared to MRD patients (1-year 29% vs. 76%, 2-year 29% vs. 68%, respectively, P = 0.003) [143]. Specifically, patients with undetectable MRD at the end of consolidation ICIs therapy demonstrated a 2-year OS of 91%. However, all patients with increasing ctDNA levels after two cycles of ICIs treatment experienced disease progression within 10.8 months of starting ICIs treatment. Similarly, a study applied ctDNA analysis to 218 samples from 65 patients with LA-NSCLC receiving CRT, and 28 patients receiving consolidation ICIs were included [144]. The results revealed that patients with undetectable ctDNA after CRT (no MRD) had excellent outcomes, regardless of whether they received consolidation ICIs. In contrast, patients with detectable ctDNA showed significant benefits with ICIs consolidation ICIs treatment. All these data suggest that MRD detection after CRT might be capable of distinguishing the cured population from the population who require enhanced ICIs consolidation. In addition, changes in ctDNA levels during consolidation treatment could serve as an early biomarker of disease progression and long-term outcomes. In an ongoing clinical trial (NCT04585490), consolidation ICIs will be personalized according to MRD after CRT. MRD-positive patients will receive four cycles of platinum doublet chemotherapy and durvalumab, whereas MRD patients will only receive durvalumab monotherapy.
Oncogenic addicted tumors
To date, there is no current SoC for patients with oncogenic driven stage III NSCLC. In the stage IV setting, ICIs have not achieved the desired effect in the treatment of oncogene-driven NSCLC, such as EGFR exon 19 and 21 mutations and ALK and ROS-1 rearrangements, either first- or second-setting [145, 146]. Although the data are derived mostly from subgroup analyses of prospective trials and retrospective studies, the mainstream does not support the use of ICIs in unresectable stage III NSCLC [147]. The PACIFIC trial enrolled 43 EGFR-mutant patients, the results of which were not promising, with a PFS HR of 0.84 (95% CI: 0.40–1.75) and OS HR of 0.97 (95% CI: 0.40–2.33) [9]. Recently, a post hoc exploratory analysis of the PACIFIC trial evaluated the efficacy and safety of durvalumab in 35 EGFR-mutant patients [148]. In this subgroup, neither the PFS nor the OS was observed to improve with durvalumab, compared with the placebo. The safety profile of durvalumab was consistent with that of the overall population. The incidence of RP was 42% in the durvalumab arm versus 36% in the placebo arm, and that of pneumonitis was 17% and 18%, respectively. A multicenter retrospective study enrolled 323 patients treated with CRT and consolidation durvalumab, 43 (23%) of whom had oncogenic driver alterations, mainly KRAS (n = 26), followed by EGFR (n = 8), BRAF (n = 5), and ALK (n = 4) [149]. They observed limited activity in patients with EGFR mutations (mPFS, 8.1 months) and BRAF V600E mutation/ALK rearrangements (mPFS, 7.8 months). Only those KRAS-mutant tumors (n = 26) benefited from durvalumab maintenance. Another multi-institutional retrospective analysis (n = 37) also showed no statistically significant benefit in EGFR-mutant patients treated with durvalumab after cCRT [150]. Furthermore, real-world data including 16 EGFR-mutant patients in all 61 patients who received consolidation durvalumab found that the presence of an EGFR-mutation was the only independent predictive factor for unfavorable PFS (6.5 vs. 33.63 months in EGFR wild-type or unknown tumors; P < 0.001) [151]. In EGFR/HER2-mutant tumors, a significantly shorter DFS with durvalumab was also obtained (7.5 vs. NR; P = 0.04) compared with wild-type tumors [152]. Poor survival was independent of PD-L1 expression. For stage III ALK rearranged NSCLC patients, a retrospective analysis (n = 20) of patients with stage III ALK-rearranged NSCLC reached a similar conclusion [153]. Notably, the evidence against the use of ICIs consolidation therapy in this setting is also due to severe irAEs. Nearly 40% of patients experienced severe irAEs [150]. On the other hand, receiving tyrosine kinase inhibitors (TKIs) such as EGFR TKIs, during or after ICIs is also associated with increased toxicity. Up to 15% of patients receiving sequential osimertinib after ICIs treatment were reported to develop severe irAEs, and most of them required hospitalization [154]. When osimertinib is combined with durvalumab, the risk of interstitial lung disease may be higher [155].
Although larger prospective studies are urgently needed to confirm these findings, based on published data, in a recent ESMO consensus, over 90% of experts did not recommend the use of consolidation ICIs therapy after curative-intent CRT in EGFR-positive NSCLC [155]. As for alterations that might benefit from ICIs consolidation, as mentioned before, patients with a KRAS mutation (n = 26) may benefit from consolidation durvalumab (PFS not reached vs. 8.1 months in EGFR-mutant patients), similar to the metastatic setting [149]. In addition, durvalumab consolidation after cCRT significantly improves local–regional control in KEAP1/NFE2L2 mutant NSCLC tumors (1-year regional failure of 62% vs. 25%, P = 0.021), which correlates with a chemoradiation-resistant phenotype, with a higher risk of locoregional failure [156]. These retrospective findings certainly add to the complexity of whether oncogenic addicted stage III NSCLC could derive benefits from ICIs consolidation, including some rare mutations, co-mutations, and other specifics. However, in summary, patients harboring driver mutations face an underwhelming prognosis with ICIs consolidation, with a hindered survival and unfavorable safety profile. Therefore, a better consolidative strategy for patients with EGFR-mutations and other oncogenic drivers is urgently needed. In this context, the role of targeted therapies is anticipated. The feasibility of RT combined with EGFR-TKIs has been reported in unresectable stage III EGFR mutation-positive NSCLC, although the sample size was limited [157, 158]. We should await the results of the randomized phase III LAURA trial, investigating the efficacy of adjuvant osimertinib after cCRT in patients with the most common EGFR sensitizing mutations (Ex19Del and L858R), which will provide evidence on the benefit of targeted therapy instead of ICIs [159]. In addition, another recruiting phase III multicenter study (NCT05170204) will evaluate the efficacy and safety of multiple therapies (alectinib, entrectinib, pralsetinib, and durvalumab) in cohorts of patients with ALK-positive, ROS-1-positive, or RET fusion-positive mutations in this setting [160]. Clearly, there is still a long way to go for this population, since genomic alterations are extraordinarily complex, and we cannot be satisfied with extrapolating data from the stage IV setting.
Patient selection and biomarkers
Despite the increasing use of ICIs in patients with NSCLC, most patients do not benefit from such therapy. In addition, irAEs occurred in half of the patients, and the significant economic burden of ICIs cannot be ignored. Thus, it is imperative to develop suitable predictive biomarkers for efficacy and toxicity to select appropriate patients. In fact, multiple biomarkers have emerged as a research hotspot, which can be summarized into five categories: tumor itself, TME, liquid biopsies for circulating biomarkers, imaging biomarkers, and patient characteristics.
Previous studies have mainly focused on PD-L1 expression, tumor mutation burden (TMB), and microsatellite instability (MSI), but it is still far from the accurate screening of patients most likely to benefit. Tumoral PD-L1 immunohistochemistry was the first biomarker approved by the FDA and has been widely used to assist clinical decisions in the treatment of ICIs [8]. Multiple clinical trials have demonstrated that advanced NSCLC patients with relatively high tumoral PD-L1 expression tended to show improved responses to ICIs and longer survival [15, 161, 162]. Some studies have also reported the predictive role of soluble or exosomal PD-L1 expression [44, 163]. However, PD-L1 expression alone is not a perfect biomarker. First, several randomized trials disputed PD-L1 as a viable predictive biomarker for ICIs treatment, especially combined treatment [162]. For instance, the PEMBRO-RT trial revealed that PD-L1 negative patients had a much better response to iRT treatment than those positive [10]. Second, there is a lack of standardized PD-L1 assessment methods, as many variables exist in tumor sampling, testing, and assessment, as well as great heterogeneity across time and space in clinical, pathological, and TME characteristics. Notably, the assessed range, tumor, or both tumor and cells in the TME, and cut-off points remain to be unified. Third, tumoral PD-L1 expression is dynamic and influenced by multiple factors. In the non-metastatic setting, durvalumab showed PFS benefit in tumors with PD-L1 < 25% (HR, 0.59) in the PACIFIC trial [9]. However, post hoc analysis showed no OS benefit with durvalumab in tumors with PD-L1 < 1% (HR, 1.14; 95% CI: 0.71–1.84), although PFS benefit with durvalumab continued to be observed across all subgroups [50]. Consequently, durvalumab is not approved for tumors with PD-L1 < 1% in Europe, whereas it is approved irrespective of PD-L1 percentage in the US. The PACIFIC-R trial supported the feasibility of durvalumab in PD-L1-negative tumors, however, the median PFS was indeed shorter than PD-L1 ⩾1% tumors [51]. The abstract 8550 posted in ASCO 2022 demonstrated that patients with tumoral PD-L1 expression of < 1% had a significantly lower survival probability, compared to those of 1–50% and > 50% in patients with stage III unresectable NSCLC who received durvalumab post cCRT [135]. Low PD-L1 expression may originate from a lack of tumor infiltrating lymphocytes (TIL) and expression of other co-inhibitory checkpoints; therefore, strategies with dual checkpoint blockade consolidation are expected. The combinations from the COAST trial appeared to generate persistent benefits regardless of PD-L1 status; however, such reliability was limited by the number of patients available [66]. Meanwhile, BTCRC-LUN 16–081 reported significantly increased toxicity with the combination of nivolumab and ipilimumab [64]. Whether the downward trend of dual checkpoint blockade consolidation could be reversed will depend on the results of the CheckMate 73 L trial with a 1% stratification of PD-L1 expression.
Tissue TMB (tTMB), mostly determined by next-generation sequencing, is another leading candidate biomarker that has been widely evaluated in clinical trials, and the majority of the evidence comes from patients with lung cancer and melanoma [164]. Recently, the FDA approved pembrolizumab for patients with TMB ≥ 10 mutations/Mb in any tumor, following the results of the KEYNOTE-158 trial [165]. However, the reproducibility of such a cut-off ignited great controversy due to its arbitrariness and capriciousness [166]. There is no consensus on gene panel size and methodology for measurement [8]. Another major concern is that TMB assessment and bioinformatics interpretation vary across different cancer types as well as subgroups of different characteristics from a single type of cancer [167, 168]. Thus, the accuracy of blood TMB (bTMB) is worthy of verification. The role of TMB in terms of RT or iRT is uncertain. Generally, the predictive value of TMB alone is limited. One study evaluated the incorporation of TMB and PD-L1 expression into multivariable predictive models and demonstrated a greater predictive power [169]. Mismatch repair deficiency (dMMR) and MSI have also been considered to earn the competence in predicting ICIs efficacy [170, 171]. Likewise, clinical application is difficult because of the lack of higher levels of evidence and difficulties in detection, especially with repeated biopsy sampling for dynamic monitoring.
TILs, such as CTLs, reflect the TME more directly, and adequate lymphocyte infiltration is necessary for ICIs to exert anti-tumor effects. A small population of progenitor exhausted cells among exhausted CD8 + TILs mediates long-term tumor control and responses to anti-PD-1 therapy [172]. It has been found that a TIL density of over 10% could predict better survival benefits from ICIs [173]. In terms of RT, CD8 + TIL density increased after cCRT and higher density post-cCRT predicted favorable clinical outcomes [174]. The characteristics of TILs, including their composition, organization, density, and functional state, may jointly predict responses to iRT. Of note, similar to tumoral PD-L1 expression, difficulties in detection resulting from limited histologic material from the biopsy restrict direct evaluation of TIL. The predictive roles of Tregs, MDSCs, and some immunoregulatory pathways, such as CD28/B7 and TIM-3, which together constitute and regulate the TME, are also being studied [175]. In addition, multiple patient-specific gene expression profiles (GEPs) characterizing the TME have been investigated, such as targeting T-cell inflammation, antigen processing and presentation, and immunosuppressive molecules. An 18-gene profile of T cell inflammation demonstrated strong correlations with clinical outcomes in a wide variety of solid tumors treated with pembrolizumab [176]. As for RT, with further exploration of radiobiology, individualized RT with dose adjustment based on genomes is also being developed [177]. Although not nearly enough, these multigene signatures characterizing radiosensitivity, TME, and immune-related mechanisms hold tremendous potential to predict RT, ICIs, and combination therapy strategies. In addition, some specific oncogenic alterations in pivotal signaling pathways have been reported to predict responses to ICIs, such as Wnt/β-catenin, PTEN, PI3K-AKT, EGFR, c-Met, ALK, and KRAS [175].
Difficulties in detection include the use of biomarkers for restrictions in lung biopsy. Liquid biopsy, developed to solve pain, has attracted great attention and has become increasingly popular owing to its feasibility and ease of operation. Of note, it has brought about a dynamic evaluation of the responses of possibility. In principle, any tumor-derived material circulating in peripheral blood can be analyzed, including ctDNA, circulating tumor cells (CTCs), circulating tumor RNA (ctRNA), tumor endothelial cells (TRCs), and exosomes. Among these, ctDNA derived from tumor cells is the most commonly used modality [139]. The prognostic value of MRD detection in patients with LA-NSCLC and the role of dynamic ctDNA in facilitating personalized consolidation ICIs strategies have been elaborated previously, which is of great potential from our point of view. However, factors such as assay type, amount of ctDNA released, and technical and biological background can all impact ctDNA MRD results. Therefore, the clinical utility of ctDNA MRD for the personalized treatment of solid tumors, including NSCLC, remains to be fully established. Another alternative marker from non-invasive detection, CTCs, may be used to evaluate dynamic variations in immune checkpoint expression during treatment [178]. Concordance between tumoral PD-L1 expression and CTCs has been reported to be as high as 93% in advanced NSCLC. Nevertheless, a common limitation of biomarkers for LA-NSCLC is that the low tumor burden in already-treated localized diseases would impact the isolation of CTCs. In addition, peripheral blood cells and lymphocytes are thought to indirectly reflect immune responses; however, they are not clinically applicable. ctRNAs with the advantages of stability have also been recognized as potential biomarkers, which need to be validated in larger cohorts. It is conceivable that dynamic liquid biopsy will guide the duration of ICIs or combined treatment in the future, with validation in larger prospective trials. At present, including in LA-NSCLC, dynamic ctDNA is undoubtedly the most promising for clinical applications.
Imaging information is the standard assessment of treatment efficacy and image-guided RT, including CT, MR, and PET/CT was the basis for precision RT. PET/CT, which integrates metabolic and anatomical information and is also called functional imaging, naturally reflects the TME. Several studies have explored the possible correlation between [18]F-FDG-uptake and existing ICIs sensitivity markers, such as PD-L1 and CD8 + TILs in tumor tissues [179, 180]. Our previous study also indicated a correlation between [18]F-RGD uptake and tumor PD-L1 expression [181]. Notably, benefiting from its non-invasiveness, PET/CT may also reflect dynamic changes in the TME under ICIs. In addition, a predictive model based on high-throughput image characteristics, namely radiomics, is a promising method. A recent study indicated that tumor radiomics of pretreatment CT images was a prognostic factor for outcomes in patients with stage III unresectable NSCLC treated with CRT followed by durvalumab or CRT alone [182].
It is evident that a single biomarker cannot serve a powerful and comprehensive predictive function, even beyond PD-L1 expression. However, a variety of biomarkers have emerged as complementary predictors of response, including combining two or more biomarkers to increase accuracy. It is foreseeable that the integration of multi-omics information, including patient characteristics, imaging, pathology, peripheral blood, and genomic information, as a predictive tool to guide comprehensive treatment, will be the direction of development. Machine learning that integrates multimodal features can be a prospective approach for predicting treatment response [183]. Meanwhile, non-invasive and real-time monitoring for prediction requires technological advances.
Novel combination strategies
Although the combined treatment improved responses in irradiated and non-irradiated tumors, resistance was also common. Owing to the distinct mechanisms of action, dual or triple checkpoint blockade is expected to work synergistically. Except for the molecules mentioned before, there are also some other co-inhibitory receptors including Lag-3, Tim-3 and B7-H3 are being explored [184, 185]. Costimulatory molecules such as the CD122 agonists NKTR-214, 4-1BB, OX-40, GITR, TLR9, and STING have also been exploited to enhance anti-tumor activity [186]. In practice, these agents are usually first tested in advanced NSCLC, and only successful applications will help them move forward to LA-NSCLC. Notably, bispecific antibodies (BsAbs) targeting two different checkpoints are emerging, which have significant advantages over combination therapy using two different mAbs, including reduced development and therapeutic costs, higher binding specificity and obligate effects [187].
Antiangiogenic agents targeting the vascular endothelial growth factor and its receptor are promising agents in combination with ICIs and RT. It is assumed that anti-angiogenic agents promote the trafficking of immune effector cells, drive DC maturation, reduce MDSCs and Tregs, and limit hypoxia partly via vessel re-normalization, thereby functioning as ideal partners for ICIs and superior radiosensitizers [186]. A quadruple combination regimen of antiangiogenic atezolizumab and carboplatin–paclitaxel chemotherapy in non-squamous NSCLC (ABCP regimen) has achieved great success in advanced non-squamous NSCLC [188]. Based on this clinical and preclinical evidence, RT is expected to further potentiate the anti-tumor effects of ICI and angiogenesis dual blockade. Such a triple combination therapy for LA-NSCLC is a promising direction for future research. In contrast, molecular-targeted agents combined with ICIs have been hampered by severe toxicities, and their addition to the treatment of LA-NSCLC seems a long way off.
Hypoxia is an important obstacle contributing to resistance to RT and immunosuppression, facilitating tumor recurrence and metastasis [189]. Radiosensitizers can sensitize hypoxic or radioresistant tumors to RT. With advancements in nanotechnology, the application of nanoparticles to overcome resistance to RT and enhance RT efficacy against tumors has become an area of intense research, as radiosensitizers are usually loaded or engineered into nanocarriers for delivery [190]. For example, a multifunctional nanoprobe, based on quantum dots emitting in the near-infrared IIb window, can effectively aggregate at the tumor site to precisely image the tumor region with high resolution, promote the radio-sensitivity and immunogenicity of cancer cells, and relieve intratumoral hypoxia to enhance RT-based therapy strategies [191]. Nanoprode-mediated immunogenic RT can exert a more intense and enduring anti-tumor effect when combined with immunotherapy. Several nanoscale metal–organic frameworks also notably enhance the effects of ionizing radiation, serving as a powerful adjuvant therapy to synergize with iRT, termed RT–radiodynamic therapy [192, 193]. Although more clinical trials are needed, novel materials, especially nanomaterials, are rapidly emerging as the frontier of cancer treatment and have immense potential. We summarize the challenges, strategies and auspicious orientations of iRT in LA-NSCLC in Fig. 4.
Challenges, strategies, and auspicious orientations of iRT in LA-NSCLC. The existing challenges of iRT in LA-NSCLC include optimal dose and fractionation of RT, optimal RT target and target volume, application of charged particle therapy, optimal timing and duration of ICIs, iRT in oncogenic addicted tumors, optimal biomarkers and novel combinations, which are also strategies and auspicious orientations. Abbreviations: RT Radiotherapy, ICIs Immune checkpoint inhibitors, iRT ICIs combined with RT
Conclusions and future perspectives
Definitive RT plays a major role as a local therapy in unresectable LA-NSCLC. In addition, RT promotes tumor regression via ICD, whereas the recruitment of immunosuppressive cell populations and exacerbation of tumor hypoxia can engender a radioresistant phenotype. Boosting these positive effects while mitigating undesirable effects can be exploited to improve clinical responses. Combining ICIs with RT is a proven and potential synergy through the mutually beneficial remodeling of the TME, which is expected to overcome resistance to RT and ICIs and augment the abscopal effect of RT and the immune memory effect. However, issues remain regarding the rational dosage and fractionation of RT and timing of combined ICIs, relying on further understanding of the paradoxical effects of RT on TME and direct comparison both in preclinical and clinical studies. Notably, hypofractionated regimens and SBRT combined with LDI are superior. In addition, TME heterogeneity within and between patients directly influences the effects of combined therapy. Effective biomarkers guiding patient selection assist precision therapy, and the integration of multi-omics information for prediction is the way forward. Finally, novel RT technologies and innovative combination strategies are promising approaches to exploit the untapped therapeutic potential. In general, iRT is a proven and potential strategy in LA-NSCLC, with multiple promising approaches to further improve the efficacy.
Availability of data and materials
Not appliable.
Abbreviations
- ICIs:
-
Immune checkpoint inhibitors
- RT:
-
Radiotherapy
- LA-NSCLC:
-
Locally advanced non-small cell lung cancer
- iRT:
-
RT combined with ICIs
- NSCLC:
-
Non-small cell lung cancer
- LA:
-
Locally advanced
- DNA:
-
Deoxyribonucleic acid
- cCRT:
-
Concurrent chemoradiotherapy
- SoC:
-
Standard of care
- OS:
-
Overall survival
- ORR:
-
Objective response rate
- TME:
-
Tumor microenvironment
- SBRT:
-
Stereotactic body radiation therapy
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed cell death-ligand 1
- CTLA-4:
-
Cytotoxic T-lymphocyte associated antigen 4
- PFS:
-
Progression-free survival
- DAMPs:
-
Damage-associated molecular patterns
- HMGB1:
-
High mobility group box 1
- ATP:
-
Adenosine triphosphate
- ICD:
-
Immunogenic cell death
- ROS:
-
Reactive oxygen species
- TAAs:
-
Tumor-associated antigens
- dsDNA:
-
Double-stranded DNA
- DCs:
-
Dendritic cells
- APCs:
-
Antigen-presenting cells
- CTLs:
-
Cytotoxic lymphocytes
- PRRs:
-
Pattern recognition receptors
- TLR:
-
Toll-like receptor
- MHC:
-
Major histocompatibility complex
- TCR:
-
T-cell receptor
- GMP:
-
Cyclic guanosine monophosphate
- AMP:
-
Adenosine monophosphate
- cGAS:
-
Cyclic GMP-AMP synthase
- STING:
-
Stimulator of interferon genes
- mtDNA:
-
Mitochondrial DNA
- AIM2:
-
Absent in melanoma 2
- IFI16:
-
Interferon-inducible protein 16
- IRF:
-
Interferon regulatory factor
- TAMs:
-
Tumor-associated macrophages
- PMNs:
-
Polymorphonuclear neutrophils
- MDSCs:
-
Myeloid-derived suppressor cells
- CAFs:
-
Cancer-associated fibroblasts
- SDF-1:
-
Stromal-derived factor 1
- CSF-1:
-
Colony stimulating factor-1
- Tregs:
-
The regulatory T cells
- HIF-1α:
-
Hypoxia-inducible factor 1-α
- HR:
-
Hazard ratio
- sCRT:
-
Sequential chemoradiotherapy
- CRT:
-
Chemoradiotherapy
- TRAEs:
-
Treatment-related adverse events
- AEs:
-
Adverse events
- TDLNs:
-
Tumor-draining lymph nodes
- TMDD:
-
Time to metastatic disease or death
- DCR:
-
Disease control rate
- PARP:
-
Poly adenosine diphosphate-ribose polymerase
- PR:
-
Partial response
- PET/CT:
-
Positron emission tomography/computed tomography
- irAES:
-
Immune-related adverse events
- CIP:
-
ICIs-related pneumonitis
- HyperRT:
-
Hyperfractionation RT
- HypoRT:
-
Hypofractionated RT
- SABR:
-
Stereotactic ablative radiotherapy
- Trex1:
-
Three prime repair exonuclease 1
- ENI:
-
Elective nodal irradiation
- LDI:
-
Low dose irradiation
- CIRT:
-
Carbon-ion RT
- ctDNA:
-
Circulating tumor DNA
- MRD:
-
Minimal residual disease
- NGS:
-
Next-generation sequencing
- TKIs:
-
Tyrosine kinase inhibitors
- TMB:
-
Tumor mutation burden (MSI)
- MSI:
-
Microsatellite instability
- TIL:
-
Tumor infiltrating lymphocytes
- tTMB:
-
Tissue TMB
- bTMB:
-
Blood TMB
- dMMR:
-
Mismatch repair deficiency
- GEPs:
-
Gene expression profiles
- CTCs:
-
Circulating tumor cells
- ctRNA:
-
Circulating tumor RNA
- TRCs:
-
Tumor endothelial cells
- LDH:
-
Lactate dehydrogenase
- LIPI:
-
Lung immune prognostic index
- NLR:
-
Neutrophil to lymphocyte ratio
- PLR:
-
Platelet to lymphocyte ratio
- LMR:
-
Lymphocyte to monocyte ratio
References
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–54.
Cheema PK, Rothenstein J, Melosky B, Brade A, Hirsh V. Perspectives on treatment advances for stage III locally advanced unresectable non-small-cell lung cancer. Curr Oncol. 2019;26(1):37–42.
Citrin DE. Recent Developments in Radiotherapy. N Engl J Med. 2017;377(11):1065–75.
Kaplan HS. Historic milestones in radiobiology and radiation therapy. Semin Oncol. 1979;6(4):479–89.
Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther. 2022;7(1):258.
Auperin A, le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–90.
Auperin A, le Pechoux C, Pignon JP, Koning C, Jeremic B, Clamon G, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol. 2006;17(3):473–83.
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(5):497–530.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377(20):1919–29.
Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5(9):1276–82.
Mole RH. Whole Body Irradiation—Radiobiology or Medicine? Br J Radiol. 1953;26(305):234–41.
Donlon NE, Power R, Hayes C, Reynolds JV, Lysaght J. Radiotherapy, immunotherapy, and the tumour microenvironment: Turning an immunosuppressive milieu into a therapeutic opportunity. Cancer Lett. 2021;502:84–96.
McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20(4):203–17.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33.
Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218.
Theelen WSME, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts JGJV, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9(5):467–75.
Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-Year Overall Survival for Patients With Advanced NonSmall-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol. 2019;37(28):2518–27.
Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40(1):25–37.
Pevzner AM, Tsyganov MM, Ibragimova MK, Litvyakov NV. Abscopal effect in the radio and immunotherapy. Radiat Oncol J. 2021;39(4):247–53.
Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–70.
Kwon J, Bakhoum SF. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020;10(1):26–39.
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95.
Ablasser A, Chen ZJ. cGAS in action: Expanding roles in immunity and inflammation. Science. 2019;363(6431):eaat8657.
Yang Y, Wu M, Cao D, Yang C, Jin J, Wu L, Hong X, Li W, Lu L, Li J, Wang X, Meng X, Zhang Z, Cheng J, Ye Y, Xiao H, Yu J, Deng L. ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation. Sci Adv. 2021;7(41):eabf6290.
Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553(7689):467–72.
Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550(7676):402–6.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22.
Li MO, Wolf N, Raulet DH, Akkari L, Pittet MJ, Rodriguez PC, et al. Innate immune cells in the tumor microenvironment. Cancer Cell. 2021;39(6):725–9.
Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, et al. Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2017;23(1):137–48.
Wennerberg E, Lhuillier C, Vanpouille-Box C, Pilones KA, Garcia-Martinez E, Rudqvist NP, et al. Barriers to Radiation-Induced In Situ Tumor Vaccination. Front Immunol. 2017;8:229.
Liu SC, Alomran R, Chernikova SB, Lartey F, Stafford J, Jang T, et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro Oncol. 2014;16(1):21–8.
Spiotto M, Fu YX, Weichselbaum RR. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci Immunol. 2016;1(3):EAAG1266.
Stafford JH, Hirai T, Deng L, Chernikova SB, Urata K, West BL, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18(6):797–806.
Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev. 2017;276(1):121–44.
Sharabi AB, Lim M, Deweese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498-509.
Chen Y, Gao M, Huang Z, Yu J, Meng X. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol. 2020;13(1):105.
Kachikwu EL, Iwamoto KS, Liao YP, Demarco JJ, Agazaryan N, Economou JS, et al. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81(4):1128–35.
Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223–49.
Karasarides M, Cogdill AP, Robbins PB, Bowden M, Burton EM, Butterfield LH, et al. Hallmarks of Resistance to Immune-Checkpoint Inhibitors. Cancer Immunol Res. 2022;10(4):372–83.
Kluger HM, Tawbi HA, Ascierto ML, Bowden M, Callahan MK, Cha E, et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J Immunother Cancer. 2020;8(1):e000398.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Ruiz-Banobre J, Areses-Manrique MC, Mosquera-Martinez J, Cortegoso A, Afonso-Afonso FJ, De Dios-Alvarez N, et al. Evaluation of the lung immune prognostic index in advanced non-small cell lung cancer patients under nivolumab monotherapy. Transl Lung Cancer Res. 2019;8(6):1078–85.
Xie F, Xu M, Lu J, Mao L, Wang S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer. 2019;18(1):146.
Twyman-Saint VC, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7.
Du SS, Chen GW, Yang P, Chen YX, Hu Y, Zhao QQ, et al. Radiation Therapy Promotes Hepatocellular Carcinoma Immune Cloaking via PD-L1 Upregulation Induced by cGAS-STING Activation. Int J Radiat Oncol Biol Phys. 2022;112(5):1243–55.
Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou F, et al. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12(7):1085–97.
Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–68.
Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 2022;40(12):1301–11.
Paz-Ares L, Spira A, Raben D, Planchard D, Cho BC, Ozguroglu M, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020;31(6):798–806.
Girard N, Bar J, Garrido P, Garassino MC, McDonald F, Mornex F, et al. Treatment Characteristics and Real-World Progression-Free Survival in Patients with Unresectable Stage III NSCLC who Received Durvalumab After Chemoradiotherapy: Findings from the PACIFIC-R Study. J Thorac Oncol. 2023;18(2):181–93.
Durm GA, Jabbour SK, Althouse SK, Liu Z, Sadiq AA, Zon RT, et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14–179. Cancer. 2020;126(19):4353–61.
Cortiula F, Reymen B, Peters S, van Mol P, Wauters E, Vansteenkiste J, et al. Immunotherapy in unresectable stage III non-small-cell lung cancer: state of the art and novel therapeutic approaches. Ann Oncol. 2022;33(9):893–908.
Zhu D, Ding R, Ma Y, Chen Z, Shi X, He P. Comorbidity in lung cancer patients and its association with hospital readmission and fatality in China. BMC Cancer. 2021;21(1):557.
Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23(2):209–19.
Wu YL, Zhou Q, Chen M, Pan Y, Jian O, Hu D, et al. OA02.05 Sugemalimab vs Placebo after cCRT or sCRT in pts with Unresectable Stage III NSCLC: Final PFS Analysis of a Phase 3 Study. J Thorac Oncol. 2022;17(9_suppl):S7–8.
Garassino MC, Mazieres J, Reck M, Chouaid C, Bischoff H, Reinmuth N, et al. Durvalumab After Sequential Chemoradiotherapy in Stage III, Unresectable NSCLC: The Phase 2 PACIFIC-6 Trial. J Thorac Oncol. 2022;17(12):1415–142.
Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375–86.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535–46.
Motzer RJ, Tannir NM, McDermott DF, Arén FO, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–90.
Yofe I, Landsberger T, Yalin A, Solomon I, Costoya C, Demane DF, et al. Anti-CTLA-4 antibodies drive myeloid activation and reprogram the tumor microenvironment through FcgammaR engagement and type I interferon signaling. Nat Cancer. 2022;3(11):1336–50.
Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845–51.
Khalifa J, Mazieres J, Gomez-Roca C, Ayyoub M, Moyal EC. Radiotherapy in the Era of Immunotherapy With a Focus on Non-Small-Cell Lung Cancer: Time to Revisit Ancient Dogmas? Front Oncol. 2021;11: 662236.
Durm GA, Mamdani H, Althouse SK, Jabbour SK, Ganti AK, Jalal SI, et al. Consolidation nivolumab plus ipilimumab or nivolumab alone following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: BTCRC LUN 16–081. J Clin Oncol. 2022;40:8509.
de Ruysscher D, Ramalingam S, Urbanic J, Gerber DE, Tan DSW, Cai J, et al. CheckMate 73L: A Phase 3 Study Comparing Nivolumab Plus Concurrent Chemoradiotherapy Followed by Nivolumab With or Without Ipilimumab Versus Concurrent Chemoradiotherapy Followed by Durvalumab for Previously Untreated, Locally Advanced Stage III Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2022;23(3):e264–8.
Herbst RS, Majem M, Barlesi F, Carcereny E, Chu Q, Monnet I, et al. COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non–Small-Cell Lung Cancer. J Clin Oncol. 2022;40(29):3383–93.
Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8(2):e000957.
Banta KL, Xu X, Chitre AS, Au-Yeung A, Takahashi C, O’Gorman WE, et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8(+) T cell responses. Immunity. 2022;55(3):512–26 e9.
Rudin CM, Liu SV, Lu S, Soo RA, Hong MH, Lee JS, et al. SKYSCRAPER-02: Primary results of a phase III, randomized, double-blind, placebo-controlled study of atezolizumab (atezo) + carboplatin + etoposide (CE) with or without tiragolumab (tira) in patients (pts) with untreated extensive-stage small cell lung cancer (ES-SCLC). J Clin Oncol. 2022;40(17_suppl):LBA8507.
Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol. 2021;16(5):860–7.
Jabbour SK, Lee KH, Frost N, Breder V, Kowalski DM, Pollock T, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol. 2021;7(9):1–9.
Reck M, Lee KH, Frost N, Breder VV, Kowalski D, Levchenko E, et al. Two-year update from KEYNOTE-799: Pembrolizumab plus concurrent chemoradiation therapy (cCRT) for unresectable, locally advanced, stage III NSCLC. J Clin Oncol. 2022;40(16_suppl):8508.
Peters S, Felip E, Dafni U, Belka C, Guckenberger M, Irigoyen A, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung Cancer. 2019;133:83–7.
Peters S, Felip E, Dafni U, Tufman A, Guckenberger M, Alvarez R, et al. Progression-Free and Overall Survival for Concurrent Nivolumab With Standard Concurrent Chemoradiotherapy in Locally Advanced Stage IIIA-B NSCLC: Results From the European Thoracic Oncology Platform NICOLAS Phase II Trial (European Thoracic Oncology Platform 6–14). J Thorac Oncol. 2021;16(2):278–88.
Lin SH, Lin Y, Yao L, Kalhor N, Carter BW, Altan M, et al. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J Thorac Oncol. 2020;15(2):248–57.
Ross HJ, Kozono D, Urbanic JJ, Williams TM, Dufrane C, Bara I, et al. AFT-16: Phase II trial of neoadjuvant and adjuvant atezolizumab and chemoradiation (CRT) for stage III non-small cell lung cancer (NSCLC). JCO. 2021;39:8513.
Ohri N, Jolly S, Cooper BT, Kabarriti R, Bodner WR, Klein J, et al. The Selective Personalized Radioimmunotherapy for Locally Advanced NSCLC Trial (SPRINT): Initial results. J Oncol Clin. 2022;40:8510.
Eichkorn T, Bozorgmehr F, Regnery S, Dinges LA, Kudak A, Bougatf N, et al. Consolidation Immunotherapy After Platinum-Based Chemoradiotherapy in Patients With Unresectable Stage III Non-Small Cell Lung Cancer-Cross-Sectional Study of Eligibility and Administration Rates. Front Oncol. 2020;10: 586449.
Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2016;34(9):953–62.
Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–43.
Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing anti-tumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30:219–35.
Jabbour SK, Berman AT, Decker RH, Lin Y, Feigenberg SJ, Gettinger SN, et al. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol. 2020;6(6):848–55.
Marcus D, Lieverse RIY, Klein C, Abdollahi A, Lambin P, Dubois LJ, et al. Charged Particle and Conventional Radiotherapy: Current Implications as Partner for Immunotherapy. Cancers (Basel). 2021;13(6):1468.
Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med. 2022;386(21):1973–85.
Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020;17(8):504–15.
June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med. 2017;23(5):540–7.
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018;4(3):374–8.
Nobashi TW, Nishimoto Y, Kawata Y, Yutani H, Nakamura M, Tsuji Y, et al. Clinical and radiological features of immune checkpoint inhibitor-related pneumonitis in lung cancer and non-lung cancers. Br J Radiol. 2020;93(1115):20200409.
Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378(2):158–68.
Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest. 2017;152(2):271–81.
Anscher MS, Arora S, Weinstock C, Amatya A, Bandaru P, Tang C, et al. Association of Radiation Therapy With Risk of Adverse Events in Patients Receiving Immunotherapy: A Pooled Analysis of Trials in the US Food and Drug Administration Database. JAMA Oncol. 2022;8(2):232–40.
Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro-Sibilot D, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50(2):1700050.
Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2(12):1607–16.
Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol. 2018;36(16):1611–8.
Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903.
Tian S, Switchenko JM, Buchwald ZS, Patel PR, Shelton JW, Kahn SE, et al. Lung Stereotactic Body Radiation Therapy and Concurrent Immunotherapy: A Multicenter Safety and Toxicity Analysis. Int J Radiat Oncol Biol Phys. 2020;108(1):304–13.
Zhai X, Zhang J, Tian Y, Li J, Jing W, Guo H, et al. The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non-small cell lung cancer patients. Cancer Biol Med. 2020;17(3):599–611.
Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki VS, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134.
Ngamphaiboon N, Ithimakin S, Siripoon T, Sintawichai N, Sriuranpong V. Patterns and outcomes of immune-related adverse events in solid tumor patients treated with immune checkpoint inhibitors in Thailand: a multicenter analysis. BMC Cancer. 2021;21(1):1275.
Tamiya A, Tamiya M, Nakahama K, Taniguchi Y, Shiroyama T, Isa SI, et al. Correlation of Radiation Pneumonitis History Before Nivolumab with Onset of Interstitial Lung Disease and Progression-free Survival of Patients with Pre-treated Advanced Non-small Cell Lung Cancer. Anticancer Res. 2017;37(9):5199–205.
Hwang WL, Niemierko A, Hwang KL, Hubbeling H, Schapira E, Gainor JF, et al. Clinical Outcomes in Patients With Metastatic Lung Cancer Treated With PD-1/PD-L1 Inhibitors and Thoracic Radiotherapy. JAMA Oncol. 2018;4(2):253–5.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol. 2019;5(10):1411–20.
Shentzer Kutiel T, Shamai S, Waissengrin B, Urban D, Daher S, Sorotzky H, et al. 41P Progression after chemo-IO and durvalumab in stage III non-small cell lung cancer (NSCLC): What should be the next line? Real-world study. Ann Oncol. 2022;33(2_suppl):S51.
Durm G, Althouse S, Sadiq A, Jalal S, Jabbour S, Zon R, et al. P1.18-05 ChemoXRT W/ Consolidation Pembrolizumab in Unresectable Stage III NSCLC: Long-Term Survival Update and Analysis of Post-Progression Therapy. J Thorac Oncol. 2019;14(10_suppl):S627.
Counago F, Luna J, Guerrero LL, Vaquero B, Guillen-Sacoto MC, Gonzalez-Merino T, et al. Management of oligometastatic non-small cell lung cancer patients: Current controversies and future directions. World J Clin Oncol. 2019;10(10):318–39.
Rheinheimer S, Heussel CP, Mayer P, Gaissmaier L, Bozorgmehr F, Winter H, et al. Oligoprogressive Non-Small-Cell Lung Cancer under Treatment with PD-(L)1 Inhibitors. Cancers (Basel). 2020;12(4):1046.
Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell. 2020;37(4):443–55.
Gettinger SN, Wurtz A, Goldberg SB, Rimm D, Schalper K, Kaech S, et al. Clinical Features and Management of Acquired Resistance to PD-1 Axis Inhibitors in 26 Patients With Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2018;13(6):831–9.
Xu Y, Li H, Fan Y. Progression Patterns, Treatment, and Prognosis Beyond Resistance of Responders to Immunotherapy in Advanced Non-Small Cell Lung Cancer. Front Oncol. 2021;11:642883.
Reynders K, Illidge T, Siva S, Chang JY, de Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–10.
Chen D, Patel RR, Verma V, Ramapriyan R, Barsoumian HB, Cortez MA, et al. Interaction between lymphopenia, radiotherapy technique, dosimetry, and survival outcomes in lung cancer patients receiving combined immunotherapy and radiotherapy. Radiother Oncol. 2020;150:114–20.
Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618.
Sun B, Brooks ED, Komaki RU, Liao Z, Jeter MD, McAleer MF, et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer. 2017;123(16):3031–9.
Chang JY, Mehran RJ, Feng L, Verma V, Liao Z, Welsh JW, et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021;22(10):1448–57.
Welsh J, Menon H, Chen D, Verma V, Tang C, Altan M, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer. 2020;8(2).
Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 2020;38(7):706–14.
Jin JY, Hu C, Xiao Y, Zhang H, Paulus R, Ellsworth SG, et al. Higher Radiation Dose to the Immune Cells Correlates with Worse Tumor Control and Overall Survival in Patients with Stage III NSCLC: A Secondary Analysis of RTOG0617. Cancers (Basel). 2021;13(24):6193.
Ramroth J, Cutter DJ, Darby SC, Higgins GS, McGale P, Partridge M, et al. Dose and Fractionation in Radiation Therapy of Curative Intent for Non-Small Cell Lung Cancer: Meta-Analysis of Randomized Trials. Int J Radiat Oncol Biol Phys. 2016;96(4):736–47.
Lin SH, Pugh SL, Tsao AS, Edelman MJ, Doemer A, Simone CB, et al. Safety results of NRG-LU004: Phase I trial of accelerated or conventionally fractionated radiotherapy combined with durvalumab in PD-L1–high locally advanced non-small cell lung cancer. J Clin Oncol. 2022;40(16_suppl):8513.
Kubicek GJ, Khrizman P, Squillante C, Callahan K, Xu Q, Abouzgheib W, et al. Stereotactic Body Radiotherapy and Systemic Dose Chemotherapy for Locally Advanced Lung Cancer: Single Arm Phase 2 Study. Am J Clin Oncol. 2022;45(3):129–33.
Welsh JW, Tang C, de Groot P, Naing A, Hess KR, Heymach JV, et al. Phase II Trial of Ipilimumab with Stereotactic Radiation Therapy for Metastatic Disease: Outcomes, Toxicities, and Low-Dose Radiation-Related Abscopal Responses. Cancer Immunol Res. 2019;7(12):1903–9.
Arnold KM, Flynn NJ, Raben A, Romak L, Yu Y, Dicker AP, et al. The Impact of Radiation on the Tumor Microenvironment: Effect of Dose and Fractionation Schedules. Cancer Growth Metastasis. 2018;11:1179064418761639.
Barsoumian HB, Sezen D, Menon H, Younes AI, Hu Y, He K, et al. High Plus Low Dose Radiation Strategy in Combination with TIGIT and PD1 Blockade to Promote Systemic Antitumor Responses. Cancers (Basel). 2022;14(1):221.
Cummings MA, Ma SJ, Hermann G, Serra L, Syed Y, Malhotra HK, et al. Comparison of Single- and Five-fraction Regimens of Stereotactic Body Radiation Therapy for Peripheral Early-stage Non-small-cell Lung Cancer: A Two-institution Propensity-matched Analysis. Clin Lung Cancer. 2018;19(6):511–7.
Nguyen QN, Chun SG, Chow E, Komaki R, Liao Z, Zacharia R, et al. Single-Fraction Stereotactic vs Conventional Multifraction Radiotherapy for Pain Relief in Patients With Predominantly Nonspine Bone Metastases: A Randomized Phase 2 Trial. JAMA Oncol. 2019;5(6):872–8.
Marciscano AE, Ghasemzadeh A, Nirschl TR, Theodros D, Kochel CM, Francica BJ, et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin Cancer Res. 2018;24(20):5058–71.
Zhu Y, Jiang C, Gu F, Lin Q, Sun X, Xu Y. The Estimate of Shrinking Field and SIB Radiotherapy Guided by 18F-FDG PET/CT in Locally Advanced NSCLC Patients: A Phase 2 Randomized Clinical. J Thorac Oncol. 2018;13:S319–42.
Kong FM S, Hu C, Haken RT, Xiao Y, Matuszak M, Hirsh V, et al. NRG-RTOG 1106/ACRIN 6697: A phase IIR trial of standard versus adaptive (mid-treatment PET-based) chemoradiotherapy for stage III NSCLC—Results and comparison to NRG-RTOG 0617 (non-personalized RT dose escalation). J Clin Oncol. 2021;39(15_suppl):8548.
Chang JY, Jabbour SK, de Ruysscher D, Schild SE, Simone CB 2nd, Rengan R, et al. Consensus Statement on Proton Therapy in Early-Stage and Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2016;95(1):505–16.
Contreras J, Srivastava A, Samson P, Dewees T, Govindan R, Baggstrom MQ, et al. Phase I Study of Accelerated Hypofractionated Proton Therapy and Chemotherapy for Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2022;113(4):742–8.
Oike T, Niimi A, Okonogi N, Murata K, Matsumura A, Noda SE, et al. Visualization of complex DNA double-strand breaks in a tumor treated with carbon ion radiotherapy. Sci Rep. 2016;6:22275.
Chen D, Menon H, Verma V, Guo C, Ramapriyan R, Barsoumian H, et al. Response and outcomes after anti-CTLA4 versus anti-PD1 combined with stereotactic body radiation therapy for metastatic non-small cell lung cancer: retrospective analysis of two single-institution prospective trials. J Immunother Cancer. 2020;8(1).
Wegner RE, Abel S, Hasan S, White R, Finley GG, Monga D, et al. Time from stereotactic body radiotherapy to immunotherapy as a predictor for outcome in metastatic non small cell lung cancer. J Clin Oncol. 2019;37(15_suppl):9024.
Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS ONE. 2016;11(6): e0157164.
Thurlapati A, Dhaliwal LS, Chennapragada S, Gutta P, Song D, Ralla D, et al. Effect of durvalumab in patients with unresectable stage 3 non–small cell lung cancer post-chemoradiotherapy. J Clin Oncol. 2022;40(16_suppl):8550.
Nakamichi S, Kubota K, Misumi T, Murakami S, Kondo T, Okamoto I, et al. A phase II study of durvalumab (MEDI4736) immediately after completion of chemoradiotherapy in unresectable stage III non–small cell lung cancer: TORG1937 (DATE study). J Clin Oncol. 2022;40((16_suppl)):8536.
Shaverdian N, Beattie J, Thor M, Offin M, Shepherd AF, Gelblum DY, et al. Safety of thoracic radiotherapy in patients with prior immune-related adverse events from immune checkpoint inhibitors. Ann Oncol. 2020;31(12):1719–24.
Bryant AK, Sankar K, Zhao L, Strohbehn GW, Elliott D, Moghanaki D, et al. De-escalating adjuvant durvalumab treatment duration in stage III non-small cell lung cancer. Eur J Cancer. 2022;171:55–63.
Moding EJ, Nabet BY, Alizadeh AA, Diehn M. Detecting Liquid Remnants of Solid Tumors: Circulating Tumor DNA Minimal Residual Disease. Cancer Discov. 2021;11(12):2968–86.
Thompson JC, Carpenter EL, Silva BA, Rosenstein J, Chien AL, Quinn K, et al. Serial Monitoring of Circulating Tumor DNA by Next-Generation Gene Sequencing as a Biomarker of Response and Survival in Patients With Advanced NSCLC Receiving Pembrolizumab-Based Therapy. JCO Precis Oncol. 2021;5:PO.20.00321.
Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017;7(12):1394–403.
Yang Y, Zhang T, Wang J, Wang J, Xu Y, Zhao X, et al. The clinical utility of dynamic ctDNA monitoring in inoperable localized NSCLC patients. Mol Cancer. 2022;21(1):117.
Jun S, Shukla N, Durm GA, Hui AB, Cao S, Kunder C, et al. Analysis of circulating tumor DNA in the phase 2 BTCRC LUN 16–081 trial of consolidation nivolumab with or without ipilimumab after chemoradiation in stage III non–small cell lung cancer. J Clin Oncol. 2022;40:8534.
Moding EJ, Liu Y, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB, et al. Circulating Tumor DNA Dynamics Predict Benefit from Consolidation Immunotherapy in Locally Advanced Non-Small Cell Lung Cancer. Nat Cancer. 2020;1(2):176–83.
Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321–8.
Wu L, Ke L, Zhang Z, Yu J, Meng X. Development of EGFR TKIs and Options to Manage Resistance of Third-Generation EGFR TKI Osimertinib: Conventional Ways and Immune Checkpoint Inhibitors. Front Oncol. 2020;10: 602762.
Aredo JV, Hellyer JA, Neal JW, Wakelee HA. Consolidation Durvalumab Should Not Be Administered to Patients With Stage III EGFR-Mutant NSCLC. J Thorac Oncol. 2021;16(12):1994–8.
Naidoo J, Antonia SJ, Wu YL, Cho BC, Thiyagarajah P, Mann H, et al. Durvalumab (durva) after chemoradiotherapy (CRT) in unresectable, stage III, EGFR mutation-positive (EGFRm) NSCLC: A post hoc subgroup analysis from PACIFIC. J Clin Oncol. 2022;40(16_suppl):8541.
Riudavets M, Auclin E, Mosteiro M, Dempsey N, Majem M, Lobefaro R, et al. Durvalumab consolidation in patients with unresectable stage III non-small cell lung cancer with driver genomic alterations. Eur J Cancer. 2022;167:142–8.
Aredo JV, Mambetsariev I, Hellyer JA, Amini A, Neal JW, Padda SK, et al. Durvalumab for Stage III EGFR-Mutated NSCLC After Definitive Chemoradiotherapy. J Thorac Oncol. 2021;16(6):1030–41.
Wang CC, Chiu LC, Ju JS, Lin YC, Fang YF, Yang CT, et al. Durvalumab as Consolidation Therapy in Post-Concurrent Chemoradiation (CCRT) in Unresectable Stage III Non-Small Cell Lung Cancer Patients: A Multicenter Observational Study. Vaccines (Basel). 2021;9(10):1122.
Hellyer JA, Aredo JV, Das M, Ramchandran K, Padda SK, Neal JW, et al. Role of Consolidation Durvalumab in Patients With EGFR- and HER2-Mutant Unresectable Stage III NSCLC. J Thorac Oncol. 2021;16(5):868–72.
Schmid S, Garcia M, Cheng S, Zhan L, Chotai S, Balaratnam K, et al. Treatment patterns and outcomes in early-stage ALK-rearranged non-small cell lung cancer. Lung Cancer. 2022;166:58–62.
Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal AN, Gadgeel SM, Girshman J, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019;30(5):839–44.
Passaro A, Leighl N, Blackhall F, Popat S, Kerr K, Ahn MJ, et al. ESMO expert consensus statements on the management of EGFR mutant non-small-cell lung cancer. Ann Oncol. 2022;33(5):466–87.
Shaverdian N, Offin M, Shepherd AF, Simone CB 2nd, Gelblum DY, Wu AJ, et al. The Impact of Durvalumab on Local-Regional Control in Stage III NSCLCs Treated With Chemoradiation and on KEAP1-NFE2L2-Mutant Tumors. J Thorac Oncol. 2021;16(8):1392–402.
Akamatsu H, Murakami H, Harada H, Shimizu J, Hayashi H, Daga H, et al. Gefitinib With Concurrent Thoracic Radiotherapy in Unresectable Locally Advanced NSCLC With EGFR Mutation; West Japan Oncology Group 6911L. J Thorac Oncol. 2021;16(10):1745–52.
Xing L, Wu G, Wang L, Li J, Wang J, Yuan Z, et al. Erlotinib Versus Etoposide/Cisplatin With Radiation Therapy in Unresectable Stage III Epidermal Growth Factor Receptor Mutation-Positive Non-Small Cell Lung Cancer: A Multicenter, Randomized, Open-Label, Phase 2 Trial. Int J Radiat Oncol Biol Phys. 2021;109(5):1349–58.
Lu S, Casarini I, Kato T, Cobo M, Ozguroglu M, Hodge R, et al. Osimertinib Maintenance After Definitive Chemoradiation in Patients With Unresectable EGFR Mutation Positive Stage III Non-small-cell Lung Cancer: LAURA Trial in Progress. Clin Lung Cancer. 2021;22(4):371–5.
Roche HL. A Phase I-III, Multicenter Study Evaluating the Efficacy and Safety of Multiple Therapies in Cohorts of Patients Selected According to Biomarker Status, With Locally Advanced, Unresectable, Stage III Non-Small Cell Lung Cancer. clinicaltrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT05170204. Accessed 15 Oct 2022.
Spigel D, de Marinis F, Giaccone G, Reinmuth N, Vergnenegre A, Barrios CH, et al. LBA78 - IMpower110: Interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1–selected NSCLC. Ann Oncol. 2019;30: v915.
Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front Immunol. 2022;13: 823618.
Mahoney KM, Ross-Macdonald P, Yuan L, Song L, Veras E, Wind-Rotolo M, et al. Soluble PD-L1 as an early marker of progressive disease on nivolumab. J Immunother Cancer. 2022;10(2):e003527.
Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30(1):44–56.
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65.
Prasad V, Addeo A. The FDA approval of pembrolizumab for patients with TMB >10 mut/Mb: was it a wise decision? No Ann Oncol. 2020;31(9):1112–4.
Merino DM, McShane LM, Fabrizio D, Funari V, Chen SJ, White JR, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. 2020;8(1):e000147.
McGrail DJ, Pilie PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661–72.
Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol. 2018;36(7):633–41.
Chae YK, Pan A, Davis AA, Raparia K, Mohindra NA, Matsangou M, et al. Biomarkers for PD-1/PD-L1 Blockade Therapy in Non-Small-cell Lung Cancer: Is PD-L1 Expression a Good Marker for Patient Selection? Clin Lung Cancer. 2016;17(5):350–61.
Becht E, Giraldo NA, Dieu-Nosjean MC, Sautès-Fridman C, Fridman WH. Cancer immune contexture and immunotherapy. Curr Opin Immunol. 2016;39:7–13.
Miller BC, Sen DR, AL Abosy R, Bi K, Virkud YV, Lafleur MW, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20(3):326–36.
Gataa I, Mezquita L, Rossoni C, Auclin E, Kossai M, Aboubakar F, et al. Tumour-infiltrating lymphocyte density is associated with favourable outcome in patients with advanced non-small cell lung cancer treated with immunotherapy. Eur J Cancer. 2021;145:221–9.
Yoneda K, Kuwata T, Kanayama M, Mori M, Kawanami T, Yatera K, et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer. 2019;121(6):490–6.
Yan X, Zhang S, Deng Y, Wang P, Hou Q, Xu H. Prognostic Factors for Checkpoint Inhibitor Based Immunotherapy: An Update With New Evidences. Front Pharmacol. 2018;9:1050.
Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–40.
Scott JG, Berglund A, Schell MJ, Mihaylov I, Fulp WJ, Yue B, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202–11.
Ilié M, Szafer-Glusman E, Hofman V, Chamorey E, Lalvée S, Selva E, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018;29(1):193–9.
Togo M, Yokobori T, Shimizu K, Handa T, Kaira K, Sano T, et al. Diagnostic value of (18)F-FDG-PET to predict the tumour immune status defined by tumoural PD-L1 and CD8(+)tumour-infiltrating lymphocytes in oral squamous cell carcinoma. Br J Cancer. 2020;122(11):1686–94.
Kaira K, Higuchi T, Naruse I, Arisaka Y, Tokue A, Altan B, et al. Metabolic activity by (18)F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45(1):56–66.
Wu L, Liu J, Wang S, Bai M, Wu M, Gao Z, et al. Negative Correlation Between (18)F-RGD Uptake via PET and Tumoral PD-L1 Expression in Non-Small Cell Lung Cancer. Front Endocrinol (Lausanne). 2022;13: 913631.
Jazieh K, Khorrami M, Saad A, Gad M, Gupta A, Patil P, et al. Novel imaging biomarkers predict outcomes in stage III unresectable non-small cell lung cancer treated with chemoradiation and durvalumab. J Immunother Cancer. 2022;10(3):e003778.
Vanguri RS, Luo J, Aukerman AT, Egger JV, Fong CJ, Horvat N, et al. Multimodal integration of radiology, pathology and genomics for prediction of response to PD-(L)1 blockade in patients with non-small cell lung cancer. Nat Cancer. 2022;3(10):1151–64.
Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44(5):989–1004.
Aggarwal C, Prawira A, Antonia S, Rahma O, Tolcher A, Cohen RB, et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer. 2022;10(4):e004424.
Zhu L, Yu X, Wang L, Liu J, Qu Z, Zhang H, et al. Angiogenesis and immune checkpoint dual blockade in combination with radiotherapy for treatment of solid cancers: opportunities and challenges. Oncogenesis. 2021;10(7):47.
Ma L, Gai J, Qiao P, Li Y, Li X, Zhu M, et al. A novel bispecific nanobody with PD-L1/TIGIT dual immune checkpoint blockade. Biochem Biophys Res Commun. 2020;531(2):144–51.
Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401.
Song G, Cheng L, Chao Y, Yang K, Liu Z. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Adv Mater. 2017;29(32):1700996.
Fan W, Tang W, Lau J, Shen Z, Xie J, Shi J, et al. Breaking the Depth Dependence by Nanotechnology-Enhanced X-Ray-Excited Deep Cancer Theranostics. Adv Mater. 2019;31(12): e1806381.
Li H, Wang M, Huang B, Zhu SW, Zhou JJ, Chen DR, et al. Theranostic near-infrared-IIb emitting nanoprobes for promoting immunogenic radiotherapy and abscopal effects against cancer metastasis. Nat Commun. 2021;12(1):7149.
Ni K, Xu Z, Culbert A, Luo T, Guo N, Yang K, et al. Synergistic checkpoint-blockade and radiotherapy-radiodynamic therapy via an immunomodulatory nanoscale metal-organic framework. Nat Biomed Eng. 2022;6(2):144–56.
Lu K, He C, Guo N, Chan C, Ni K, Lan G, et al. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018;2(8):600–10.
Acknowledgements
We thank all individuals who participated in this research.
Funding
The study was supported by the Three Years Action to Accelerate the Development of Traditional Chinese Medicine Plan (ZY[2018–2020]-FWTX-3004), Start-up Fund for Talent Introduction of Shanghai Pulmonary Hospital (grant No. 20180101), 2021 Development Fund of Discipline-Department of Radiotherapy, 2022 Development Fund of Discipline-Department of Radiotherapy, 2023 Development Fund of Discipline-Department of Radiotherapy, A randomized, open, controlled, multicenter Phase II study of continuing concurrent chemoradiotherapy after induction carelizumab combined with chemotherapy followed by carelizumab maintenance therapy versus standard chemoradiotherapy in locally advanced small cell lung cancer (FKLY20006), the foundation of National Natural Science Foundation of China (81627901, 81972863 and 82030082) and the Academic Promotion Program of Shandong First Medical University (2019ZL002).
Author information
Authors and Affiliations
Contributions
Leilei Wu: Conceptualization, Methodology, Writing-Original drafe preparation; Zhenshan Zhang: Data curation, Writing-Original draft preparation, Software, Validation. Menglin Bai: Investigation, Visualization, Writing-Review & Editing; Yujie Yan: Visualization, Writing-Review & Editing; Jinming Yu: Supervision, Funding acquisition; Yaping Xu: Funding acquisition, Project administration. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Shanghai Pulmonary Hospital. Written informed consent was taken from all the patients.
Competing interests
All authors have no conflicts of interest to declare. The figures were created with the help of BioRender.com.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, L., Zhang, Z., Bai, M. et al. Radiation combined with immune checkpoint inhibitors for unresectable locally advanced non-small cell lung cancer: synergistic mechanisms, current state, challenges, and orientations. Cell Commun Signal 21, 119 (2023). https://doi.org/10.1186/s12964-023-01139-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-023-01139-8