Abstract
Background
Although treatment and control of diabetes can prevent complications and reduce morbidity, few data sources exist at the state level for surveillance of diabetes comorbidities and control. Surveys and electronic health records (EHRs) offer different strengths and weaknesses for surveillance of diabetes and major metabolic comorbidities. Data from self-report surveys suffer from cognitive and recall biases, and generally cannot be used for surveillance of undiagnosed cases. EHR data are becoming more readily available, but pose particular challenges for population estimation since patients are not randomly selected, not everyone has the relevant biomarker measurements, and those included tend to cluster geographically.
Methods
We analyzed data from the National Health and Nutritional Examination Survey, the Health and Retirement Study, and EHR data from the DARTNet Institute to create state-level adjusted estimates of the prevalence and control of diabetes, and the prevalence and control of hypertension and high cholesterol in the diabetes population, age 50 and over for five states: Alabama, California, Florida, Louisiana, and Massachusetts.
Results
The estimates from the two surveys generally aligned well. The EHR data were consistent with the surveys for many measures, but yielded consistently lower estimates of undiagnosed diabetes prevalence, and identified somewhat fewer comorbidities in most states.
Conclusions
Despite these limitations, EHRs may be a promising source for diabetes surveillance and assessment of control as the datasets are large and created during the routine delivery of health care.
Trial Registration: Not applicable.
Similar content being viewed by others
Background
Control of Hemoglobin A1c, Blood Pressure, and Cholesterol (ABCs) is essential for preventing micro- and macro-vascular diabetes-related complications. The lack of state-level estimates of the prevalence and control of diabetes and major metabolic comorbidities, such as hypertension and high cholesterol, limits the ability of public health agencies to monitor diabetes prevention and management at the state level [1]. Data from electronic health records (EHRs), along with novel uses of survey data, may be able to fill gaps in the nation’s diabetes surveillance system [2,3,4]. However, more work is needed to validate estimates from these non-traditional methods and data sources. Data from EHRs are challenging to analyze for population-based studies because they are generated from the routine delivery of clinical care. Therefore, they cover partial, sometimes non-representative subpopulations, do not always include the same measurements on all individuals, and include limited variables for case finding and adjustment [5].
We could find no previous studies that examined metabolic comorbidities and control within the diabetes population at the state level. Researchers in New York City validated EHR-based estimates in the general adult population relative to population-based survey and chart review data. The EHR-based estimates performed well for diabetes and hypertension, although the authors recommended using EHR data for high cholesterol with caution [6, 7]. Several studies examined EHR data from participating health systems to describe metabolic risk factors among diabetes patients, but they do not address the representativeness of this group in the broader population [8, 9]. Other analysts looked at control of the ABCs within the diabetes population at the national level, but did not examine state-level data [10,11,12,13]. In follow-up work, the methods developed in this paper were applied to NHANES data to create estimates of ABC control for each state in the USA. [14]
The purpose of this analysis is to compare EHR-based and survey-based estimates of the prevalence of comorbid hypertension and high cholesterol, and ABC control, in the diabetes population at the state level.
Methods
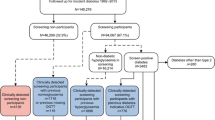
We compared EHR data from the DARTNet Institute, a collaboration of practice-based research networks, with estimates derived from two surveys: (1) the National Health and Nutrition Examination Survey (NHANES), used to create synthetic state-level estimates by adjusting national estimates to State demographic population counts, and (2) the Health and Retirement Study (HRS), used to create synthetic state-level estimates based on within-state or neighboring-state data. We analyzed five states representing diverse geography and populations (Alabama, California, Florida, Louisiana, and Massachusetts). We focused on those age 50 and over who had an office visit in the past year because they are included in each dataset.
Data sources
DARTNet EHR data 2012–2013
The DARTNet Institute is a national collaboration of practice-based research networks that has built a collection of data from electronic health records, claims, and patient-reported outcomes. Eight participating networks contributed diagnosis and prescription information, patient vitals, and ABC measurements. We used ABC results from 2012 and 2013 for the control analysis to better match the survey timeframes, and we used diagnosis, biomarker, and prescription information from 2010 to 2013 for case finding. The EHR data did not include fasting plasma glucose (FPG) values.
NHANES 2011–2012
NHANES is a non-institutionalized population-based survey that includes ABC measurements on all participants, as well as variables useful for adjustment such as age, sex, race/ethnicity, insurance type, education, marital status, income, healthcare visit in the past 12 months, and general health status. We used the public-use file, which does not include a state residence variable. NHANES is not designed for state-level estimation, and the dataset may not contain respondents from each state. By design, the NHANES data included A1c values for the whole sample and FPG values for a random half of the sample. We used a previously developed imputation model for diabetes status that performed well and accounted for non-measured FPG values [15].
HRS 2010–2012
HRS is a longitudinal panel study that surveys a representative sample of approximately 20,000 non-institutionalized Americans over the age of 50 every 2 years. HRS is not designed for direct state-level estimation. In addition to self-reported data on diabetes and other chronic diseases, HRS includes ABC measurements on all participants, as well as many variables for adjustment. HRS does not include FPG values, but it had similar covariates to NHANES so we applied the diabetes status imputation model developed using NHANES data to account for non-measured FPG [15].
Dataset preparation
Depending upon the data source, the information available in addition to the ABC values includes survey responses, diagnosis codes, prescriptions written, and other variables useful for modeling and adjustment. Prior to analysis, we harmonized variables across datasets so similar concepts were coded as consistently as possible.
For the analysis of the NHANES data, we created synthetic state-level estimates. We adjusted NHANES national weights to reflect the demographic characteristics of each target state by raking and propensity modeling [16,17,18,19,20,21]. This resulted in person-level adjusted weights that match the state-level distributions of selected demographic variables from the American Community Survey, and health status from the Behavioral Risk Factor Surveillance System. For HRS, we were able to create estimates for the two largest states (California and Florida) based on respondents from those states alone. For the other three states, we created synthetic state estimates using respondents in the census division in a manner similar to the NHANES adjustment. Additional details of the adjustments for NHANES and HRS are provided elsewhere [15].
The EHR data were originally in six datasets containing multiple records per person for visits, diagnoses, laboratory tests, prescription drugs ordered, providers, and demographic characteristics. We merged records from these files to create an analytic file with a single comprehensive record for each individual that included variables representing disease status, ABC results, prescription history, and age and sex. The EHR data preparation process is illustrated in Additional file 1: Online Appendix Figure A1.
The EHR dataset includes clinic ZIP code, allowing us to assess the geographic distribution of clinic visits. In each state, visits were highly clustered by county. For example, Fig. 1 illustrates the geographic clustering in Florida. There are clusters in several urban areas, and relatively few visits in other areas. We designed a two-step weighting strategy to improve the geographic representation within each state. First, cases within each county were weighted to county-level American Community Survey totals of age and sex cross-tabulations. This ensures that each county in the dataset receives a total weight proportional to its total population. Second, the weighted totals of age by sex groups for the counties represented in the data were raked to the corresponding state totals for those with an office visit in the past year.
Significance testing and precision of estimates
We quantified the precision of the NHANES and HRS estimates with confidence intervals (CIs) calculated using standard errors that take into account the sample design, and used a t test to calculate whether differences in estimates across the datasets were statistically significant. For the EHR data, we calculated CIs and t tests that incorporate the geographic weights described above.
Case and disease control definitions
We developed case definitions for diabetes, hypertension, and high cholesterol, and for control of the ABCs, that could be applied across the data sources. Additional details can be found in Additional file 2: Online Appendix Tables A1, A2, and A3.
Diabetes
We classified individuals into three categories: diagnosed diabetes, undiagnosed diabetes, or no diabetes. Pregnant women were excluded from all analyses. For NHANES and HRS, diagnosed diabetes was based on self-report of ever being diagnosed by a healthcare provider, excluding gestational diabetes. Patients in the EHR data with at least one ABC result in 2012–2013 were assigned a diabetes status using diagnosis codes and drug prescriptions recorded in 2013 or earlier [22,23,24,25]. We used the Healthcare Effectiveness Data and Information Set Comprehensive Diabetes Care pharmacy list as our source for anti-diabetic prescription drugs. Individuals in each dataset who were not diagnosed were classified as undiagnosed cases if they had A1c ≥ 6.5% or FPG ≥ 126 mg/dl in any laboratory result [26].
Hypertension
We required at least one hypertension diagnosis code, two or more elevated systolic or diastolic blood pressure readings (systolic ≥ 140 mmHg or diastolic ≥ 90 mmHg), or a prescription for blood pressure medication to identify cases of hypertension [27, 28]. We did not distinguish between diagnosed and undiagnosed hypertension.
High cholesterol
We required at least one high cholesterol diagnosis code, a self-report or documentation of drugs to lower cholesterol, or a laboratory result of non-HDL-C ≥ 130 mg/dL to identify cases of high cholesterol. We used non-HDL-C, calculated as total cholesterol minus HDL-C, as the lipid measure since the HRS dataset does not include low-density lipoprotein cholesterol (LDL-C) values, and only 50% of those in the NHANES dataset had an LDL-C value by design [29]. Evidence suggests that non-HDL-C is a good marker of risk in both primary and secondary prevention studies of atherosclerotic cardiovascular disease [30], and the cut point of non-HDL-C ≥ 130 mg/dL to identify high cholesterol has been established in the literature [31]. We did not distinguish between diagnosed and undiagnosed high cholesterol.
ABC control
For this study, we used an A1c cut point of < 9% to represent “not-poorly controlled” diabetes (1, [32]. This target is less stringent than the American Diabetes Association’s Standards of Medical Care’s target of A1c < 7.0% for most people with diabetes [26]. Less stringent A1c goals may be appropriate for patients with a history of severe hypoglycemia, the very elderly, extensive comorbid conditions, or long-standing diabetes [33]. Consistent with National Committee for Quality Assurance’s 2016 criteria for the Comprehensive Diabetes Care measure and the ADA’s guidelines, we defined systolic blood pressure of < 140 and diastolic blood pressure < 90 as “adequate control” [22, 34, 35]. While cholesterol control targets for patients with diabetes may be individualized based on personal risk, we used a control target of non-HDL-C < 130 mg/dL.
Results
As shown in Table 1, the diagnosed diabetes prevalence estimates were higher in the HRS-adjusted data than in the NHANES-adjusted data in four out of five states, significantly so in California and Florida (p < 0.01). There were no significant differences between the surveys for undiagnosed diabetes. The EHR prevalence estimates for diagnosed diabetes were more highly variable, being significantly higher than the surveys in some states and significantly lower in others. The EHR-based estimates for undiagnosed diabetes were consistently low across all of the states.
As shown in Table 2, the prevalence of major metabolic comorbidities within the diabetes population is high. The adjusted NHANES and HRS estimates are not significantly different except in Alabama, where the hypertension and “Both” condition estimates are significantly higher in HRS (p < 0.01). In most states, the EHR data show significantly fewer diabetes patients with one or both comorbidities. Massachusetts is a notable exception in that the EHR-based prevalence is significantly higher for both hypertension and high cholesterol. Figure 2 illustrates the variability across states in the prevalence of one or both comorbidities by data source.
Proportion of the diabetes population with hypertension and/or high cholesterol by data source and state among adults age 50-plus with an office visit in the past year. NHANES National Health and Nutrition Examination Survey, HRS Health and Retirement Study, EHR electronic health record. All NHANES estimates are synthetically derived, based on adjusted data
Nearly all of the individuals in the NHANES or HRS survey samples had all three ABCs measured. In the EHR data, only 52% of the patients with diagnosed diabetes had A1c measurements, 96% had blood pressure measurements, 46% had cholesterol measurements, and 41% had all three measurements. The EHR-based control percentages shown in Table 3 are based on only those patients who had the relevant test(s). In Alabama, the EHR estimates for control of all three ABCs are lower, significantly so for cholesterol and all three indicators (p < 0.01). In addition, the EHR estimates for blood pressure control are significantly lower in Florida and Louisiana. In California, the HRS rates for blood pressure and cholesterol control are significantly low, driving a lower rate for control of all three ABCs. In addition, the A1c control rate is significantly higher in HRS in several states. Otherwise, there is no consistent pattern across the other data sources and states, with most of the differences not significant.
To facilitate comparisons across datasets, we analyzed those aged 50-plus who had contact with the healthcare system in the past year, the subpopulation represented in all three datasets. For completeness, we also looked at pair-wise comparisons of the datasets for larger subpopulations. Specifically, we compared adjusted EHR and NHANES results for those age 18-plus and not pregnant who had healthcare contact in the past 12 months, and we compared adjusted HRS and NHANES results for those age 50-plus regardless of whether they had healthcare contact. The patterns of prevalence and control were similar in those analyses (data not shown).
Discussion
Results across data sources
We tested methods for improved diabetes surveillance by exploring three novel data sources for state-level estimation of diabetes comorbidities and ABC control. We did not expect the prevalence and control estimates to align completely, even after adjustment. We cannot reach conclusions about which dataset is most accurate; however, we can describe the patterns across data sources, which illuminate their strengths and weaknesses. While almost every general statement about consistency across datasets has an exception in at least one of the states examined, the two surveys—NHANES and HRS—generally aligned well when using them to make state-adjusted synthetic estimates. The adjusted HRS estimates may be more accurate as the larger HRS sample allowed for the analysis of respondent subsets with closer geographic representation to the target states, especially in California and Florida which, in this analysis, did not involve adjustments to any out-of-state data.
Although one prior study documents good performance of EHR data for estimating diabetes prevalence at the local level [7], we found several differences between the EHR estimates and those derived from the surveys; notably that the EHR-based prevalence estimates were consistently lower for undiagnosed diabetes, significantly so in most states. A1c results were not present for 85% of the EHR population age 50 and older, likely because there was no clinical reason to order the test. Some of the untested individuals likely have undiagnosed diabetes, cases that cannot be identified using EHR data, a conclusion that would hold even if other types of laboratory tests were considered. For diagnosed diabetes, the EHR data produced more variable prevalence estimates than the surveys. Because the EHR data are not population-based and are geographically clustered within each state, it is possible that this variability is more influenced by local patterns in healthcare utilization, diagnostic, or coding practices than the survey data.
EHR-based prevalence estimates for hypertension and high cholesterol within the diabetes population were generally below the estimates from the surveys, except in Massachusetts. The lower EHR comorbidity rates in most states may be due to undercounting of undiagnosed cases, as with diabetes. It is possible that individuals in Massachusetts, a state with near full health insurance coverage and citizens who seek healthcare more often [36], are more likely to have chronic conditions diagnosed. For ABC control, the EHR-based rates were again variable, although there were fewer significant differences than in the prevalence analyses. In all three datasets, rates of A1c control were almost always highest, followed by blood pressure control, and non-HDL-C control.
Implications for surveillance
EHRs are a promising data source for diabetes surveillance as the datasets are large and created at low cost during the routine delivery of health care. However, any EHR-based data source represents only those who sought care in that provider network. The geographic coverage of the data tends to be clustered in certain localities, and there are limited covariates available for adjustments. Further, clinician decisions to administer the tests needed to assess ABC control, or detect undiagnosed cases of chronic diseases, are non-random. In addition, it is possible that some patients were treated at multiple practices and are double counted. All of these factors introduce the potential for bias in EHR-based surveillance estimates, and are likely responsible for the observed variability in the EHR estimates relative to survey-based estimates. Propensity modeling or other approaches for handling missing data may reduce this bias [4, 37, 38]; however, the optimal adjustment for any particular measure A, B, or C, may not be optimal for the other measures since different subgroups of patients are measured for each indicator. In addition, it is difficult to quantify the uncertainty in EHR-based estimates when the goal is population surveillance.
We found relatively high consistency in the prevalence of diabetes, hypertension, and high cholesterol among those 50 years and older between the NHANES and HRS surveys. This was reassuring since they use similar case ascertainment and data collection methodologies. These surveys did not have state-based samples among our five states for our study years, although NHANES has produced a California file that aggregates and reweights data from four NHANES cycles (2007–2014). We did not analyze this dataset as it did not match the time period of this analysis [39]. The lack of state-based samples necessitated the use of statistical adjustments to create state-level estimates. In previous studies, synthetic adjustment of NHANES data noticeably changed state estimates [15], and improved accuracy relative to a gold standard [4]. A description of alternative methods that might be used can be found in Rao and Molina [40].
Conclusions
EHR data availability for research and epidemiology, and methods for analyzing it, are likely to improve over time as the use of these systems expands. Inclusion of key covariates such as race would allow for reductions in their biases. There are few sources of EHR data available for research outside of the health systems that generate the data. The DARTNet Institute, which compiles data from numerous providers and makes it available for research, is one notable exception. As the use of EHRs continues to expand, organizations that compile and standardize the data for research will be integral to their use for state-level diabetes surveillance. While our analysis is limited to five states, it suggests areas for future research that could enhance national surveillance efforts and provide state-level estimates of measures obtained primarily in clinical or laboratory settings.
Availability of data and materials
NHANES data used in the study are publically available at https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2011. HRS and DARTNet EHR data are available from the Health and Retirement Study and DARTNet Institute respectively. Restrictions apply to the use of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the licensors.
Abbreviations
- ABC:
-
Hemoglobin A1c
- CI:
-
Confidence interval
- EHR:
-
Electronic health record
- FPG:
-
Fasting plasma glucose
- HDL-C:
-
High-density lipoprotein cholesterol
- HRS:
-
Health and Retirement Study
- LDL-C:
-
Low-density lipoprotein cholesterol
- NHANES:
-
National Health and Nutrition Examination Survey
References
Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. [Published erratum in: N Engl J Med 2013;369(6):587.]. N Engl J Med 2013;368(17):1613–24.
Birkhead GS, Klompas M, Shah NR. Uses of electronic health records for public health surveillance to advance public health. Annu Rev Public Health. 2015;36(1):345–59.
Nichols GA, Desai J, Elston Lafata J, Lawrence JM, O’Connor PJ, Pathak RD, et al. Construction of a multisite DataLink using electronic health records for the identification, surveillance, prevention, and management of diabetes mellitus: the SUPREME-DM project. Prev Chronic Dis. 2012;9:E110.
Marker DA, et al. 2018. State-level Estimation of Diabetes and Prediabetes Prevalence: Combining National and Local Survey Data and Clinical Data. Statistics in Medicine. In press.
Casey JA, Schwartz BS, Stewart WF, Adler NE. Using electronic health records for population health research: a review of methods and applications. Annu Rev Public Health. 2016;37:61–81.
Perlman SE, et al. Innovations in population health surveillance: using electronic health records for chronic disease surveillance. Am J Public Health. 2017;2017:e1-5. https://doi.org/10.2105/AJPH.2017.303813.
Thorpe, L.E., et.a.l 2016. Monitoring Prevalence, Treatment, and Control of Metabolic Conditions in New York City Adults Using 2013 Primary Care Electronic Health Records: A Surveillance Validation Study. eGEMs. Vol. 4: Iss. 1, Article 28.
Lafata JE, et al. Medication adherence does not explain black-white differences in cardiometabolic risk factor control among insured patients with diabetes. J Gen Intern Med. 2015;31(2):188–95.
Shah AD, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–13.
Fang M, Want D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. Adults, 1999–2018. NEJM. 2021;384(23):2219–28.
Saaddine JB, et al. Improvements in diabetes processes of care and intermediate outcomes: United State, 1988–2002. Ann Intern Med. 2006;144:465–74.
Zhang X, et al. Access to health care and control of ABCs of diabetes. Diabetes Care. 2012;35(7):1566–71.
Casagrande SS, et al. The prevalence of meeting A1c, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–9.
Chen Y, Rolka D, Xie H, Saydah S. Imputed state-level prevalence of achieving goals to prevent complications of diabetes in adults with self-reported diabetes—United States, 2017–2018. MMWR. 2020;69(45):1665.
Mardon R, Marker D, Nooney J, Campione J, Jenkins F, Johnson M, et al. Novel methods and data sources for surveillance of state-level diabetes and prediabetes prevalence. Prev Chronic Dis. 2017;14: 160572.
Battaglia, M.P.; Hoaglin, D.C.; Frankel, M.R. Practical Considerations in Raking Survey Data. Survey Practice, [S.l.], v. 2, n. 5, Aug. 2013. ISSN 2168-0094.
Kalton, G. 1983. Compensating for Missing Survey Data. Survey Research Center, Institute for Social Research, University of Michigan.
DuGoff E, Schuler M, Stuart E. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res J. 2014;49(1):284–303.
Duncan KB, Stasny EA. Using propensity scores to control coverage bias in telephone surveys. Surv Methodol. 2001;27:121–30.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
National Center for Health Statistics: Synthetic State Estimates of Disability. PHS Pub. No. 1759. Public Health Services, Washington. U.S. government Printing office, 1968.
Klompas M, Eggleston E, et al. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care. 2013;36(914–921):2013.
National Committee for Quality Assurance. The HEDIS 2016 Criteria for the Comprehensive Diabetes Care. NCQA HEDIS 2016 Technical Specifications, Volume 2.
Williamson, T., et al. CPCSSN Disease Definitions: Canadian Primary Care Sentinel Surveillance Network (CPCSSN). June 15, 2014. http://cpcssn.ca/research-resources/case-definitions.
Peng M, Chen G, Kaplan GG, Lix LM, Drummond N, Lucyk K, Garies S, Lowerison M, Weibe S, Quan H. Methods of defining hypertension in electronic medical records: validation against national survey data. J Public Health (Oxf). 2016;38(3):e392–9 (Epub 2015 Nov 6).
American Diabetes Association. Standards of Medical Care in Diabetes—2017. Diabetes Care. Volume 40, Supplement 1, January 2017
Wang J, Geiss L, Cheng Y, Imperatore G, Saydah S, James C, Gregg E. Long-term and recent progress in blood pressure levels among U.S. adults with diagnosed diabetes, 1988–2009. Diabetes Care. 2011;34(7):1579–81.
Quan H, et al. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54:1423–8.
See https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/TRIGLY_H.htm, accessed March 15, 2018.
The Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000.
Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr. ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68:92–125.
Healthy People 2020. Accessed June 26, 2017. https://www.healthypeople.gov/2020/topics-objectives/topic/diabetes/objectives
American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities. Diabetes Care. 2017;40(Suppl. 1):S25–32.
American Diabetes Association. Cardiovascular disease and risk management. Sec. 9. In: Standards of Medical Care in Diabetes - 2017. Diabetes Care 2017;40(Suppl. 1):S75–S87.
Lipman ML, Schiffrin EL. What is the ideal blood pressure goal for patients with diabetes mellitus and nephropathy? Curr Cardiol Rep. 2012;14(6):651–9.
Miller S. The effect of the Massachusetts reform on health care utilization. Inquiry. 2012;49:317–26.
Haneuse S, Daniels M. A general framework for considering selection bias in EHR-based studies: what data are observed and why? eGEMs. 2016;4(1):1203.
Goldstein, B.A., Bhavsar, N.A., Phelan, M., and Pencina M.J. Controlling for Informed Presence bias due to the Number of Health Encounters in an Electronic Health Record. 2016. Am J. of Epidemiology. Advanced Access, Nov 16.
See https://wwwn.cdc.gov/Nchs/Nhanes/limited_access/CDEMO_EH.htm for more information, accessed March 15, 2018.
Rao JNK, Molina I. Small area estimation. 2nd ed. Hoboken: Wiley; 2015.
Acknowledgements
We thank the Technical and Stakeholder Workgroup, representing state-level chronic disease and epidemiology programs, for useful conversations and feedback on this work.
Funding
This work was funded through CDC contract 2002014F61238. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The manuscript was reviewed by CDC and has been cleared for submission.
Author information
Authors and Affiliations
Contributions
All of the authors made substantial contributions to the conception and design of the study. The Westat authors RM, JC, JN, LM, MJ, DM, and FJ acquired and analyzed the data. All authors interpreted the results. RM, JC, and JN took the lead in drafting and revising the article, and all authors reviewed it critically and provided edits and comments.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work was reviewed by the Westat IRB and was determined not to involve human subjects. This paper does not contain any individual person’s data in any form.
Consent for publication
All authors give approval for this manuscript to be published.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure A1.
EHR Data File Preparation Workflow. *Required 2 blood pressure readings in range to quality as a case of hypertension
Additional file 2: Table A1.
Diabetes Case Definition Details for Each Data Source. Table A2. Hypertension Case Definition Details for Each Data Source. Table A3. High Cholesterol Case Definition Details for Each Data Source.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mardon, R., Campione, J., Nooney, J. et al. State-level metabolic comorbidity prevalence and control among adults age 50-plus with diabetes: estimates from electronic health records and survey data in five states. Popul Health Metrics 20, 22 (2022). https://doi.org/10.1186/s12963-022-00298-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12963-022-00298-z